Abstract
Pediatric irritability is prevalent and impairing, yet little is known about its pathophysiology and treatment. In this article, we build on our and others’ previous work to posit core mechanisms of irritability operating across the brain, behavior, and environment. Specifically, we propose proximal processes that surround the symptomatology of irritability and are potential targets for an exposure-based cognitive-behavioral therapy (CBT) for irritability that our group has developed. The heart of this model focuses on neurocognitive processes: youth’s encoding of nonreward and threat stimuli, which involves prediction error signaling in the brain, and cognitive control in the context of frustration. Alterations in these processes are theorized to be central to chronic, severe irritability. Environmental responses to youth’s symptom expression are also examined. Exposure-based CBT for irritability utilizes controlled, in vivo exposure to nonreward and threat stimuli with the aim to engage cognitive control and target top-down regulation of frustration. This intervention integrates selected parent management training (PMT) techniques to target symptom reinforcement processes. Continued pathophysiological and treatment studies of irritability will not only refine our emerging understanding of the phenotype, but also inform broader questions on the brain and behavioral mechanisms of CBT efficacy.
Keywords: irritability, frustrative nonreward, threat, exposure, cognitive-behavioral therapy, functional magnetic resonance imaging
Irritability is a common and consequential domain of pediatric psychopathology (Brotman, Kircanski, Stringaris, Pine, & Leibenluft, 2017; Leibenluft, 2011). Chronic, severe irritability in youth is associated with functional impairment and increased risk for depression, anxiety, and suicidality (Copeland, Angold, Costello, & Egger, 2013; Orri et al., 2018; Vidal-Ribas, Brotman, Valdivieso, Leibenluft, & Stringaris, 2016). Despite this clinical significance, relatively little is known about the pathophysiology of pediatric irritability. Further, there are no well-established treatments for chronic, severe irritability as seen in disruptive mood dysregulation disorder (DMDD) (Kircanski, Clayton, Leibenluft, & Brotman, 2018; Stringaris, Vidal-Ribas, Brotman, & Leibenluft, 2018). Here, we build on our recent pathophysiological model of irritability that posits core mechanisms operating across the brain, behavior, and environment (Brotman et al., 2017). We specify components of the model that can serve as targets for an exposure-based cognitive-behavioral therapy (CBT) for irritability that our group has developed, and we hypothesize how this exposure-based CBT targets the proposed mechanisms. We conclude by outlining key questions for future research.
Brief Background and Rationale
Definition and Assessment of Irritability
Irritability refers to an increased proneness to anger, compared to one’s peers (Brotman et al., 2017; Leibenluft, 2017; Leibenluft & Stoddard, 2013). Proneness to anger is a continuously-distributed (Copeland, Brotman, & Costello, 2015) and stable (Caprara, Paciello, Gerbino, & Cugini, 2007) individual difference in youth. However, there are normative developmental variations such that certain behaviors (e.g., temper tantrums) are more frequent in early childhood and decrease thereafter (Wakschlag et al., 2012, 2015). Here, we focus on severe, impairing, and developmentally-atypical irritability in later childhood and adolescence that necessitates clinical intervention. Children and adolescents with this high level of irritability exhibit temper outbursts that are more frequent, behaviorally intense, longer, and contextually atypical relative to their peers (Brotman et al., 2017). These youth also exhibit a sustained angry mood over many days, which may manifest as sullenness or annoyance at minor provocations (Brotman et al., 2017).
Clinically-significant irritability is codified in the DSM-5 as the defining symptom of disruptive mood dysregulation disorder (DMDD) (American Psychiatric Association, 2013). DMDD is placed in the mood disorders section of the DSM-5, in recognition of its primary affective component and associations with unipolar depression and anxiety. Diagnostic criteria for DMDD include: (a) severe, recurrent temper outbursts occurring at least three times per week that are out of proportion to the situation and inconsistent with developmental level; and (b) persistently irritable mood between outbursts, for most of the day, nearly every day. Symptoms must begin by age 10 and be present for at least 1 year, but DMDD cannot be diagnosed before age 6. Because DMDD is a new diagnosis, current prevalence data relies on post-hoc analyses of datasets that asses irritability using a variety of methods. Community-based prevalence estimates from this work range from 0.12% to 5%, with the estimate for DMDD criteria converging at 3% (Althoff et al., 2016; Brotman et al., 2006; Copeland et al., 2013).
The symptoms of DMDD can be assessed using a specialized module of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS), developed by Leibenluft and colleagues (see Wiggins et al., 2016). In addition, the Affective Reactivity Index (ARI; Stringaris et al., 2012) is a brief child- and parent-report questionnaire designed to provide a dimensional measurement of irritability. Recently, our research group developed a clinician-administered ARI (CL-ARI), which probes temper outbursts, irritable mood, and irritability-related impairment in a more fine-grained, but similarly transdiagnostic and dimensional, manner as with the original ARI (Haller et al., under review). Of course, in clinical settings, irritability should always be assessed and conceptualized in the context of any other co-occurring symptoms or syndromes to inform treatment decisions.
Irritability in the Setting of Other Psychopathologies
The symptom criteria for DMDD share certain features with the criteria for oppositional defiant disorder (ODD), a disruptive behavior disorder characterized by at least 6 months of angry mood and/or defiant behavior (American Psychiatric Association, 2013). Diagnostic guidelines indicate that DMDD supersedes ODD when both are applicable, given that the severity of the irritability required is greater for DMDD. Studies have examined the ‘irritable’ symptom dimension within ODD (versus the oppositional behavior dimension) and found that irritability is uniquely related to depression and anxiety (Burke, Hipwell, & Loeber, 2010; Drabick & Gadow, 2012; Stringaris & Goodman, 2009a,b; Vidal-Ribas et al., 2016). DMDD is also frequently comorbid with attention-deficit/hyperactivity disorder (ADHD) (Brotman et al., 2006; Roy et al., 2013). Importantly, interventions involving parent management training (PMT) (Barkley, 2013; Kazdin, 2010) have demonstrated efficacy in reducing disruptive behavior in ODD and ADHD, as most commonly applied in young children or in adolescent conduct disorder (Comer, Show, Chan, & Cooper-Vince, 2013; Furlong et al., 2012; NICE, 2013). Despite this established literature, there is a paucity of data on PMT efficacy specifically for the irritability symptom dimension. Therefore, future research is needed to test whether PMT is effective specifically for chronic, severe irritability.
Irritability as a symptom transects multiple diagnoses in youth, particularly mood and anxiety disorders (American Psychiatric Association, 2013). However, irritability does not relate to all domains of psychopathology uniformly. In fact, during the 1990s it was proposed that pediatric bipolar disorder can present as chronic, severe irritability without distinct episodes of hypo/mania. Subsequent research on longitudinal course, family history, and pathophysiology documented that pediatric irritability is not a developmental phenotype of bipolar disorder (Leibenluft, 2011). Irritability in youth was found to predict unipolar, but not bipolar, depression and anxiety in adulthood (Vidal-Ribas et al., 2016), partly due to shared genetic liability among irritability, anxiety, and unipolar depression (Savage et al., 2015; Stringaris, Zavos, Leibenluft, Maughan, & Eley, 2012). These findings have important treatment implications, in that first-line treatments for bipolar disorder are not indicated for pediatric irritability across the spectrum of pediatric psychopathology. Indeed, currently there are no established treatments specifically for DMDD (Brotman et al., 2017; Kircanski, Clayton, et al., 2018; Stringaris et al., 2018). The need for intervention development is underscored by the prevalence and public health burden of irritability.
