Abstract
Aphids are agricultural pest insects that transmit viruses and cause feeding damage on a global scale. Current pest control practices involving the excessive use of synthetic insecticides over many years have resulted in aphid resistance to a number of pesticides. In nature, plants produce secondary metabolites during their interaction with insects and these metabolites can act as toxicants, antifeedants, anti-oviposition agents and deterrents towards the insects. In a previous study, we demonstrated that the butanol fraction from a crude methanolic extract of an important plant species, Isodon rugosus showed strong insecticidal activity against the pea aphid, Acyrthosiphon pisum. To further explore this finding, the current study aimed to exploit a bioactivity-guided strategy to isolate and identify the active compound in the butanol fraction of I. rugosus. As such, reversed-phase flash chromatography, acidic extraction and different spectroscopic techniques were used to isolate and identify the new compound, rosmarinic acid, as the bioactive compound in I. rugosus. Insecticidal potential of rosmarinic acid against A. pisum was evaluated using standard protocols and the data obtained was analyzed using qualitative and quantitative statistical approaches. Considering that a very low concentration of this compound (LC90 = 5.4 ppm) causes significant mortality in A. pisum within 24 h, rosmarinic acid could be exploited as a potent insecticide against this important pest insect. Furthermore, I. rugosus is already used for medicinal purposes and rosmarinic acid is known to reduce genotoxic effects induced by chemicals, hence it is expected to be safer compared to the current conventional pesticides. While this study highlights the potential of I. rugosus as a possible biopesticide source against A. pisum, it also provides the basis for further exploration and development of formulations for effective field application.
Introduction
Aphids are among the most important agricultural pest insects of many crops worldwide. They feed exclusively on plant phloem sap by inserting their needle-shaped mouthparts into sieve elements, usually resulting to plant discoloration, stunting and deformation. Honey dew produced by aphids promotes the growth of sooty molds which further reduces the economic value of the crop [1, 2]. Moreover, aphids are also vectors of many important plant viruses [3–5]. The pea aphid, Acyrthosiphon pisum (Hemiptera: Aphididae), adversely affects economically important legume crops worldwide. It is oligophagous, comprising of a number of biotypes or races living on a number of legume hosts (red clover, pea, broad bean and alfalfa races) [6–9]. Current aphid control strategies predominantly rely on the use of insecticides such as carbamates, organophosphates, pyrethroids, neonicotinoids and pymetrozine [10]. However, the repeated use of these insecticides for many years has resulted in aphid resistance to most insecticides, making it very difficult to control aphids [11].
The use of botanical pesticides could present a safe alternative compared to the use of broad spectrum chemical insecticides in crop protection [12, 13]. In nature, plants produce secondary metabolites during their interaction with insects and these metabolites can act as toxicants [14], antifeedants [15], anti-oviposition agents and deterrents towards insects [16]. Because of such wide insecticidal properties, the study of secondary metabolites and the development of new potent formulations based on them have become increasingly important. For the discovery of bioactive natural products against insect pests, the screening of plant extracts followed by bioactivity-guided fractionation, isolation and identification of active principles is considered to be one of the most successful strategies [17].
Isodon rugosus (Wall. ex Benth.) Codd (syn. Plectranthus rugosus Wall. ex. Benth.) is an aromatic branched shrub, belonging to the Lamiaceae family. The plant is used in Pakistani traditional medicine for many diseases as an antiseptic, hypoglycemic, antidiarrheal and as bronchodilator [18, 19]. Among many other traditional medicinal uses, the plant extracts and different solvent fractions are known to be effective as antifungal [20], antibacterial, phytotoxic [21] and antioxidant agents [22] and are able to show lipoxygenase inhibitory activities [23]. Based on phytochemical studies, this plant is known to contain steroids, terpenoids, saponins, flavonoids, tannins, coumarins, cardiac glycosides, β-cyanin and reducing sugars [24]. Diterpenoids (effusanin-A, rugosinin, effusanin-B, oridonin, effusanin-E and lasiokaurin) [25] and triterpenoids (acetyl plectranthoic acid, plectranthoic acid A and B and plectranthadiol) have also been successfully isolated from this plant [26]. However, despite several studies on the bioactivity of I. rugosus, where most efforts were focused towards human health, none of these have isolated and analyzed the insecticidal activity of compounds from this plant.
In a previous study, we evaluated the aphicidal properties of the hexane, dichloromethane, butanol and ethyl acetate fractions of a crude methanolic extract from I. rugosus, and confirmed that the butanol fraction showed the best activity against the pea aphid, A. pisum [27]. To further explore this finding, a bioactivity-guided strategy against A. pisum was used to isolate and identify the active compound in the butanol fraction of I. rugosus.
Materials and methods
Insects
A continuous colony of A. pisum was maintained on faba bean plants (Vicia faba) in the Laboratory of Agrozoology at Ghent University, Belgium at 23–25 °C and 65±5% relative humidity (RH) under a 16:8 h light: dark photoperiod [28]. All the bioassays were performed under these conditions. Neonates (< 24 h old) of A. pisum were used for all the bioassays. Mortality was observed after 24 h of treatment by slight probing of the aphids with the help of a brush and also by analyzing post-mortem color change of the body.
Plant collection and extraction
The aerial parts of I. rugosus were collected from lower Northern areas of Pakistan in the month of October, 2012. The plant material was shade-dried for up to 3 months and ground to powder using an electric grinder. Extracts were prepared as described by Khan et al. [27, 29]. Briefly, 1 kg of the dried powder was soaked in a glass jar containing 3 L of methanol at room temperature. After two days, the solvent layer was filtered with a Whatman filter paper No. 1 and this procedure was repeated three times. By using a rotary evaporator, the obtained filtrate was concentrated at 35 °C and the resulting crude methanolic extract was stored at 4 °C. For fractionation, 90 g dried crude methanolic extract was mixed with five parts of water and then extracted successively by n-hexane (4 × 150 mL), dichloromethane (4 × 150 mL), ethyl acetate (4 × 150 mL) and n-butanol (4 × 150 mL) as described by Khan et al. [27]. All the fractions were concentrated using a rotary evaporator under reduced pressure at 40 °C. The resulting extracts were stored in a refrigerator at 4 °C until further use.
