Abstract
Background
Chewing ability is often compromised in patients with oral cancer. The aim of this study was to identify which factors affect masticatory performance in these patients.
Methods
Patients with primary oral cancer were assessed for up to 5 years after primary treatment. Healthy controls were assessed once. A mixed‐model analysis was performed, with masticatory performance as outcome measure.
Results
A total of 123 patients were included in the study. Factors positively associated with masticatory performance were number of occlusal units (OU), having functional dentures, and maximum mouth opening (MMO). The impact of tumor location and maximum bite force (MBF) differed per assessment moment. Masticatory performance declined for up to 1 year but recovered at 5 years after treatment.
Conclusion
Masticatory performance in patients treated for oral cancer is affected by MBF, MMO, number of OU, and dental status. These should be the focus of posttreatment therapy.
Keywords: bite force, head and neck neoplasms, mastication, mixed model analysis, prospective cohort
1. INTRODUCTION
The aim of curative treatment for oral malignancies is disease remission achieved by radical resection and, on indication, postoperative irradiation. A great challenge for this curative treatment is trying to maintain or restore acceptable functional and esthetical outcomes after treatment. Oral function (eating, speaking, drinking, and swallowing) is known to be lower in patients who have had tumor surgery in the oral cavity than in healthy subjects.1, 2, 3 Masticatory performance is an important aspect of posttreatment quality of life in patients treated for head and neck cancer.4, 5, 6
Oncological surgery removes malignancies at the cost of functional anatomy.7 After ablation of the tumor, where primary closure is impossible, reconstruction is necessary either immediately after the ablation of the tumor or in a secondary stage and is achieved using a local flap, bone grafts, microvascular free‐tissue transfer, and/or implant surgery. Often, dental rehabilitation with a prosthesis is used to restore oral function and esthetics8; however, different phases of mastication may still be affected, such as the transportation, trituration, and consolidation of the food bolus.9, 10 Patients who undergo a glossectomy may, for example, have difficulty with the transportation of food, whereas patients who have had a mandibulectomy can experience difficulty in trituration and vertical mobility during mastication.11 Radiotherapy can cause masticatory impairment due to tooth loss, mucositis, fibrosis, trismus, or xerostomia. Additionally, a lack of saliva can make patients unable to tolerate dentures.3, 12, 13
Oral rehabilitation can improve masticatory performance. To achieve optimal oral rehabilitation, it is important to identify factors that affect masticatory performance in patients treated for oral cancer. Previous studies on this patient group focused mainly on the subjective appraisal of chewing ability using questionnaires; very few studies have assessed masticatory performance with an objective method.14, 15, 16
The primary aim of this study was to identify and quantify factors involved in objective masticatory performance for patients who have been treated for oral cavity malignancies, with a follow‐up of 5 years after treatment. We also compared these results to healthy controls.
2. SUBJECTS AND METHODS
2.1. Patients
In this multicenter prospective cohort research, the study population consisted of patients with a primary malignant tumor involving the oral cavity who were referred to University Medical Center Utrecht (UMCU) or Radboud University Medical Center (Radboudumc) between January 2007 and August 2009. Patients were included if they were being treated with a curative intent, using surgery or surgery followed by radiotherapy. Exclusion criteria included inoperability, a previous and/or current second primary malignancy, cognitive impairment, or the inability to understand Dutch. Sixty age‐matched controls were also recruited, whose details were published previously.17 The experimental protocol was approved by the Ethics Committees of the UMCU and Radboudumc. All patients received written information and provided their signed informed consent.
Pretreatment oral screening and dental management were performed for all patients. Adjuvant radiotherapy, when given, started within 4‐6 weeks after surgery, in accordance with the Dutch Head and Neck Society treatment guidelines, with a total radiation dosage of 64‐70 Gy.
Tumor locations included in this study were included codes C00, C02‐C06, and C31, defined by the WHO International Classification of Diseases Oncology, third edition.18 Maxillary tumors included the upper alveolar process, tuber maxillae, palate, and maxillary sinus (C03.0, C05, C31.0). Mandibular tumors included the lower alveolar process, the retromolar trigonum, the buccal mucosa, and the lower lip (C00.4, C03.1, C06.0, C06.1, C06.2). Tongue and/or floor of the mouth (TFM) tumors included the tongue and the anterior floor of the mouth (C02, C04).
Patient information, including sex, tumor location and size (pT of TNM‐classification19), resection site, and details of reconstruction, was extracted from medical records. Age, smoking habit, and alcohol consumption were charted at the pretreatment session. Smoking habit was scored as “No” for nonfrequent or infrequent smokers and “Yes” for daily smokers. Alcohol consumption scored “Yes” if it exceeded 1 unit per day on average.
2.2. Assessments
Patients were assessed no more than 4 weeks before primary treatment (baseline, t0), then at 4‐6 weeks after surgery (t1a) and/or 4‐6 weeks after radiotherapy (t1b), 6 months (t2), 1 year (t3), and 5 years (t5) after their primary treatment. At every assessment, masticatory performance, maximum bite force (MBF), and maximum mouth opening (MMO) was evaluated, as well as dental status and the presence of an obturator prosthesis. Healthy controls were assessed once.