Despite its presence across multiple diagnoses, irritability is not a general severity marker or risk factor for all forms of psychopathology. In addition to its lack of prediction of bipolar disorder, early irritability does not significantly predict later conduct disorder or substance use disorder. Finally, irritability accounts for unique variance in functional impairment, even when co-occurring diagnoses are covaried (Vidal-Ribas et al., 2016). As we discuss below with respect to future directions, further studies will need to interrogate the brain and behavioral correlates of irritability in the settings of diverse co-occurring symptoms. Future treatment studies should also test whether exposure targeting irritability may be incrementally beneficial for youth with primary diagnoses other than DMDD. Here, we focus on chronic, severe irritability as seen in DMDD.
Exposure-Targeted Pathophysiological Model of Irritability
We (Brotman et al., 2017) developed a translational neuroscience model of pediatric irritability that integrates the available pathophysiological research. Synthesizing previous findings, the model proposes that aberrant brain and behavioral responses to frustrative nonreward and threat are key mechanisms driving the development and maintenance of severe, chronic irritability. Frustrative nonreward, first articulated in rodent research (Amsel, 1958), is the state induced when an organism does not receive a reward that it has been conditioned to expect (i.e., blocked goal attainment). Threats are stimuli that signal increased potential for harm to an organism (LeDoux & Pine, 2016). Studies of frustrative nonreward and threat processing in irritability have most consistently implicated disruptions in fronto-striatal-amygdala circuitry (Deveney et al., 2013; Kircanski, White, et al., 2018; Stoddard et al., 2017; Tseng et al., 2018). However, the available literature is still limited and some findings are mixed, so replication and further research is needed to support reliable neural correlates of irritability (Brotman et al., 2017; Leibenluft, 2017).
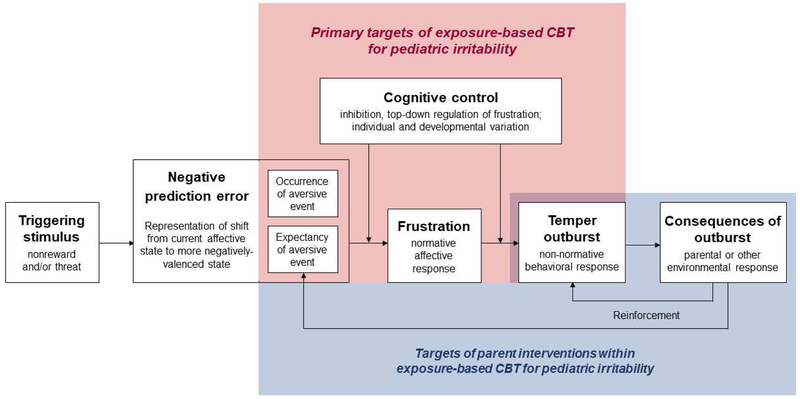
Our initial pathophysiological model (Brotman et al., 2017) proposed a range of risk and maintaining factors in irritability. Here, we focus on specific, proximal processes within this broader framework that are potential targets for an exposure-based CBT for irritability. Figure 1 presents this new exposure-targeted model. This conceptualization includes a series of proximal processes that surround a clinically-significant temper outburst and typically occur within a timescale of minutes. Below, we describe each of the component processes.
Figure 1. Exposure-targeted model of pediatric irritability.
Visual representation of proposed proximal processes that surround a clinically-significant temper outburst in pediatric irritability, typically occurring within a timescale of minutes. White boxes denote key constructs in bold text. Within each box, additional text briefly describes the construct. Arrows denote direct or moderating influences of these components on one another. Boxes encompassed by red background indicate the primary targets of exposure-based CBT for irritability. Boxes encompassed by blue background indicate targets of parent interventions within this CBT.
Triggering Stimulus
Consistent with prior work, our published model posits that a temper outburst is elicited by one or more triggering stimuli that can be characterized as nonrewarding and/or threatening (Brotman et al., 2017; see also Berkowitz, 1993; Buss & Durkee, 1957; Caprara et al., 1985). Importantly, both nonreward and threat stimuli signal an aversive event. Indeed, Wagner (1969) postulated that frustrative nonreward serves as a punishment or threat akin to shock. Here, we use the abbreviated term, nonreward, to refer to a broad class of stimuli or situations involving the “withdrawal or prevention of reward” (https://www.nimh.nih.gov/research-priorities/rdoc/constructs/frustrative-nonreward.shtml). Of note, frustrative nonreward was defined as resulting from a very specific combination of conditioned reward associations that were then violated (Amsel, 1958). The more general term, nonreward, is more inclusive. Common clinical examples of nonreward triggers include: removal of a preferred activity or state (e.g., parent telling child to stop playing a video game); withholding of a desired activity or state (e.g., parent telling child that s/he cannot play a video game); and transitioning from a preferred to a nonpreferred activity or state (e.g., parent telling child to stop playing a video game and start doing homework). In severe irritability, outbursts occur in response to multiple nonreward triggers across home, school, and peer contexts.
As noted, threat stimuli convey increased potential for harm. Threat stimuli vary in proximity; imminent threats normatively provoke flight or attack behavior in the service of survival and safety (Blair, 2010; Fanselow, 1994). However, youth with significant irritability exhibit atypical approach behavior toward non-imminent threats in the form of temper outbursts (Brotman et al., 2017; see also Crick & Dodge, 1994). That is, in many instances, the behavior that defines an outburst clinically (e.g., verbal or physical aggression) manifests as active approach toward and engagement with a threat. Common triggers of this type include: stimuli indicating potential for danger (e.g., peer looking at child with an angry facial expression); and stimuli indicating potential for personal failure (e.g., parent telling child to complete challenging homework). Perceptions of threat in the environment can be heightened by individuals’ cognitive biases. Not surprisingly, youth with high levels of irritability tend to exhibit such threat-relevant cognitive biases, including differential attention toward threat vs. neutral stimuli (Hommer et al., 2014; Salum et al., 2017) and more threat-based interpretations of neutral or ambiguous stimuli (Brotman et al., 2010; Dodge, 1980; Stoddard et al., 2016).
Some situations that trigger outbursts may involve both nonreward and threat (e.g., teacher telling child to transition from a preferred state [open study time] to a nonpreferred state [math work], the latter of which also involves increased potential for failure). Last, while we view nonreward and threat as two distinct constructs, our current model is agnostic as to whether temper outbursts may differ meaningfully based on the type or combination of triggers. This is an interesting question for future clinical research.
Negative Prediction Error
The second component of the model, prediction error (PE), refers to the difference between an expected and a received outcome (Schultz & Dickinson, 2000). Outcomes that are better than expected result in positive PEs, whereas outcomes that are worse than expected result in negative PEs (Schultz, 2016). Foundational animal studies have shown that midbrain dopamine neurons encode PEs across a range of situations (Tobler, Fiorillo, & Schultz, 2005). Further work has shown that PE-guided reward learning involves the amygdala as well as the striatum (Averbeck & Costa, 2017), and ventral and dorsal circuits through the striatum differentially process stimulus-based and action-based reward learning, consistent with our model’s focus on triggering stimuli (Rothenhoefer et al., 2017).
