Abstract
Fatigue loading is a primary cause of tendon degeneration, which is characterized by the disruption of collagen fibers and the appearance of abnormal (e.g., cartilaginous, fatty, calcified) tissue deposits. The formation of such abnormal deposits, which further weakens the tissue, suggests that resident tendon cells acquire an aberrant phenotype in response to fatigue damage and the resulting altered mechanical microenvironment. While fatigue loading produces clear changes in collagen organization and molecular denaturation, no data exist regarding the effect of fatigue on the local tissue mechanical properties. Therefore, the objective of this study was to identify changes in the local tissue stiffness of tendons after fatigue loading. We hypothesized that fatigue damage would reduce local tissue stiffness, particularly in areas with significant structural damage (e.g., collagen denaturation). We tested this hypothesis by identifying regions of local fatigue damage (i.e., collagen fiber kinking and molecular denaturation) via histologic imaging and by measuring the local tissue modulus within these regions via atomic force microscopy (AFM). Counter to our initial hypothesis, we found no change in the local tissue modulus as a consequence of fatigue loading, despite widespread fiber kinking and collagen denaturation. These data suggest that changes in topography and tissue structure – but not local tissue mechanics – initiate the early changes in tendon cell phenotype as a consequence of fatigue loading that ultimately culminates in tendon degeneration.
Keywords: tendon, fatigue, microscale mechanics, atomic force microscopy, second harmonic generation imaging
INTRODUCTION
Tendon degeneration and injury account for 20–30% of all musculoskeletal disorders and result in impaired function and persistent pain (Badley and Tennant, 1993; Fleming et al., 2005). A primary cause of tendon degeneration is overuse (i.e., fatigue loading), which produces repeated microscale damage of the load-bearing collagen fibrils (Cook and Purdam, 2009; Kujala et al., 2005; Gibbon et al., 1999; Soslowsky et al., 2000; Fung et al., 2010; Nakama et al., 2005). Coincident with (and possibly due to) this direct mechanical damage, tendons accumulate atypical tissue components (e.g., cartilaginous, fat, and calcium deposits) during degeneration, which further weakens the tissue and drives disease progression (Arya and Kulig, 2010; Kannus and Józsa, 1991; Aström and Rausing, 1995; Hashimoto et al., 2003; Archambault et al., 2007; Scott et al., 2008; Samiric et al., 2009; Corps et al., 2012). While it is generally believed that tenocytes produce these atypical matrix deposits during fatigue in response to the excessive loading and resultant local tissue damage, the specific biophysical cues that mediate this response are unknown. Uncovering the mechanisms that induce tenocytes to exacerbate tendinopathy via the production of atypical matrix deposits may inform new strategies that can prevent or reverse tendon degeneration.
Experimental studies at the tissue and cellular level demonstrate that tenocytes are mechanosensitive (Lavagnino et al., 2015; Wang et al., 2017). For example, unloading tendons leads to deterioration in tissue structure and mechanics mediated by an increase in protease secretion (Hannafin et al., 1995; Arnoczky et al., 2004, 2007b; Abreu et al., 2008; Leigh et al., 2008). These findings suggest that there is a minimal loading level necessary to maintain tendon homeostasis (Lavagnino and Arnoczky, 2005; Arnoczky et al., 2008; Wang et al., 2015). Additionally, tendon overuse or fatigue loading produces structural and mechanical deterioration, atypical matrix components, and increased protease activity (Shepherd and Screen, 2013; Fung et al., 2010; Archambault et al., 2007; Attia et al., 2012; Sun et al., 2008; Thorpe et al., 2014; Nakama et al., 2006). However, it is unclear what changes in the mechanical microenvironment alter tenocyte behavior in response to fatigue loading. On one hand, tenocytes may respond to the history of excessive mechanical stimulation that occurs with repetitive loading. Indeed, when isolated tenocytes are exposed to elevated strains they produce atypical matrix components (Zhang and Wang, 2010, 2013), as well as inflammatory and pleiotropic cytokines (e.g., PGE2, IL-1β, TGF-β, BMP-2) (Wang et al., 2003; Tsuzaki et al., 2003; Jones et al., 2013; Rui et al., 2011) that may instigate further tendon degeneration (Khan et al., 2005; Zhang and Wang, 2014; K. Zhang et al., 2015; de Mos et al., 2009; Bell et al., 2013; Rui et al., 2013). Alternatively, it is possible that fatigue loading damages the pericellular matrix surrounding tenocytes, leading to microscale unloading rather than over-stimulation (Arnoczky et al., 2007a, 2008; Lavagnino et al., 2006; Hakimi et al., 2017; Maeda et al., 2011; Mehdizadeh et al., 2017). To identify the altered mechanical stimuli that drive tendon degeneration, the magnitude and time course of changes in the local mechanical microenvironment must be elucidated.
Currently, limited data exist regarding the specific changes in local tissue structure and mechanics that result from tendon fatigue damage. Clear changes in collagen organization (Fung et al., 2010; Sereysky et al., 2012; Herod and Veres, 2017) and molecular damage in the form of denaturation (Veres et al., 2014) have been identified. Surprisingly, these structural changes do not seem to alter tissue strains at the cellular level (Shepherd et al., 2014). However, no data exist regarding the effect of fatigue damage on the local tissue mechanical properties. The stiffness of the local mechanical microenvironment has dramatic effects on cell behavior, including morphology, motility, contractility, proliferation, survival, and progenitor cell differentiation (Engler et al., 2006; Hadjipanayi et al., 2009; Oakes et al., 2014; Pelham and Wang, 1997; Wang et al., 2000; Yeung et al., 2005). Therefore, it is possible that fatigue-induced changes in tendon stiffness at the cellular level alter mechanotransduction pathways in tenocytes or tendon progenitor/stem cells, resulting in their adoption of abnormal (i.e., non-tenogenic) phenotypes and production of atypical matrix deposits.
