Abstract
BACKGROUND:
In humans, accumulated adverse experiences during childhood increase the risk of anxiety disorders and attention-deficit hyperactivity disorder. In rodents, the ventral hippocampus (vHIP) is associated with anxiety regulation, and lesion of this region alters both anxiety-like behavior and activity levels. Neuronal oscillations in the vHIP of the theta frequency range (4–12 Hz) have been implicated in anxious states and derive in part from the activity of inhibitory interneurons in the hippocampus, some of which are enwrapped with perineuronal nets (PNNs), extracellular matrix structures that regulate plasticity. Here we sought to investigate the associations among early life stress-induced anxiety and hyperactivity with vHIP neuronal oscillations, inhibitory interneurons and PNNs in mice.
METHODS:
We used repeated maternal separation with early weaning (MSEW) to model accumulated early life adversity in mouse offspring and studied the underlying behavioral and cellular level changes in the vHIP associated with early life adversity.
RESULTS:
We found increased anxiety-like behavior and activity levels in MSEW adult males, along with increased theta power and enhanced theta-gamma coupling in the vHIP. MSEW mice showed reduced intensity of parvalbumin as well as increased PNN intensity around parvalbumin+ interneurons in the vHIP. We further observed that MSEW increased OTX2, a transcription factor promoting PNN development, in the choroid plexus where it is produced, as well as in parvalbumin+ interneurons, where it is sequestered.
CONCLUSION:
These findings raise the possibility of causal links among parvalbumin+ interneurons, PNNs, OTX2, and MSEW-induced anxiety and hyperactivity.
Keywords: ventral hippocampus, anxiety, early life stress, theta rhythm, interneurons, perineuronal nets
Introduction
Childhood maltreatment is known to substantially increase the risk of developing neuropsychiatric conditions in adulthood, including anxiety and mood disorders (1–3). In fact, more than 50% of diagnosed cases of anxiety disorders and clinical depression occur in individuals who have experienced early life adversity (4). Attention-deficit hyperactivity disorder (ADHD) is often comorbid with anxiety disorders in adults and childhood maltreatment has been shown to increase susceptibility to both ADHD and excessive anxiety (5). Studies in humans suggest that exposure to stress during a sensitive period in development may be predictive of negative outcomes (6–8), but the preponderance of evidence, including a recent large study, indicates that the accumulation of multiple bouts of early life stress exposure is most damaging in terms of long-term psychiatric problems (7–10). To cover both the sensitive period and cumulative aspects of early life adversity, we selected a multiple-hit model of early life stress in mice, maternal separation with early weaning (MSEW), where mouse pups are subjected to daily maternal separations of increasing length during the first 16 days of life followed by weaning four days earlier than typically reared laboratory mice (11; 12). This developmental manipulation has been shown to increase both anxiety levels and hyperactivity in adulthood (11), suggesting it has strong translational validity (see Supplemental Materials for further discussion).
The ventral hippocampus (vHIP) of rodents has been linked to anxiety regulation; bilateral lesions of this area have been shown to reduce anxiety-like behavior in rodents (13–15) while optogenetic or pharmacological stimulation of the region has the opposite effect (16–18). Several studies suggest that neuronal oscillations in the theta frequency (4–12 Hz) range in the vHIP and mPFC are important for anxiety regulation, with decreases in theta power in the vHIP occurring after anxiolytic drug administration (19) and increases in theta power in the vHIP and mPFC as well as theta synchrony between the vHIP and mPFC during anxious states (20). Furthermore, in humans, increased self-reported anxiety during threat is associated with increased theta rhythm in the anterior hippocampus (21), a region that is considered analogous to the vHIP in rodents (22). Despite these associations, the electrophysiological profile of rodents that display increased anxious behavior after early life adversity has not been studied.
Inhibitory interneuron subtypes have been implicated in theta rhythm in the hippocampus and neocortex (23–27). Optogenetic studies on the hippocampus have shown that parvalbumin (PV)+ interneurons are the main effectors of intrinsic theta rhythm while somatostatin (SST)+ interneurons modulate the entrainment of intrinsic theta by controlling external inputs to the hippocampus (25). Many PV+ interneurons are encapsulated by specialized extracellular matrix structures termed perineuronal nets (PNNs). PNNs are thought to limit plasticity and are responsible for the closure of the critical period for ocular dominance in the visual cortex (28). PNN degradation has been shown to reduce PV+ interneuron activity (29), as well as reduce theta frequency synchrony between brain regions involved in fear memory (30). Experience has been shown to alter PNN expression but the effects are complex, depending on the type of experience, the developmental stage examined and the brain region. For example, early life trauma has been shown to reduce PNN expression in the basolateral amygdala (31), enriched environment rearing reduces PNNs in the cerebellum (32) but increases them in the CA2 region of the hippocampus (33), while enriched environment exposure in adulthood increases or decreases PNN expression depending on the brain region and presence of other experiences (34). PNNs have been linked to the transcription factor orthodenticle homeobox protein 2 (OTX2) (35), the expression of which coincides with the maturation of PV+ cells and the closure of the critical period of plasticity in mice. In adults, OTX2 is produced in the choroid plexus, released into the CSF and accumulates in PV+ cells where it facilitates PNN formation. No previous studies have investigated whether PNNs and OTX2 are altered in PV+ cells of the hippocampus after early life adversity.
Studies have shown that adult-generated neurons in the hippocampus have the ability to modulate neuronal oscillations in that their elimination by focal irradiation or by transgenic means increases rhythmic firing (36). Some previous studies have reported that early life adversity reduces the production of new neurons in the dentate gyrus (37), raising the possibility that changes in neuronal oscillations and potentially anxiety may involve reductions in this form of plasticity. Additionally, adult-generated neurons also form connections with PV+ inhibitory interneurons (38; 39). Despite these compelling associations, no previous studies have investigated whether changes in inhibitory interneurons, PNNs and adult-generated neurons occur in association with early life stress-induced alterations in neuronal oscillations and anxiety-like behavior.
Here, we sought to investigate these possibilities by performing behavioral analyses and local field potential (LFP) recordings in the vHIP of adult mice subjected to MSEW and found consistent increases in anxiety-like behavior and hyperactivity along with increases in theta power in a novel environment, as well as enhanced theta-gamma coupling in both familiar and novel environments. This was accompanied by a reduction in densities of PV+ and SST+ interneurons, but no decrease in immature neurons, in the granule cell layer (GCL) of MSEW mice. Further analysis of PV+ interneurons revealed increased PNN intensity surrounding PV+ cells as well as increases in transcription factor OTX2 within these cells. These findings suggest interrelationships among PV+ interneurons, PNNs, OTX2, and MSEW-induced anxiety and hyperactivity.