We speculate that negative PE may be a common pathway through which nonreward and threat stimuli are represented in the brain. Specifically, negative PE may represent the shift from one’s current affective state to a more negatively-valenced state, due to the occurrence or expectancy of an aversive event. As reviewed above, both nonreward and threat stimuli signal an aversive event (i.e., the shift to a less positive and/or more negative state or the prediction of such a shift). We hypothesize that, relative to their peers, severely irritable youth encode more extreme negative PEs in response to nonreward or threat. Negative PE as a common pathway may also aid in understanding outburst triggers that entail a change to the child’s status quo or expectation, but are not related overtly to nonreward or threat. For example, a child may expect to be driven home from school one way, and instead be driven another way, triggering an outburst. The negative PE framework suggests that, for this child, the loss of or threat of losing the status quo or an expected feature of one’s environment is an aversive event. This may be related to threat-relevant cognitive biases. For example, the child may disproportionately attend to slight changes in certain features of the environment (e.g., slightly more traffic) and/or disproportionately interpret those changes as frustrating (e.g., thinking that this will delay a preferred activity at home). It is also possible that because the child is often in an irritable state, the neural circuitry engaged in PE-guided learning may link aspects of the environment with the irritable state. That is, the child’s irritable state itself may drive learning, erroneously linking neutral environmental cues to the irritability. Moreover, dopamine neurons have been shown to change their firing rate in response to shifts in sensory features of the environment (i.e., sensory or identity PEs) (Takahashi et al., 2017). While this neural marker has not been investigated with respect to irritability, we might speculate that more irritable youth overweight or encode more strongly changes in sensory or identity features of the environment per se, which would be evidenced by stronger PEs.
While some neural substrates of positive PE are established, the mechanisms involved in generating and responding to negative PE are not well known. This gap in understanding is partly due to the low baseline firing rate of midbrain dopamine neurons, which can make inhibition difficult to detect (Bayer & Glimcher, 2005). In addition, fewer studies have examined PE during learning from losses or punishments vs. learning from rewards. In a lesion experiment with non-human primates, Taswell and colleagues (2018) found that the ventral striatum contributed uniquely to learning from rewards, but not to learning from losses. Similarly, a recent study in youth reported that insular, but not striatal, activation varied as a function of negative PE (Keren et al., 2018). Schultz’s (2016) two-component theory of PE argues that aversive stimuli primarily impact an initial, nonspecific response to novelty in midbrain dopamine neurons, rather than a true PE with respect to computation of the subjective value of the stimulus. Thus, the little available evidence on negative PE remains inconclusive.
Further, no studies of irritability have investigated PE signaling per se. In one study, youth with severe mood dysregulation (SMD), the precursor to DSM-5 DMDD, exhibited reduced striatal activity (greater deactivation) in response to nonreward relative to healthy volunteer youth (Deveney et al., 2013). This finding is consistent, but not synonymous, with an enhanced negative PE in irritability. Youth with SMD also showed striatal dysfunction during reward learning as compared to healthy youth (Adleman et al., 2011). Youth with conduct problems and oppositionality, a related but distinct phenotype, showed atypical modulation of striatal activity based on stimulus expected value (White et al., 2016). Adolescents with anxiety disorders showed similar striatal dysfunction (Guyer et al., 2012). Finally, youth at risk for depression were characterized by reduced striatal activity to loss, again consistent with an enhanced negative PE (Luking, Pagliaccio, Luby, & Barch, 2016). Clearly, much more research is needed to interrogate the neural basis of negative PE, both from a basic science perspective and in potential clinical applications. Our research group is currently developing and conducting functional magnetic resonance imaging (fMRI) experiments designed to identify neural substrates of positive and negative PEs in youth along the dimension of irritability.
Frustration
In our exposure-targeted model, negative PE in response to nonreward or threat generates frustration. Amsel (1958) conceptualized frustration as a normative response to blocked goal attainment that is associated with increased motor activity and aggression as the animal tries to obtain the reward. Since that time, the definition of frustration has expanded to include affective responses not only to nonreward, but also to failed attempts to avoid punishment, thus implicating threats (Berkowitz, 1989; Berkowitz & Harmon-Jones, 2004). Consistent with this, we view frustration as an adaptive, normative response to negative PE triggered by nonreward and/or threat. We propose negative PE as a neural mechanism through which these triggering stimuli come to elicit frustration.
As we describe below, our model also suggests that cognitive control may moderate the link between negative PE and level of frustration (relevant literature is reviewed below). Specifically, impairments in cognitive control may place youth at risk for (a) increased frustration in response to negative PE, and (b) increased temper outbursts in response to frustration, thus manifesting as chronic, severe irritability. Below, following a description of temper outbursts, we further discuss how cognitive control may moderate these associations.
Temper Outburst
Compared to their peers, youth with clinically significant irritability have a lower threshold for experiencing outbursts (i.e., their outbursts are more frequent and occur in more diverse contexts). Their outbursts are also more severe on average, including greater motor activity (e.g., stomping, waving arms), verbal aggression (e.g., yelling), and sometimes physical aggression (e.g., hitting) (Brotman et al., 2017). Such temper outbursts can be viewed as non-normative behavioral responses to frustration.
Although temper outbursts often manifest as approach behavior toward nonreward or threat stimuli, outbursts can ultimately serve to neutralize or avoid the triggering stimuli, akin to behavioral avoidance in anxiety disorders. We postulate that a temper outburst, while unpleasant to the child in the moment, can function to return the child to a less negatively-valenced affective state than that which was originally triggered (i.e., can generate a positive or less negative PE). This is partly because, as discussed below, a temper outburst can lead to environmental responses that remove the initial trigger (e.g., parent giving child what s/he wants, thus removing nonreward) (Barkley, 2013; Kazdin, 2010).
Cognitive Control
Cognitive control is a multifaceted construct. It refers to the capacity to deploy cognitive resources flexibly in response to changing environmental contingencies (McTeague et al., 2017). Cognitive control involves top-down (primarily prefrontal cortex [PFC] and parietal) regulation of lower-level brain processes to achieve goal-driven behavior. Inhibitory control, a key subcomponent of cognitive control, is the ability to suppress behaviors that are maladaptive or incompatible with current goals (e.g., the ability to stop an automatic or prepotent behavior) (Friedman & Miyake, 2017). Therefore, it is plausible that impairments in cognitive control, in particular inhibitory control, increase the behavioral expression of (a) frustration in response to negative PE, and (b) temper outbursts in response to frustration. In both cases (a) and (b), impairments in inhibitory control would manifest as difficulties stopping affective or behavioral impulses to the preceding input, resulting in increased frustration and temper outbursts (Schall, Palmeri, & Logan, 2017). Consistent with this idea, youth with clinically-significant irritability often (but not always) report higher levels of frustration than healthy youth following nonreward (Deveney et al., 2013; Rich et al., 2007, 2011; but see Grabell et al., 2018; Tseng et al., 2018).
Support for the role of cognitive control in modulating the expression of temper outbursts comes from normative development and developmental psychopathology. Temper outbursts occur most commonly in the preschool years, and then decrease steadily in frequency from mid-childhood through adulthood (Wakschlag et al., 2012, 2015; Wiggins, Mitchell, Stringaris, & Leibenluft, 2014). This temporal pattern follows, inversely, maturation of the PFC and development of inhibitory control (Luna, Marek, Larsen, Tervo-Clemmens, & Chahal, 2015). Research on irritability has implicated brain regions known to mediate cognitive control. In particular, Perlman and colleagues have been studying cognitive control, frustration, and irritability in young children. Findings included associations between higher irritability and decreased activation in regulatory PFC regions during frustration (Grabell et al., 2018; Perlman, Luna, Hein, & Huppert, 2014; Perlman et al., 2015). However, other reports from this group indicated increased PFC activation during cognitive control in relation to higher irritability (Fishburn et al., 2019; Li, Grabell, Wakschlag, Huppert, & Perlman, 2017). The specific ability to control one’s attention in emotional contexts (i.e., executive attention) also has been related to developmental and individual differences in youth’s regulation of mood (Posner & Rothbart, 1998; Rueda, Posner, & Rothbart, 2005). We recently found that, in children and adolescents, higher levels of irritability were associated with increased PFC engagement when performing an attention orienting task immediately following frustration. In some PFC regions, the irritability-related increased activation was specific to younger children. This increased PFC engagement could reflect a compensatory mechanism that allows irritable youth to recruit these regions to a greater extent and thus be able to meet task goals (Tseng et al., 2018). Last, Karim and Perlman (2017) showed that, in irritable youth, PFC activation to negatively-valenced images increased with advancing age.