The objective of this study was to identify changes in the local tissue stiffness of tendons that had been subjected to fatigue loading. We hypothesized that fatigue damage would reduce local tissue stiffness, particularly in areas with significant structural damage (e.g., collagen denaturation). This hypothesis was tested by identifying regions of local fatigue damage (i.e., collagen fiber kinking and molecular denaturation) via histologic imaging and by measuring the local tissue modulus within these same regions via atomic force microscopy (AFM). In contrast to our hypothesis, we found no change in the local tissue modulus after fatigue loading, despite widespread fiber kinking and collagen denaturation. These data suggest that changes in topography and tissue structure – but not local tissue mechanics – initiate early changes in tendon cell phenotype as a consequence of fatigue loading that ultimately culminates in tendon degeneration.
MATERIALS AND METHODS
Mechanical Testing and Fatigue Loading
Thirty-five flexor carpi ulnaris tendons (FCU), including the pisiform and muscle (Fig. 1), were harvested from fresh-frozen 6 month-old male Sprague Dawley rats with approval from the Institutional Animal Care and Use Committee. Tendon cross-sectional area was determined using a non-contact laser transducer (micro-epsilon, optoNCDT1800-20). All muscle was stripped from each tendon via blunt scraping and sandpaper tabs were affixed to the tendon end with cyanoacrylate glue. Samples were placed in a bath of phosphate buffered saline (PBS) heated to 37°C with the pisiform and sandpaper tabs clamped in custom fixtures of a uniaxial tensile testing device (Instron, Model 5848). A preload of 2 mN was applied and the sample gauge length was measured. Six tendons were ramped to failure at a strain rate of 0.5%/s to determine the average ultimate tensile strength (UTS). Seven additional samples were then sinusoidally loaded between 1 and 20% of the average UTS at 1 Hz until failure with the grip-to-grip strain and applied load recorded at 100 Hz. Plots of the peak strain and secant modulus for each cycle exhibited a typical triphasic fatigue response (Fig. 2), with an initial rapid increase in peak cyclic strain and secant modulus (primary phase), a period of slowly increasing strains and reductions in stiffness (secondary phase), and finally rapid changes in strain and stiffness prior to failure (tertiary phase). The secondary phase was defined as the cycles in which the creep rate (change in peak strain per cycle) was within one order-of-magnitude of the median creep rate. Eleven additional samples were then fatigue loaded while the peak strain was monitored in real-time to determine the beginning of the secondary phase. Loading ceased when the peak strain increased 6% beyond this point, which represents approximately 70% of the total creep strain that occurred during the secondary phase (9.0 ± 2.9%). These eleven fatigue-loaded samples, as well as eleven fresh (unloaded) samples, were then cut to remove the pisiform and sandpaper tabs, embedded in OCT medium, and frozen for subsequent analysis.
Figure 1.
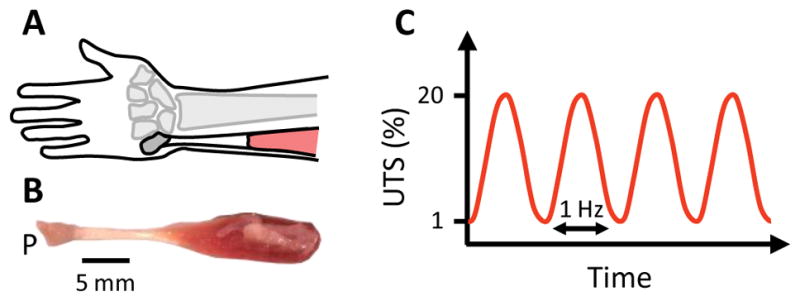
(A) Schematic of anatomical location of FCU. (B) Image of rat FCU tendon including adjacent pisiform (P) and muscle. Note: Anatomical locations of the FCU are identical between rats and humans. (C) Fatigue loading protocol.
Figure 2.
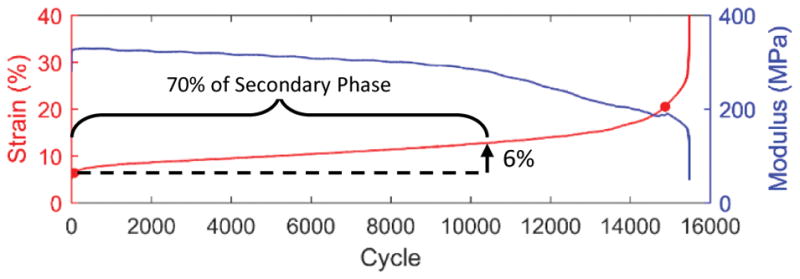
Change in peak strain and secant modulus during fatigue of representative sample loaded to failure. Loading of subsequent samples was terminated prior to failure at a creep strain of 6%, which represents approximately 70% of the secondary creep phase.
Cryosectioning and Staining
Samples were cut using standard techniques on a cryotome into 20 μm thick sections and placed on Superfrost Plus Gold slides (Fisher Scientific, 15-188-48). Sections were washed with PBS and then incubated overnight at 4°C with a fluorescein-labeled collagen hybridizing peptide (CHP) (Echelon, C-660F) that specifically binds to denatured collagen (Li et al., 2013), diluted 1:10 in PBS with protease inhibitors (Sigma Aldrich, P8340). Sections were then washed 3× with PBS prior to imaging and AFM.
Imaging
Full tile-scan images of single sections from eleven fatigue and fresh tendons were captured using a multiphoton microscope (Nikon, A1R MP+) to visualize CHP fluorescence as well as fibrillar collagen via forward-scatter second harmonic generation (SHG) detection. The SHG images were divided into 36×36 pixel (18×18 μm) subregions, and local collagen fiber alignment was measured via Fourier transform analysis (Fig. 3) (Sereysky et al., 2010). Damage in the form of fiber kinking was defined as a >30° difference in fiber angles between neighboring subregions.