Methods and Materials
Maternal Separation with Early Weaning
Twenty litters of C57BL/6J mice from two breeding cohorts were exposed to one of two rearing conditions at P2: 1) non-handled controls; or 2) maternal separation for 4h daily from P2-P5 and 8h daily from P6-P16. Pups in the latter group were weaned at P17 while control litters were weaned normally at P21 (11). At age P60-P70, mice underwent behavior testing. See supplemental methods for more details.
Behavior
Anxiety and activity testing were carried out as detailed in supplemental methods.
Histochemistry
Two hours after behavioral analyses were conducted, mice were perfused and sections through the hippocampus were stained for doublecortin, Ki67, calretinin, PV, SST, OTX2, cfos and WFA using immunohistochemical and histochemical methods. Antibodies are detailed in Table S1. See supplemental methods for more details.
Cell count and density analyses
The vHIP was analyzed separately as it is functionally distinct from the dHIP and has been implicated in anxiety regulation (22). DCX+, Ki67+ and GAD67+ cells from the GCL and interneuron subtypes across layers of the dentate gyrus (DG), CA1 and CA3 were counted using Stereo Investigator software (Microbrightfield Bioscience). Densities were determined by dividing the total number of positive cells by the volume of the region outlined.
WFA and OTX2 intensity analyses
Confocal images were analyzed on ImageJ where every PV+/WFA+ cell was outlined and the area and maximum intensity (mean gray value of WFA or OTX2 x area of the WFA stain) for each WFA+/PV+ cell was determined according to previously published protocols (40; 41). See supplemental methods for more details.
Surgery
Insulated stainless steel electrodes (0.005”, Plastics One) were implanted in the vHIP CA1; (3.3 mm posterior, 3.45 mm lateral and 4.2 mm depth). Placement of electrode was in the left hemisphere as this hemisphere has been specifically shown to be affected in MSEW mice (42).
LFP Recordings and Analysis
One week after surgery, mice from cohort 2 underwent habituations and behavioral testing. LFP recording data were collected on the fourth day from 2 separate recording sessions. First, mice were exposed to a pre-habituated familiar environment for a period of 10 min followed by an hour of rest and then exposed to a novel environment for a period of 10 min. Power spectra were calculated from data acquired from mice during segments of movement only (4–15 cm/s). Any animal without a minimum of 5 seconds of movement during the task was excluded from analysis. For phase amplitude coupling analyses (43), all animals regardless of movement (moving or stationary) were considered for analysis. See supplemental methods for details.
Statistical analysis
Electrophysiology data were analyzed as described above using custom Matlab and R scripts (code available upon request). For all other measures, unpaired two tailed Student’s t-tests or Mann-Whitney U tests were performed on each data set following determination of homogeneity of variance with Levene’s test. All statistical tests and p values are listed in Tables S2 and S3.
Results
MSEW results in increased anxiety-like behaviors in males in the elevated plus maze
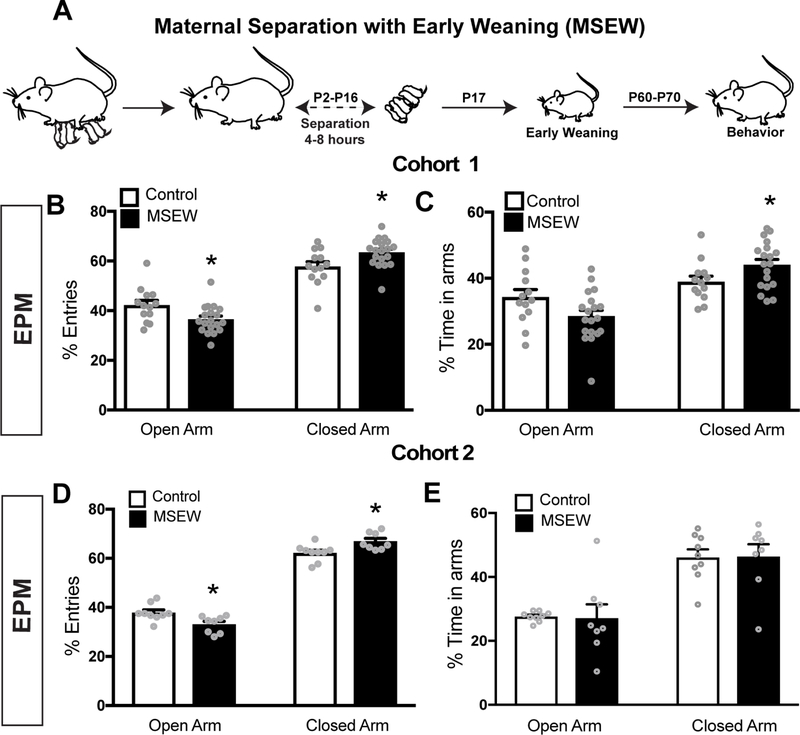
We observed increased anxiety-like behavior in 2 cohorts of MSEW male mice compared to male controls. MSEW mice in cohort 1 showed increased anxiety in the EPM with significantly lower percentage of entries into the open arms (Fig 1B). MSEW mice in cohort 1 also spent significantly more time in the closed arms (Fig 1C). To verify the reliability of this model, we repeated behavioral tests with a second cohort of mice. MSEW mice from cohort 2 also displayed greater anxiety in the EPM as they made significantly lower percent entries into the open arms (Fig 1D), although no differences were observed in the amount of time spent in the open or closed arms between groups (Fig 1E). No measurable changes in anxiety-like behavior were observed in MSEW female mice compared to same sex controls (Fig S1). Because our aim was to search for neural correlates of MSEW-increased anxiety, we focused our subsequent studies on males.
Figure 1.
(A) Timeline depicting the MSEW paradigm. (B) MSEW male mice from cohort 1 displayed increased anxiety-like behavior in the EPM, making significantly lower percent entries in to the open and higher entries into closed arms. (C) MSEW mice spent significantly more time in the closed arms of the EPM. (D) MSEW male mice from cohort 2 displayed increased anxiety-like behavior in the EPM, making significantly lower percent entries into the open arms and higher percent entries into closed arms. (E) MSEW mice from cohort 2 did not show any differences in percent time spent in the open or close arms. *p<0.05
MSEW results in increased activity levels in a novel environment
Consistent with previous work (11), we found that MSEW male mice were more active in a novel environment than controls. MSEW male mice exhibited greater locomotion in a novel testing arena in that they covered a larger distance (Fig 2A) as well as an increase in the percentage of time spent moving during the duration of test (Fig 2B).