Thus, there is evidence for both irritability and age effects on dysfunction in brain regions known to mediate cognitive control. However, the precise direction of these neural effects has been mixed, and neural effects have been reported in the absence of behavioral deficits in cognitive control (e.g., Tseng et al., 2018). A large body of research documents behavioral impairments in cognitive control in externalizing disorders that are frequently comorbid with DMDD (e.g., ADHD) (Nigg, 2000; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). Importantly, such behavioral impairments have not yet been isolated to irritability vs. other symptom dimensions in these populations. In contrast, anxiety has been associated with excessive inhibitory control (Moser, Moran, Schroder, Donnellan, & Yeung, 2013), although findings may vary across development (Meyer et al., 2018). We are currently conducting further behavioral and fMRI research to characterize the nature of inhibitory control in irritability, both at baseline (i.e., cold cognitive control) and in the context of frustration, across a range of ages from mid-childhood through late adolescence. It will be particularly important to distinguish between baseline inhibitory control vs. inhibitory control specifically during frustration. Last, while our model proposes moderating effects of cognitive control on symptom expression, some neurocognitive theories of child psychopathology (e.g., ADHD; Nigg & Casey, 2005) propose that perturbed cognitive control can contribute directly to PE signaling deficits. In future work, it will be important to test differing conceptualizations of how these neurocognitive functions relate to one another.
Consequences of Outburst
As the final component of the model, consequences of temper outbursts include parental or other environmental responses that influence the likelihood and/or intensity of future negative PEs and outbursts. This component is based on extensions of basic behavioral principles (Mowrer, 1960) to research on disruptive psychopathology, as reflected in longstanding parent-child interaction research with relevance to therapeutics (e.g., Patterson, DeBaryshe, & Ramsey, 1989; Patterson, 1982; Patterson, Reid, & Dishion, 1992). Specifically, the treatment literature indicates a causal effect of parenting interventions on child behavior (e.g., Comer, Chow, Chan, Cooper-Vince, & Wilson, 2013; Furlong et al., 2012), including the prevention of aggressive behavior in youth at high risk (e.g., Brotman et al., 2008). Parents are also key participants in CBT for child anxiety disorders, which often includes PMT components (Higa-McMillan, Francis, Rith-Najarian, & Chorpita, 2016). Proximal environmental responses to temper outbursts may be reinforcing (i.e., increase future negative PEs and outbursts) or punishing (i.e., decrease future negative PEs and outbursts). Common examples of responses that can be reinforcing are: differential attention (e.g., parent increasing attention to child); and removal of triggering stimuli (e.g., parent giving child what s/he wants). Differential attention and removal of triggering stimuli most likely reinforce outbursts directly through response-outcome learning (Barkley, 2013; Kazdin, 2010). Here, there are parallels to pediatric anxiety, in which overprotective parental responses (i.e., attention to potential threats and removing the child from anxiety-producing stimuli or situations) may reinforce youth’s anxiety and avoidant behavior (Rapee, Schniering, & Hudson, 2009). In addition, parents of irritable youth may engage in negative affective responses to temper outbursts (e.g., parent displaying anger or anxiety), serving to differentially reinforce negative PEs. Specifically, such responses can increase the attentional salience of nonreward and/or threat events, enhancing their encoding (Stoddard & Jones, personal communication). Negative affective responses may also increase the subjective aversiveness of nonreward and/or threat events for youth, making future negative PEs even more extreme. In this manner, learning history could lead to amplification of negative PEs in youth with irritability.
Summary
In sum, this conceptualization of specific, proximal mechanisms in irritability includes elevated negative PEs to nonreward and/or threat stimuli, which lead to increased frustration. Increased frustration, along with impaired cognitive control in the context of frustration, generates more frequent and severe temper outbursts. Temper outbursts, in turn, lead to environmental responses that may reinforce future negative PEs or outbursts. Here, specific component processes represent key targets for intervention.
Exposure-Based CBT for Irritability
In this section, we describe the exposure-based CBT that our group has been developing for chronic, severe irritability such as that seen in DMDD. We postulate how this treatment targets key mechanisms of irritability proposed above, and we discuss how we are testing predictions regarding target engagement and clinical efficacy in an ongoing trial (ClinicalTrials.gov: NCT02531893).
Overview of the Treatment
Exposure is a behavioral technique grounded in the phobia and anxiety disorder treatment literature. Patients confront and tolerate (i.e., no longer avoid) stimuli that they perceive as threatening, and learn that expected adverse outcomes do not occur (Craske et al., 2008; Foa & McLean, 2016). Our initial consideration of exposure as an intervention for irritability began with several broad observations. First, pediatric irritability and anxiety disorders are highly comorbid (Cornacchio, Crum, Coxe, Pincus, & Comer, 2016; Stoddard et al., 2014), partly due to shared genetics (Savage et al., 2015). Second, irritability and anxiety are both characterized by threat-relevant cognitive biases (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007; Hommer et al., 2014; Lau & Waters, 2017; Rozenman, Amir, & Weersing, 2015; Salum et al., 2017; Stoddard et al., 2016; Waters & Craske, 2016; White, Suway, Pine, Bar-Haim, & Fox, 2011), and there is an established neural circuit mediating threat responding including the ventromedial PFC, amygdala, hypothalamus, and periaqueductal gray (Blair, 2010). Third, both irritability and anxiety are characterized by high-arousal negative affective states in response to a triggering stimulus (Rothbart, 2007; Watson & Clark, 1984), therefore potentially suitable for exposure. In the adult anger treatment literature, a pilot study of imaginal exposure appeared promising (Grodnitzky & Tafrate, 2000). Thus, we began to discuss whether in vivo exposure to stimuli that evoke temper outbursts within a safe, supportive therapeutic context could generate corrective learning in patients with clinically-significant irritability.
The treatment that we developed focuses on controlled, graduated exposure to nonreward and/or threat stimuli with therapeutic support. The current manualized CBT protocol (Brotman, Kircanski, Gold, & Leibenluft, 2018) includes 12 weekly outpatient therapy sessions, each 60-90 minutes in duration. The first half of each session (30-45 minutes) involves the patient completing exposure exercises. The second half of each session (30-45 minutes) involves selected parent management training (PMT) techniques (Barkley, 2013; Kazdin, 2010). While therapists initially facilitate the exposures, parents are encouraged to facilitate the exposures both in session and during weekly practices once PMT skills are sufficiently demonstrated. Common examples of in vivo exposures include: child having an electronic device taken away and withstanding not taking it back; transitioning from a preferred activity to a nonpreferred activity; interacting with a confederate who is “acting out” a peer who the child perceives as threatening; and completing challenging schoolwork. The therapy also includes imaginal exposures when appropriate (e.g., imagining interacting with a peer who is perceived as threatening).