2.3. Masticatory performance
The mixing ability test (MAT) measures how well a subject mixes a 2‐colored wax tablet by chewing on it.17, 20 The wax is a soft material (Plasticine modeling wax, non‐toxic DIN EN‐71) that forms a compact bolus during chewing. The tablet was offered to patients at room temperature (20°C). The tablet had a diameter of 20 mm and consisted of two 3‐mm layers of bright red and blue wax. Chewing mixes the colors to yield intermediate shades of red and blue. After being chewed, the wax was flattened to a thickness of 2.0 mm and photographed on both sides using a high‐quality scanner (Epson V750; Long Beach, California). The wax images were analyzed using Adobe Photoshop CS3 (San Jose, California) to generate a measure for the spread of red and blue intensities: the Mixing Ability Index (MAI). A lower index implies a better mixed tablet, hence better masticatory performance. The MAI ranges from 0 to 30.
2.4. Maximum bite force measurement
MBF was measured using a bite force transducer.21 The device consists of a unilateral strain gauge with a surface area of 100 mm2 and a vertical height of 2.8 mm. It was covered with a hard putty for dental protection and mounted on a mouthpiece. The strain gauge element was placed between the first molars to measure the occlusal forces when subjects clenched their jaws together as hard as possible. Two measurements each were taken from the left and right sides. The mean of the highest measurements on the left and right sides is presented as the MBF.
2.5. Maximum mouth opening measurement
MMO was measured extraorally using a previously published protocol.22 Briefly, the distance between applied markings on the inferior border of the chin and the tip of the nose was measured in patients in a resting position, as well as when opening the mouth as far as possible. Both positions were measured twice at every assessment. The difference between the average of the 2 resting positions and the highest value of the 2 maximum opening positions was defined as the MMO.
2.6. Dental status
Dental status was assessed and stratified into the following groups: edentulous (ED), full denture in upper and lower jaw (FD), full denture in upper or lower jaw combined with implant retention in upper or lower jaw (FD&FDI), full denture in upper or lower jaw and dentate in the other jaw (FD&D), full denture with implant retention in upper and lower jaw (FDI&FDI), full denture with implant retention in upper or lower jaw and dentate in the other jaw (FDI&D), or dentate upper and lower jaw (D&D). Partially dentate jaws were classified as dentate. Additionally, pairs of natural occluding premolars and molars were counted and scored, respectively, as 1 and 2 occlusal units (OU).23
2.7. Obturator prosthesis
When maxillary defects could not be closed primarily, a temporary obturator was fabricated based on preoperative assessments and dental casts. After approximately 1 year, the patient was provided with the definite obturator, made of acrylic resin, based on Beumer's method.24 The presence or absence of an obturator prosthesis was scored as “Yes” or “No,” respectively.
2.8. Statistical analysis
Chi‐square tests were used to analyze differences in patient characteristics with respect to nominal and ordinal variables, such as tumor location, whereas a one‐way analysis of variance was used to examine the age differences among the groups. Independent t tests were used to calculate the differences between mean values. The mean values of MAI, MMO, MBF, OU (paired t test), and prosthetic status (Wilcoxon signed rank test) did not differ between the t 1a and t 1b time points, thus only the t 1b values were presented when the patient had undergone both these assessments (t 1).
A linear mixed‐effects model with the MAI as outcome was constructed to assess both the changes over time and the effect of patient characteristics and clinical parameters. To account for within‐patient correlations, a random patient factor was added. Fixed‐effect factors such as age, sex, tumor location, alcohol consumption at baseline, tumor extent (pT classification), treatment modality, surgical reconstruction, assessment moment (t 0‐t 5), smoking habit, dental status, number of OU, the presence of an obturator prosthesis, MMO, and MBF during the follow up, as well as all 2‐way interactions of the factors during the assessment period, were assessed. The factors that were not significant at a P < .05 level were removed in a backward fashion, beginning with the interactions, to build a parsimonious model with sufficient fit while maintaining a hierarchical structure, meaning that if an interaction was included in the model, the main effects were also represented in the model. When an interaction with the assessment moment was found for a specific variable, there was a different coefficient for all levels of the variable at each assessment moment. The coefficients of the significant covariates, together with the value of the intercept of the mixed model analysis, were combined into a formula for the estimated MAI. The intercept is the value of the estimated MAI in the event that all following coefficients remain zero. The addition of coefficients of the significant covariates will, depending on the coefficient being positive or negative, either increase or decrease the estimated MAI. The formula was used to compare the chewing performance of patients with tumors in the 3 location groups during the follow‐up period. For each time point, we filled the formula with the average variable values for the significant coefficients in the 3 tumor location groups, as calculated by a restricted maximum likelihood approach.
A P‐value less than .05 was considered statistically significant. The mixed‐model analysis was performed using SAS version 9.2 (SAS Institute, Cary, North Carolina). The remaining tests were performed using SPSS 21 (IBM Corp., Armonk, New York).