Figure 2.
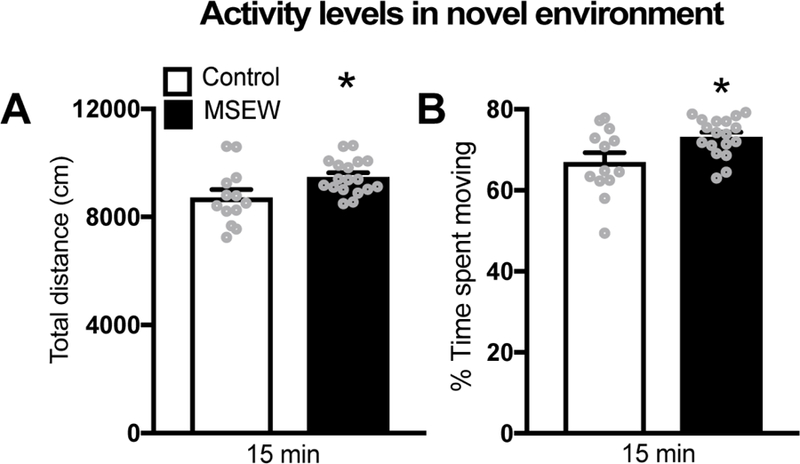
(A) MSEW male mice display greater activity in a novel environment as they showed increased locomotion (total distance) compared to controls. (B) MSEW mice show increased time spent being active (%time spent moving) during the testing period compared to controls.
MSEW alters vHIP neuronal oscillations in both familiar and novel environments
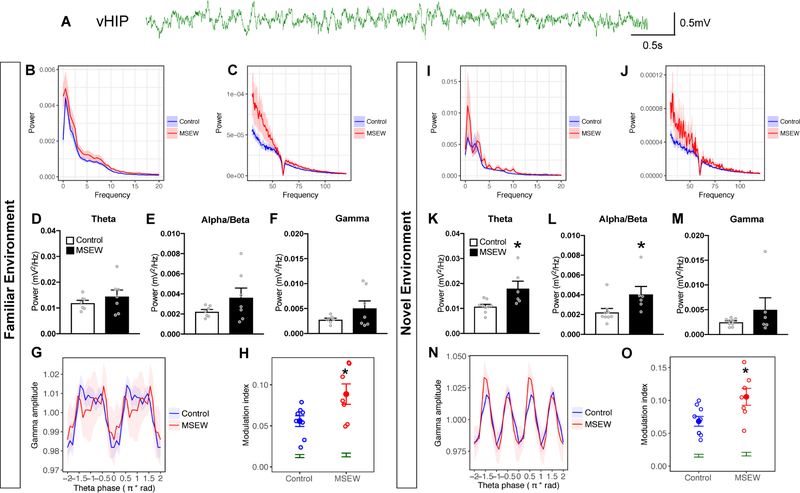
When tested in a familiar environment, no differences were observed between MSEW and control groups in theta (4–12Hz), beta (12–20Hz) or gamma (30–80 Hz) power (Fig 3D–F). Additionally, delta (1–4 Hz) power was not altered (Fig S2A). By contrast, phase amplitude coupling analysis in the familiar environment showed that MSEW male mice displayed greater modulation of gamma oscillations by theta phase (Fig 3G–H). When tested in a novel environment, MSEW mice showed significantly greater theta power (Fig 3K) as well as alpha/beta power (Fig 3L) compared to controls. Delta (Fig S2B) and gamma power (Fig 3M) were not different between groups. Phase amplitude coupling analysis showed that amplitude of gamma oscillations was also significantly modulated by the phase of theta oscillations in MSEW mice in a novel environment when compared to controls (Fig 3N–O). These data suggest vHIP theta-gamma coupling is inherently enhanced in MSEW mice compared to controls, occurring in both familiar and novel environments, but increases in theta and alpha/beta power are observed only when MSEW mice are in a novel environment.
Figure 3.
(A) Raw LFP trace from the vHIP. (B,C) High and low frequency power spectra plots from MSEW mice during 10 minutes in the familiar environment (F.E). In the F.E, there were no power differences in either theta (D), alpha/beta (E) or gamma (F) power between groups. (G) Phase amplitude coupling analysis plotting normalized gamma power as a function of theta phase in the F.E (H) Analysis of theta-gamma phase amplitude coupling in the F.E. revealed that MSEW mice had significantly more modulation compared to controls. (I, J) High and low frequency power spectra plots from MSEW mice during 10 minutes in the novel environment (N.E). (K,L) In the N.E, theta and alpha/beta frequency power was significantly increased in MSEW mice compared to controls. (M) No changes in gamma power were observed between groups in the N.E. (N) Phase amplitude coupling analysis plotting normalized gamma power as a function of theta phase in the N.E. (O) Permutation tests showed significantly greater modulation above chance for both treatment conditions (green). MSEW significantly increased theta-gamma modulation relative to controls in the N.E, suggesting enhanced theta-gamma coupling in the vHIP due to early life adversity. *p<0.05
MSEW leads to a reduction in the densities of PV+ and SST+ cells but no overall decrease in interneuron densities
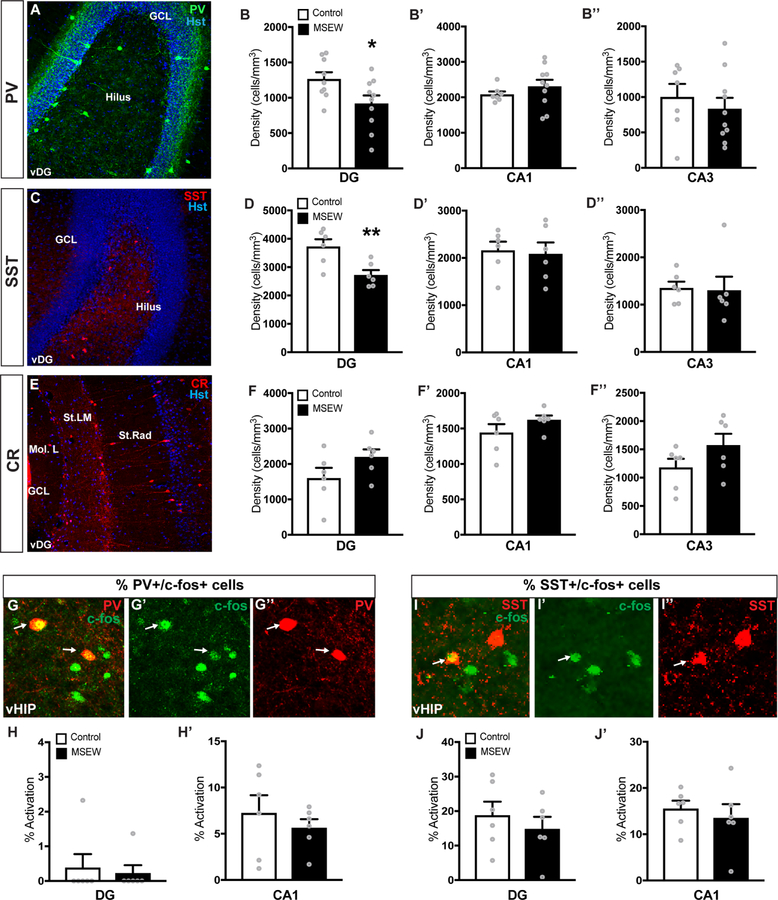
