Abstract
Aims
To evaluate adherence, persistence, glycaemic control and costs at 12‐month follow‐up for patients initiating dulaglutide versus liraglutide or exenatide once weekly.
Materials and methods
The present retrospective observational claims study included patients with type 2 diabetes (T2D) and ≥ 1 pharmacy claim for dulaglutide, liraglutide or exenatide once weekly from the HealthCore Integrated Research Database. Adherence was defined as proportion of days covered ≥80%, and persistence was measured by time to discontinuation of index therapy. Change from baseline in glycated haemoglobin (HbA1c) concentration was assessed in a subset with pre‐ and post‐index HbA1c results. Propensity scores were used to match the cohorts.
Results
The baseline characteristics were balanced for the matched cohorts, dulaglutide versus liraglutide (n = 2471) and dulaglutide versus exenatide once weekly (n = 1891). Among those initiating dulaglutide there was a significantly higher proportion of adherent patients compared with the groups initiating liraglutide (51.2% vs. 38.2%; P < 0.001) and exenatide once weekly (50.7% vs. 31.9%; P < 0.001). At 12 months, 55% of patients in the dulaglutide group versus 43.8% in the liraglutide group (P < 0.001), and 54.9% in the dulaglutide versus 34.4% in the exenatide once‐weekly group (P < 0.001) were persistent. The dulaglutide group had a significantly greater reduction in HbA1c than the liraglutide group (−34.24 vs. −31.94 mmol/mol; P = 0.032), and a greater, but nonsignificant, reduction in HbA1c than the exenatide once‐weekly group (−34.46 vs. −31.94 mmol/mol; P = 0.056). The diabetes‐related total costs were not significantly different between the dulaglutide and the liraglutide group ($16,174 vs. $16,694; P = 0.184), and were significantly higher for dulaglutide than for exenatide once weekly ($15,768 vs. $14,615; P = 0.005).
Conclusions
Adherence and persistence are important considerations in patient‐centric treatment selection for patients with T2D. Higher adherence and persistence for dulaglutide compared with liraglutide or exenatide once weekly are relevant criteria when choosing glucagon‐like peptide‐1 receptor agonist treatment for patients with T2D.
Keywords: adherence, dulaglutide, effectiveness, GLP‐1 receptor agonists, HbA1c
1. INTRODUCTION
In a recent consensus statement, the American Diabetes Association and European Association for the Study of Diabetes recommended a patient‐centred glycaemic management approach for patients with type 2 diabetes (T2D).1 Glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) are given increased emphasis in the treatment approach after metformin because of the low associated risk of hypoglycaemia, potential for weight loss, and proven cardiovascular effects for some of these agents. The consensus report also recommends considering GLP‐1RAs prior to insulin as a first injectable option for treatment intensification in asymptomatic patients with T2D not at glycaemic goal.1
In the United States, exenatide twice daily, liraglutide, exenatide once weekly (as pen and auto injector), dulaglutide, and semaglutide are the GLP‐1RAs approved and available for management of glycaemic control in patients with T2D. Controlled clinical trials have shown that GLP‐1RAs differ in the magnitude of glycated haemoglobin (HbA1c) reduction, weight loss, and possible cardiovascular benefits, as well as in the frequency of gastrointestinal adverse events, including nausea, vomiting and diarrhoea.2, 3 Currently, liraglutide is the only agent indicated for reduction of cardiovascular risk in patients with established cardiovascular disease.4 In addition to differences in clinical profiles, these GLP‐1RAs have substantial differences in dosing regimen, need/length of dose titration, and administration device features such as need for reconstitution, single‐dose vs. multi‐dose devices, needle handling, and needle size (Appendix S1, Table S1). These differences in profile of GLP‐1RAs may play an important role in treatment adherence, persistence and eventually effectiveness.
Increased adherence to anti‐hyperglycaemic medications is associated with better glycaemic control, fewer complications, and lower healthcare utilization.5, 6, 7 The change in HbA1c with long‐acting GLP‐1RAs in real‐world settings is approximately half that observed in randomized clinical trials.8 Approximately 75% of that gap is attributed to poor adherence.8 Additionally a decrease in adherence may worsen long‐term health outcomes and consequently increase associated healthcare costs.9, 10, 11 A study conducted by Alatorre et al12 showed that patients initiating treatment with dulaglutide had higher adherence and persistence compared with patients initiating treatment with liraglutide or exenatide once weekly at 6‐month follow‐up. In a comparative glycaemic effectiveness study also using 6 months' follow‐up, Unni et al13 reported no significant differences between exenatide once weekly, dulaglutide, and albiglutide in HbA1c change from baseline; however, published literature on longer‐term treatment adherence and persistence with GLP‐1RAs and their impact on glycaemic effectiveness and healthcare costs is limited. Because treatment adherence remains a major challenge among patients with T2D, it is important to evaluate the long‐term real‐world treatment patterns, including adherence, persistence and discontinuation, along with comparative glycaemic effectiveness, among agents in this promising class.
The aim of the present retrospective observational study in a US real‐world setting, therefore, was to compare adherence, persistence, glycaemic control and cost outcomes at 12‐month follow‐up among commercially insured patients with T2D initiating treatment with one of the three long‐acting GLP‐1RAs commonly used in the United States at the time of the study: dulaglutide, liraglutide, or exenatide once‐weekly pen.