3. RESULTS
At t 0, a total of 123 patients were enrolled in this study. After 5 years, 69 patients were still in the study; 30 patients died during the follow‐up period and 24 patients chose to stop participating for various reasons (Figure 1). Baseline demographics and clinical characteristics categorized on primary tumor location are displayed in Table 1.
Figure 1.
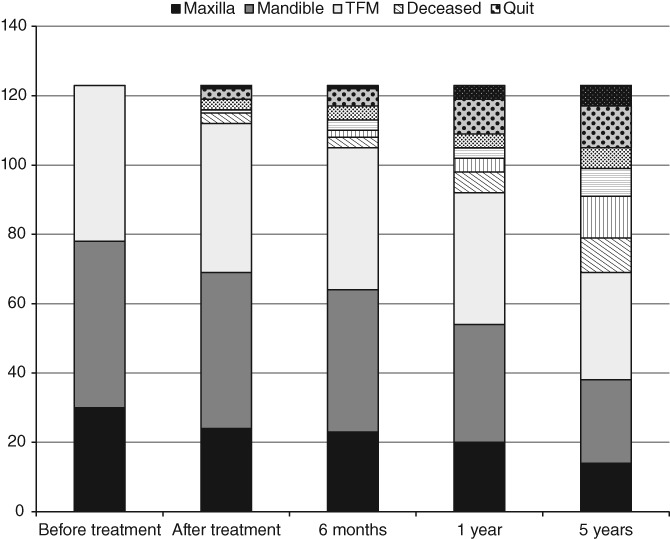
Flowchart showing the number of subjects (n) at each assessment stage and the average time since the primary oncological treatment. TFM, Tongue/Floor of the Mouth
Table 1.
Baseline demographics, clinical characteristics, and assessments categorized by tumor location
| Categorical variables, n (%) | Controls | Maxilla | Mandible | TFM | |
|---|---|---|---|---|---|
| (n = 60) | (n = 30) | (n = 48) | (n = 45) | P‐valuea | |
| Sex | |||||
| Male | 31 (51) | 14 (47) | 25 (52) | 30 (67) | .29 |
| Female | 29 (49) | 16 (53) | 23 (48) | 15 (33) | |
| Smoker | |||||
| Yes | 13 (22) | 8 (27) | 18 (38) | 16 (36) | .25 |
| No | 47 (78) | 22 (73) | 30 (62) | 29 (64) | |
| Alcohol use | |||||
| Yes | 30 (50) | 8 (27) | 15 (31) | 19 (42) | .09 |
| No | 30 (50) | 22 (73) | 33 (69) | 26 (58) | |
| pT‐stage | |||||
| T1 | NA | 5 (17) | 14 (29) | 23 (51) | .000** |
| T2 | NA | 11 (37) | 13 (27) | 14 (31) | |
| T3 | NA | 1 (3) | 3 (6) | 4 (9) | |
| T4 | NA | 13 (43) | 18 (38) | 4 (9) | |
| Dental status | |||||
| ED | 0 | 7 (23) | 13 (27) | 5 (11) | .000** |
| FD | 20 (33.3) | 7 (23) | 8 (17) | 13 (29) | |
| FD&FDI | 20 (33.3) | 0 (0) | 2 (4) | 4 (9) | |
| FD&D | 0 | 4 (14) | 8 (17) | 3 (7) | |
| FDI&FDI | 0 | 0 (0) | 0 (0) | 0 (0) | |
| FDI&D | 0 | 1 (3) | 0 (0) | 0 (0) | |
| D | 20 (33.3) | 11 (37) | 17 (35) | 20 (44) | |
| Treatment | |||||
| Surgery | NA | 12 (40) | 24 (50) | 23 (51) | .000** |
| Surgery and radiotherapy | NA | 18 (60) | 24 (50) | 22 (49) | |
| Surgical reconstruction | |||||
| No surgery | 60 | 0 (0) | 0 (0) | 0 (0) | .000** |
| Primary closure | NA | 17 (57) | 16 (33) | 23 (51) | |
| Local flap | NA | 1 (3) | 2 (4) | 1 (2) | |
| Fasciocutaneous free flap | NA | 12 (40) | 12 (25) | 19 (42) | |
| Bone graft/flap | NA | 0 (0) | 18 (38) | 2 (4) |
| Categorical variables, n (%) | Controls | Maxilla | Mandible | TFM | P‐valueb |
|---|---|---|---|---|---|
| (n = 60) | (n = 30) | (n = 48) | (n = 45) | ||
| Age (y) | 60.3 (7.2) | 68.7 (12.3) | 66.7 (12.7) | 61.4 (13.1) | .001* |
| Number of occlusal units | 3.8 (5.4) | 2.4 (4.1) | 2.3 (3.9) | 3.8 (5.1) | .26 |
| Maximum mouth opening (mm) | 53.7 (7.5) | 52.9 (11.8) | 46.6 (11.4) | 56.0 (9.8) | .000** |
| Maximum bite force (Newton) | 446 (384) | 224 (233) | 257 (330) | 377 (344) | .006* |
| Mixing ability index | 18.5 (3.7) | 24.1 (5.9) | 23.4 (5.0) | 21.0 (4.6) | .000** |
Abbreviations: D, dentate; ED, edentulous; FD, full denture; FDI, full denture on implants; NA, not applicable; TFM, tongue/floor of mouth.