Because inhibitory interneurons play roles in theta rhythm and theta-gamma coupling (23; 25), we examined their presence in hippocampus of MSEW and control mice. Analyses of the DG, CA1 and CA3 revealed that the density of PV+ interneurons in MSEW mice was significantly reduced in the ventral DG (vDG) compared to controls (Fig 4B), while the density in the CA1 and CA3 remained unaltered (Fig 4B’–B’’). PV intensity within PV+ neurons of the vDG was also reduced (Fig. S5A) suggesting that the decrease in cell density was due to reduced expression of PV, as opposed to a loss of interneurons due to cell death. SST+ interneuron density was also significantly reduced in the vDG of MSEW mice (Fig 4D), while the CA1 and CA3 regions showed no differences in this measure (Fig 4D’–D’’). It is likely that MSEW-induced decreases in densities of PV+ and SST+ interneurons are not due to cell death because there was no overall difference in the density of cells stained with the pan-interneuron marker GAD67 (Fig S5B). No significant change in density was observed in the CR+ subpopulation in either the DG, CA1 or CA3 regions (Fig 4F–F’’).
Figure 4.
(A) Image of PV+ interneuron distribution in the vDG. (B-B’’) MSEW significantly reduced PV+ interneuron densities in the vDG, but not in the CA1 or CA3. (C) SST+ interneuron distribution in the vDG. (D-D’’’) MSEW significantly reduced SST interneuron densities in the vDG, but not in the CA1 or CA3. (E) Calretinin (CR) interneuron distribution in the vDG. (F-F’) MSEW did not alter CR densities in the DG, CA1, or CA3 regions of the vHIP. (G-G’’) Images depicting c-fos activation of PV+ cells in the vDG after exposure to the EPM. (H-H’) No difference in PV+ interneuron activation in the DG or CA1 was observed. (I-I’’) Images depicting c-fos activation of SST+ cells in the vDG after anxiety-like behavior tests. (J-J’) No difference in SST+ interneuron activation in the DG or CA1 was observed. *p <0.05, **p<0.01
Mice were perfused 2h after the EPM test to allow for maximal accumulation of the protein products of c-fos, an immediate early gene used as a proxy for neuronal activation, in interneurons (44). No differences in the percentage of PV+ or SST+ cells co-labeled with c-fos in the DG or CA1 were observed (Fig 4H–H’, 4J–J’’), indicating that overt differences in the population of PV+ or SST+ interneurons activated are not likely the cause of altered neuronal oscillations and behavior.
MSEW increases the intensity of PNN labeling around PV+ interneurons
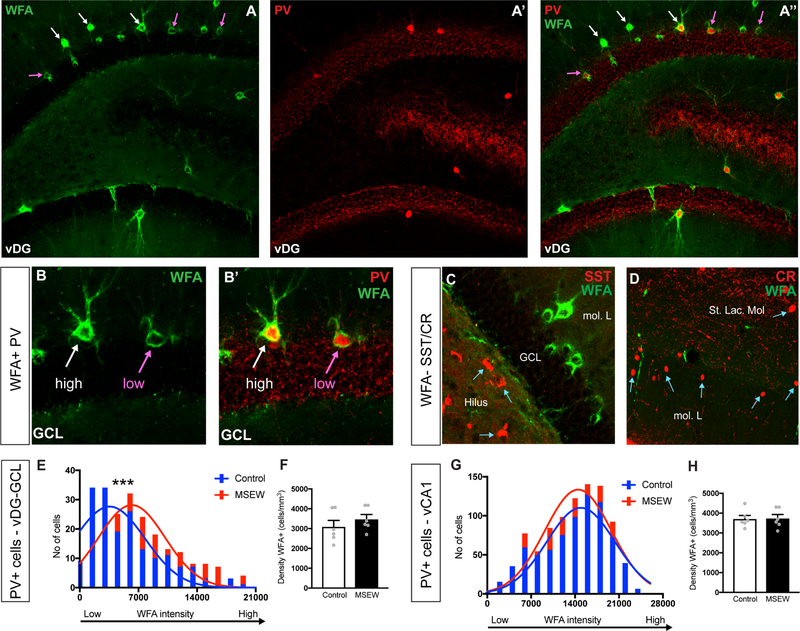
PNNs are involved in the plasticity and activity of inhibitory interneurons (45). Wisteria Floribunda Agglutinin (WFA), a plant based lectin stain commonly used to label PNNs, along with PV immunolabeling was used to visualize PNNs around PV+ cells in the brain (Fig 5A–B). SST+ and CR+ interneurons were not found to be encapsulated by WFA+ PNNs (Fig 5C–D). In the GCL of the vDG, we observed that PV+ cells in MSEW mice had significantly greater WFA intensity (Fig 5E) compared to controls. However, no significant differences in intensity of WFA was observed between groups in the vCA1 (Fig 5G). The overall density of WFA+ cells in either region was also not affected between groups (Fig 5F,H). No differences were observed in the percentage of PV+ cells that were WFA+ in the vCA1 or the vDG, between control and MSEW mice (Fig S4).
Figure 5.