2. MATERIALS AND METHODS
2.1. Study design
In this retrospective observational study, patients with at least one pharmacy claim for dulaglutide, liraglutide, or exenatide once‐weekly pen between 1 November 2014 and 31 May 2016 (the index period) were identified from the HealthCore Integrated Research Database (HIRD). The HIRD contains longitudinal administrative claims data for members of 14 major commercial health plans in the Northeastern, Mid‐Atlantic, Southeastern, Midwestern, Central and Western regions of the United States, and includes members in each of the 50 states. Data include professional, facility and outpatient pharmacy claims, outpatient laboratory results, and health plan enrolment information. The study analysis included all medical and pharmacy claims, as well as outpatient laboratory data submitted to the HIRD between 1 May 2014 and 31 May 2017.
2.2. Patient population
Patients aged ≥18 years were assigned to one of the three cohorts based on the earliest fill date (index date) for one of the index GLP‐1RAs prescribed (dulaglutide, liraglutide or exenatide once weekly). For each index GLP‐1RA cohort, all other available GLP‐1RAs were considered non‐index GLP‐1RAs. All patients had continuous medical and pharmacy plan enrolment (either commercial or Medicare Advantage health plans) for at least 6 months pre‐index (baseline period) and at least 12 months post‐index (follow‐up period). Patients were also required to have at least one medical claim for T2D (International Classification of Diseases, 9th revision Clinical Modification [ICD‐9‐CM] diagnoses codes 250.x0 or 250.x2; ICD‐10‐CM diagnosis code E11) during the 6 months pre‐index. Patients with a diagnosis of type 1 diabetes or secondary diabetes during the baseline period, a diagnosis of pregnancy‐ or childbirth‐related diabetes during the entire study period, or a claim for an index drug during the baseline period (to ensure identification of new initiators) were excluded. The main analysis population (MAP) consisted of patients meeting inclusion and exclusion criteria with complete information on pharmacy costs. Patients in the MAP were new initiators of the index GLP‐1RAs but were not necessarily new users of the GLP‐1RA class, as they may have had a prescription of a non‐index GLP‐1RA during the baseline period.
The HbA1c analysis population (HAP) was a subset of all patients, irrespective of pharmacy cost completeness, with at least one pre‐ and one post‐index (at 12 months) laboratory HbA1c result available with which to assess mean change from baseline in HbA1c. Patients in the HAP were GLP‐1RA‐naïve, that is, they did not have a prescription for any GLP‐1RA in the 6‐month baseline period.
2.3. Study measures
Patient demographics and clinical characteristics were assessed at baseline, including the specialty of the prescribing physician for the index GLP‐1RA agent. Baseline comorbidities were captured by ICD‐9/10 codes, the Quan‐Charlson Comorbidity Index,14 and the adapted Diabetes Complications Severity Index.15 Antidiabetic medication usage was assessed at baseline, including use of: any type of insulin; oral antidiabetic drugs (OADs), such as metformin, thiazolidinediones, sulphonylureas, dipeptidyl peptidase‐4 inhibitors or sodium‐glucose co‐transporter‐2 inhibitors; and non‐insulin injectables, such as GLP‐1RAs.
Adherence, measured by proportion of days covered (PDC), was defined as the sum of the number of days with the index GLP‐1RA available divided by the number of days in the observation period. The start date of each new prescription fill was adjusted whenever the patient had overlapping days' supply from the previous fill. For this study, it was assumed that patients finished the supply of the preceding fill before starting the new supply.16 Patients with PDC ≥80% were considered adherent.
Persistence was calculated as the number of days of continuous therapy from the point of initiation until the end of 12 months' follow‐up, allowing a maximum gap of 45 days between fills.12 To determine the duration of continuous therapy, dates of early refills were adjusted to the day after finishing the supply from the previous fill. A patient with a gap between the run‐out date of the previous fill and the next fill of >45 days was considered to have discontinued treatment. The index dose of GLP‐1RA and the percentage of patients with second fills were also reported.
All‐cause and diabetes‐related healthcare costs were calculated as the sum of patient‐paid and plan‐paid costs. Diabetes‐related medical costs were aggregated costs for any medical claims that had diagnosis codes for any diabetes type; diabetes‐related pharmacy costs were summed costs for any pharmacy claims that had antidiabetic medication generic product identifier codes. The MAP was used for adherence, persistence and cost analyses.
The HbA1c level closest to the respective assessment time point was used in analysis: baseline (range 183 days before index date to 14 days after, with index date being the assessment time point) and 12 months (range 275 days to 410 days after the index date, with index date +365 days being the assessment time point). Glycaemic control was assessed by the change in HbA1c levels from baseline to 12 months' follow‐up, and by the percentage of patients reaching an HbA1c target of <53 mmol/mol at 12 months. The HAP was used for HbA1c analysis. Additionally, the average cost at 12 months' follow‐up per 1% HbA1c reduction from baseline was calculated by dividing the mean 12‐months' follow‐up costs by the mean percentage reduction in HbA1c from baseline to 12‐month follow‐up. A subset of HAP with complete pharmacy cost data was used in this analysis and no statistical testing was performed.
2.4. Statistical analysis
All variables were summarized using descriptive statistics, with mean, SD and median used for continuous variables, and counts and percentages used for categorical variables. Differences in continuous variables were tested using t‐tests or Wilcoxon rank sum tests; χ2 tests were used for categorical variables. An α level of 0.05 was used to identify statistical significance. No adjustments for multiple comparisons were made in this study.
Because of the observational nature of the study and lack of randomization of patients into the three study cohorts, it was necessary to control for treatment selection bias that may impact outcome. The propensity‐score method to control for potential differences in characteristics influencing treatment selection has been widely accepted.17, 18 Propensity scores, defined as the probability of initiating dulaglutide (vs. initiating liraglutide or exenatide once weekly) given the baseline characteristics, were calculated using logistic regression.