Figure 3.
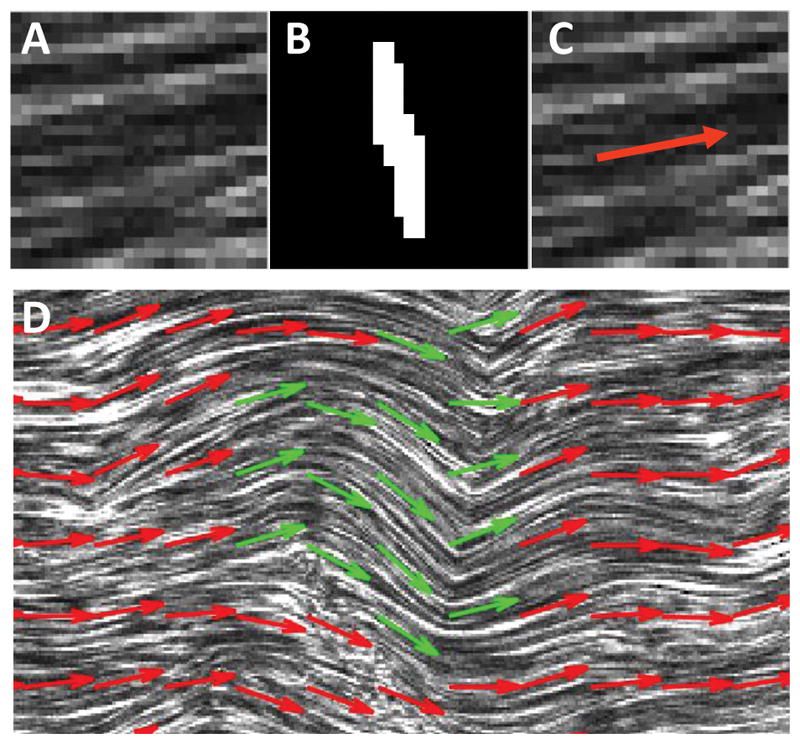
Measurement of local collagen fiber orientation. (A) Representative subregion of SHG image (18×18 μm). (B) Binarized image of two-dimensional Fourier transform for the subregion. (C) Average collagen fiber orientation shown based on direction perpendicular to image of Fourier transform. (D) Representative image of fiber kinking (green arrows), which was defined as a >30 ° change in fiber orientation between adjacent subregions.
Atomic Force Microscopy
Single sections from ten fatigue and fresh tendons were bathed in PBS supplemented with protease inhibitors (Sigma Aldrich, P8340) while the local tissue modulus was measured using an Asylum MFP-3D mounted onto a fluorescent microscope. Specifically, for each fatigue-loaded sample, six locations within the CHP-positive and CHP-negative regions were probed 3–5 times with a 10 μm diameter polystyrene microsphere (Polysciences, 17136-5) mounted on a 0.6 N/m tipless cantilever (NanoAndMore, HQ:NSC36/tipless/Cr-Au) at 1 μm/s (Fig. S1). To obtain the average local tissue modulus for each location, force-indentation curves were successfully fit with AtomicJ (Hermanowicz et al., 2014) to a depth of 0.54 ± 0.15 μm (Fig. S2) using a thickness-corrected Hertzian model (Dimitriadis et al., 2002) and assuming a Poisson’s ratio of 0.5 for transverse compression of tendon (Salisbury et al., 2016). These measurements were then averaged over the six locations to generate a single value for the CHP-positive and CHP-negative regions. For the fresh samples, twelve random locations were probed and the measurements were averaged.
Statistics
Unpaired Student’s t-tests with Dunnett’s correction were used to compare the amount of kinked fibers and the local tissue modulus between fresh and fatigue-loaded samples. Paired t-tests were used to compare the modulus values and overlap with kinked fiber regions between the CHP-positive and CHP-negative regions of the fatigue-loaded samples. All data are presented as mean ± standard deviation.
RESULTS
Preliminary quasi-static tensile testing demonstrated that rat FCU tendons had an UTS of 45 ± 17 MPa. Based on these data, subsequent fatigue protocols loaded samples between 0.45 and 9.0 MPa. This resulted in a characteristic tri-phasic change in strain and modulus as a function of loading cycle (Fig. 2). On average, the secondary creep phase accounted for 96 ± 2% of the loading cycles, during which the tendons elongated 9.0 ± 2.9% strain at a rate of 0.001 ± 0.0005% per cycle. The number of cycles and peak strain at failure were 10,273 ± 5,580 and 31.3 ± 11.4%, respectively, and the maximum secant modulus was 299 ± 65 MPa.