Chi‐square test.
Analysis of variance
P < .01.
P < .001.
Thirty patients had maxillary tumors, 48 had maxillary tumors, and 45 had TFM tumors. Of the 30 patients who underwent a maxillectomy, 20 received an obturator prosthesis. In 11 of these 20 patients, the obturator was placed without further surgical intervention covering the defect; in 1 patient, it was combined with a local flap; and in the other 9 patients, it was combined with a fasciocutaneous free flap. Of the 48 patients with mandibular tumors, 18 had segmental defects. Two patients with primary floor of the mouth tumors had mandibular invasion to an extent that necessitated segmental resection. Nine patients were reconstructed using a reconstruction plate, of whom 3 combined with a fasciocutaneous flap due to soft tissue deficiency. Seven patients were reconstructed with a free vascularized bone flap and 1 with a nonvascularized iliac crest graft. One patient's segmental defect was not reconstructed due to comorbidity. Sixty‐four patients (52%) received postoperative radiotherapy (Table 1).
At baseline, sex, smoking status, alcohol usage, and the number of OU did not differ between patients with tumors in different locations; however, significant differences were observed between their MBF and masticatory performance, dental status, pT‐classification, treatment, reconstruction, and age (Table 1).
The mixed‐model analysis showed that age, sex, smoking and alcohol usage, pT classification, treatment modality, presence of an obturator prosthesis, and the type of reconstruction did not significantly contribute to the MAI; therefore, these factors were removed from the model. The assessment moment, dental status, number of OU, and MMO did significantly affect MAI. The location of the tumor and the MBF also contributed significantly to the MAI, but the relative effects differed at every assessment moment.
The following formula for the estimated MAI shows the significant variables and their coefficients that were retained in the model:
| (1) |
Factors with a coefficient above 0 will increase the MAI and thus reflect a worse masticatory performance. The contribution of a single coefficient can only be interpreted when all other variables remain stable. Figure 2 shows the relative impact of all significant variables and can be interpreted as a visual presentation of the formula.
Figure 2.
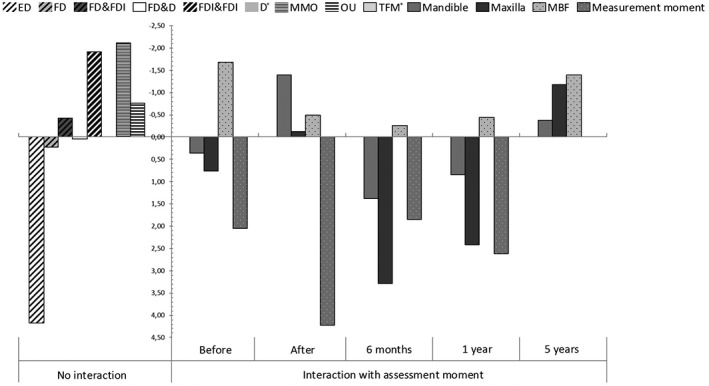
Visual presentation of the significant variables and their coefficients of the estimated mixing ability index (MAI) formula. Variables without an interaction with time are presented left of the y‐axis. Impact on the intercept (MAI = 23.9) is shown. The variables without interaction with the assessment moment are shown separately. The continuous variables (MMO, OU, MBF) are presented as the impact for the mean values of those variables. The mean number of occlusal units was 2.9 and the mean MMO was 52 mm. Mean MBF differs per assessment moment and is 326 N before intervention, 145 N directly after intervention, 193 N at 6 months, 194 N at 1 year, and 283 N at 5 years. Factors with positive outcomes increase the mixing ability outcome, reflecting a deterioration of masticatory performance; for convenience, the y‐axis has been inverted. *Reference category in mixed model, equals 0. D, Dentate; ED, edentulous; FD, full denture; FDI, full denture on implants; max: maxilla; Mand, mandible; MBF, maximum bite force; MAI, mixing ability index; MMO, maximum mouth opening; OU, occlusal units; TFM, tongue and floor of the mouth
All assessment moments except t 5 (t 0, t 1, t 2, and t 3) increased the MAI (thus worse masticatory performance), with the assessment moment immediately after surgery (t 1) having the highest coefficient. A dental status better than edentulous, a higher number of OU, and an increased MMO lowered the MAI (thus improve masticatory performance). An edentulous state without a prosthesis increased the MAI than all other dental states. A higher MBF lowered the MAI; however, its impact was greatest before treatment and 5 years after treatment. The influence of the tumor location differed between the assessment stages; The TFM location was used as reference category and equaled zero at every assessment moment. At the baseline, 6 months, and 1 year after treatment, maxillary and mandibular tumor location increased the MAI, thus the TFM location performs better at those moments. However, mandibulary tumor location and maxillary tumor location lowered the MAI at the assessments immediately after treatment and after 5 years.