Isolation of the bioactive principle
Based on bioassays conducted by Khan et al. [27], the butanol extract presented the best biological activity against A. pisum and was hence selected in this study for further bioactivity-guided fractionation and identification of the active principle. The butanol extract (500 mg) was eluted with a Reveleris automated flash chromatography instrument on a 12 g C18 pre-packed column (GRACE, Columbia, MD, US) starting with 100% water. The gradient was ramped to 100% methanol over 60 column volumes (CV) and after collection of 95 fractions, the solid phase was flushed with 5 CV acetonitrile. The flow rate was set to 30 mL/min (S1 Table). Based on the UV spectral data, the 95 fractions were combined into a total of 14 subfractions. These combined fractions were evaporated under reduced pressure at 45 °C and finally under high vacuum, resulting in 14 subfractions (1A- 14A, S2 Table). The 14 subfractions were evaluated for their bioactivity against A. pisum, of which, one active fraction was selected on the basis of maximum bioactivity for further fractionation through preparative liquid chromatography (prep-LC). A 10% solution of this active fraction was prepared in methanol. Two solvents were used, water (solvent A) and acetonitrile (solvent B). A gradient was set starting with 100% solvent A from 0 to 100 min. From 100 min to 110 min, solvent B went from 18% to 100% and stayed at 100% until 128 min, and then to 0% at 128.10 min and stayed at 0% until 132.10 min. After concentration under reduced pressure with a rotary evaporator and finally under high vacuum, three fractions, 3A-1, 3A-2 and 3A-3 were obtained. Fraction 3A-3 was selected for active compound identification (NMR and LC-MS) on the basis of the bioactivity against A. pisum. This compound was obtained in pure form by doing a second flash chromatographic separation of 5 g of butanol extract and by using the run conditions as mentioned in S3 Table. From the second flash chromatography, a total of 354 fractions were collected which were combined into six fractions, 1B, 2B, 3B, 4B, 5B and 6B on the basis of UV spectra and were further analyzed for their bioactivity after concentration with a rotary evaporator under reduced pressure and high vacuum (S4 Table). The most active fraction was selected for further purification on the basis of best bioactivity. On the basis of knowledge regarding the acidic compound present in sub fraction 3A-3 (from 1H NMR and HPLC-MS analysis), an extraction under acidic conditions was done to isolate the active compound from the active fraction from the second flash chromatography. For this purpose, 200 mg of this fraction was dissolved in 10 mL of distilled water and acidified with 4 drops of hydrochloric acid (12 M). Following extraction with ethyl acetate (four times 5 mL), two phases, ethyl acetate and aqueous, were obtained. Both the ethyl acetate and the aqueous phase were concentrated and analyzed for their bioactivity. Last traces of ethyl acetate were removed azeotropically with toluene and evaporation under high vacuum of the residues resulted in 60 mg from the ethyl acetate phase and 60 mg from the aqueous phase. The purified active principle was identified through different spectroscopic techniques.
Identification of the bioactive compound
Mass spectra were recorded using a HPLC-MS instrument consisting of an Agilent (Waldbronn, Germany) model 1100 liquid chromatograph with a diode array detector coupled with a mass spectrometer with electrospray ionization geometry (Agilent MSD 1100 series). The prep-LC consisted of an Agilent 1100 Series liquid chromatograph using a Supelco Ascentis C18 column (I.D. x L 21.2 mm x 150 mm, 5 μm particle size) connected to an UV-VIS variable wavelength detector (VWD) and automatic fraction collector. Flash chromatography was performed with the Reveleris Flash System (GRACE). 1H and 13C NMR spectra were obtained on a BRUKER Advance III 400 spectrometer. All the solvents and chemicals used were of analytical grade. Optical rotation was taken with a JASCO P-2000 series polarimeter.
Insecticidal bioactivity
For the bioassays, artificial diet test cages were constructed according to Sadeghi et al. [30]. Between two layers of parafilm, 100 μL of liquid artificial diet was sealed. On these layers of parafilm, ten neonate aphids were placed and to avoid the escape of aphids, the cages were covered with a hollow plastic ring incorporating a ventilated lid. In six aerated well plates, these cages were kept in an inverted position. Five concentrations were used for each treatment against the aphids. A stock solution of 1% was prepared by mixing 1 mg of each fraction in 100 μL of water. For reversed-phase flash fractions, five concentrations of 50, 25, 12.5, 6.3 and 3.1 ppm and for prep-LC and acidic extraction fractions, five concentrations of 5, 2.5, 1.3, 0.7 and 0.3 ppm, were prepared by diluting the stock solution with the artificial diet of aphids. For each concentration, a final volume of 300 μL was made to carry out three replications of each treatment (100 μL for each replication). Pure isolated and identified active compound was analyzed in eight different concentrations, including 50, 25, 12.5, 6.3, 3.1, 1.6, 0.8 and 0.4 ppm, by using a stock solution of 1 mg of the compound in 100 μL of water. The untreated artificial diet was used as a control and for each treatment three replications were used in all the bioassays. Mortality was analyzed after 24 h of each treatment.
Additionally, the growth of the surviving aphids exposed to 0.4 ppm of the active compound for 24 h was followed for 9 days (on the same treated diet) in comparison to the untreated aphids.
Data analysis
For statistical analysis, Probit analysis of mortality vs. concentration using POLO-Plus program version 2 was conducted and the lethal concentrations (LC50, LC90) and their corresponding 95% confidence intervals (95% CI) were estimated for each fraction. LC’s were considered to be significantly different when the 95% CI’s did not overlap.
Results
Bioactivity of fractions from the butanol extract of I. rugosus
Bioactivity of the fourteen fractions (1A-14A) obtained through the first reversed-phase flash chromatography of 500 mg of butanol extract of I. rugosus was analyzed for 24 h against A. pisum. Except fractions 8A, 9A, 11A, 13A and 14A, all the other fractions showed considerable toxic effects against A. pisum. Among all the fractions, fraction 3A was the most active fraction with lower LC’s values (Table 1).