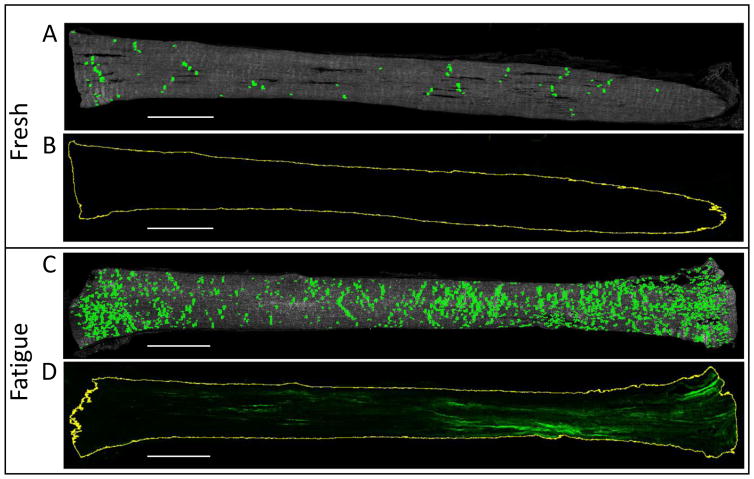
Histological analysis revealed marked changes in tendon samples after fatigue loading. Significantly larger areas of collagen fiber kinking (Fig. 4) were seen in fatigue-loaded samples compared to fresh controls (14.9 ± 6.0% vs 4.0 ± 1.9% tendon area; p<0.0001). Additionally, CHP staining of denatured collagen was observed only in fatigue-loaded samples (13.2 ± 10.8% tendon area). Positive CHP staining generally was most intense near the tendon end clamped using sandpaper tabs; however, denatured collagen was not isolated to one location and could be found throughout the fatigue-loaded tissue. Fiber kinking was evenly distributed across the fatigue-loaded samples, with regions of fiber kinking comprising 14.9 ± 10.5% of the CHP-positive regions versus 14.3 ± 5.4% of the CHP-negative regions (Fig. 5). Surprisingly, mechanical analysis of these regions with AFM showed no difference in tissue modulus between the fresh and fatigue-loaded samples, and no difference between CHP-positive and CHP-negative regions within the fatigue-loaded sections (Fig. 6).
Figure 4.
Minimal (A) collagen fiber kinking and (B) CHP staining was observed for fresh tendons, which sharply contrasts the significant (C) kinking and (D) denaturation seen in the fatigue-loaded samples. Scale bar = 1 mm.
Figure 5.
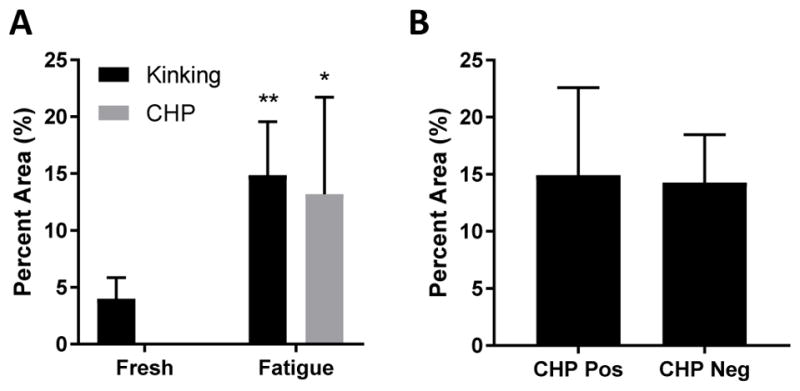
(A) Significantly more collagen fiber kinking and CHP staining was observed in the fatigue-loaded samples. (B) Fiber kinking was evenly distributed between the CHP-positive and CHP-negative regions, suggesting no correlation between fiber kinking and collagen denaturation. * p<0.001 & ** p<0.0001 vs Fresh
Figure 6.
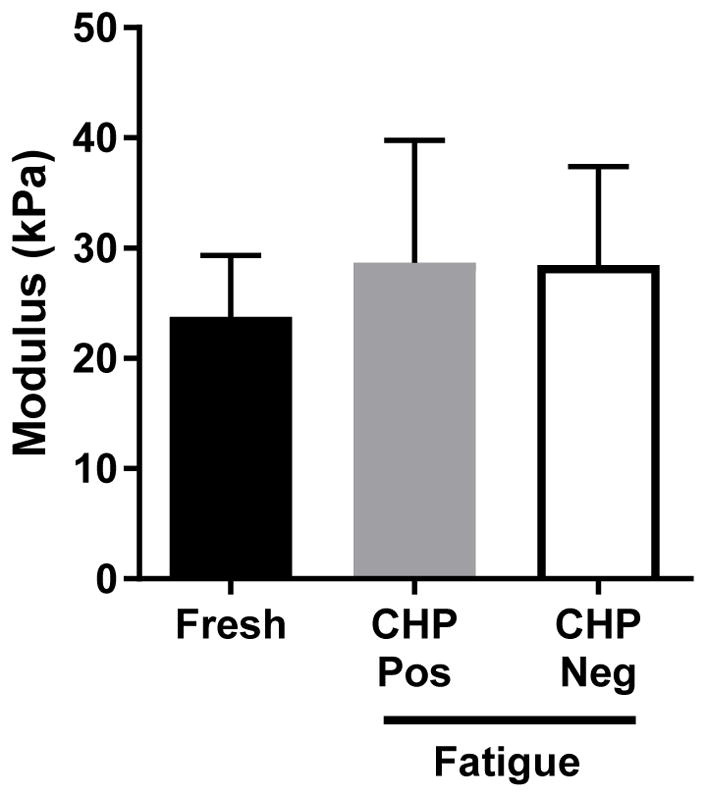
No significant difference was found in the local tissue modulus either between fresh and fatigue-loaded samples or between CHP-positive and CHP-negative regions.
DISCUSSION
This study investigated the effect of fatigue damage on tendon microstructure and microscale mechanical properties. Consistent with previous work (Fung et al., 2010; Shepherd et al., 2014; Veres et al., 2014), we found that fatigue loading resulted in widespread disruption of collagen fiber organization (i.e., fiber kinking) and collagen denaturation at the molecular level (Fig. 4). Interestingly, the degree of fiber kinking was independent of the presence of collagen denaturation (Fig. 5B), suggesting that these two structural defects may result from independent damage mechanisms. Despite the widespread structural damage observed in the fatigue-loaded samples, there was no difference in the local tissue modulus compared to fresh controls (Fig. 6). This was even true when comparing the modulus between regions with and without collagen denaturation in the fatigue-loaded samples. These data suggest that changes in topography and tissue structure – but not local tissue mechanics – initiate early changes in tendon cell phenotype during fatigue loading that lead to tendon degeneration.