The mean MAI before treatment was significantly lower (thus better masticatory performance) in healthy controls (=18.5 ± 3.7) than in all location groups (maxilla =24.1 ± 5.9, mandible =23.4 ± 5.0, both P = .000, and TFM =21.0 ± 4.6, P = .04). The general course of the mean MAI scores for all primary tumor locations is an initial deterioration after treatment, followed by a recovery over the 5 years after treatment. The recovery was mainly achieved between 1 and 5 years after treatment (Figure 3). No recovery plateau phase was observed for any of the groups.
Figure 3.
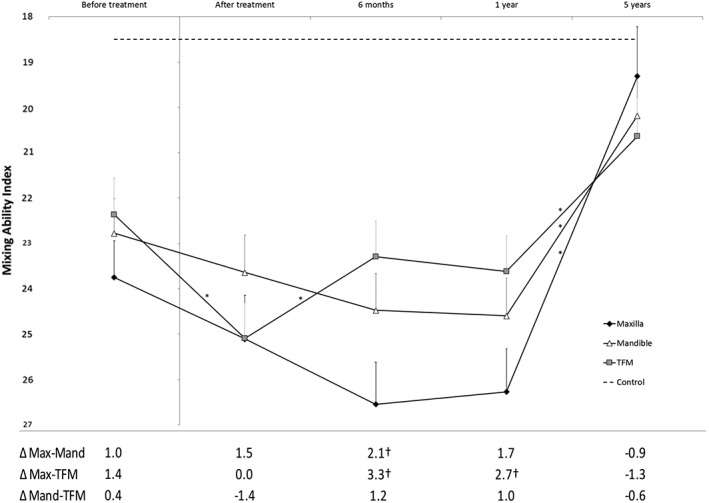
Estimates of mixing ability index (MAI) and SE rendered using a mixed‐model analysis. The y‐axis inverted for more values entered into the model was that of the mean patient in the cohort, calculated using a least‐squares method. The outcome presented is divided by the location of the primary tumor over a 5‐year follow up. Differences between groups are presented in the table under the figure. MAI ranges from 0 to 30, where 0 represents the best and 30 the worst possible outcome; thus for convenience, the y‐axis has been inverted. *A significant difference (P < .05) between that and the next assessment; † A significant difference (P < .05) between 2 groups at the same assessment time point, calculated using a restricted maximum likelihood approach. Only the assessment at 5 years after treatment of the maxillary tumor group was not significantly different from the control group (P = .27). Max, Maxilla; Mand, Mandible; TFM, Tongue/Floor of the Mouth
Five years after treatment, the mean MAI of patients who had a maxillary primary tumor (MAI 19.3 ± 1.1) was not significantly different from those who had a tumor in the mandible (MAI 20.2 ± 0.9, P = .46) or TFM (MAI 20.6 ± 0.9, P = .25). At 5 years after treatment, patients who had a mandibulary tumor or TFM tumor had worse MAI scores than the healthy controls (P = .01 and P = .001, respectively); however, patients who had a maxillary tumor showed no significant difference compared with the healthy controls at the end of the 5‐year follow‐up period (P = .27; Figure 3).
Although pT‐classification was not a significant factor according to our mixed‐model analysis, subjects who survived for 5 years after the treatment more often had lower pT‐classification outcomes (1 and 2) at the baseline than those who died during the follow‐up period (chi‐squared test: P = .04). The proportion of patients who died before the end of the assessment period was not significantly different between the patients with different tumor locations (chi‐squared test: P = .45).
4. DISCUSSION
In this 5‐year prospective study, factors with a significant contribution to masticatory performance were identified for patients who underwent a curative oncological intervention because of an oral carcinoma. The relative impacts of these factors on masticatory performance, assessed using the MAT, were also quantified. Increased numbers of OU, MBF, and MMO had a positive influence on masticatory performance, whereas the absence of a functional prosthesis in edentulous subjects had a negative impact.
The mean masticatory performance for patients with TFM, mandible, and maxilla tumors was higher at 5 years after treatment than it was before the treatment, which might partially be explained by the fact that the tumor itself, especially in advanced stages, may cause pain and discomfort. Also, between 1 and 5 years after treatment, rehabilitated patients received their definitive prosthetics.25, 26
4.1. Comparison with existing literature
In contrast to the results of our 5‐year prospective study, 2 previous cohort studies and 1 cross‐sectional study reported a lower masticatory performance compared with a healthy control group, but study groups were small.11, 15, 27
In contrast to our longitudinal results, a negative effect of age on masticatory performance in healthy subjects was reported in 2 cross‐sectional studies,28, 29 but in another cross‐sectional study, no clear relationship was found between these factors in healthy subjects.10
Using a comminution test in patients treated for all head and neck cancer locations, a significant and clinically relevant increase in bite force and objective masticatory performance was demonstrated after definitive prosthodontic rehabilitation.28 In the same study, the number of occluding pairs of (pre)molars were found to influence masticatory performance. Bite force has been previously reported to account for up to 60% of the variance in masticatory performance in healthy subjects.30 Other objective data in patients with both head and neck cancer and healthy subjects found beneficial effects of adequate prosthetic rehabilitation.17, 31, 32 In a systematic review, the importance of rehabilitating dentition, including the use of dental implants to support a fixed prosthesis, add OU, or support a removable prosthesis, was confirmed.33 Studies on patient‐reported masticatory ability, however, found little11 to no effect31, 34 of prosthodontic treatment.