We recently published an open active pilot of the treatment in 10 youth diagnosed with DMDD, through which we manualized therapy procedures and documented preliminary feasibility (Kircanski, Clayton, et al., 2018). The pilot study data did not indicate any evidence of DMDD symptom worsening in any patient as a result of the treatment; this is important as some theorists have cautioned that exposure for anger may be contraindicated or may not lead to reduction in affective responding (Abramowitz, 2013). For a full description of the treatment, see Kircanski, Clayton, et al. (2018). Here, we elaborate our hypotheses on how specific techniques within the treatment target the mechanisms of irritability outlined above.
Primary Targets of Exposure
As shown in Figure 1, therapeutic exposure to nonreward and/or threat stimuli is posited to engage cognitive control circuitry and target top-down regulation of frustration. Specifically, we hypothesize that repeated exposure to and toleration of negative PE-driven frustration (i.e., shifts from one’s current affective state to a more negatively-valenced state) will increase patients’ inhibitory control capacity in this context. For inhibitory control to be successfully deployed, therapists must work with patients to block prepotent outburst behaviors (e.g., yelling or grabbing at parent to not take away video game). Therefore, prior to initiating exposures, therapists and patients discuss the precise behaviors to be inhibited. Over the course of treatment, it is expected that increasing top-down regulation will be associated with (a) decreased frustration in response to negative PE, and (b) decreased temper outbursts in response to frustration.
Previous work in fear and anxiety disorders documents engagement of the ventromedial PFC to downregulate amygdala activity during extinction (e.g., Maren & Quirk, 2004; Sotres-Bayon, Cain, & LeDoux, 2006) and exposure therapy (e.g., Hauner, Mineka, Voss, & Paller, 2012). Based on the reviewed findings for cognitive control dysfunction in irritability (Fishburn et al., 2018; Li et al., 2017; Tseng et al., 2018), we predict that regional activation including the ventromedial and dorsolateral PFC will be more normalized from pre- to post-CBT. To test this prediction, we are currently conducting a within-subjects multiple baseline trial of the CBT, which includes pre-, mid-, and post-treatment fMRI scanning on the task that assesses attention orienting in the context of frustration (Tseng et al., 2018). As noted above, higher irritability was related to increased PFC engagement on that task, potentially reflecting a compensatory mechanism to meet task goals despite frustration. Therefore, an intriguing possibility is that PFC activation may decrease from pre- to post-CBT, indicating a normalization or recalibration of top-down and bottom-up processes. The fMRI assessment at mid-treatment will be used to interrogate neural changes as mediators of subsequent clinical changes.
In addition to engaging cognitive control to regulate behavior, exposure may also have downstream effects on negative PE. This is because, as noted above, temper outbursts may serve an avoidant function in which the child does not actually confront the aversive event (e.g., parent gives child what s/he wants, thus removing the nonreward event). By inhibiting temper outbursts and thus their avoidant function, patients can learn through exposures that the nonreward or threat stimuli are not as aversive as they expected, or that they can tolerate their level of frustration while completing the aversive tasks (Craske et al., 2008). That is, patients’ elevated negative PEs will no longer be instrumentally reinforced, and thereby reduced at post-treatment. To test this hypothesis, CBT patients complete a reward learning fMRI task pre- and post-treatment. This task assesses reward learning both in a neutral affective context and following an acute frustration induction. Using this task, we will computationally model the neural substrates of positive and negative PE in a whole-brain analysis.
Primary Targets of Parent Interventions
As noted above, the selected PMT interventions within exposure-based CBT for irritability are theorized to target reinforcement mechanisms (Barkley, 2013; Kazdin, 2010). Indeed, PMT is based in principles of operant conditioning in which behavior is influenced by its consequences (Mowrer, 1960). With respect to our current model of treatment targets in irritability, interventions designed to reduce parents’ attention to temper outbursts and parents’ tendency to remove the triggering stimuli are expected to decrease direct reinforcement of outbursts. In addition, therapists conducting the CBT focus heavily on parents’ affective responses to outbursts. Therapists work with parents to minimize their negative affect in response to outbursts, thus reducing reinforcement of patients’ negative PEs posited above. This component of the treatment is predicted to decrease the likelihood or intensity of future negative PEs by reducing the associative strength between parents’ negative affective responses and the triggering stimuli. Parents participating in the trial complete pre-, mid-, and post-treatment measures of parenting behaviors in response to child temper outbursts. These measures include ecological momentary assessment (EMA), in which parents use smartphones to report on their responses to child outbursts in real time (e.g., attention vs. active ignoring). Parents also complete behavioral tasks that parallel the patient tasks described above, assessing attention orienting and reward learning in the context of frustration. We will examine these data in relation to the patients’ data, to investigate how changes in parental responses to temper outbursts relate to neural, behavioral, and clinical changes in patients.
Key Questions for Future Research
In the coming years, we plan to test the hypotheses described here through continued pathophysiological and treatment-related studies. As work in this area progresses, it will be important to focus on several specific questions.
First, how might the mechanisms of exposure-based CBT for irritability compare to mechanisms of exposure therapy for anxiety disorders? As noted above, our initial consideration of exposure as an intervention for irritability stemmed from observations of its comorbidity and shared features with anxiety. Similar to the model of irritability proposed here, anxiety disorders involve phasic affective responses to threats and associated non-normative behavior (LeDoux & Pine, 2016). However, whereas irritability involves aberrant approach behavior toward threats, anxiety disorders involve avoidance behavior. These differing behavioral responses to threat may implicate different neural circuitry and, therefore, distinct potential targets for intervention (Brotman et al., 2017; Kircanski, White, et al., 2018). In particular, while exposure therapy for anxiety disorders is thought to enhance ventromedial PFC regulation of amygdala activation (Craske et al., 2008; Hauner et al., 2012), we have postulated that one mechanism of exposure-based CBT for irritability involves broader improvements in inhibitory control, mediated by dorsolateral and ventromedial PFC engagement. Thus, it will be interesting to examine how exposure-driven changes at the brain and behavioral levels compare across these two phenotypes. It is possible that both forms of exposure target negative PE in response to threats (i.e., neural encoding of the expectancy of aversive events), but only exposure for irritability also targets cognitive control (i.e., active inhibition of outburst behavior in response to frustration).
Second, could this pathophysiological and treatment model apply to irritability transdiagnostically, in the setting of other psychopathologies besides DMDD? As reviewed, irritability is an associated symptom of many clinical presentations, including anxiety disorders and depression (Cornacchio et al., 2016; Stoddard et al., 2014; Stringaris, Zavos, Leibenluft, Maughan, & Eley, 2012; Toohey & DiGiuseppe, 2017). Recently, our group reported an interaction between irritability and anxiety symptoms in relation to functional connectivity between the amygdala and ventromedial PFC when viewing threatening faces (Stoddard et al., 2017). In contrast, we found distinct neural correlates of irritability in youth with DMDD vs. bipolar disorder (Wiggins et al., 2016); these contrasting findings may reflect the stronger clinical and genetic links between irritability and anxiety (Savage et al., 2015; Stringaris et al., 2012) than between irritability and bipolar disorder (Sparks et al., 2014). Similar research questions should be explored across multiple phenotype contexts. Moreover, it is important to test whether exposure targeting irritability symptoms provides incremental benefit for youth with other primary diagnoses such as anxiety or depression. To the extent that youth with such comorbidities experience frustration and temper outbursts in response to nonreward or threat stimuli, and those outbursts are distressing or impairing, exposure for irritability may be worth considering.