Table 1. Toxicity of subfractions of the butanol fraction from first reversed-phase flash chromatography against newborn (< 24 h old) Acyrthosiphon pisum nymphs following 24 h exposure to artificial diet containing different concentrations of the subfractions.
| Fractions | LC50 (95% CI) ppm | Ratio | LC90 (95% CI) ppm | Ratio | Slope ± SE | Chi-Square | HF |
|---|---|---|---|---|---|---|---|
| 1A | 5.5 (3–8) a | 2.6 | 66 (37–211) a | 2.2 | 1.1 ± 0.3 | 7.1 | 0.5 |
| 2A | 8.9 (6.1–12) a | 4.2 | 81 (47–231) a | 2.7 | 1.3 ± 0.3 | 5.6 | 0.4 |
| 3A | 2.1 (0.6–3.8) a | 1.0 | 30 (18–85) a | 1.0 | 1.1± 0.3 | 7.5 | 0.6 |
| 4A | 6.8 (3.8–10) a | 3.2 | 112.2 (54–561) a | 3.8 | 1.1 ± 0.3 | 4.6 | 0.4 |
| 5A | 3.3 (1.3–5.4) a | 1.6 | 50 (28–176) a | 1.7 | 1.1 ± 0.3 | 10.1 | 0.8 |
| 6A | 18 (13–27) b | 8.5 | 187 (90–808) a | 6.3 | 1.3 ± 0.3 | 3.8 | 0.3 |
| 7A | 74 (52–169) c | 35.3 | 267 (131–1651) a | 9.1 | 2.3 ± 0.6 | 8.1 | 0.6 |
| 8A | - | - | - | - | 1.7 ± 0.7 | 7.0 | 0.5 |
| 9A | - | - | - | - | 2.0 ± 1.3 | 4.7 | 0.4 |
| 10A | 36 (33–40) d | 17.2 | 52.5 (46–64) a | 1.8 | 8.0 ± 1.4 | 2.8 | 0.2 |
| 11A | - | - | - | - | 1.6 ± 0.6 | 8.5 | 0.7 |
| 12A | 51 (43–71) c | 24.5 | 109 (77–241) a | 3.7 | 3.9 ± 1.0 | 2.2 | 0.2 |
| 13A | - | - | - | - | 1.5 ± 1.2 | 6.6 | 0.5 |
| 14A | - | - | - | - | 2 ± 1.3 | 4.7 | 0.4 |
Data is presented as lethal concentration values, 50% (LC50) and 90% (LC90) (both in ppm) together with their particular 95% confidence interval (95% CI), the slope ± SE of the toxicity vs concentration curve, and the Chi-Square and heterogeneity factor HF as accuracy of data fitting to probit analysis in POLO-PlusV2. Due to non-overlapping of 95% CI, different letters in the same column indicate significant differences. Ratio, LCx, fraction/LCx, 3A
Bioactivity of subfractions from fraction 3A collected through prep-LC
The three collected subfractions (3A-1, 3A-2 and 3A-3) of 3A were analyzed against A. pisum for 24 h. Fraction 3A-1 and fraction 3A-2 gave negligible toxic effects (no LC50 and LC90). Fraction 3A-3 was the most toxic fraction analyzed against A. pisum with low LC’s values (Table 2).
Table 2. Toxicity of subfractions of fraction 3A against newborn (< 24 h old) Acyrthosiphon pisum nymphs following 24 h exposure to artificial diet containing different concentrations of the subfractions.
| Fractions | LC50 (95% CI) ppm | Ratio | LC90 (95% CI) ppm | Ratio | Slope ± SE | Chi-Square | HF |
|---|---|---|---|---|---|---|---|
| 3A-1 | - | - | - | - | 2.0 ± 1.3 | 4.9 | 0.4 |
| 3A-2 | - | - | - | - | 1.5 ± 1.2 | 6.6 | 0.5 |
| 3A-3 | 1 (0.6–1.6) | 1 | 14 (6.1–97) | 1 | 1.1± 0.3 | 14.8 | 1.1 |
Data is presented as lethal concentration values, 50% (LC50) and 90% (LC90) (both in ppm) together with their particular 95% confidence interval (95% CI), the slope ± SE of the toxicity vs concentration curve, and the Chi-Square and heterogeneity factor HF as accuracy of data fitting to probit analysis in POLO-PlusV2. Ratio, LCx, fraction/LCx, 3A-3
Spectroscopic analysis of fraction 3A-3
Out of three subfractions of 3A (3A-1, 3A-2 and 3A-3), fraction 3A-3 was the most bioactive fraction against A. pisum. This fraction 3A-3 was analyzed through 1H NMR which confirmed that it contained rosmarinic acid. Different gradients were used to purify the compound but during different Prep-LC runs, the chromatographic behavior, that is, peak shape and position, of this fraction was inconsistent. Therefore, the reversed-phase flash chromatography was repeated with 5 g of butanol fraction of I. rugosus in order to get the most bioactive compound in pure form.
Bioactivity of fractions of butanol extract from the second reversed-phase flash chromatography
Six fractions (1B-6B) obtained through a second reversed-phase flash chromatography of the butanol extract of I. rugosus, were analyzed against A. pisum for 24 h. Out of the six fractions analyzed, fraction 4B, 5B and 6B showed negligible toxicity (no LC50 and LC90). Fraction 1B was more toxic and moderate toxicity was observed for fraction 2B. Lower toxicity was found for fraction 3B (Table 3).