(A-A’’) Images of PV+ cells and WFA+ perineuronal nets (PNNs) in the vDG of MSEW mice. (B-B’) Higher magnification images depicting high and low intensity WFA stained PNNs surrounding PV+ cells. (C-D) SST and CR interneurons are not encapsulated by WFA+ PNNs. (E) MSEW mice showed a significant increase in intensities of WFA labelled PNNs in the vDG-GCL. (F) Graph showing no differences in the density of WFA+ PNNs in the vDG-GCL. (G) Graph showing no differences between groups in the intensities of WFA labelled PNNs in the vCA1. (H) Graph showing no differences in the density of WFA+ PNNs in the vCA1. *p<0.05, ***p<0.001
MSEW increases OTX2 labeling intensity
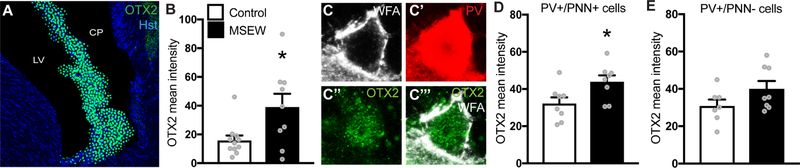
OTX2 produced in the choroid plexus binds to PNNs in the visual cortex where it facilitates the expression of PNNs (28). MSEW mice showed a significant increase in immunofluorescence intensity of OTX2 when compared to controls in the choroid plexus where it is produced (Fig 6B). To verify if the increase in OTX2 was also present in PV+/WFA+ cells in the vDG, sections were tripled labelled for OTX2, PV and WFA and OTX2 intensity in PV+/WFA+ cells in the vDG was analyzed (Fig 6C–C’’’). Similar to the choroid plexus, MSEW mice showed increased intensity of OTX2 in PV+/WFA+ cells (Fig 6D) while PV+/WFA- cells showed no such increase (Fig 6E). Since previous studies have shown that OTX2 is not synthesized in the vHIP (46), our findings suggest that PV+/PNN+ cells in the vHIP sequester OTX2 in greater amounts after MSEW.
Figure 6.
(A) Images of the choroid plexus stained with OTX2 in the lateral ventricle. (B) Graph showing increased OTX2 labeling intensity in the choroid plexus of MSEW mice. (C-C’’’) Images showing a cell colabelled for WFA, PV and OTX2 in the vDG-GCL. (D) Graph showing increased OTX2 labeling intensity in PV+WFA+ cells in the vDG of MSEW mice. (E) PV+WFA- cells do not show increase in OTX2 labeling. *p<0.05
MSEW has no effect on the density of new neurons or proliferating cells
Because adult-generated neurons affect neuronal oscillations in the hippocampus (36), and have been linked to stress-induced anxiety-like behavior (47–49), we examined the numbers of DCX+ immature neurons and Ki67+ proliferating cells in the vDG of MSEW and control mice. No differences were detected in DCX+ or Ki67+ cell densities between MSEW and control mice (Fig S3A, S3B).
Discussion
The findings presented here confirmed previous studies that adult male mice subjected to MSEW exhibit an increase in anxiety-like behavior and activity levels (11). We further showed that MSEW increased theta power in the vHIP when in a novel environment, but not in a familiar environment, as well as increased phase amplitude coupling between theta and gamma in both settings. Analysis of cellular subtypes within the vHIP revealed MSEW resulted in reduced densities of PV+ and SST+ interneurons in the vDG, but no change in densities of CR+ interneurons, GAD67+ interneurons, DCX+ immature neurons or Ki67+ proliferating cells. Further analysis of PV+, SST+ and CR+ subtypes revealed that only PV+ interneurons were encapsulated by PNNs, which were present in increased intensities around PV+ interneurons in the vDG of MSEW mice. Reduced PV expression in PV+ cells was also observed following MSEW. These changes were accompanied by an increase in labeling intensity of the transcription factor OTX2 in the choroid plexus as well as in PV+ interneurons in the vDG. Taken together, our findings show that early life adversity produces altered neuronal oscillations in the vHIP of adult male mice and further demonstrate cellular changes in interneurons that may be causally linked to the electrophysiological and behavioral alterations.
MSEW is an adaptation of the maternal separation paradigm developed to produce reliable increases in anxiety-like behavior in adult mice (11). Early weaning by itself results in increased anxiety in both mice and rats (50;51). The combination of early weaning with maternal separation also results in increased hyperactivity in mice (11). We did not observe any changes in anxiety-like behavior in females subjected to MSEW. The lack of an increase in anxiety among female rodents subjected to early life stress is consistent with several other studies (reviewed in 52) and surprising given that women are more susceptible to anxiety and depressive disorders than men (53). These inconsistent findings raise questions about whether traditional methods of assessing anxiety in male rodents may not be adequate for females (52). It is also relevant to note that while overall significant differences in behavior were observed in 2 separate cohorts of male mice, within each study it was clear that not all mice were adversely affected by MSEW. These findings are also consistent with the work of others (reviewed in 52) and raise interesting questions about individual differences in resilience and susceptibility, the mechanisms of which will be the focus of future work.
Our data showing increased vHIP theta power in the novel environment are consistent with reports showing increased theta power and increased coherence between the mPFC and vHIP during heightened states of anxiety (20). Additionally, enhanced theta-phase-gamma-amplitude coupling in MSEW mice when in either environmental setting indicates an inherent alteration to the electrophysiological profile. Behavioral tasks can modulate phase amplitude coupling which has been implicated in sensory integration, memory processes, and attentional selection previously (54–56). Specifically, theta-phase gamma-amplitude coupling in the dorsal hippocampus has been implicated in cognitive function (57), and in the amygdala has been implicated in periods of heightened anxiety (58). Increased theta-gamma coupling has been associated with greater dysfunctional attention/arousal behaviors in ADHD children (59). While the involvement of PV+ interneurons in theta-gamma coupling has been previously established (23), our study is the first to report enhanced theta-gamma coupling in the vHIP in the context of early life adversity and anxiety/hyperactivity.
Adult neurogenesis in the hippocampus has been implicated in some anxiety- and depression-related behaviors (60; 61). Previous work showed maternal separation stress reduced adult neurogenesis in the rat hippocampus (37; 62–64), although other studies failed to show this (65). New neurons may be involved in modulating neuronal oscillations in the hippocampus as ablation of neurogenesis destabilizes network activity in vivo (36). While we did not observe differences in DCX+ and Ki67+ cell densities between control and MSEW mice, postsynaptic connections of new neurons to other cell types may be altered. Immature neurons project to interneurons in the DG and reports have documented the role of interneurons in modulating neuronal circuitry controlling hippocampal neurogenesis, as well as the activity of new neurons in vivo (38;39). It is possible that altered connections between new neurons and interneurons in the DG adversely affect neuronal networks resulting in the altered electrophysiological profile of MSEW mice.