Third, how does exposure-based CBT for irritability compare to established treatments for disruptive behavior, particularly PMT? As a parent-delivered treatment, PMT intervenes at the environmental level to modify maladaptive parent-child interactions. This approach focuses on the consequences of child outbursts as noted above, as well as antecedent processes such as the ways in which parents structure the child’s environment and give commands for behavior (Barkley, 2013; Kazdin, 2010). Given that PMT typically focuses on disruptive behavior in preschool-aged children (e.g., Comer et al., 2013) or adolescents with primary antisocial behavior (e.g., Furlong et al., 2012), it is unclear whether PMT would be effective for youth with chronic, severe irritability. Indeed, the vast majority of studies of PMT have not reported on irritability as a baseline or outcome variable (Waxmonsky et al., 2008, 2013). Future randomized controlled trials might compare exposure-based CBT to traditional PMT for youth with irritability.
Of course, there are many more open, tractable research questions to pursue on pediatric irritability in the years to come. It will be critical to conduct multi-modal studies that refine our understanding of the links among brain, behavior, and environment in relation to this phenotype. As research on pediatric irritability continues to advance, new studies can have a significant impact on both scientific understanding and clinical care.
Highlights.
We propose mechanisms of pediatric irritability across the brain, behavior, and environment
Key neurocognitive processes include prediction error and cognitive control
These may be targets for exposure-based cognitive-behavioral therapy (CBT) for irritability
Exposure-based CBT for irritability is currently being tested in a clinical trial
This research may inform understanding of the brain and behavior mechanisms of CBT
Acknowledgements:
This research was supported by the Intramural Research Program (IRP) of the National Institute of Mental Health, National Institutes of Health (NIMH/NIH), ZIAMH002786 (Leibenluft) and ZIAMH002781 (Pine), and was conducted under NIH Clinical Study Protocols 00-M-0021, 01-M-0192, and 15-M-0182 (ClinicalTrials.gov IDs: NCT00025935, NCT00018057, and NCT02531893).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We further confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email.
Signed by all authors on November 30, 2018 as follows:
Katharina Kircanski, Ph.D.
Michelle G. Craske, Ph.D.
Bruno B. Averbeck, Ph.D.
Daniel S. Pine, M.D.
Ellen Leibenluft, M.D.
Melissa A. Brotman, Ph.D.
References
- Abramowitz JS (2013). The practice of exposure therapy: Relevance of cognitive-behavioral theory and extinction theory. Behavior Therapy, 44, 548–558. [DOI] [PubMed] [Google Scholar]
- Adleman NE, Kayser R, Dickstein D, Blair RJR, Pine D, & Leibenluft E (2011). Neural correlates of reversal learning in severe mood dysregulation and pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 50, 1173–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althoff RR, Crehan ET, He J-P, Burstein M, Hudziak JJ, & Merikangas KR (2016). Disruptive mood dysregulation disorder at ages 13-18: Results from the National Comorbidity Survey-Adolescent Supplement. Journal of Child and Adolescent Psychopharmacology, 26, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders. 5th ed Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Amsel A (1958). The role of frustrative nonreward in noncontinuous reward situations. Psychological Bulletin, 55, 102–119. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, & Costa VD (2017). Motivational neural circuits underlying reinforcement learning. Nature Neuroscience, 20, 505–512. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, & van IJzendoorn MH (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin, 133, 1–24. [DOI] [PubMed] [Google Scholar]
- Barkley RA (2013). Defiant children: A clinician’s manual for assessment and parent training. New York: Guilford Press. [Google Scholar]
- Bayer HM, & Glimcher PW (2005). Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron, 47, 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz L (1989). Frustration-aggression hypothesis: Examination and reformulation. Psychological Bulletin, 106, 59–73. [DOI] [PubMed] [Google Scholar]
- Berkowitz L (2012). A different view of anger: The cognitive-neoassociation conception of the relation of anger to aggression. Aggressive Behavior, 38, 322–333. [DOI] [PubMed] [Google Scholar]
- Berkowitz L, & Harmon-Jones E (2004). Toward an understanding of the determinants of anger. Emotion, 4, 107–130. [DOI] [PubMed] [Google Scholar]
- Blair RJR (2010). Psychopathy, frustration, and reactive aggression: the role of ventromedial prefrontal cortex. British Journal of Psychology, 101, 383–399. [DOI] [PubMed] [Google Scholar]
- Brotman LM, Gouley KK, Huang K-Y, Rosenfelt A, O’Neal C, Klein RG, & Shrout P (2008). Preventive intervention for preschoolers at high risk for antisocial behavior: Long-term effects on child physical aggression and parenting practices. Journal of Clinical Child and Adolescent Psychology, 37, 386–396. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Kircanski K, Gold AL, & Leibenluft E (2018). Exposure-based cognitive-behavioral therapy for irritability and disruptive mood dysregulation disorder. Unpublished therapy manual. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA, Kircanski K, Stringaris A, Pine DS, & Leibenluft E (2017). Irritability in youths: A translational model. The American Journal of Psychiatry, 174, 520–532. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, … Leibenluft E (2010). Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. The American Journal of Psychiatry, 167, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA, Schmajuk M, Rich BA, Dickstein DP, Guyer AE, Costello EJ, … Leibenluft E (2006). Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biological Psychiatry, 60, 991–997. [DOI] [PubMed] [Google Scholar]
- Burke JD, Hipwell AE, & Loeber R (2010). Dimensions of oppositional defiant disorder as predictors of depression and conduct disorder in preadolescent girls. Journal of the American Academy of Child and Adolescent Psychiatry, 49, 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss AH, & Durkee A (1957). An inventory for assessing different kinds of hostility. Journal of Consulting Psychology, 21, 343–349. [DOI] [PubMed] [Google Scholar]
- Caprara GV, Cinanni V, D’Imperio G, Passerini S, Renzi P, & Travaglia G (1985). Indicators of impulsive aggression: Present status of research on irritability and emotional susceptibility scales. Personality and Individual Differences, 6, 665–674. [Google Scholar]
- Caprara Gian Vittorio, Paciello M, Gerbino M, & Cugini C (2007). Individual differences conducive to aggression and violence: Trajectories and correlates of irritability and hostile rumination through adolescence. Aggressive Behavior, 33, 359–374. [DOI] [PubMed] [Google Scholar]
- Comer JS, Chow C, Chan PT, Cooper-Vince C, & Wilson LAS (2013). Psychosocial treatment efficacy for disruptive behavior problems in very young children: a meta-analytic examination. Journal of the American Academy of Child and Adolescent Psychiatry, 52, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Angold A, Costello EJ, & Egger H (2013). Prevalence, comorbidity, and correlates of DSM-5 proposed disruptive mood dysregulation disorder. American Journal of Psychiatry, 170, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Brotman MA, & Costello EJ (2015). Normative irritability in youth: Developmental findings from the Great Smoky Mountains Study. Journal of the American Academy of Child and Adolescent Psychiatry, 54, 635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornacchio D, Crum KI, Coxe S, Pincus DB, & Comer JS (2016). Irritability and anxiety severity among youth with anxiety. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, & Baker A (2008). Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy, 46, 5–27. [DOI] [PubMed] [Google Scholar]
- Crick NR, & Dodge KA (1994). A review and reformulation of social information-processing mechanisms in children’s social adjustment. Psychological Bulletin, 115, 74–101. [Google Scholar]
- Deveney CM, Connolly ME, Haring CT, Bones BL, Reynolds RC, Kim P, … Leibenluft E (2013). Neural mechanisms of frustration in chronically irritable children. The American Journal of Psychiatry, 170, 1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA (1980). Social cognition and children’s aggressive behavior. Child Development, 51, 162–170. [PubMed] [Google Scholar]