Two separate propensity‐score models were created for the MAP, one for each comparison (dulaglutide vs. liraglutide and dulaglutide vs. exenatide once weekly; see Table 1 footnote for list of covariates). Nearest‐neighbour 1:1 matching with calipers was used. The caliper width was set to 0.2 of the SD of the logit of the propensity score. Additionally, patients were matched exactly on prior use of non‐index GLP‐1RAs at baseline.19 Absolute standardized differences of <0.10 were considered to denote balance in baseline characteristics between the cohorts.20 Separate propensity‐score models were created for the HbA1c analyses, and patients were matched exactly on the HbA1c result categories (<53, 53 to <64, 64 to <75, and >75). All propensity‐score matching was finalized before the outcome analyses were conducted.
Table 1.
Post‐matchinga patient demographic and clinical characteristics at baseline: Main analysis population
| Characteristics | Dulaglutide n = 2427 | Liraglutide n = 2427 | Standardized difference | Dulaglutide n = 1808 | Exenatide once weekly n = 1808 | Standardized difference |
|---|---|---|---|---|---|---|
| Age in years, mean (SD) | 54.1 (9.54) | 54.3 (9.44) | −0.017 | 54.3 (9.77) | 54.3 (9.61) | −0.006 |
| Female, n (%) | 1171 (48.2) | 1154 (47.5) | 0.014 | 891 (49.3) | 873 (48.3) | 0.020 |
| Health plan type, n (%) | ||||||
| PPO | 1538 (63.4) | 1552 (63.9) | −0.012 | 1111 (61.4) | 1101 (60.9) | 0.011 |
| HMO | 637 (26.2) | 600 (24.7) | 0.035 | 496 (27.4) | 505 (27.9) | −0.011 |
| CDHP/other/ missing/unknown | 252 (10.4) | 275 (11.3) | −0.030 | 201 (11.1) | 202 (11.2) | −0.002 |
| Medicare advantage, n (%) | 161 (6.6) | 172 (7.1) | −0.018 | 147 (8.1) | 150 (8.3) | −0.006 |
| Geographic region, n (%) | ||||||
| Northeast | 343 (14.1) | 348 (14.3) | −0.006 | 193 (10.7) | 195 (10.8) | −0.004 |
| Midwest | 727 (30.0) | 762 (31.4) | −0.031 | 621 (34.3) | 633 (35.0) | −0.014 |
| South | 1006 (41.5) | 969 (39.9) | 0.031 | 743 (41.1) | 719 (39.8) | 0.027 |
| West | 351 (14.5) | 348 (14.3) | 0.004 | 251 (13.9) | 261 (14.4) | −0.016 |
| Prescribing HCP specialty, n (%) | ||||||
| Endocrinologist | 829 (34.2) | 825 (34.0) | 0.003 | 466 (25.8) | 462 (25.6) | 0.005 |
| Primary care physician | 621 (25.6) | 603 (24.8) | 0.017 | 551 (30.5) | 563 (31.1) | −0.014 |
| Otherb | 941 (38.8) | 948 (39.1) | −0.006 | 766 (42.4) | 754 (41.7) | 0.013 |
| Missing | 36 (1.5) | 51 (2.1) | −0.047 | 25 (1.4) | 29 (1.6) | −0.018 |
| aDCSI score, mean (SD) | 0.7 (1.18) | 0.7 (1.24) | −0.012 | 0.7 (1.17) | 0.7 (1.22) | −0.023 |
| QCI score, mean (SD) | 1.7 (1.21) | 1.7 (1.23) | 0.004 | 1.7 (1.23) | 1.7 (1.24) | 0.000 |
| Comorbid conditions | ||||||
| Cardiovascular disease | 333 (13.7) | 355 (14.6) | −0.026 | 258 (14.3) | 269 (14.9) | −0.017 |
| Dyslipidaemia | 1801 (74.2) | 1816 (74.8) | −0.014 | 1313 (72.6) | 1329 (73.5) | −0.020 |
| Hypertension | 1770 (72.9) | 1786 (73.6) | −0.015 | 1331 (73.6) | 1306 (72.2) | 0.031 |
| Nephropathy | 245 (10.1) | 241 (9.9) | 0.005 | 175 (9.7) | 181 (10.0) | −0.011 |
| Neuropathy | 436 (18.0) | 426 (17.6) | 0.011 | 308 (17.0) | 300 (16.6) | 0.012 |
| Obesity | 682 (28.1) | 703 (29.0) | −0.019 | 528 (29.2) | 533 (29.5) | −0.006 |
| Retinopathy | 160 (6.6) | 181 (7.5) | −0.034 | 110 (6.1) | 117 (6.5) | −0.016 |
| Endocrinologist visit during pre‐index period, n (%) | 875 (36.1) | 888 (36.6) | −0.011 | 467 (25.8) | 465 (25.7) | 0.003 |
| Number of endocrinologist visits, mean (SD) | 0.6 (1.02) | 0.6 (1.01) | 0.003 | 0.4 (0.87) | 0.4 (0.89) | 0.011 |
| Number of diabetes‐related prescription drug fills during pre‐index period, mean (SD) | 7.0 (4.95) | 7.0 (5.01) | 0.002 | 6.9 (5.07) | 6.8 (4.86) | 0.028 |
| Antidiabetic medications during pre‐index period, n (%) | 2314 (95.3) | 2302 (94.8) | 0.023 | 1720 (95.1) | 1712 (94.7) | 0.020 |
| DPP‐4 inhibitors | 705 (29.0) | 680 (28.0) | 0.023 | 518 (28.7) | 509 (28.2) | 0.011 |
| GLP‐1RA agents | 166 (6.8) | 166 (6.8) | 0.000 | 114 (6.3) | 114 (6.3) | 0.000 |
| Insulin | 796 (32.8) | 797 (32.8) | −0.001 | 566 (31.3) | 552 (30.5) | 0.017 |
| Metformin | 1802 (74.2) | 1766 (72.8) | 0.034 | 1353 (74.8) | 1326 (73.3) | 0.034 |
| SGLT2 inhibitors | 479 (19.7) | 485 (20.0) | −0.006 | 288 (15.9) | 285 (15.8) | 0.005 |
| Sulphonylureas | 779 (32.1) | 858 (35.4) | −0.069 | 589 (32.6) | 623 (34.5) | −0.040 |
| Thiazolidinediones | 227 (9.4) | 201 (8.3) | 0.038 | 168 (9.3) | 179 (9.9) | −0.021 |
| Number of oral antidiabetic medication classes, mean (SD) | 1.7 (1.05) | 1.7 (1.06) | 0.001 | 1.6 (1.03) | 1.6 (1.06) | −0.002 |
aDCSI, adapted Diabetes Complication Severity Index; CDHP, consumer‐driven health plan; DPP‐4, dipeptidyl peptidase‐4; GLP‐1RA, glucagon‐like peptide receptor agonist; HCP, healthcare provider; HMO, health maintenance organization; PPO, preferred provider organization; QCI, Quan‐Charlson Comorbidity Index; SGLT2, sodium‐glucose co‐transporter‐2.