Although trismus has previously been identified in a systematic review as a negative influencer of masticatory performance in head and neck oncology, however, from which severity of MMO‐restriction this negative impact occurs is unknown.35 In our model, each millimeter of mouth opening provides a 0.04 improvement in the MAI, so a reduction of MMO from 55 to 30 mm deteriorates the MAI by 1.0 points, which is a clinically relevant impact independent of all other factors. In another prospective study using a logistic regression analysis on the same patient population, plus 20 patients with oropharyngeal tumors, the prognostic factors of trismus development have been presented. Lower pretreatment MMO, maxillary or mandibulary tumor location and (postoperative) radiotherapy increased the risk of the development of trismus.13
Other studies found a 1‐year postoperative improvement in objective masticatory efficacy, determined using ATP‐grains and the MAT, respectively, in patients with a TFM tumor, when compared with preoperative values. This effect might be due to the effect of primary tumor discomfort on the masticatory performance.15, 36
In a cross‐sectional study, patients who underwent a maxillectomy showed better masticatory performance after full oral rehabilitation than those who had a mandibulectomy, but their chewing ability was still inferior to healthy full‐denture wearers. However, the treatment groups in this study were small.16
4.2. Clinical implications
Masticatory performance is an important factor in posttreatment quality of life for patients treated for head and neck cancer.37 In general, functional outcomes are not the sole contributors to quality of life, but they form a significant part of a patient's well‐being and are therefore important issues to address.4, 5, 6 This study showed a number of factors that impact masticatory performance during the rehabilitation of patients treated for oral malignancies. The clinical significance of the formula can calculate the estimated masticatory performance for any patient with oral cavity, although information such as bite force is usually not readily available in daily practice. The factors found to significantly affect masticatory performance such as OU count, prosthetic status, bite force, and mouth opening are all factors that need to be considered when constructing the initial treatment plan. Primary consideration of reconstructive and rehabilitative options in a multidisciplinary setting might ensure adequate management of these factors. This includes the consideration of digital planning for reconstruction and primary implant placement by the head and neck surgeon and prosthodontist. Additionally, beneficial effects of orofacial physiotherapy have been reported in orthognatic surgery,38 but have yet to be proven in patients with head and neck cancer.35 Finally, thoughtful consideration should be given in maintaining OU when clearing the dentition of potential foci in osteoradionecrosis prevention. However, the presence of natural teeth after radiotherapy necessitates patients to commit to lifelong meticulous oral hygiene and frequent self‐application of fluoride, either as neutral sodium fluoride or a 1% gel applied at least every other day to prevent radiation caries.39 We found that the initial tumor location has an effect on masticatory performance. This factor cannot be influenced but can be addressed during the pretreatment counseling to optimize a patient's expectations regarding masticatory performance.
4.3. Strengths and limitations
The strengths of this study are its long follow‐up, prospective design, large study population (N = 123), meticulous data generation through testing, and thorough statistical analysis. The latter provided us the opportunity to correct for missing values (participants who dropped out or died) and adjust for repeated measures and an unequal distribution of baseline clinical characteristics between groups. In this cohort study, no subgroup analyses were performed on other possible influential factors, such as lingual nerve removal, the extent of surgery, or the specific location of radiotherapy, due to the small subgroup sample sizes.
The mean estimate of the MAI was higher for patients at 5 years after treatment than before treatment, regardless of the location of the tumor. A possible explanation for this is that the mixed‐model outcome is based on the remaining participants at every assessment moment, which in this case could influence the outcome. In addition, those who survived for 5 years after their treatment had smaller tumors, and thus smaller resections, than patients who died or dropped out of the study before the 5‐year assessment. This could have influenced the mean outcome of masticatory performance.
Unfortunately, the healthy controls were only assessed once, so changes in their masticatory performance over the course of the follow‐up could not be compared to the treatment groups.
4.4. Future research
Future research should focus on possible further recovery, or a secondary decline, of masticatory performance after the 5‐year posttreatment period.
Tongue function is of great importance in mastication40, 41; however, until now the influence of disabled tongue function on masticatory performance was unknown for patients treated for oral cancer. The clinical effects of standardized posttreatment physical therapy protocols have yet to be evaluated.
4.5. Conclusion
Oral cancer and its treatment drastically affect masticatory performance, but it recovers to pretreatment levels in patients who survive for 5 years. Masticatory performance in patients with oral cancer is positively affected by having full dentures or better, a higher number of OU, increased MMO, elevated MBF, and having a maxillary rather than mandibular or TFM tumor location.