Table 3. Toxicity of subfractions of the butanol fraction from a second reversed-phase flash chromatography against newborn (<24 h old) Acyrthosiphon pisum nymphs following 24 h exposure to artificial diet containing different concentrations of the subfractions.
| Fractions | LC50 (95% CI) ppm | Ratio | LC90 (95% CI) ppm | Ratio | Slope ± SE | Chi-Square | HF |
|---|---|---|---|---|---|---|---|
| 1B | 2.5 (1–4.1) a | 1.0 | 28 (18–69) a | 1 | 1.2 ± 0.3 | 11.4 | 0.9 |
| 2B | 7.5 (4.3–11) b | 3.0 | 71 (38–280) a | 2.5 | 1.3 ± 0.3 | 16.5 | 1.3 |
| 3B | 16 (11–26) c | 6.5 | 101 (52–417) a | 3.6 | 1.6± 0.3 | 22.3 | 1.7 |
| 4B | - | - | - | - | 1.0 ± 0.3 | 25.3 | 2.0 |
| 5B | - | - | - | - | 1.5 ± 1.2 | 6.6 | 0.5 |
| 6B | - | - | - | - | 1.8 ± 0.7 | 6.5 | 0.5 |
Data is presented as lethal concentration values, 50% (LC50) and 90% (LC90) (both in ppm) together with their particular 95% confidence interval (95% CI), the slope ± SE of the toxicity vs concentration curve, and the Chi-Square and heterogeneity factor HF as accuracy of data fitting to probit analysis in POLO-PlusV2. Due to non-overlapping of 95% CI, different letters in the same column indicate significant differences. Ratio, LCx, fraction/LCx, 1B
Bioactivity of the ethyl acetate and aqueous phase of acidic extraction
Both collected phases of acidic extraction were analyzed for their insecticidal potential through bioassays against A. pisum for 24 h. The aqueous phase caused negligible toxic effects (no LC50 and LC90) while the ethyl acetate phase caused more toxicity (Table 4).
Table 4. Toxicity of ethyl acetate and aqueous phase of acidic extraction against newborn (< 24 h old) Acyrthosiphon pisum nymphs following 24 h exposure to artificial diet containing different concentrations of both phases.
| Fractions | LC50 (95% CI) ppm | Ratio | LC90 (95% CI) ppm | Ratio | Slope ± SE | Chi-Square | HF |
|---|---|---|---|---|---|---|---|
| Aqueous | - | - | - | - | 1.5 ± 1.2 | 6.6 | 0.5 |
| Ethyl acetate | 0.2 (0.04–0.5) | 1 | 9.2 (3.9–13) | 1 | 0.8 ± 0.3 | 4.2 | 0.3 |
Data is presented as lethal concentration values, 50% (LC50) and 90% (LC90) (both in ppm) together with their particular 95% confidence interval (95% CI), the slope ± SE of the toxicity vs concentration curve, and the Chi-Square and heterogeneity factor HF as accuracy of data fitting to probit analysis in POLO-PlusV2. Ratio, LCx, fraction/LCx, ethyl acetate
Identification of the most bioactive compound
Out of the two phases of acidic extraction, the ethyl acetate phase fraction was the most active. After removing ethyl acetate azeotropically, this fraction was analyzed and the active compound was identified as rosmarinic acid through HPLC-MS, optical rotation measurement and 1H and 13C NMR spectroscopy.
HPLC-MS
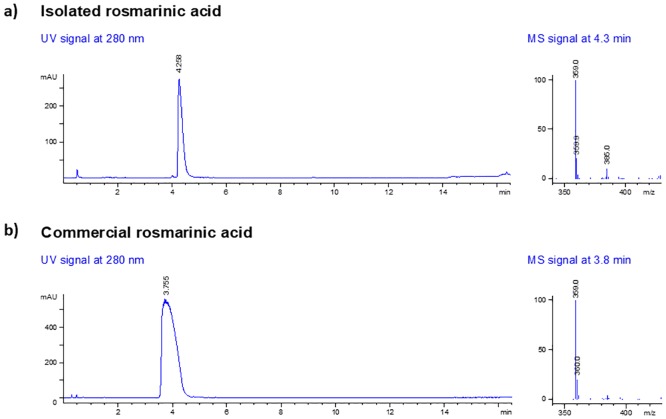
Both isolated and commercial rosmarinic acid (Sigma Aldrich) had the same peak appearance in the HPLC-MS chromatograms with the same solvent gradient. Both had a pseudo-molecular ion with an m/z value of 359 with negative mode electrospray ionization which confirmed that it was rosmarinic acid (Fig 1).
Fig 1. Mass spectra (negative mode electrospray ionization) of rosmarinic acid obtained via HPLC-MS with a pseudo molecular ion at m/z value of 359 (a) isolated rosmarinic acid (b) commercial rosmarinic acid.
Optical rotation and 1H and 13C NMR
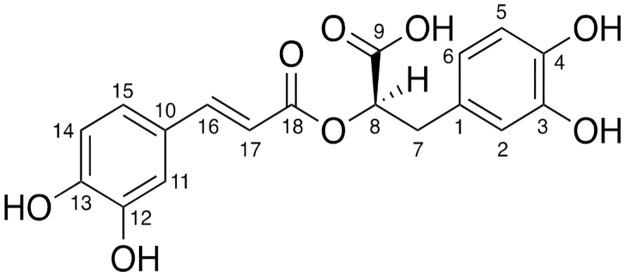
Brown crystals; (c 0.233, MeOH); 1H NMR (400 MHz, CD3OD): δ°3.01 (1H, dd, J = 8.3, 14.3 Hz, H7a), 3.10 (1H, dd, J = 4.4, 14.3 Hz, H7b), 5.19 (1H, dd, J = 4.4, 8.3 Hz, H8), 6.27 (1H, d, J = 15.9, H17), 6.61 (1H, dd, J = 2.0, 8.0 Hz, H6), 6.70 (1H, d, J = 8.0 Hz, H5), 6.75 (1H, d, J = 2.0 Hz, H2), 6.78 (1H, d, J = 8.2 Hz, H14), 6.95 (1H, dd, J = 2.0, 8.2 Hz, H15), 7.04 (1H, d, J = 2.0 Hz, H11), 7.55 (1H, d, J = 15.9 Hz, H16);13C NMR (100 MHz, CD3OD): δ°37.9 (C7), 74.6 (C8), 114.4 (C17), 115.2 (C11), 116.3 (C5), 116.5 (C14), 117.6 (C2), 121.8 (C6), 123.2 (C15), 127.7 (C10), 129.2 (C1), 145.3 (C4), 146.2 (C3), 146.8 (C12), 147.7 (C16), 149.7 (C13), 168.4 (C18), 173.5 (C9); ESI-MS: m/z (%) 359 (M-H+, 100). Optical rotation and NMR data were in accordance with the literature (Fig 2) [31, 32].