Propensity scores were calculated using logistic regression with baseline covariates age, gender, geographic location, Medicare Advantage coverage, health plan type, and prescribing health care provider specialty (endocrinologist vs. PCP vs. others/missing) on the index date; aDCSI, presence of cardiovascular disease, presence of obesity, diabetes‐related pharmacy costs (combined amount paid by the health plan, the patient, and third parties), presence and number of endocrinologist visits, number of diabetes‐related prescription drug fills, number of oral antidiabetic medication classes, use of non‐index GLP‐1 (exact match), insulin, SGLT2 inhibitors, DPP‐4 inhibitors, and diabetes supplies during the baseline period.
Includes physicians with other specialties and non‐physician healthcare professionals that can prescribe, eg, nurse practitioner.
Cost comparison was carried out using generalized linear model analysis with gamma distribution and log link function or t‐tests.
Kaplan‐Meier survival analysis was used to assess the predicted probability of persistence to index GLP‐1RA agent at 12‐month follow‐up. Comparisons were carried out between cohorts using log‐rank tests. Hazard ratios for discontinuation were calculated using Cox proportional hazard models. Relative risk of discontinuation was also calculated.
3. RESULTS
3.1. Study population
Prior to propensity‐score matching, the MAP contained 11 211 patients (2471 dulaglutide initiators, 6849 liraglutide initiators, and 1891 exenatide once weekly initiators). After matching, the dulaglutide versus liraglutide comparison consisted of 2427 pairs and the dulaglutide versus exenatide once‐weekly comparison consisted of 1808 pairs (Appendix S1, Figure S1). The HAP consisted of 2387 patients before propensity‐score matching: 617 dulaglutide initiators, 1291 liraglutide initiators, and 479 exenatide once‐weekly initiators. The post‐matching HAP for dulaglutide versus liraglutide comparison consisted of 585 pairs; the dulaglutide versus exenatide once‐weekly comparison consisted of 422 pairs.
3.2. Main analysis population
3.2.1. Demographics and clinical characteristics
Demographics, baseline clinical characteristics and antidiabetic drug utilization were evaluated for both the pre‐matching (Appendix S1, Table S2) and post‐matching MAP (Table 1). In the matched dulaglutide and liraglutide cohort, the mean age was 54 years and 52% were men. The matched dulaglutide and exenatide once‐weekly cohort was similar, with a mean age of 54 years and ~51% men. Demographics and clinical characteristics were balanced in the two matched cohorts (Table 1).
In the dulaglutide and liraglutide matched cohort, ~87% of patients used an OAD at baseline, with slightly more than half having used at least two classes of OAD. Similar baseline characteristics were observed in the dulaglutide and exenatide once‐weekly matched cohort.
3.2.2. Adherence and persistence
In the dulaglutide and liraglutide matched cohorts, 1462 patients (60.2%) received dulaglutide 0.75 mg/wk, 965 (39.8%) received dulaglutide 1.5 mg/wk as the index dose; 1112 (45.8%) received liraglutide 0.6 mg/d or 1.2 mg/d, and 1315 (54.2%) received liraglutide 1.8 mg/d as the index dose. Second fills of the index GLP‐1RA were obtained by 2183 (89.9%) and 2068 patients (85.2%) in the dulaglutide and liraglutide matched cohorts, respectively, and by 1622 (89.7%) and 1465 patients (81.0%) in the dulaglutide and exenatide once‐weekly matched cohorts, respectively.
Table 2 shows the adherence and persistence outcomes for the two matched cohorts, dulaglutide versus liraglutide, and dulaglutide versus exenatide once weekly. Dulaglutide initiators had significantly higher mean PDC at 12 months compared with liraglutide initiators (0.67 vs. 0.60; P < 0.001) and exenatide once‐weekly initiators (0.67 vs. 0.51; P < 0.001). In addition, significantly more patients were adherent (ie, achieved a PDC of ≥80%) to dulaglutide than to liraglutide (51.2% vs. 38.2%; P < 0.001) or to exenatide once weekly (50.7 vs. 31.9%; P < 0.001).
Table 2.