GABAergic interneurons, especially the PV+ subtype, play an important role in the regulation of neuronal oscillations in the brain (24–27) and have also been implicated in neuropsychiatric disease (66; 67). PV+ interneurons appear to be the main generators of intrinsic theta oscillations in the hippocampus (24). In fact, disconnecting PV cell activity from the fast spiking inhibitory network in the hippocampus reduces both theta power and theta-gamma coupling (23) These reports along with our data suggest that increased rhythmic inhibitory inputs to PV+ cells may underlie the increased theta power and theta-gamma coupling observed in MSEW mice. The reduction in PV+ interneuron densities we observed in the MSEW hippocampus is likely due to decreased PV protein levels rather than loss of neurons given our data on PV expression and GAD67+ cell densities, as well as previous studies showing reductions in PV protein levels due to early life adversity in the mPFC of juvenile mice (68; 69). Along these lines, it is worth noting that experimentally induced theta bursts reduce PV expression in other brain regions (70; 71), raising the possibility that the changes we observed in PV intensity are the result of, as opposed to being responsible for, the altered electrophysiological profile observed with MSEW. Assessing this possibility will be the focus of future studies.
SST+ interneurons on the other hand have been demonstrated to indirectly contribute to the entrainment of theta oscillations in the hippocampus (25) and are responsible for preferentially driving beta (15–30 Hz) frequency oscillations in other brain regions (72). We observed an increase in alpha/beta frequency oscillation power in the vHIP of MSEW mice, which could be related to decreased SST+ interneuron intensities and possibly activity of this cell type. However, the exact role of SST+ interneurons in MSEW-induced changes in neuronal oscillations needs to be more completely investigated in future studies.
Our findings reporting increased intensities of WFA labelled PNNs around PV+ interneurons suggest altered plasticity of PV+ cells as PNNs have been known to regulate synaptic and cellular plasticity of the cells they encapsulate (45) and digestion of PNNs around PV+ cells has been shown to reduce the fast spiking properties and excitability of PV+ cells (73) In fact, the maturation of PNNs around PV+ cells in the visual cortex coincides with the closure of the critical period for the development of binocular vision; degradation of PNNs has been shown to reopen this critical period in the visual cortex (74; 75). PNN degradation also reduces PV+ interneuron activity and alters gamma and theta range neuronal oscillations in rats (29). PNN degradation has also been shown to reduce theta synchrony between the amygdala and the association visual cortex; brain regions involved in fear memory recall (30). However, no previous studies have investigated how early life adversity impacts PNNs and our findings raise the possibility that increased PNNs around PV+ cells may drive the increase in theta oscillations, as well as elevated anxiety and hyperactivity observed in MSEW mice.
Orthodenticle homeobox protein 2 (OTX2) is a transcription factor known to be important in the regulation of the critical period of plasticity in the visual cortex of developing mice (46) and its expression coincides with the maturation of PV+ cells and the closure of the critical period (35). Additionally, OTX2 has been shown to specifically accumulate in PV+ cells through exogenous transfer from the choroid plexus, the region where it is produced (35; 46). Our findings showed an increase in intensity of labeling of OTX2 in the choroid plexus suggesting an increase in OTX2 production in MSEW mice. We hypothesized that this could lead to an increase in accumulation of OTX2 in cells of the vHIP, where OTX2 is not made (46) but may work in a non-cell autonomous way as it does elsewhere in the brain (28). Our observations showed that MSEW mice displayed increases in OTX2 labeling intensity in PV+ PNN+ cells in the vHIP. Continual binding of OTX2 to PNNs has been shown to be necessary for synaptic stability in the adult visual cortex and blocking OTX2 uptake into PV+ cells in adults has been demonstrated to reduce PNN expression and reinstate juvenile levels of plasticity (28). Our results are consistent with an OTX2-mediated mechanism of action for modulating PNNs and hence, PV+ cell plasticity in the vHIP. Additional discussion of the role of cell autonomous OTX2 in stress resilience (76) can be found in the supplemental materials.
These findings are the first to report that early life adversity results in an altered electrophysiological profile in the vHIP in adulthood, which is associated with an increase in anxiety and hyperactivity. The data presented here further indicate that OTX2, PNNs and PV+ interneurons are affected by MSEW and these changes may participate in altering neuronal oscillations underlying anxiety and hyperactivity. Understanding such mechanisms is important in the identification of novel targets to develop circuit level interventions for countering the negative impact of early life adversity on neuropsychiatric disease.
Supplementary Material
Acknowledgments:
We thank Adam T. Brockett and Patrick K. Monari for their advice and help with surgeries and experiments, Brandy A. Briones and Elise C. Cope for their helpful comments on the manuscript. This work was supported by the CV Starr Fellowship (SM) and by grant NIH MH117459-01 (EG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1).Heim C, Shugart M, Craighead WE, Nemeroff CB (2010): Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol 52:671–690. [DOI] [PubMed] [Google Scholar]
- 2).Fuller-Thomson E, Mehta R, Valeo A (2014): Establishing a link between attention deficit disorder/attention deficit hyperactivity disorder and childhood physical abuse. J Aggress Maltreat T 23:188–198. [Google Scholar]
- 3).Gallo EAG, Munhoz TN, Loret de Mola C, Murray J (2018): Gender differences in the effects of childhood maltreatment on adult depression and anxiety: A systematic review and meta-analysis. Child Abuse Negl 79:107–114. [DOI] [PubMed] [Google Scholar]
- 4).Li M, D’Arcy C, Meng X (2016): Maltreatment in childhood substantially increases the risk of adult depression and anxiety in prospective cohort studies: systematic review, meta-analysis, and proportional attributable fractions. Psychol Med 46:717–730. [DOI] [PubMed] [Google Scholar]
- 5).Mao AR, Findling RL (2014): Comorbidities in adult attention-deficit/hyperactivity disorder: a practical guide to diagnosis in primary care. Postgrad Med 126:42–51. [DOI] [PubMed] [Google Scholar]
- 6).Marshall AD (2016): Developmental timing of trauma exposure relative to puberty and the nature of psychopathology among adolescent girls. J Am Acad Child Adolesc Psychiatry 55:25–32 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Dunn EC, Soare TW, Raffeld MR, Busso DS, Crawford KM, Davis KA, et al. (2018): What life course theoretical models best explain the relationship between exposure to childhood adversity and psychopathology symptoms: recency, accumulation, or sensitive periods? Psychol Med 1–11. [DOI] [PMC free article] [PubMed]