Fig 2. Structure of rosmarinic acid isolated from I. rugosus.
Bioactivity of I. rugosus rosmarinic acid and commercial rosmarinic acid
Rosmarinic acid isolated from I. rugosus and commercial rosmarinic acid (Sigma Aldrich) were analyzed against A. pisum for their pesticidal activity for 24 h. Both I. rugosus rosmarinic acid (RA) and commercial RA caused comparable toxic effects in the treated aphids (Table 5).
Table 5. Toxicity of isolated rosmarinic acid (RA) and commercial rosmarinic acid (RA) against newborn (< 24 h old) Acyrthosiphon pisum nymphs following 24 h exposure to artificial diet containing different concentrations of isolated rosmarinic acid and commercial rosmarinic acid.
| Compound | LC50 (95% CI) ppm | Ratio | LC90 (95% CI) ppm | Ratio | Slope ± SE | Chi-Square | HF |
|---|---|---|---|---|---|---|---|
| Commercial RA | 0.2 (0.05–0.5) a | 1 | 14 (7.4–42) a | 2.6 | 0.7 ± 0.2 | 15.5 | 0.7 |
| I. rugosus RA | 0.2 (0.04–0.4) a | 1 | 5.4 (3.3–12) a | 1 | 0.8 ± 0.2 | 10.5 | 0.5 |
Data is presented as lethal concentration values, 50% (LC50) and 90% (LC90) (both in ppm) together with their particular 95% confidence interval (95% CI), the slope ± SE of the toxicity vs concentration curve, and the Chi-Square and heterogeneity factor HF as accuracy of data fitting to probit analysis in POLO-PlusV2. Due to overlapping of 95% CI, same letter in the same column indicate no significant differences. Ratio, LCx, compound/LCx, Isodon rugosus RA
Comparison of the growth of surviving aphids exposed to rosmarinic acid-treated and untreated diet after 24 h of bioassay
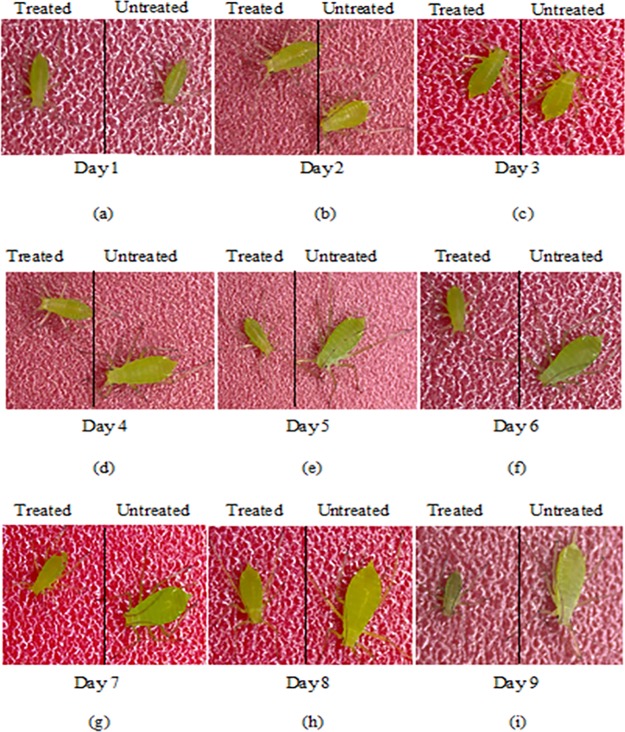
After incorporating rosmarinic acid into the aphid’s diet at a concentration of 0.4 ppm, its effect on A. pisum that survived after 24 h treatment, was analyzed every day for up to 9 days (on same treated diet). It was confirmed that rosmarinic acid had a drastic effect on their growth. Firstly, most aphids exposed to treated diet were dead while the survivors did not grow further to become adults and were thus not able to reproduce further. Fig 3 shows a comparison between treated and untreated aphids. There was a clear difference between untreated and treated aphids after day 4, and by day 9 the treated aphids were all dead, while the untreated aphids were still alive.
Fig 3. Comparison between growth of surviving aphids exposed to rosmarinic acid-treated and untreated diet after 24 h of bioassay, (a) to (i) comparison observed for up to 9 days, all treated aphids died by day 9.
Discussion
Screening candidate plants, purifying active ingredients, isolating and identifying the active plant constituents is required to discover new bioactive natural products [33]. We applied this methodology to identify rosmarinic acid as an active principle from the plant I. rugosus. Based on our previous study on the insecticidal activity of botanical extracts from various plant species, we found that the extract from I. rugosus was the most toxic to A. pisum [27] Further fractionation showed that the butanol fraction most likely contained the active principle. In this study we used the bioactivity-guided strategy to isolate and identify the active compound as rosmarinic acid. This strategy is interesting and has been used in previous studies to identify bioactive compounds. For example, the butanol fraction from Citrullus colocynthis was reported to be active against the black legume aphid, Aphis craccivora, and through the bioactivity-guided isolation strategy, the active principle, 2-O-ß-D-glucopyranosylcucurbitacin E, was successfully isolated [34]. Similarly, in another study involving bioactivity-guided isolation, the active principle, ailanthone, was isolated from the aqueous fraction of Ailanthus altissima against A. pisum [35].