Post‐index adherence and persistence outcomes at 12‐month follow‐up in propensity‐score‐matched cohort: Main analysis population
| Matched dulaglutide vs. liraglutide | Matched dulaglutide vs. exenatide once weekly | |||||
|---|---|---|---|---|---|---|
| Outcome variable | Dulaglutide n = 2427 | Liraglutide n = 2427 | P | Dulaglutide n = 1808 | Exenatide once weekly n = 1808 | P |
| Adherence | ||||||
| PDC (%), mean (SD) | 67.3 (32.06) | 59.5 (32.63) | <0.001 | 66.8 (32.24) | 51.3 (34.62) | <0.001 |
| PDC ≥0.80, n (%) | 1242 (51.2) | 927 (38.2) | <0.001 | 917 (50.7) | 576 (31.9) | <0.001 |
| Persistence | ||||||
| Days of persistent index GLP‐1RA use, mean (SD) | 252.8 (136.41) | 218.2 (143.85) | <0.001 | 251.4 (137.45) | 192.5 (140.22) | <0.001 |
| Patients who were persistent to index GLP‐1RA, n (%) | 1334 (55.0) | 1064 (43.8) | <0.001 | 992 (54.9) | 622 (34.4) | <0.001 |
Abbreviations: GLP‐1RA, glucagon‐like peptide receptor agonist; PDC, proportion of days covered.
At 12 months, patients on dulaglutide were significantly more persistent than those on liraglutide (55.0% vs. 43.8%; P < 0.001). The mean number of days on dulaglutide and liraglutide therapy was 252.8 days and 218.2 days (median 365 days and 231 days), respectively. Similar results were seen in the dulaglutide and exenatide once‐weekly cohorts, where 54.9% of the dulaglutide initiators and 34.4% of the exenatide once‐weekly initiators were persistent with index therapy (P < 0.001). The mean number of days on dulaglutide was 251.4 days (median 365 days), whereas the mean number of days for exenatide once‐weekly use was 192.5 days (median 150 days) during the 12 months' follow‐up (Table 2). Similar trends in adherence and persistence results were observed at 6‐month follow‐up (Appendix S1, Table S3).
Cox proportional hazard models showed that patients initiating dulaglutide were significantly less likely to discontinue therapy than those initiating liraglutide or exenatide once weekly (Figure 1A,B). Compared with liraglutide or exenatide once weekly, the relative risk reduction of treatment discontinuation with dulaglutide was 20% (hazard ratio [HR] 0.74, 95% confidence interval [CI] 0.68‐0.80; P < 0.001) and 31% (HR 0.57, 95% CI 0.52‐0.62; P < 0.001), respectively.
Figure 1.
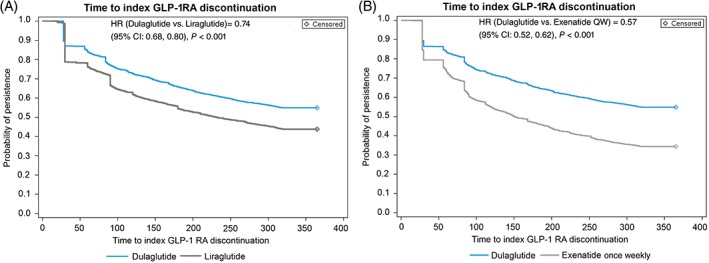
A, Survival analysis for persistence to index glucagon‐like peptide receptor agonist (GLP‐1RA): dulaglutide vs. liraglutide matched cohort: main analysis population (MAP). B, Survival analysis for persistence to index GLP‐1 RA: dulaglutide vs. exenatide once‐weekly matched cohort: MAP. CI, confidence interval; HR, hazard ratio
3.2.3. Healthcare costs
At 12‐month follow‐up, patients using dulaglutide had lower diabetes‐related medical costs compared with those using liraglutide ($6077 vs. $7026; P = 0.001), and higher diabetes‐related pharmacy costs ($10 097 vs. $9668; P = 0.025). The mean diabetes‐related total costs for dulaglutide versus liraglutide were $16 174 and $16 694, respectively (P = 0.184; Appendix S1, Table S4A).
Diabetes‐related pharmacy costs were higher for dulaglutide than for exenatide once weekly ($9694 vs. $8827; P < 0.001). Diabetes‐related medical costs were slightly higher for dulaglutide ($6074) than for exenatide once weekly ($5787), although the difference was not statistically significant (P = 0.322). The mean diabetes‐related total costs for dulaglutide versus exenatide once weekly was $15 768 and $14 615, respectively (P = 0.005; Appendix S1, Table S4B).
3.3. HbA1c analysis population
3.3.1. Glycaemic control
The baseline demographic and clinical characteristics for the HAP were balanced after propensity‐score matching (Appendix S1, Tables S5A,B). Change in HbA1c was significantly greater for initiators of dulaglutide than liraglutide at 12‐month follow‐up (−0.98% vs. −0.77%; P = 0.032 [Figure 2]). The proportion of patients achieving at least a 1% reduction in HbA1c was also greater for dulaglutide than for liraglutide (41.9% vs. 34.7%; P = 0.012).
Figure 2.