de Groot RJ, Wetzels J‐W, Merkx MAW, et al. Masticatory function and related factors after oral oncological treatment: A 5‐year prospective study. Head & Neck. 2019;41:216–224. 10.1002/hed.25445
REFERENCES
- 1. Moroi HH, Okimoto K, Terada Y. The effect of an oral prosthesis on the quality of life for head and neck cancer patients. J Oral Rehabil. 1999;26:265‐273. [DOI] [PubMed] [Google Scholar]
- 2. Burlage FR, Coppes RP, Meertens H, Stokman MA, Vissink A. Parotid and submandibular/sublingual salivary flow during high dose radiotherapy. Radiother Oncol. 2001;61(3):271‐274. [DOI] [PubMed] [Google Scholar]
- 3. Specht L. Oral complications in the head and neck radiation patient. Support Care Cancer. 2002;10(1):36‐39. [DOI] [PubMed] [Google Scholar]
- 4. Korfage A, Schoen PJ, Raghoebar GM, et al. Five‐year follow up of oral functioning and quality of life in patients with oral cancer with implant‐retained mandibular overdentures. Head Neck. 2011;33:831‐839. [DOI] [PubMed] [Google Scholar]
- 5. Wells M, Swartzman S, Lang H, et al. Predictors of quality of life in head and neck cancer survivors up to 5 years after end of treatment: a cross‐sectional survey. Support Care Cancer. 2016;24(6):2463‐2472. [DOI] [PubMed] [Google Scholar]
- 6. Morimata J, Otomaru T, Murase M, Haraguchi M, Sumita Y, Taniguchi H. Investigation of factor affecting health‐related quality of life in head and neck cancer patients. Gerodontology. 2013;30(3):194‐200. [DOI] [PubMed] [Google Scholar]
- 7. Speksnijder CM, van der Glas HW, van der Bilt A, van Es RJJ, van der Rijt E, Koole R. Oral function after oncological intervention in the oral cavity: a retrospective study. J Oral Maxillofac Surg. 2010;68(6):1231‐1237. [DOI] [PubMed] [Google Scholar]
- 8. Wetzels JW, Koole R, Meijer GJ, de Haan AFJ, Merkx MAW, Speksnijder CM. Functional benefits of implants placed during ablative surgery: a 5‐year prospective study on the prosthodontic rehabilitation of 56 edentulous oral cancer patients. Head Neck. 2016;38(S1):E2103‐E2111. [DOI] [PubMed] [Google Scholar]
- 9. Thexton AJ. Mastication and swallowing: an overview. Br Dent J. 1992;173(6):197‐206. [DOI] [PubMed] [Google Scholar]
- 10. Hatch JP, Shinkai RSA, Sakai S, Rugh JD, Paunovich ED. Determinants of masticatory performance in dentate adults. Arch Oral Biol. 2001;46(7):641‐648. [DOI] [PubMed] [Google Scholar]
- 11. Curtis DA, Plesh O, Miller AJ, et al. A comparison of masticatory function in patients with or without reconstruction of the mandible. Head Neck. 1997;19(4):287‐296. [DOI] [PubMed] [Google Scholar]
- 12. Ammajan RR, Joseph R, Rajeev R, Choudhary K, Vidhyadharan K. Assessment of periodontal changes in patients undergoing radiotherapy for head and neck malignancy: a hospital‐based study. J Cancer Res Ther. 2013;9(4):630‐637. [DOI] [PubMed] [Google Scholar]
- 13. Wetzels JGH, Merkx MAW, de Haan AFJ, Koole R, Speksnijder CM. Maximum mouth opening and trismus in 143 patients treated for oral cancer: a 1‐year prospective study. Head Neck. 2014;36(12):1754‐1762. [DOI] [PubMed] [Google Scholar]
- 14. Ono T, Kohda H, Hori K, Nokubi T. Masticatory performance in postmaxillectomy patients with edentulous maxillae fitted with obturator prostheses. Int J Prosthodont. 2007;20(2):145‐150. [PubMed] [Google Scholar]
- 15. Namaki S, Matsumoto M, Ohba H, Tanaka H, Koshikawa N, Shinohara M. Masticatory efficiency before and after surgery in oral cancer patients: comparative study of glossectomy, marginal mandibulectomy and segmental mandibulectomy. J Oral Sci. 2004;46(2):113‐117. [DOI] [PubMed] [Google Scholar]
- 16. Reitemeier B, Unger M, Richter G, Ender B, Range U, Markwardt J. Clinical test of masticatory efficacy in patients with maxillary/mandibular defects due to tumors. Onkologie. 2012;35(4):170‐174. [DOI] [PubMed] [Google Scholar]
- 17. Speksnijder CM, Abbink JH, van der Glas HW, Janssen NG, Van Der Bilt A. Mixing ability test compared with a comminution test in persons with normal and compromised masticatory performance. Eur J Oral Sci. 2009;117(5):580‐586. [DOI] [PubMed] [Google Scholar]
- 18. Fritz AG. International Classification of Diseases for Oncology (ICD‐O). Geneva: World Health Organization; 2000:240. [Google Scholar]
- 19. Patel SG, Shah JP. TNM staging of cancers of the head and neck: striving for uniformity among diversity. CA Cancer J Clin. 2005;55(4):242‐258. [DOI] [PubMed] [Google Scholar]
- 20. van der Bilt A, Speksnijder CM, de Liz Pocztaruk R, Abbink JH. Digital image processing versus visual assessment of chewed two‐colour wax in mixing ability tests. J Oral Rehabil. 2012;39(1):11‐17. [DOI] [PubMed] [Google Scholar]