- Drabick DA, & Gadow KD (2012). Deconstructing oppositional defiant disorder: clinic-based evidence for an anger/irritability phenotype. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS (1994). Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review, 1, 429–438. [DOI] [PubMed] [Google Scholar]
- Foa EB, & McLean CP (2016). The efficacy of exposure therapy for anxiety-related disorders and its underlying mechanisms: The case of OCD and PTSD. Annual Review of Clinical Psychology, 12, 1–28. [DOI] [PubMed] [Google Scholar]
- Friedman NP, & Miyake A (2017). Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex, 86, 186–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong M, McGilloway S, Bywater T, Hutchings J, Smith SM, & Donnelly M (2012). Behavioural and cognitive-behavioural group-based parenting programmes for early-onset conduct problems in children aged 3 to 12 years. The Cochrane Database of Systematic Reviews, 2, CD008225. [DOI] [PubMed] [Google Scholar]
- Grabell AS, Li Y, Barker JW, Wakschlag LS, Huppert TJ, & Perlman SB (2018). Evidence of non-linear associations between frustration-related prefrontal cortex activation and the normal:abnormal spectrum of irritability in young children. Journal of Abnormal Child Psychology, 46, 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodnitzky GR, & Tafrate RC (2000). Imaginal exposure for anger reduction in adult outpatients: A pilot study. Journal of Behavior Therapy and Experimental Psychiatry, 31, 259–279. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, … & Ernst M (2012). Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. American Journal of Psychiatry, 169, 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller SP, Kircanski K, Stringaris A, Clayton M, Bui H, Agorsor C, Cardenas SI, Towbin KE, Pine DS, Leibenluft E, & Brotman MA (under review). The Clinician Affective Reactivity Index: Validity and reliability of a clinician-rated assessment of irritability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauner KK, Mineka S, Voss JL, & Paller KA (2012). Exposure therapy triggers lasting reorganization of neural fear processing. Proceedings of the National Academy of Sciences of the United States of America, 109, 9203–9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa-McMillan CK, Francis SE, Rith-Najarian L, & Chorpita BF (2016). Evidence base update: 50 years of research on treatment for child and adolescent anxiety. Journal of Clinical Child and Adolescent Psychology, 45, 91–113. [DOI] [PubMed] [Google Scholar]
- Hommer RE, Meyer A, Stoddard J, Connolly ME, Mogg K, Bradley BP, … Brotman MA (2014). Attention bias to threat faces in severe mood dysregulation. Depression and Anxiety, 31, 559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim HT, & Perlman SB (2017). Neurodevelopmental maturation as a function of irritable temperament: Insights from a naturalistic emotional video viewing paradigm. Human Brain Mapping, 38, 5307–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE (2010). Problem-solving skills training and parent management training for oppositional defiant disorder and conduct disorder In Evidence-based psychotherapies for children and adolescents, second edition (pp. 211–226). New York: Guilford Press. [Google Scholar]
- Keren H, Chen G, Benson B, Ernst M, Leibenluft E, Fox NA, … Stringaris A (2018). Is the encoding of reward prediction error reliable during development? NeuroImage, 178, 266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K, Clayton ME, Leibenluft E, & Brotman MA (2018). Psychosocial treatment of irritability in youth. Current Treatment Options in Psychiatry, 5, 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K, White LK, Tseng W-L, Wiggins JL, Frank HR, Sequeira S, … Brotman MA (2018). A latent variable approach to differentiating neural mechanisms of irritability and anxiety in youth. JAMA Psychiatry, 75, 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, & Waters AM (2017). Annual Research Review: An expanded account of information-processing mechanisms in risk for child and adolescent anxiety and depression. Journal of Child Psychology and Psychiatry, 58, 387–407. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, & Pine DS (2016). Using neuroscience to help understand fear and anxiety: a two-system framework. The American Journal of Psychiatry, 173, 1083–1093. [DOI] [PubMed] [Google Scholar]
- Leibenluft E (2011). Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. The American Journal of Psychiatry, 168, 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E (2017). Pediatric irritability: A systems neuroscience approach. Trends in Cognitive Sciences, 21, 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, & Stoddard J (2013). The developmental psychopathology of irritability. Development and Psychopathology, 25, 1473–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Pagliaccio D, Luby JL, & Barch DM (2016). Depression risk predicts blunted neural responses to gains and enhanced responses to losses in healthy children. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, & Chahal R (2015). An integrative model of the maturation of cognitive control. Annual Review of Neuroscience, 38, 151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, & Quirk GJ (2004). Neuronal signalling of fear memory. Nature Reviews. Neuroscience, 5, 844–852. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, & Etkin A (2017). Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. The American Journal of Psychiatry, 174, 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey-Newman D, Kujawa A, Olino TM, Dyson M, & Klein DN (2018). Early temperamental fearfulness and the developmental trajectory of error-related brain activity. Developmental Psychobiology, 60, 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser J, Moran T, Schroder H, Donnellan B, & Yeung N (2013). On the relationship between anxiety and error monitoring: A meta-analysis and conceptual framework. Frontiers in Human Neuroscience, 7, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowrer OH (1960). Learning theory and behavior. Hoboken, NJ: Wiley & Sons. [Google Scholar]
- Nigg JT (2000). On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin, 126, 220–246. [DOI] [PubMed] [Google Scholar]
- Nigg JT, & Casey BJ (2005). An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology, 17, 785–806. [DOI] [PubMed] [Google Scholar]
- Orri M, Galera C, Turecki G, Forte A, Renaud J, Boivin M, … Geoffroy M-C (2018). Association of childhood irritability and depressive/anxious mood profiles with adolescent suicidal ideation and attempts. JAMA Psychiatry, 75, 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GR, DeBaryshe BD, & Ramsey E (1989). A developmental perspective on antisocial behavior. The American Psychologist, 44, 329–335. [DOI] [PubMed] [Google Scholar]
- Patterson GR (1982). Coercive family process. Eugene: Castalia Pub. Co. [Google Scholar]
- Patterson GR, Reid JB, & Dishion TJ (1992). Antisocial boys. Eugene: Castalia Pub. Co. [Google Scholar]
- Perlman SB, Jones BM, Wakschlag LS, Axelson D, Birmaher B, & Phillips ML (2015). Neural substrates of child irritability in typically developing and psychiatric populations. Developmental Cognitive Neuroscience, 14, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Luna B, Hein TC, & Huppert TJ (2014). fNIRS evidence of prefrontal regulation of frustration in early childhood. NeuroImage, 85, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, & Rothbart MK (1998). Attention, self-regulation and consciousness. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 353, 1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapee RM, Schniering CA, & Hudson JL (2009). Anxiety disorders during childhood and adolescence: origins and treatment. Annual review of clinical psychology, 5, 311–341. [DOI] [PubMed] [Google Scholar]
- Rich BA, Carver FW, Holroyd T, Rosen HR, Mendoza JK, Cornwell BR, … Leibenluft E (2011). Different neural pathways to negative affect in youth with pediatric bipolar disorder and severe mood dysregulation. Journal of Psychiatric Research, 45, 1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Schmajuk M, Perez-Edgar KE, Fox NA, Pine DS, & Leibenluft E (2007). Different psychophysiological and behavioral responses elicited by frustration in pediatric bipolar disorder and severe mood dysregulation. The American Journal of Psychiatry, 164, 309–317. [DOI] [PubMed] [Google Scholar]
- Rothbart MK (2007). Temperament, development, and personality. Current Directions in Psychological Science, 16, 207–212. [Google Scholar]