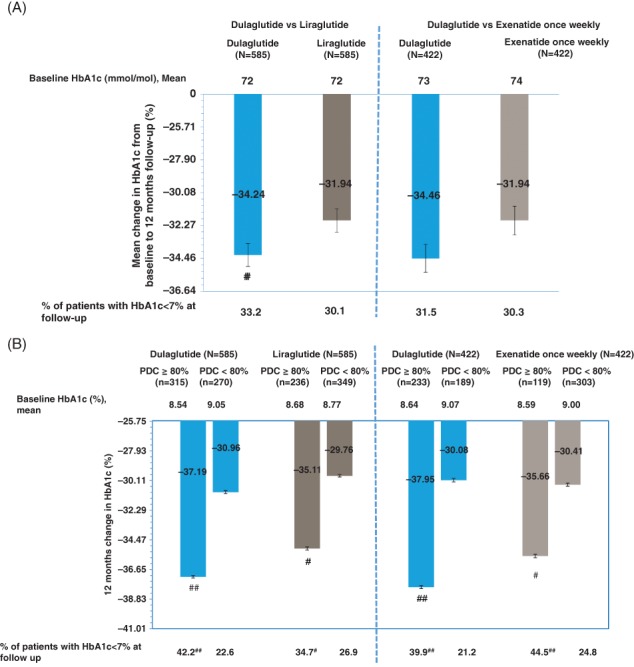
A, Change in glycated haemoglobin (HbA1c) from baseline to 12‐month follow‐up and stratified by adherence among the matched cohorts: HbA1c analysis population (HAP). B, Change in HbA1c from baseline to 12‐month follow‐up and stratified by adherence among the matched cohorts: HAP. #Significant difference between adherent and non‐adherent patients within each cohort with P value <0.05. ##Significant difference between adherent and non‐adherent patients within each cohort with P value <0.001. Error bars represent standard error. Adherent patients were those with proportion of days covered (PDC) ≥ 0.80; non‐adherent patients were those with PDC <0.80
In the dulaglutide versus exenatide once‐weekly propensity‐score‐matched cohort, change in HbA1c was greater but nonsignificant for initiators of dulaglutide than exenatide once weekly at 12‐month follow‐up (−1.00% vs. −0.77%; P = 0.056 [Figure 2]), with 42.7% of dulaglutide initiators and 36.5% exenatide once‐weekly initiators achieving at least a 1% reduction in HbA1c (P = 0.067). There were no differences between either the dulaglutide versus the liraglutide cohort or the dulaglutide versus the exenatide once‐weekly cohort in the proportion of patients achieving the HbA1c target of <53 mmol/mol (Figure 2).
In all cohorts, patients who were adherent at 12‐month follow‐up had a greater reduction in HbA1c levels than patients who were non‐adherent. Furthermore, a higher percentage of patients who were adherent achieved the HbA1c target of <53 mmol/mol than patients who were non‐adherent (Figure 2).
Similar trends in HbA1c results were observed at 6‐month follow‐up (Appendix S1, Figure S2) among a subset of the patients in the HAP with HbA1c results available at the 6‐month follow‐up.
3.4. Cost associated with glycaemic control: subset of the HAP with complete pharmacy cost
The average diabetes‐related total costs at 12‐month follow‐up per 1% HbA1c reduction were $13 988 and $19 779 for patients initiating dulaglutide versus liraglutide, respectively; and were $13 241 and $16 496 for dulaglutide initiators versus exenatide once‐weekly initiators, respectively (Figure 3).
Figure 3.
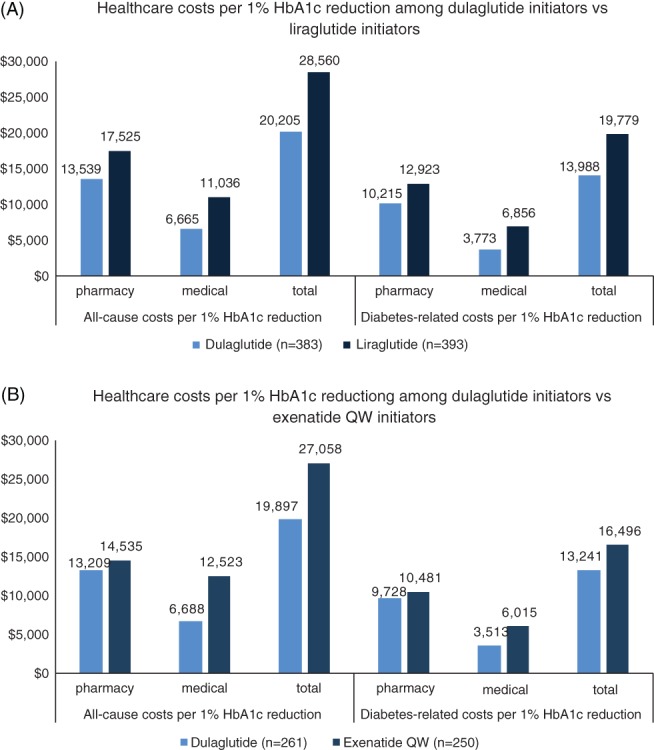
A, Post‐index healthcare costs per 1% glycated haemoglobin (HbA1c) reduction at 12 months among patients initiating a GLP‐1RA: HbA1c Analysis Population (HAP). B, Post‐index healthcare costs per 1% HbA1c reduction at 12 months among patients initiating a GLP‐1RA: HAP
4. DISCUSSION
The results of the present real‐world analysis showed that patients with T2D initiating dulaglutide treatment had significantly higher adherence and persistence at 12‐month follow‐up than patients initiating either liraglutide or exenatide once weekly. The observed adherence rates were consistent with previously published research. Previous US studies reported 6‐month dulaglutide adherence rates of 54% to 57%,12, 21 which are similar to the 6‐month adherence rate reported in the present study (59%; Appendix S1, Table S3). Similarly, our observed 6‐month adherence rates of 46% for liraglutide and 41% for exenatide once weekly were within the ranges previously reported (ie, 29% to 48% for liraglutide12, 19, 22, 23, 24, 25 and 29% to 51% for exenatide once weekly12, 19, 23, 25). When limiting the comparison to prior studies reporting outcomes at 12 months, adherence rates in the present study were higher than those reported in the limited data for both liraglutide (29% to 34%22, 23) and exenatide once weekly (29%23).
It is worth noting that a higher proportion of patients who initiated liraglutide discontinued therapy than those who initiated dulaglutide during the initial 30‐day treatment period, and the difference in probability of persistence between dulaglutide and liraglutide initiators plateaued after ~90 days of treatment. By contrast, the difference in probability of persistence between dulaglutide and exenatide once weekly continued to separate over time.