In this study, the butanol fraction was subfractionated through reversed-phase flash chromatography. After bioactivity based screening of all the resulting subfractions (1A-14A) against A. pisum, fraction 3A with lower LC values was selected for further fractionation. Through prep-LC, fraction 3A was subfractionated and the resulting subfractions (3A-1, 3A-2 and 3A-3) were analyzed for their bioactivity. Fraction 3A-3 with lower LC values was subjected to spectroscopic analysis. 1H NMR spectroscopy confirmed that the isolated fraction contained rosmarinic acid. However, due to the inconsistent chromatographic behavior during prep-LC, not enough compound could be collected to record 13C NMR data. The inconsistent chromatographic behavior with peak splitting observed could have arisen from several causes; a contamination on guard or analytical column inlet, a blocked frit or a small void at the column inlet (~wear). The problem of peak shifting (variable retention times) could have been due to small changes in mobile composition, temperature fluctuations, column overloading or a combination of these problems which could have led to different UV patterns for each run. Due to this problem, the reversed-phase flash chromatography was repeated with a larger amount of the butanol fraction. Out of all the resulting subfractions (1B-6B), 1B was selected with lower LC values against A. pisum. Fraction 1B was subjected to acidic extraction to get two phases, aqueous and ethyl acetate. The ethyl acetate phase fraction was more active with lower LC values. After removing ethyl acetate, the active principle was identified through different spectroscopic techniques as rosmarinic acid. Similarly in another study, Chakraborty et al. [36] reported the isolation of caffeic acid and rosmarinic acid from Basilicum polystachyon through acidic extraction with HCl followed by partitioning with ethyl acetate and analyzed their antimicrobial activities.
This study reports the isolation and purification of rosmarinic acid (RA) from I. rugosus and its bioactivity against A. pisum for the first time. Feeding bioassays were used to analyze the toxicity of rosmarinic acid. For evaluating the insecticidal activity, incorporation of these products into a food source is a standard technique. Under controlled conditions, the use of artificial diet permits testing of a small amount of insecticidal product and stimulates aphids to oral exposure easily. This technique is fast, easy to handle, inexpensive and gives results in a short period of time including effects of insecticides on aphids [30]. In the current study, it was observed that the aphids were feeding on the treated diet, which is why it was concluded that aphids were dying because of toxic effects of rosmarinic acid.
In this study, there was no significant difference observed between the bioactivity depicted by both isolated and commercial rosmarinic acid. Isodon rugosus rosmarinic acid gave LC values of LC50 = 0.2 ppm and LC90 = 5.4 ppm. These are very low LC values depicted after 24 h of bioassay and such low LC values have not been previously reported in any study with compounds against A. pisum using the same feeding bioassay methodology [30, 37–39, 28, 40, 41]. This means that a very low amount of rosmarinic acid can cause significant toxic effects against A. pisum in 24 h. Furthermore, many plant essential oils have caused over 90% mortality in A. pisum upon application of 2 μl l-1 of oil in air using fumigation assays, with most of the plant species belonging to the Lamiacea family [42]. I. rugosus also belongs to the Lamiacea family, indicating that plants from this family could have a high potential to control the aphid, A. pisum through integral valorization of different plant constituents. Based on this observation, the essential oil from I. rugosus should be studied as well for possible control of these aphids.
Very few insecticidal activities have been reported for rosmarinic acid. Regnault-Roger et al, [43] investigated the insecticidal activities of polyphenolic compounds, isolated from five plants belonging to Lamiaceae family against Acanthoscelides obtectus (Say) and observed that among all the polyphenolic compounds, rosmarinic acid and luteolin-7-glucoside were more toxic. However, in another study, rosmarinic acid exhibited negligible toxicity against the red palm weevil larvae, Rhynchophorus ferrugineus [44]. Although the mode of action of rosmarinic leading to toxicity in insects is unknown, one could postulate that the low toxicity observed in the red palm weevil could have arisen from several possible reasons probably linked to its genotype and gene expression profile as observed in other insects tolerant to some classical insecticides.
Additionally, a comparison between the growth of surviving aphids exposed to rosmarinic acid-treated and untreated diet after 24 h of bioassay was analyzed. It was clearly observed that the growth of surviving A. pisum nymphs stopped after 48 h of exposure to rosmarinic acid-treated diet, resulting in a size reduction and ultimately death as compared to aphids exposed to an untreated diet. A similar observation was made by Sadeghi et al. [30] who observed that the aphid size was reduced after 48 h of exposure to novel biorational insecticides, flonicamid and pymetrozine, and mortality was observed after 72 h.
Conclusion
In this study, I. rugosus was identified as an interesting source for a botanical insecticide against A. pisum. Following bioactivity-guided selection, rosmarinic acid was isolated and identified through spectroscopic analysis as the bioactive compound in the I. rugosus extract for the first time. Based on the bioassay results, either the extracts from I. rugosus or the isolated insecticidal compound, rosmarinic acid could be used to develop effective aphicides, because of the high mortality of aphids caused at very low rosmarinic acid concentrations in 24 h. This potential botanical insecticide may fit well in integrated pest management programs intended to control aphids. Considering that I. rugosus is known to be used for medicinal purposes, it is expected to be safer as compared to the current conventional pesticides used for controlling aphids. Also, rosmarinic acid is known to reduce genotoxic effects induced by chemicals, which is contrary to some currently used toxic synthetic pesticides that could induce genotoxic effects in consumers. An interesting avenue to follow for future studies will be the analyses of the underlying molecular mechanisms responsible for the cause of mortality in rosmarinic acid-treated aphids. While this study highlights the potential of I. rugosus as a possible biopesticide source against a notorious insect pest such as A. pisum, it also provides the basis for further exploration and development of a formulation for effective field application. However, more experiments under field conditions are required to further verify the applicability of rosmarinic acid for the insect’s control and in future contact application tests can be performed to broaden its effects on crops under field conditions. From an implementation point of view, genes involved in the biosynthesis of rosmarinic acid could be transformed under the control of a phloem-specific promoter to produce resistant crops towards aphids.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors are highly thankful to the PhD scholar, Kleber Pereira at the Faculty of Bioscience Engineering, Ghent University, Ghent, for maintaining and taking care of aphid colonies in the laboratory. We are grateful to Dr. Irum Shehzadi and other colleagues at the Department of Environmental Sciences, COMSATS University Islamabad, Abbottabad Campus, Pakistan, for their helpful support in the laboratory work throughout this research project.
Data Availability
All relevant data are within the manuscript and its supporting information files.