The findings from this work are consistent with previous data regarding tendon fatigue damage. Similar to the lack of colocalization between fiber kinking and molecular denaturation, prior ultrastructural imaging of fatigue-loaded tendons showed that while fiber/fibril kinking contains highly local points of fibril disruption at the kink point (Herod and Veres, 2017), they largely lack the so-called “discrete plasticity” patterns resulting from molecular denaturation spread across the fibril length (Veres et al., 2013, 2014). Additionally, while the lack of change in local tissue modulus with fatigue is surprising, it is consistent with data demonstrating that microscale tissue strains are also unchanged in fatigue-damaged tendon (Shepherd et al., 2014). Finally, even in tendons with extensive discrete plasticity or positive CHP staining, only about 4% of the total collagen is denatured (Veres et al., 2014; Zitnay et al., 2017), suggesting that the bulk of the collagen molecules (and hence of the fibrils) are still mechanically unaffected. Together, these data suggest that structural damage in fatigue-loaded tendons precedes changes in local tissue mechanical properties.
Existing data also supports the idea that local changes in tissue organization and topography are sufficient for initiating tendon degeneration or aberrant tissue remodeling. Aligned biomaterials better maintain tendon cell phenotype in vitro compared to flat or randomly organized substrates (Yin et al., 2010; Zhu et al., 2010). Similarly, aligned nanofibrous scaffolds improve tenogenic differentiation of mesenchymal or induced pluripotent stem cells (Maharam et al., 2015; Younesi et al., 2014; C. Zhang et al., 2015). Interestingly, nuclear localization of the mechanosensitive transcriptional co-activator YAP and cell contractility are similar in cells on aligned and non-aligned nanofibrous scaffolds (Heo et al., 2017), suggesting that the local stiffness of the fiber networks are independent of fiber alignment, which is consistent with the AFM data in this work. Changes in fiber crimping (e.g., formation of kinking) also change tenocyte behavior even when stimulated by the same substrate strain (Chao et al., 2014), suggesting that fiber crimping/kinking may influence the mechano-perception of tenocytes (Szczesny et al., 2017). Indeed, our own preliminary data suggest that severe fiber kinking distorts and possibly disrupts local tenocyte nuclei (Fig. S3), which likely alters their behavior. Therefore, it is possible that fatigue-induced changes in tissue structure may be the initial stimulus for aberrant tissue remodeling, which may lead to later changes in local tissue mechanics and drive tendon degeneration.
One limitation to this work is that AFM measurements of sectioned samples may not accurately reflect the properties of intact tissue. Nevertheless, the values obtained in this study were comparable to those obtained from AFM of intact tendon samples (Connizzo and Grodzinsky, 2017). Additionally, AFM measures the tissue transverse compressive properties and not the tensile properties important for tendon function. However, the purpose of this work was to estimate the local tissue mechanics in the tenocyte microenvironment. By using a large colloidal probe, the modulus values presented here represent the overall collagen fiber network modulus on a length scale that likely matches the mechanical stimuli presented to cells within tendon. A second limitation is that unfixed tissue sections were prepared using a cryotome, which could introduce cutting artifacts. Indeed, some of the fiber kinking observed in the fresh control samples was likely due to sectioning (Fig. 4). Nevertheless, clear differences were observed between fresh and fatigue-loaded samples in terms of the extent of fiber kinking. Furthermore, no CHP staining was observed in fresh samples.
Another limitation is that random variability in the tissue modulus data between measurement locations required averaging across all locations within a given sample. This prevented more precise regional comparisons of the local tissue modulus (e.g., distal vs proximal locations in control samples, kinked vs non-kinked regions). Additionally, a post hoc power analysis demonstrated that our methods had a power of 0.8 for measuring a 30% change in tissue modulus. Therefore, while we can conclude that there is no gross change in local tissue mechanics with fatigue damage, there may be finer changes that could indeed affect tenocyte mechanotransduction. Finally, this study used FCU tendons from only male rats, which may accumulate fatigue damage more quickly than female tendons (Lepley et al., 2018; Pardes et al., 2016). In the future, it would be interesting to investigate the gender differences that may exist in the local tissue properties of fatigue-loaded tendons. Together, these data represent a first step at evaluating the pertinent changes in local tissue structure and mechanics with fatigue loading that may drive alterations in cellular behavior leading to tendon degeneration.
Supplementary Material
Acknowledgments
This work was funded by the National Institutes of Health (R01 EB002425, F32 AR070562).