Current treatment algorithms recommended by the American Diabetes Association/European Association for the Study of Diabetes promote a patient‐centred approach that takes into account the pharmacological properties of drugs, including glucose‐lowering action, presence of atherosclerotic cardiovascular disease, effects on body weight, correction on multiple pathophysiological defects, tolerability, and long‐term safety.26, 27 In addition to efficacy and safety, patient preference plays a particularly important role in treatment adherence and persistence. A simple dosing regimen as well as an easy‐to‐use delivery device have been shown to improve adherence28, 29; thus, the higher adherence rates associated with dulaglutide treatment, in addition to its effectiveness, may also be a function of patient preference for device characteristics, such as ease of use, convenient dosing and administration.
In the subset of patients with available HbA1c results, those initiating dulaglutide had a greater reduction in HbA1c from baseline. The HbA1c results observed for dulaglutide initiators in the present study are consistent with those in the AWARD programme, ie, 0.7% to 1.6% for the 0.75‐mg dose and 0.8% to 1.6% for the 1.5‐mg dose.30, 31, 32, 33, 34, 35, 36, 37, 38, 39 In the AWARD‐6 trial, dulaglutide 1.5 mg was non‐inferior to liraglutide 1.8 mg, and both treatments lowered HbA1c by 1.4% from baseline at 26 weeks.31 The slightly higher HbA1c reduction for dulaglutide compared with liraglutide initiators could be attributable to the higher adherence among dulaglutide initiators in the present real‐world study.
Treatment adherence is a key component in achieving target glycaemic levels. Lack of patient adherence explains 75% of the efficacy gap in HbA1c reduction between clinical trial and real‐world data.8 The present study results show that, in all cohorts, adherent patients had a greater reduction in HbA1c compared with non‐adherent patients, highlighting the importance of adherence in achieving glycaemic control.
Improved adherence might be a contributing factor to higher diabetes‐related pharmacy costs, as demonstrated in the present and previous studies40, 41; however, consistent with previous research,40, 41 the higher pharmacy costs of dulaglutide were offset by lower diabetes‐related medical costs compared with liraglutide. Even though the mean total costs for dulaglutide were similar to those for liraglutide and significantly higher than for exenatide once weekly, when accounting for the reduction in HbA1c, the all‐cause and diabetes‐related 12‐month follow‐up cost per 1% reduction in HbA1c was the lowest for the dulaglutitide cohort.
Several limitations should be considered when interpreting these results. Propensity‐score matching was used to reduce confounding of treatment selection by ensuring balance in measured baseline characteristics; however, as in other observational studies, the present study was limited by the potential for bias attributable to unmeasured confounders. Data were derived from medical and pharmacy claims, which may have contained undetected coding errors. Also, certain patient (such as duration of diabetes, weight, education, or patient out‐of‐pocket costs) and provider characteristics that may be associated with the outcomes of interest were unavailable for analysis. Pharmacy claims indicate only that a prescription was filled; it is unknown whether patients used the medication as prescribed. Furthermore, pharmacy claims do not capture medications purchased in cash or over the counter. Lastly, all patients included in the study were enrolled in commercial health insurance plans in the United States and satisfied all the inclusion/exclusion criteria. The results may not be generalizable to patients not selected, or to those with other types of health insurance or who are uninsured, or to those outside the United States.
This analysis examined real‐world usage of three GLP‐1RAs in a large diverse population. The results of the study showed that patients receiving dulaglutide for treatment of T2D were more adherent and persistent at 12 months compared with those receiving liraglutide or exenatide once weekly. Patients using dulaglutide also exhibited a significantly greater HbA1c reduction compared with those using liraglutide and a greater but nonsignificant HbA1c reduction compared with those using exenatide once weekly. In summary, given the importance of adherence and its role in glycaemic control, dulaglutide may be an important treatment option for improving outcomes in patients with T2D.
CONFLICTS OF INTEREST
R.M., M.Y., H.P. and L.F.L. are employees and stockholders of Eli Lilly and Company. Q.H. and M.G. are employees of HealthCore, Inc., an independent research organization that received funding from Eli Lilly and Company for the conduct of the study. R.Z. was an employee of HealthCore, Inc. when the study was conducted.
Author contributions
Author contributiosn were as follows: R.M.: study concept and design, data interpretation, manuscript preparation and critical review, final approval of the manuscript. Q.H.: study concept and design; data collection; data interpretation; manuscript preparation and critical review; final approval of the manuscript.
M.Y.: study concept and design; data interpretation; manuscript preparation and critical review; final approval of the manuscript. R.Z.: study concept and design; data collection; data interpretation; manuscript preparation and critical review; final approval of the manuscript. H.P.: study concept and design; data interpretation; manuscript preparation and critical review; final approval of the manuscript. M.G.: study concept and design; data collection; data interpretation; manuscript preparation and critical review; final approval of the manuscript. L.F.L.: study concept and design; data interpretation; manuscript preparation and critical review; final approval of the manuscript.
Supporting information
Appendix S1.
ACKNOWLEDGMENTS
Cheryl Jones, an employee of HealthCore Inc., provided writing and editorial support for this manuscript.
Mody R, Huang Q, Yu M, et al. Adherence, persistence, glycaemic control and costs among patients with type 2 diabetes initiating dulaglutide compared with liraglutide or exenatide once weekly at 12‐month follow‐up in a real‐world setting in the United States. Diabetes Obes Metab. 2019;21:920–929. 10.1111/dom.13603
Funding information Funding for this study was provided by Eli Lilly and Company.