Funding Statement
This work was supported by the Higher Education Commission, Pakistan (IRSIP) to SK (http://www.hec.gov.pk/english/pages/home.aspx). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kim SK, Kim YC, Lee S, Kim JC, Yun MY, Kim IS. Insecticidal activity of rhamnolipid isolated from Pseudomonas. EP-3 against green peach aphid (Myzus persicae). J Agric Food Chem. 2011; 59: 934–938 10.1021/jf104027x [DOI] [PubMed] [Google Scholar]
- 2.Baek MY, Park HJ, Kim GM, Lee DY, Lee GY, Moon SJ et al. Insecticidal alkaloids from the seeds of Macleaya cordata on cotton aphid (Aphis gossypii). J Korean Soc Appl Biol Chem. 2013; 56: 135–140. [Google Scholar]
- 3.Sylvester ES. In Aphids, Their Biology, Natural Enemies and Control (2C): Viruses Transmitted by Aphids; Minks A. K., Harrewijn P., Eds.; Elsevier: Amsterdam, The Netherlands: 1989; 65–87. [Google Scholar]
- 4.James CKNG, Perry KL. Transmission of plant viruses by aphid vectors. Mol Plant Pathol. 2004; 5: 505–511. 10.1111/j.1364-3703.2004.00240.x [DOI] [PubMed] [Google Scholar]
- 5.Dedryver CA, Le Ralec A, Fabre F. The conflicting relationships between aphids and men: A review of aphid damage and control strategies. C R Biol. 2010; 333(6–7): 539–553. 10.1016/j.crvi.2010.03.009 [DOI] [PubMed] [Google Scholar]
- 6.Via S, Shaw AJ. Clonal genetic variability and short term evolution in the size and shape of pea aphids. Evolution. 1996; 50: 163–173. [DOI] [PubMed] [Google Scholar]
- 7.Via S. Reproductive isolation between sympatric races of pea aphids. I Gene flow restriction and habitat choice. Evolution. 1999; 53:1446–1457. 10.1111/j.1558-5646.1999.tb05409.x [DOI] [PubMed] [Google Scholar]
- 8.Peccoud J, Simon JCH, McLaughlin HJ, Moran NA. Postpleistocene radiation of the pea aphid complex revealed by rapidly evolving endosymbionts. Proc Natl Acad Sci USA. 2009a; 106: 16315–16320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peccoud J, Ollivier A, Plantegenest M, Simon JCHA. Continuum of genetic divergence from sympatric host races to species in the pea aphid complex. Proc Natl Acad Sci USA. 2009b; 106: 7495–7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sparks TC, Nauen R. IRAC: mode of action classification and insecticide resistance management. Pestic Biochem Physiol. 2015; 121: 122–128. 10.1016/j.pestbp.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 11.Jaouannet M, Rodriguez PA, Thorpe P, Lenoir CJ, MacLeod R, Escudero-Martinez C, Bos JI. Plant immunity in plant–aphid interactions. Front Plant Sci. 2014; 5: p.663 10.3389/fpls.2014.00663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isman MB. The role of botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol. 2006; 51: 45–66. 10.1146/annurev.ento.51.110104.151146 [DOI] [PubMed] [Google Scholar]
- 13.Akhtar Y, Yu Y, Isman MB, Plettner E. Dialkoxybenzene and dialkoxyallylbenzene feeding and oviposition deterrents against the cabbage looper, Trichoplusiani: potential insect behavior control agents. J Agric Food Chem. 2010; 58: 4983–4991. 10.1021/jf9045123 [DOI] [PubMed] [Google Scholar]
- 14.Pavela R. Larvicidal effects of various Euro-Asiatic plants against Culex quinquefasciatus Say larvae (Diptera: Culicidae). Parasitol Res, 2008; 102(3): p.555 10.1007/s00436-007-0821-3 [DOI] [PubMed] [Google Scholar]
- 15.Akhtar Y; Isman MB. Comparative growth inhibitory and antifeedant effects of plant extracts and pure allelochemicals on four phytophagous insect species. J Appl Entomol. 2004; 128(1): pp.32–38. [Google Scholar]
- 16.Basukriadi A. Wilkins RM. Oviposition deterrent activities of Pachyrhizus erosus seed extract and other natural products on Plutella xylostella (Lepidoptera: Plutellidae). J Insect Sci. 2014; 14(1): p.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archer TL, Segarra E, Bynum ED. Greenbug resistance management on sorghum with insecticide mixtures: a biological and economic analysis. J Econ Entomol. 1999; 92: 794–803. [Google Scholar]
- 18.Sher Z, Khan Z, Hussain F. Ethnobotanical studies of some plants of Chagharzai valley, district Buner, Pakistan. Pak J Bot. 2011; 43: 1445–1452. [Google Scholar]
- 19.Ajmal SM, Mohammad S, Zahid K, Bakht Z. Ethnomedicinal and phytoeconomic elaboration of Lilownai valley, district Shangla Pakistan. IRJP3. 2012; 164–169.