Footnotes
CONFLICT OF INTEREST STATEMENT
None of the authors have any conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu EL, Leigh D, Derwin KA. Effect of altered mechanical load conditions on the structure and function of cultured tendon fascicles. J Orthop Res. 2008;26:364–373. doi: 10.1002/jor.20520. [DOI] [PubMed] [Google Scholar]
- Archambault JM, Jelinsky SA, Lake SP, Hill AA, Glaser DL, Soslowsky LJ. Rat supraspinatus tendon expresses cartilage markers with overuse. J Orthop Res. 2007;25:617–624. doi: 10.1002/jor.20347. [DOI] [PubMed] [Google Scholar]
- Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int J Exp Pathol. 2007a;88:217–226. doi: 10.1111/j.1365-2613.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoczky SP, Lavagnino M, Egerbacher M, Caballero O, Gardner K. Matrix metalloproteinase inhibitors prevent a decrease in the mechanical properties of stress-deprived tendons: an in vitro experimental study. Am J Sports Med. 2007b;35:763–769. doi: 10.1177/0363546506296043. [DOI] [PubMed] [Google Scholar]
- Arnoczky SP, Lavagnino M, Egerbacher M, Caballero O, Gardner K, Shender MA. Loss of homeostatic strain alters mechanostat “set point” of tendon cells in vitro. Clin Orthop. 2008;466:1583–1591. doi: 10.1007/s11999-008-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoczky SP, Tian T, Lavagnino M, Gardner K. Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J Orthop Res. 2004;22:328–333. doi: 10.1016/S0736-0266(03)00185-2. [DOI] [PubMed] [Google Scholar]
- Arya S, Kulig K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol. 2010;108:670–675. doi: 10.1152/japplphysiol.00259.2009. [DOI] [PubMed] [Google Scholar]
- Aström M, Rausing A. Chronic Achilles tendinopathy. A survey of surgical and histopathologic findings. Clin Orthop. 1995:151–164. [PubMed] [Google Scholar]
- Attia M, Scott A, Duchesnay A, Carpentier G, Soslowsky LJ, Huynh MB, Van Kuppevelt TH, Gossard C, Courty J, Tassoni M-C, Martelly I. Alterations of overused supraspinatus tendon: a possible role of glycosaminoglycans and HARP/pleiotrophin in early tendon pathology. J Orthop Res. 2012;30:61–71. doi: 10.1002/jor.21479. [DOI] [PubMed] [Google Scholar]
- Badley EM, Tennant A. Impact of disablement due to rheumatic disorders in a British population: estimates of severity and prevalence from the Calderdale Rheumatic Disablement Survey. Ann Rheum Dis. 1993;52:6–13. doi: 10.1136/ard.52.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R, Li J, Gorski DJ, Bartels AK, Shewman EF, Wysocki RW, Cole BJ, Bach BR, Mikecz K, Sandy JD, Plaas AH, Wang VM. Controlled treadmill exercise eliminates chondroid deposits and restores tensile properties in a new murine tendinopathy model. J Biomech. 2013;46:498–505. doi: 10.1016/j.jbiomech.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Chao PG, Hsu HY, Tseng HY. Electrospun microcrimped fibers with nonlinear mechanical properties enhance ligament fibroblast phenotype. Biofabrication. 2014;6:035008. doi: 10.1088/1758-5082/6/3/035008. [DOI] [PubMed] [Google Scholar]
- Connizzo BK, Grodzinsky AJ. Tendon exhibits complex poroelastic behavior at the nanoscale as revealed by high-frequency AFM-based rheology. J Biomech. 2017;54:11–18. doi: 10.1016/j.jbiomech.2017.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43:409–416. doi: 10.1136/bjsm.2008.051193. [DOI] [PubMed] [Google Scholar]
- Corps AN, Robinson AHN, Harrall RL, Avery NC, Curry VA, Hazleman BL, Riley GP. Changes in matrix protein biochemistry and the expression of mRNA encoding matrix proteins and metalloproteinases in posterior tibialis tendinopathy. Ann Rheum Dis. 2012;71:746–752. doi: 10.1136/annrheumdis-2011-200391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mos M, Koevoet W, van Schie HTM, Kops N, Jahr H, Verhaar JAN, van Osch GJVM. In vitro model to study chondrogenic differentiation in tendinopathy. Am J Sports Med. 2009;37:1214–1222. doi: 10.1177/0363546508331137. [DOI] [PubMed] [Google Scholar]
- Dimitriadis EK, Horkay F, Maresca J, Kachar B, Chadwick RS. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys J. 2002;82:2798–2810. doi: 10.1016/S0006-3495(02)75620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Fleming DM, Cross KW, Barley MA. Recent changes in the prevalence of diseases presenting for health care. Br J Gen Pract J R Coll Gen Pract. 2005;55:589–595. [PMC free article] [PubMed] [Google Scholar]
- Fung DT, Wang VM, Andarawis-Puri N, Basta-Pljakic J, Li Y, Laudier DM, Sun HB, Jepsen KJ, Schaffler MB, Flatow EL. Early response to tendon fatigue damage accumulation in a novel in vivo model. J Biomech. 2010;43:274–279. doi: 10.1016/j.jbiomech.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon WW, Cooper JR, Radcliffe GS. Sonographic incidence of tendon microtears in athletes with chronic Achilles tendinosis. Br J Sports Med. 1999;33:129–130. doi: 10.1136/bjsm.33.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjipanayi E, Mudera V, Brown RA. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J Tissue Eng Regen Med. 2009;3:77–84. doi: 10.1002/term.136. [DOI] [PubMed] [Google Scholar]
- Hakimi O, Ternette N, Murphy R, Kessler BM, Carr A. A quantitative label-free analysis of the extracellular proteome of human supraspinatus tendon reveals damage to the pericellular and elastic fibre niches in torn and aged tissue. PLOS ONE. 2017;12:e0177656. doi: 10.1371/journal.pone.0177656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA. Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study. J Orthop Res. 1995;13:907–914. doi: 10.1002/jor.1100130615. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Nobuhara K, Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop. 2003:111–120. doi: 10.1097/01.blo.0000092974.12414.22. [DOI] [PubMed]