- 8).Schalinski I, Breinlinger S, Hirt V, Teicher MH, Odenwald M, Rockstroh B (2017): Environmental adversities and psychotic symptoms: The impact of timing of trauma, abuse, and neglect. Schizophr Res pii: S0920–9964(17)30662-X [DOI] [PubMed]
- 9).Bjorkenstam E, Burstrom B, Vinnerljung B, Kosidou K (2016): Childhood adversity and psychiatric disorder in young adulthood: An analysis of 107,704 Swedes. J Psychiatr Res 77:67–75. [DOI] [PubMed] [Google Scholar]
- 10).Copeland WE, Shanahan L, Hinesley J, Chan R, Aberg KA, Fairbank JA, et al. (2018): Association of childhood trauma exposure with adult psychiatric disorders and functional outcomes. JAMA Netw Open 1:e184493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).George ED, Bordner KA, Elwafi HM, Simen AA (2010): Maternal separation with early weaning: a novel mouse model of early life neglect. BMC Neurosci 11:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Carlyle BC, Duque A, Kitchen RR, Bordner KA, Coman D, Doolittle E, et al. (2012): Maternal separation with early weaning: A rodent model providing novel insights into neglect associated developmental deficits. Development and Psychopathology 24:1401–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JNP (1999): Double dissociation of function within the hippocampus: A comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci 113:1170–1188. [DOI] [PubMed] [Google Scholar]
- 14).Bannerman DM, Grubb M, Deacon RMJ, Yee BK, Feldon J, Rawlins JNP (2003): Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Research 139:197–213. [DOI] [PubMed] [Google Scholar]
- 15).Weeden CS, Roberts JM, Kamm AM, Kesner RP (2015): The role of the ventral dentate gyrus in anxiety-based behaviors. Neurobiol Learn Mem 118:143–149. [DOI] [PubMed] [Google Scholar]
- 16).Bast T, Zhang WN, Feldon J (2001): Hyperactivity, decreased startle reactivity, and disrupted prepulse inhibition following disinhibition of the rat ventral hippocampus by the GABA(A) receptor antagonist picrotoxin. Psychopharmacology 156:225–233. [DOI] [PubMed] [Google Scholar]
- 17).Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM (2013): BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79:658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, et al. (2016): Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron 89:857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).McNaughton N, Gray JA (2000): Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. J Affect Disorders 61:161–176. [DOI] [PubMed] [Google Scholar]
- 20).Adhikari A, Topiwala MA, Gordon JA (2010): Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 65:257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Cornwell BR, Arkin N, Overstreet C, Carver FW, Grillon C (2012): Distinct contributions of human hippocampal theta to spatial cognition and anxiety. Hippocampus 22:1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Fanselow MS, Dong HW (2010): Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Wulff P, Ponomarenko AA, Bartos M, Korotkova TM, Fuchs EC, Bahner F, et al. (2009): Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci U S A 106:3561–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Stark E, Eichler R, Roux L, Fujisawa S, Rotstein HG, Buzsaki G (2013): Inhibition-induced theta resonance in cortical circuits. Neuron 80:1263–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Amilhon B, Huh CY, Manseau F, Ducharme G, Nichol H, Adamantidis A, et al. (2015): Parvalbumin Interneurons of Hippocampus Tune Population Activity at Theta Frequency. Neuron 86:1277–1289. [DOI] [PubMed] [Google Scholar]
- 26).Ferguson KA, Huh CYL, Amilhon B, Manseau F, Williams S, Skinner FK (2015): Network models provide insights into how oriens-lacunosum-moleculare and bistratified cell interactions influence the power of local hippocampal CA1 theta oscillations. Front Syst Neurosci 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Huh CYL, Amilhon B, Ferguson KA, Manseau F, Torres-Platas SG, Peach JP, et al. (2016): Excitatory inputs determine phase-locking strength and spike-timing of CA1 stratum oriens/alveus parvalbumin and somatostatin interneurons during intrinsically generated hippocampal theta rhythm. J Neurosci 36:6605–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Beurdeley M, Spatazza J, Lee HH, Sugiyama S, Bernard C, Di Nardo AA, et al. (2012): OTX2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci 32:9429–9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Lensjo KK, Lepperod ME, Dick G, Hafting T, Fyhn M (2017): Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. J Neurosci 37:1269–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Thompson EH, Lensjo KK, Wigestrand MB, Malthe-Sorenssen A, Hafting T, Fyhn M (2018): Removal of perineuronal nets disrupts recall of a remote fear memory. Proc Natl Acad Sci U S A 115:607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Santiago AN, Lim KY, Opendak M, Sullivan RM, Aoki C (2018): Early life trauma increases threat response of peri-weaning rats, reduction of axo-somatic synapses formed by parvalbumin cells and perineuronal net in the basolateral nucleus of amygdala. J Comp Neurol 526:2647–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Foscarin S, Ponchione D, Pajaj E, Leto K, Gawlak M, Wilczynski GM, Rossi F, Carulli D (2011): Experience-dependent plasticity and modulation of growth regulatory molecules at central synapses. PLoS One 6:e16666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Carstens KE, Phillips ML, Pozzo-Miller L, Weinberg RJ, Dudek SM (2016): Perineuronal nets suppress plasticity of excitatory synapses on CA2 pyramidal neurons. J Neurosci 36:6312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Slaker M, Barnes J, Sorg BA, Grimm JW (2016) Impact of environmental enrichment on perineuronal nets in the prefrontal cortex following early and late abstinence from sucrose self-administration in rats. PLoS One 11:e0168256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, et al. (2008): Experience-dependent transfer of OTX2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 134:508–520. [DOI] [PubMed] [Google Scholar]
- 36).Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA (2012): Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus 22:106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Mirescu C, Peters JD, Gould E (2004): Early life experience alters response of adult neurogenesis to stress. Nat Neurosci 7:841–846. [DOI] [PubMed] [Google Scholar]
- 38).Song J, Sun J, Moss J, Wen Z, Sun GJ, Hsu D, et al. (2013): Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat Neurosci 16:1728–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Drew LJ, Kheirbek MA, Luna VM, Denny CA, Cloidt MA, Wu MV, et al. (2015): Activation of local inhibitory circuits in the dentate gyrus by adult-born neurons. Hippocampus [DOI] [PMC free article] [PubMed]
- 40).Slaker ML, Harkness JH, Sorg BA (2016): A standardized and automated method of perineuronal net analysis using Wisteria floribunda agglutinin staining intensity IBRO Rep 1:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Donato F, Rompani SB, Caroni P (2013): Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning Nature 504:272–276. [DOI] [PubMed] [Google Scholar]