- 20.Rauf A, Khan A, Rasool S, Shah ZA, Saleem M. In-vitro antifungal activity of three selected Pakistani medicinal plants. MEJMPR. 2012a; 1: 41–43. [Google Scholar]
- 21.Rauf A, Muhammad N, Khan A, Uddin N, Atif M. Antibacterial and phytotoxic profile of selected Pakistani medicinal plants. WASJ. 2012b; 20: 540–544. [Google Scholar]
- 22.Rauf A, Uddin G, Ali M, Muhammad N, Gul S. Phytochemical screening and antioxidant activity of Pakistani medicinal plants. WJMP. 2013; 2: 1–6. [Google Scholar]
- 23.Janbaz KH, Arif J, Saqib F, Imran I, Ashraf M, et al. In-vitro and in-vivo validation of ethnopharmacological uses of methanol extract of Isodon rugosus Wall. ex Benth. (Lamiaceae). BMC Complement Altern Med. 2014; 14: 71 10.1186/1472-6882-14-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Razdan TK, Kachroo V, Harkar S, Koul GL. Plectrantholic acids A and B—two new triterpenoids from Plectranthus rugosus. Tetrahedron. 1982a; 38: 991–992. [Google Scholar]
- 25.Sun HD, Huang SX, Han QB. Diterpenoids from Isodon species and their biological activities. Nat Prod Rep. 2006; 23: 673–698. 10.1039/b604174d [DOI] [PubMed] [Google Scholar]
- 26.Razdan TK, Kachroo V, Harkar S, Koul GL, Dhart KL. Plectranthoic acid, acetyl plectranthoic acid and plectranthadiol-three new triterpenoids from Plectranthus rugosus. Phytochemistry. 1982b; 21: 409–412. [Google Scholar]
- 27.Khan S, Taning CNT, Bonneure E, Mangelinckx S, Smagghe G, Shah MM. Insecticidal activity of plant-derived extracts against different economically important pest insects. Phytoparasitica. 2017; 45(1): 113–124. [Google Scholar]
- 28.Nachman RJ, Hamshou M, Kaczmarek K, Zabrocki J, Smagghe G. Biostable and PEG polymer-conjugated insect pyrokinin analogs demonstrate antifeedant activity and induce high mortality in the pea aphid Acyrthosiphon pisum (Hemiptera: Aphididae). Peptides. 2012; 34: 266–273. 10.1016/j.peptides.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 29.Khan S, Shah MM, Ahmad R, Haq IU. The insecticidal potential of botanical extracts for management of Peach fruit fly, Bactrocera zonata Saunders, 1842 (Diptera: Tephritidae). Türk entomol derg. 2016; 40 (4): 445–453. [Google Scholar]
- 30.Sadeghi A, Van Damme EJM, Michiels K, Kabera A, Smagghe G. Acute and chronic insecticidal activity of a new mannose-binding lectin from Allium porrum against Acyrthosiphon pisum via an artificial diet. Can. Entomol. 2009, 141: 95–100. [Google Scholar]
- 31.Dapkevicius A, van Beek TA, Lelyveld GP, van Veldhuizen A, de Groot A, Linssen JP, Venskutonis R. Isolation and Structure Elucidation of Radical Scavengers from Thymus vulgaris Leaves. J Nat Prod. 2002; 65(6): 892–896. [DOI] [PubMed] [Google Scholar]
- 32.Hyun HBS, Hrestha S, Boo KH, Cho SK. Evaluation of antioxidant potential of ethyl acetate fraction of Rosmarinus officinalis L and its major components. J Korean Soc Appl Bio Chem. 2015; 58(5): 715–722. [Google Scholar]
- 33.Chermenskaya TD, Stepanycheva EA, Shchenikova AV, Savelieva EI, Chakaeva AS. Insecticidal effects of Ungernia severtzovii bulb extracts against the grain aphid Schizaphis graminum (Rondani). Ind Crops Prod. 2012; 36: 122–126. [Google Scholar]
- 34.Torkey HM, Abou-Yousef HM, Abdel Azeiz AZ, Hoda EAF. Insecticidal effect of cucurbitacin E glycoside isolated from Citrullus colocynthis against Aphis craccivora. Aust J Basic Appl Sci. 2009; 3(4): 4060–4066. [Google Scholar]
- 35.De Feo V, Mancini E, Voto E, Curini M, Digilio MC. Bioassay-oriented isolation of an insecticide from Ailanthus altissima. J Plant Interact. 2009; 4(2): 119–123. [Google Scholar]
- 36.Chakraborty D, Mandal SM, Chakraborty J, Bhattacharyaa PK, Bandyopadhyay A, Mitra A, Gupta K. Antimicrobial activity of leaf extract of Basilicum polystachyon (L) Moench. Indian J Exp Biol. 2007; 45 (8): 744–748. [PubMed] [Google Scholar]
- 37.Smagghe G, Mahdian K, Zubrzak P, Nachman RJ. Antifeedant activity and high mortality in the pea aphid Acyrthosiphon pisum (Hemiptera, Aphidae) induced by biostable insect kinin analogs. Peptides. 2010; 31: 498–505. 10.1016/j.peptides.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 38.Carrillo L, Martinez M, Álvarez-Alfageme F, Castanera P, Smagghe G, Díaz I, Ortego F. Abarley cysteine–proteinase inhibitor reduces the performance of two aphid species in artificial diets and transgenic Arabidopsis plants. Transg Res. 2011; 20 (2): 305–319. [DOI] [PubMed] [Google Scholar]
- 39.De Geyter E, Smagghe G, Rahbé Y, Geelen D. Triterpene saponins of Quillaja saponaria show strong aphicidal and deterrent activity against the pea aphid Acyrthosiphon pisum. Pest Manag. Sci. 2012; 68 (2): 164–169. 10.1002/ps.2235 [DOI] [PubMed] [Google Scholar]
- 40.Giner M, Avilla J, De Zutter N, Ameye M, Balcells M, Smagghe G. Insecticidal and repellent action of allyl esters against Acyrthosiphon pisum (Hemiptera: Aphididae) and Tribolium castaneum (Coleoptera: Tenebrionidae). Ind Crop Prod. 2013; 47: 63–68. [Google Scholar]
- 41.Zapata N, Van Damme EJM, Vargas M, Devotto L, Smagghe G. Insecticidal activity of a protein extracted from bulbs of Phycellaaustralis Ravenna against the aphids Acyrthosiphon pisum Harris and Myzus persicae Sulzer. Chil J Agric Res. 2016; 76(2): 188–194. [Google Scholar]
- 42.Ikbal C, Pavela R. Essential oils as active ingredients of botanical insecticides against aphids. J Pest Sci. 2019; 92: 971–986. [Google Scholar]
- 43.Regnault-Roger C, Ribodeau M, Hamraoui A, Bareau I, Blanchard P, Gil-Munoz MI, Barberan FT. Polyphenolic compounds of Mediterranean Lamiaceae and investigation of orientational effects on Acanthoscelides obtectus (Say). J Stored Prod Res. 2004; 40(4): 395–408. [Google Scholar]
- 44.AlJabr AM, Hussain A, Rizwan-ul-Haq M, Al-Ayedh H. Toxicity of plant secondary metabolites modulating detoxification genes expression for natural red palm weevil pesticide development. Molecules. 2017; 22(1): 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its supporting information files.