- 21. Slagter AP, Bosman F, van der Glas HW, van der Bilt A. Human jaw‐elevator muscle activity and food comminution in the dentate and edentulous state. Arch Oral Biol. 1993;38(3):195‐205. [DOI] [PubMed] [Google Scholar]
- 22. McCord J, Grant A. Registration: stage II‐‐intermaxillary relations. Br Dent J. 2000;188:601‐606. [DOI] [PubMed] [Google Scholar]
- 23. Witter DJ, Creugers NHJ, Kreulen CM, de Haan AFJ. Occlusal stability in shortened dental arches. J Dent Res. 2001;80(2):432‐436. [DOI] [PubMed] [Google Scholar]
- 24. Beumer J Jr, Curtis T, Marunick M. Maxillofacial Rehabilitation; Prosthodontic and Surgical Considerations. St Louis, Tokyo: Ishiyaku EuroAmerica; 1996:240‐324. [Google Scholar]
- 25. Stenson K, MacCracken E, List MA, et al. Swallowing function in patients with head and neck cancer prior to treatment. Arch Otolaryngol Head Neck Surg. 2016;126:371‐377. [DOI] [PubMed] [Google Scholar]
- 26. Nicoletti G, Soutar DS, Jackson MS, Wrench AA, Robertson G. Chewing and swallowing after surgical treatment for oral cancer: functional evaluation in 196 selected cases. Plast Reconstr Surg. 2004;114(2):329‐338. [DOI] [PubMed] [Google Scholar]
- 27. Loewen IJ, Boliek CA, Harris J, Seikaly H, Rieger JM. Oral sensation and function: a comparison of patients with innervated radial forearm free flap reconstruction to healthy matched controls. Head Neck. 2010;32(1):85‐95. [DOI] [PubMed] [Google Scholar]
- 28. Kosaka T, Ono T, Kida M, et al. A multifactorial model of masticatory performance: the Suita study. J Oral Rehabil. 2016;43(5):340‐347. [DOI] [PubMed] [Google Scholar]
- 29. Yamashita S, Sakai S, Hatch JP, Rugh JD. Relationship between oral function and occlusal support in denture wearers. J Oral Rehabil. 2000;27(10):881‐886. [DOI] [PubMed] [Google Scholar]
- 30. van der Bilt A. Assessment of mastication with implications for oral rehabilitation: a review. J Oral Rehabil. 2011;38(10):754‐780. [DOI] [PubMed] [Google Scholar]
- 31. van der Bilt A, Olthoff LW, Bosman F, Oosterhaven SP. Chewing performance before and after rehabilitation of post‐canine teeth in man. J Dent Res. 1994;73(11):1677‐1683. [DOI] [PubMed] [Google Scholar]
- 32. Sumida T, Kobayashi Y, Ishikawa A, et al. Bite force and masticatory performance using implant‐supported overdentures after treatment of mandibular cancer. Anticancer Res. 2016;36(8):4077‐4080. [PubMed] [Google Scholar]
- 33. Roumanas ED, Chang T‐L, Beumer J. Use of osseointegrated implants in the restoration of head and neck defects. J Calif Dent Assoc. 2006;34(9):711‐718. [PubMed] [Google Scholar]
- 34. Korfage A, Schoen PJ, Raghoebar GM, Roodenburg JLN, Vissink A, Reintsema H. Benefits of dental implants installed during ablative tumour surgery in oral cancer patients: a prospective 5‐year clinical trial. Clin Oral Implants Res. 2010;21(9):971‐979. [DOI] [PubMed] [Google Scholar]
- 35. Dijkstra PU, Kalk WWI, Roodenburg JLN. Trismus in head and neck oncology: a systematic review. Oral Oncol. 2004;40(9):879‐889. [DOI] [PubMed] [Google Scholar]
- 36. Speksnijder CM, van der Bilt A, Abbink JH, Merkx MAW, Koole R. Mastication in patients treated for malignancies in tongue and/or floor of mouth: a 1‐year prospective study. Head Neck. 2011;33(7):1013‐1020. [DOI] [PubMed] [Google Scholar]
- 37. Inukai M, John MT, Igarashi Y, Baba K. Association between perceived chewing ability and oral health‐related quality of life in partially dentate patients. Health Qual Life Outcomes. 2010;8(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prado DG d A, Berretin‐Felix G, Migliorucci RR, et al. Effects of orofacial myofunctional therapy on masticatory function in individuals submitted to orthognathic surgery: a randomized trial. J Appl Oral Sci. 2018;26(0):e20170164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vissink A, Burlage F, Spijkervet F, Jansma J, Coppes R. Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14(3):213‐225. [DOI] [PubMed] [Google Scholar]
- 40. Matsuo K, Palmer JB. Coordination of mastication, swallowing and breathing. Jpn Dent Sci Rev. 2009;45(1):31‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Speksnijder CM, Van Der Bilt A, Van Der Glas HW, Koole R, Merkx MAW. Tongue function in patients treated for malignancies in tongue and/or floor of mouth; a one year prospective study. Int J Oral Maxillofac Surg. 2011;40(12):1388‐1394. [DOI] [PubMed] [Google Scholar]