- 42).Duque A, Coman D, Carlyle BC, Bordner KA, George ED, Papademetris X, et al. (2012): Neuroanatomical changes in a mouse model of early life neglect. Brain Struct Funct 217:459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Tort AB, Komorowski R, Eichenbaum H, Kopell N (2010): Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol 104:1195–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Peng Z, Houser CR (2005): Temporal patterns of fos expression in the dentate gyrus after spontaneous seizures in a mouse model of temporal lobe epilepsy. J Neurosci 25:7210–7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Sorg BA, Berretta S, Blacktop JM, Fawcett JW, Kitagawa H, Kwok JC, et al. (2016): Casting a wide net: role of perineuronal nets in neural plasticity. J Neurosci 36:11459–11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Spatazza J, Lee HH, Di Nardo AA, Tibaldi L, Joliot A, Hensch TK, et al. (2013): Choroid plexus-derived OTX2 homeoprotein constrains adult cortical plasticity. Cell Rep 3:1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Seo DO, Carillo MA, Lim SCH, Tanaka KF, Drew MR (2015): Adult hippocampal neurogenesis modulates fear learning through associative and nonassociative mechanisms. J Neurosci 35:11330–11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Yun S, Donovan MH, Ross MN, Richardson DR, Reister R, Farnbauch LA, et al. (2016): Stress-induced anxiety- and depressive-like phenotype associated with transient reduction in neurogenesis in adult nestin-creER(T2)/diphtheria toxin fragment A transgenic mice. Plos One 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Glover LR, Schoenfeld TJ, Karlsson RM, Bannerman DM, Cameron HA (2017): Ongoing neurogenesis in the adult dentate gyrus mediates behavioral responses to ambiguous threat cues. Plos Biol 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Kikusui T, Takeuchi Y, Mori Y (2004): Early weaning induces anxiety and aggression in adult mice. Physiol Behav 81:37–42. [DOI] [PubMed] [Google Scholar]
- 51).Ito A, Kikusui T, Takeuchi Y, Mori Y (2006): Effects of early weaning on anxiety and autonomic responses to stress in rats. Behav Brain Res 171:87–93. [DOI] [PubMed] [Google Scholar]
- 52).Murthy S, Gould E (2018): Early life stress in rodents: animal models of illness or resilience? Front Behav Neurosci 12:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Altemus M, Sarvaiya N, Neill Epperson C (2014): Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol 35:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Lisman JE, Idiart MA (1995): Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science 267:1512–1515. [DOI] [PubMed] [Google Scholar]
- 55).Lisman J (2005): The theta/gamma discrete phase code occuring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus 15:913–922. [DOI] [PubMed] [Google Scholar]
- 56).Schroeder CE, Lakatos P (2009): Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci 32:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H (2009): Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci U S A 106:20942–20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Stujenske JM, Likhtik E, Topiwala MA, Gordon JA (2014): Fear and safety engage competing patterns of theta-gamma coupling in the basolateral amygdala. Neuron 83:919–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Kim JW, Lee J, Kim HJ, Lee YS, Min KJ (2015): Relationship between theta-phase gamma-amplitude coupling and attention-deficit/hyperactivity behavior in children. Neurosci Lett 590:12–17. [DOI] [PubMed] [Google Scholar]
- 60).Sahay A, Hen R (2007): Adult hippocampal neurogenesis in depression. Nat Neurosci 10:1110–1115. [DOI] [PubMed] [Google Scholar]
- 61).Hill AS, Sahay A, Hen R (2015): Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacology 40:2368–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Hulshof HJ, Novati A, Sgoifo A, Luiten PG, den Boer JA, Meerlo P (2011): Maternal separation decreases adult hippocampal cell proliferation and impairs cognitive performance but has little effect on stress sensitivity and anxiety in adult Wistar rats. Behav Brain Res 216:552–560. [DOI] [PubMed] [Google Scholar]
- 63).Leslie AT, Akers KG, Krakowski AD, Stone SS, Sakaguchi M, Arruda-Carvalho M, et al. (2011): Impact of early adverse experience on complexity of adult-generated neurons. Transl Psychiatry 1:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Korosi A, Naninck EF, Oomen CA, Schouten M, Krugers H, Fitzsimons C, et al. (2012): Early-life stress mediated modulation of adult neurogenesis and behavior. Behav Brain Res 227:400–409. [DOI] [PubMed] [Google Scholar]
- 65).Oomen CA, Girardi CE, Cahyadi R, Verbeek EC, Krugers H, Joels M, et al. (2009): Opposite effects of early maternal deprivation on neurogenesis in male versus female rats. PLoS One 4:e3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66).Fee C, Banasr M, Sibille E (2017): Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: cortical microcircuit and therapeutic perspectives. Biol Psychiatry 82:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ (2007): GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology 32:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Grassi-Oliveira R, Honeycutt JA, Holland FH, Ganguly P, Brenhouse HC (2016): Cognitive impairment effects of early life stress in adolescents can be predicted with early biomarkers: Impacts of sex, experience, and cytokines. Psychoneuroendocrinology 71:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Holland FH, Ganguly P, Potter DN, Chartoff EH, Brenhouse HC (2014): Early life stress disrupts social behavior and prefrontal cortex parvalbumin interneurons at an earlier time-point in females than in males. Neurosci Lett 566:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70).Mix A, Hoppenrath K, Funke K (2015): Reduction in cortical parvalbumin expression due to intermittent theta-burst stimulation correlates with maturation of the perineuronal nets in young rats. Dev Neurobiol 75:1–11. [DOI] [PubMed] [Google Scholar]
- 71).Mix A, Benali A, Eysel UT, Funke K (2010): Continuous and intermittent transcranial magnetic theta burst stimulation modify tactile learning performance and cortical protein expression in the rat differently. Eur J Neurosci 32:1575–86. [DOI] [PubMed] [Google Scholar]
- 72).Chen G, Zhang Y, Li X, Zhao X, Ye Q, Lin Y, et al. (2017): Distinct inhibitory circuits orchestrate cortical beta and gamma band oscillations. Neuron 96:1403–1418 e1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Balmer TS (2016): Perineuronal nets enhance the excitability of fast-spiking neurons. eNeuro 3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74).Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L (2002): Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298:1248–1251. [DOI] [PubMed] [Google Scholar]
- 75).Takesian AE, Hensch TK (2013): Balancing plasticity/stability across brain development. Prog Brain Res 207:3–34. [DOI] [PubMed] [Google Scholar]
- 76).Peña CJ, Kronman HG, Walker DM, Cates HM, Bagot RC, Purushothaman I, et al. (2017): Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 356:1185–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.