- Rothenhoefer KM, Costa VD, Bartolo R, Vicario-Feliciano R, Murray EA, & Averbeck BB (2017). Effects of ventral striatum lesions on stimulus-based versus action-based reinforcement learning. The Journal of Neuroscience, 37, 6902–6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Klein RG, Angelosante A, Bar-Haim Y, Leibenluft E, Hulvershorn L, … Spindel C (2013). Clinical features of young children referred for impairing temper outbursts. Journal of Child and Adolescent Psychopharmacology, 23, 588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenman M, Amir N, & Weersing VR (2014). Performance-based interpretation bias in clinically anxious youths: Relationships with attention, anxiety, and negative cognition. Behavior Therapy, 45, 594–605. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Posner MI, & Rothbart MK (2005). The development of executive attention: contributions to the emergence of self-regulation. Developmental Neuropsychology, 28, 573–594. [DOI] [PubMed] [Google Scholar]
- Salum GA, Mogg K, Bradley BP, Stringaris A, Gadelha A, Pan PM, … Leibenluft E (2017). Association between irritability and bias in attention orienting to threat in children and adolescents. Journal of Child Psychology and Psychiatry, 58, 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage J, Verhulst B, Copeland W, Althoff RR, Lichtenstein P, & Roberson-Nay R (2015). A genetically informed study of the longitudinal relation between irritability and anxious/depressed symptoms. Journal of the American Academy of Child and Adolescent Psychiatry, 54, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Palmeri TJ, & Logan GD (2017). Models of inhibitory control. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, & Dickinson A (2000). Neuronal coding of prediction errors. Annual Review of Neuroscience, 23, 473–500. [DOI] [PubMed] [Google Scholar]
- Schultz W (2016). Dopamine reward prediction-error signalling: A two-component response. Nature Reviews. Neuroscience, 17, 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, & LeDoux JE (2006). Brain mechanisms of fear extinction: Historical perspectives on the contribution of prefrontal cortex. Biological Psychiatry, 60, 329–336. [DOI] [PubMed] [Google Scholar]
- Sparks GM, Axelson DA, Yu H, Ha W, Ballester J, Diler RS, … Birmaher B (2014). Disruptive mood dysregulation disorder and chronic irritability in youth at familial risk for bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 53, 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard J, Sharif-Askary B, Harkins EA, Frank HR, Brotman MA, Penton-Voak IS, … Leibenluft E (2016). An open pilot study of training hostile interpretation bias to treat disruptive mood dysregulation disorder. Journal of Child and Adolescent Psychopharmacology, 26, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard J, Stringaris A, Brotman MA, Montville D, Pine DS, & Leibenluft E (2014). Irritability in child and adolescent anxiety disorders. Depression and Anxiety, 31, 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard J, Tseng W-L, Kim P, Chen G, Yi J, Donahue L, … Leibenluft E (2017). Association of irritability and anxiety with the neural mechanisms of implicit face emotion processing in youths with psychopathology. JAMA Psychiatry, 74, 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, & Goodman R (2009a). Longitudinal outcome of youth oppositionality: irritable, headstrong, and hurtful behaviors have distinctive predictions. Journal of the American Academy of Child and Adolescent Psychiatry, 48, 404–412. [DOI] [PubMed] [Google Scholar]
- Stringaris A, & Goodman R (2009b). Three dimensions of oppositionality in youth. Journal of Child Psychology and Psychiatry, 50, 216–223. [DOI] [PubMed] [Google Scholar]
- Stringaris A, Goodman R, Ferdinando S, Razdan V, Muhrer E, Leibenluft E, & Brotman MA (2012). The Affective Reactivity Index: A concise irritability scale for clinical and research settings. Journal of Child Psychology and Psychiatry, 53, 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Vidal-Ribas P, Brotman MA, & Leibenluft E (2018). Practitioner Review: Definition, recognition, and treatment challenges of irritability in young people. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 59, 721–739. [DOI] [PubMed] [Google Scholar]
- Stringaris A, Zavos H, Leibenluft E, Maughan B, & Eley TC (2012). Adolescent irritability: Phenotypic associations and genetic links with depressed mood. The American Journal of Psychiatry, 169, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi YK, Batchelor HM, Liu B, Khanna A, Morales M, & Schoenbaum G (2017). Dopamine neurons respond to errors in the prediction of sensory features of expected rewards. Neuron, 95, 1395–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taswell CA, Costa VD, Murray EA, & Averbeck BB (2018). The ventral striatum’s role in learning from gains and losses. Proceedings of the National Academy of Sciences, 115, E12398–E12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, & Schultz W (2005). Adaptive coding of reward value by dopamine neurons. Science, 307, 1642–1645. [DOI] [PubMed] [Google Scholar]
- Toohey MJ, & DiGiuseppe R (2017). Defining and measuring irritability: Construct clarification and differentiation. Clinical Psychology Review, 53, 93–108. [DOI] [PubMed] [Google Scholar]
- Tseng W-L, Deveney CM, Stoddard J, Kircanski K, Frackman AE, Yi JY, … Leibenluft E (2018). Brain mechanisms of attention orienting following frustration: associations with irritability and age in youths. The American Journal of Psychiatry, appiajp201818040491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Ribas P, Brotman MA, Valdivieso I, Leibenluft E, & Stringaris A (2016). The status of irritability in psychiatry: A conceptual and quantitative review. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 556–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AR (1969). Frustrative nonreward: A variety of punishment. Punishment and aversive behavior In Campbell BA & Church RM (Eds.), Punishment and aversive behavior. New York: Appleton-Century-Crofts. [Google Scholar]
- Wakschlag LS, Estabrook R, Petitclerc A, Henry D, Burns JL, Perlman SB, … Briggs-Gowan ML (2015). Clinical implications of a dimensional approach: The normal:abnormal spectrum of early irritability. Journal of the American Academy of Child and Adolescent Psychiatry, 54, 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Henry DB, Tolan PH, Carter AS, Burns JL, & Briggs-Gowan MJ (2012). Putting theory to the test: Modeling a multidimensional, developmentally-based approach to preschool disruptive behavior. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, & Craske MG (2016). Towards a cognitive-learning formulation of youth anxiety: A narrative review of theory and evidence and implications for treatment. Clinical Psychology Review, 50, 50–66. [DOI] [PubMed] [Google Scholar]
- Watson D, & Clark LA (1984). Negative affectivity: The disposition to experience aversive emotional states. Psychological Bulletin, 96, 465–490. [PubMed] [Google Scholar]
- Waxmonsky JG, Wymbs FA, Pariseau ME, Belin PJ, Waschbusch DA, Babocsai L, … Pelham WE (2013). A novel group therapy for children with ADHD and severe mood dysregulation. Journal of Attention Disorders, 17, 527–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxmonsky J, Pelham WE, Gnagy E, Cummings MR, O’Connor B, Majumdar A, … Robb JA (2008). The Efficacy and tolerability of methylphenidate and behavior modification in children with attention-deficit/hyperactivity disorder and severe mood dysregulation. Journal of Child and Adolescent Psychopharmacology, 18, 573–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LK, Suway JG, Pine DS, Bar-Haim Y, & Fox NA (2011). Cascading effects: The influence of attention bias to threat on the interpretation of ambiguous information. Behaviour Research and Therapy, 49, 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Tyler PM, Erway AK, Botkin ML, Kolli V, Meffert H, … Blair JR (2016). Dysfunctional representation of expected value is associated with reinforcement-based decision-making deficits in adolescents with conduct problems. Journal of Child Psychology and Psychiatry, 57, 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Mitchell C, Stringaris A, & Leibenluft E (2014). Developmental trajectories of irritability and bidirectional associations with maternal depression. Journal of the American Academy of Child and Adolescent Psychiatry, 53, 1191–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, & Pennington BF (2005). Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry, 57, 1336–1346. [DOI] [PubMed] [Google Scholar]