- Heo S-J, Szczesny SE, Kim DH, Saleh KS, Mauck RL. Expansion of Mesenchymal Stem Cells on Electrospun Scaffolds Maintains Stemness, Mechano-Responsivity, and Differentiation Potential. J Orthop Res. 2017 doi: 10.1002/jor.23772. In Press. [DOI] [PMC free article] [PubMed]
- Hermanowicz P, Sarna M, Burda K, Gabryś H. AtomicJ: An open source software for analysis of force curves. Rev Sci Instrum. 2014;85:063703. doi: 10.1063/1.4881683. [DOI] [PubMed] [Google Scholar]
- Herod TW, Veres SP. Development of overuse tendinopathy: A new descriptive model for the initiation of tendon damage during cyclic loading. J Orthop Res. 2017 doi: 10.1002/jor.23629. In Press. [DOI] [PubMed]
- Jones ER, Jones GC, Legerlotz K, Riley GP. Cyclical strain modulates metalloprotease and matrix gene expression in human tenocytes via activation of TGFβ. Biochim Biophys Acta. 2013;1833:2596–2607. doi: 10.1016/j.bbamcr.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannus P, Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- Khan MH, Li Z, Wang JH-C. Repeated exposure of tendon to prostaglandin-E2 leads to localized tendon degeneration. Clin J Sport Med. 2005;15:27–33. doi: 10.1097/00042752-200501000-00006. [DOI] [PubMed] [Google Scholar]
- Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15:133–135. doi: 10.1097/01.jsm.0000165347.55638.23. [DOI] [PubMed] [Google Scholar]
- Lavagnino M, Arnoczky SP. In vitro alterations in cytoskeletal tensional homeostasis control gene expression in tendon cells. J Orthop Res. 2005;23:1211–1218. doi: 10.1016/j.orthres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Lavagnino M, Arnoczky SP, Egerbacher M, Gardner KL, Burns ME. Isolated fibrillar damage in tendons stimulates local collagenase mRNA expression and protein synthesis. J Biomech. 2006;39:2355–2362. doi: 10.1016/j.jbiomech.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Lavagnino M, Wall ME, Little D, Banes AJ, Guilak F, Arnoczky SP. Tendon mechanobiology: Current knowledge and future research opportunities. J Orthop Res. 2015;33:813–822. doi: 10.1002/jor.22871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh DR, Abreu EL, Derwin KA. Changes in gene expression of individual matrix metalloproteinases differ in response to mechanical unloading of tendon fascicles in explant culture. J Orthop Res. 2008;26:1306–1312. doi: 10.1002/jor.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepley AS, Joseph MF, Daigle NR, Digiacomo JE, Galer J, Rock E, Rosier SB, Sureja PB. Gender differences in mechanical properties of the Achilles tendon: Longitudinal response to repetitive loading exercise. J Strength Cond Res. 2018 doi: 10.1519/JSC.0000000000002386. Publish Ahead of Print. [DOI] [PubMed]
- Li Y, Ho D, Meng H, Chan TR, An B, Yu H, Brodsky B, Jun AS, Michael Yu S. Direct Detection of Collagenous Proteins by Fluorescently Labeled Collagen Mimetic Peptides. Bioconjug Chem. 2013;24:9–16. doi: 10.1021/bc3005842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, Sasaki T, Stavnezer E, Iannotti J, Schweitzer R, Ilic D, Baskaran H, Sakai T. Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr Biol. 2011;21:933–941. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharam E, Yaport M, Villanueva NL, Akinyibi T, Laudier D, He Z, Leong DJ, Sun HB. Rho/Rock signal transduction pathway is required for MSC tenogenic differentiation. Bone Res. 2015;3:15015. doi: 10.1038/boneres.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdizadeh A, Gardiner BS, Lavagnino M, Smith DW. Predicting tenocyte expression profiles and average molecular concentrations in Achilles tendon ECM from tissue strain and fiber damage. Biomech Model Mechanobiol. 2017;16:1329–1348. doi: 10.1007/s10237-017-0890-x. [DOI] [PubMed] [Google Scholar]
- Nakama LH, King KB, Abrahamsson S, Rempel DM. VEGF, VEGFR-1, and CTGF cell densities in tendon are increased with cyclical loading: An in vivo tendinopathy model. J Orthop Res. 2006;24:393–400. doi: 10.1002/jor.20053. [DOI] [PubMed] [Google Scholar]
- Nakama LH, King KB, Abrahamsson S, Rempel DM. Evidence of tendon microtears due to cyclical loading in an in vivo tendinopathy model. J Orthop Res. 2005;23:1199–1205. doi: 10.1016/j.orthres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Oakes PW, Banerjee S, Marchetti MC, Gardel ML. Geometry regulates traction stresses in adherent cells. Biophys J. 2014;107:825–833. doi: 10.1016/j.bpj.2014.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardes AM, Freedman BR, Fryhofer GW, Salka NS, Bhatt PR, Soslowsky LJ. Males have Inferior Achilles Tendon Material Properties Compared to Females in a Rodent Model. Ann Biomed Eng. 2016;44:2901–2910. doi: 10.1007/s10439-016-1635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham RJ, Wang Yl. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui YF, Lui PPY, Ni M, Chan LS, Lee YW, Chan KM. Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. J Orthop Res Off Publ Orthop Res Soc. 2011;29:390–396. doi: 10.1002/jor.21218. [DOI] [PubMed] [Google Scholar]
- Rui YF, Lui PPY, Wong YM, Tan Q, Chan KM. BMP-2 stimulated non-tenogenic differentiation and promoted proteoglycan deposition of tendon-derived stem cells (TDSCs) in vitro. J Orthop Res Off Publ Orthop Res Soc. 2013;31:746–753. doi: 10.1002/jor.22290. [DOI] [PubMed] [Google Scholar]
- Salisbury STS, Buckley CP, Zavatsky AB. Transverse Compression of Tendons. J Biomech Eng. 2016;138:041002. doi: 10.1115/1.4032627. [DOI] [PubMed] [Google Scholar]
- Samiric T, Parkinson J, Ilic MZ, Cook J, Feller JA, Handley CJ. Changes in the composition of the extracellular matrix in patellar tendinopathy. Matrix Biol. 2009;28:230–236. doi: 10.1016/j.matbio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Scott A, Lian Ø, Roberts CR, Cook JL, Handley CJ, Bahr R, Samiric T, Ilic MZ, Parkinson J, Hart DA, Duronio V, Khan KM. Increased versican content is associated with tendinosis pathology in the patellar tendon of athletes with jumper’s knee. Scand J Med Sci Sports. 2008;18:427–435. doi: 10.1111/j.1600-0838.2007.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereysky JB, Andarawis-Puri N, Jepsen KJ, Flatow EL. Structural and mechanical effects of in vivo fatigue damage induction on murine tendon. J Orthop Res Off Publ Orthop Res Soc. 2012;30:965–972. doi: 10.1002/jor.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereysky JB, Andarawis-Puri N, Ros SJ, Jepsen KJ, Flatow EL. Automated image analysis method for quantifying damage accumulation in tendon. J Biomech. 2010;43:2641–2644. doi: 10.1016/j.jbiomech.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JH, Riley GP, Screen HRC. Early stage fatigue damage occurs in bovine tendon fascicles in the absence of changes in mechanics at either the gross or micro-structural level. J Mech Behav Biomed Mater. 2014;38:163–172. doi: 10.1016/j.jmbbm.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JH, Screen HRC. Fatigue loading of tendon. Int J Exp Pathol. 2013;94:260–270. doi: 10.1111/iep.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- Sun HB, Li Y, Fung DT, Majeska RJ, Schaffler MB, Flatow EL. Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subrupture fatigue damage. Clin Orthop. 2008;466:1555–1561. doi: 10.1007/s11999-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesny SE, Driscoll TP, Tseng H-Y, Liu P-C, Heo S-J, Mauck RL, Chao P-HG. Crimped Nanofibrous Biomaterials Mimic Microstructure and Mechanics of Native Tissue and Alter Strain Transfer to Cells. ACS Biomater Sci Eng. 2017;3:2869–2876. doi: 10.1021/acsbiomaterials.6b00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CT, Chaudhry S, Lei II, Varone A, Riley GP, Birch HL, Clegg PD, Screen HRC. Tendon overload results in alterations in cell shape and increased markers of inflammation and matrix degradation. Scand J Med Sci Sports. 2014 doi: 10.1111/sms.12333. [DOI] [PubMed]
- Tsuzaki M, Bynum D, Almekinders L, Yang X, Faber J, Banes AJ. ATP modulates load-inducible IL-1beta, COX 2, and MMP-3 gene expression in human tendon cells. J Cell Biochem. 2003;89:556–562. doi: 10.1002/jcb.10534. [DOI] [PubMed] [Google Scholar]
- Veres SP, Harrison JM, Lee JM. Mechanically overloading collagen fibrils uncoils collagen molecules, placing them in a stable, denatured state. Matrix Biol. 2014;33:54–59. doi: 10.1016/j.matbio.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Veres SP, Harrison JM, Lee JM. Repeated subrupture overload causes progression of nanoscaled discrete plasticity damage in tendon collagen fibrils. J Orthop Res Off Publ Orthop Res Soc. 2013;31:731–737. doi: 10.1002/jor.22292. [DOI] [PubMed] [Google Scholar]
- Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279:C1345–1350. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- Wang JH-C, Jia F, Yang G, Yang S, Campbell BH, Stone D, Woo SL-Y. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res. 2003;44:128–133. doi: 10.1080/03008200390223909. [DOI] [PubMed] [Google Scholar]
- Wang T, Chen P, Zheng M, Wang A, Lloyd D, Leys T, Zheng Q, Zheng MH. In vitro loading models for tendon mechanobiology. J Orthop Res. 2017 doi: 10.1002/jor.23752. In Press. [DOI] [PubMed]
- Wang T, Lin Z, Ni M, Thien C, Day RE, Gardiner B, Rubenson J, Kirk TB, Smith DW, Wang A, Lloyd DG, Wang Y, Zheng Q, Zheng MH. Cyclic mechanical stimulation rescues achilles tendon from degeneration in a bioreactor system. J Orthop Res Off Publ Orthop Res Soc. 2015;33:1888–1896. doi: 10.1002/jor.22960. [DOI] [PubMed] [Google Scholar]
- Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- Yin Z, Chen X, Chen JL, Shen WL, Hieu Nguyen TM, Gao L, Ouyang HW. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31:2163–2175. doi: 10.1016/j.biomaterials.2009.11.083. [DOI] [PubMed] [Google Scholar]
- Younesi M, Islam A, Kishore V, Anderson JM, Akkus O. Tenogenic Induction of Human MSCs by Anisotropically Aligned Collagen Biotextiles. Adv Funct Mater. 2014;24:5762–5770. doi: 10.1002/adfm.201400828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Yuan H, Liu H, Chen X, Lu P, Zhu T, Yang L, Yin Z, Heng BC, Zhang Y, Ouyang H. Well-aligned chitosan-based ultrafine fibers committed teno-lineage differentiation of human induced pluripotent stem cells for Achilles tendon regeneration. Biomaterials. 2015;53:716–730. doi: 10.1016/j.biomaterials.2015.02.051. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang JHC. Prostaglandin E2 (PGE2) exerts biphasic effects on human tendon stem cells. PloS One. 2014;9:e87706. doi: 10.1371/journal.pone.0087706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang JHC. The Effects of Mechanical Loading on Tendons - An In Vivo and In Vitro Model Study. PLoS ONE. 2013;8:e71740. doi: 10.1371/journal.pone.0071740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang JH-C. Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy. J Orthop Res. 2010;28:639–643. doi: 10.1002/jor.21046. [DOI] [PubMed] [Google Scholar]
- Zhang K, Asai S, Yu B, Enomoto-Iwamoto M. IL-1β irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochem Biophys Res Commun. 2015;463:667–672. doi: 10.1016/j.bbrc.2015.05.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Li J, Wang B, Zhang WJ, Zhou G, Cao Y, Liu W. The regulation of phenotype of cultured tenocytes by microgrooved surface structure. Biomaterials. 2010;31:6952–6958. doi: 10.1016/j.biomaterials.2010.05.058. [DOI] [PubMed] [Google Scholar]
- Zitnay JL, Li Y, Qin Z, San BH, Depalle B, Reese SP, Buehler MJ, Yu SM, Weiss JA. Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides. Nat Commun. 2017;8:ncomms14913. doi: 10.1038/ncomms14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.