REFERENCES
- 1. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bethel MA, Patel RA, Merrill P, et al. EXSCEL Study Group. Cardiovascular outcomes with glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes: a meta‐analysis. Lancet Diabetes Endocrinol. 2018;6(2):105‐113. [DOI] [PubMed] [Google Scholar]
- 3. Trujillo JM, Nuffer W, Ellis SL. GLP‐1 receptor agonists: a review of head‐to‐head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Victoza [package insert]. Bagsvaerd, Denmark: Novo Nordisk A/S; 2017.
- 5. Banerji MA, Dunn JD. Impact of glycemic control on healthcare resource utilization and costs of type 2 diabetes: current and future pharmacologic approaches to improving outcomes. Am Health Drug Benefits. 2013;6(7):382‐392. [PMC free article] [PubMed] [Google Scholar]
- 6. Boye KS, Curtis SE, Lage MJ, Garcia‐Perez LE. Associations between adherence and outcomes among older, type 2 diabetes patients: evidence from a Medicare Supplemental database. Patient Prefer Adherence. 2016;10:1573‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia‐Perez LE, Alvarez M, Dilla T, et al. Adherence to therapies in patients with type 2 diabets. Diabetes Ther. 2013;4(2):175‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carls GS, Tuttle E, Tan R‐D, et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real‐world use of GLP‐1 RA and DPP‐4 therapies in patients with type 2 diabetes. Diabetes Care. 2017;40:1469‐1478. [DOI] [PubMed] [Google Scholar]
- 9. Huber CA, Rapold R, Brungger B, Reich O, Roseman T. One‐year adherence to oral antihyperglycemic medication and risk prediction of patient outcomes for adults with diabetes mellitus: an observational study. Medicine. 2016;95(26):e3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rozenfield Y, Hunt JS, Plauschinat C, Wong KS. Oral antidiabetic medication adherence and glycemic control in managed care. Am J Manag Care. 2008;14:71‐75. [PubMed] [Google Scholar]
- 11. Simpson SH, Eurich DT, Majumdar SR, et al. A meta‐analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alatorre C, Lando LF, Yu M, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon‐like peptide‐1 receptor agonists: higher adherence and persistece with dulaglutide compared with once‐weekly exenatide and liraglutide. Diabetes Obes Metab. 2017;19:953‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Unni S, Wittbrodt E, Ma J, et al. Comparative effectiveness of once‐weekly glucagon‐like peptide‐1 receptor agonists with regard to 6‐month glycaemic control and weight outcomes in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20(2):468‐473. [DOI] [PubMed] [Google Scholar]
- 14. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130‐1139. [DOI] [PubMed] [Google Scholar]
- 15. Chang H‐Y, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted diabetes complications severity index in claims data. Am J Manag Care. 2012;19(11):721‐726. [PubMed] [Google Scholar]
- 16. Nau DP. Proportion of days covered (PDC) as a preferred method of measuring medication adherence. http://ep.yimg.com/ty/cdn/epill/pdcmpr.pdf. Accessed October 25, 2018.
- 17. Fairies DE, Leon AC, Haro JM, Obenchain RL. Analysis of Observational Health Care Data using SAS. Cary, NC: SAS Institute, Inc.; 2010. [Google Scholar]
- 18. Stuart EA. Matching methods for casual inference: a review and a look forward. Stat Sci. 2010;25(1):1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu M, Xie J, Lando LF, Kabul S, Swindle RW. Liraglutide versus exenatide once weekly: persistence, adherence, and early discontinuation. Clin Ther. 2016;38:149‐160. [DOI] [PubMed] [Google Scholar]
- 20. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mody R, Yu M, Fernandez Lando L, et al. Real world effectiveness of dulaglutide among patients with type 2 diabetes. International Society for Pharmacoeconomics and Outcomes Research; May 20‐24, 2017; Boston, MA. [Google Scholar]
- 22. Buysman EK, Liu F, Hammer M, Langer J. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther. 2015;32:341‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cai J, Wang Y, Baser O, Xie L, Chow W. Comparative persistence and adherence with newer anti‐hyperglycemic agents to treat patients with type 2 diabetes in the United States. J Med Econ. 2016;19(12):1175‐1186. [DOI] [PubMed] [Google Scholar]
- 24. Durden E, Lenhart G, Lopez‐Gonzalez L, Hammer M, Langer J. Predictors of glycemic control and diabetes‐related costs among type 2 diabetes patients initiating therapy with liraglutide in the United States. J Med Econ. 2016;19(4):403‐413. [DOI] [PubMed] [Google Scholar]
- 25. Nguyen H, Dufour R, Caldwell‐Tarr A. Glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) therapy for patients with type 2 diabetes in a Medicare population. Adv Ther. 2017;41(suppl 1):S1‐S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. American Diabetes Association . Pharmacologic approaches to glycemic treatment standards of medical care in diabetes – 2018. Diabetes Care. 2018;41(suppl 1):S73‐S85. [DOI] [PubMed] [Google Scholar]
- 27. American Diabetes Association . Standards of medical care in diabetes – 2018. Diabetes Care. 2018;41(suppl 1):S1‐S159.29222369 [Google Scholar]
- 28. Gelhorn HL, Poon JL, Davies EW, Paczkowski R, Curtis SE, Boye KS. Evaluating preferences for profiles of GLP‐1 receptor agonists among injection‐naive type 2 diabetes patients in the UK. Patient Prefer Adherence. 2015;9:1611‐1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xie L, Zhou S, Wei W, Gill J, Pan C, Baser O. Does pen help? A real‐world outcomes study of switching from vial to disposable pen among insulin glargine‐treated patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2013;15(3):230‐236. [DOI] [PubMed] [Google Scholar]
- 30. Blonde L, Jendle J, Gross J, et al. Once‐weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD‐4): a randomised, open‐label, phase 3, non‐inferiority study. Lancet. 2015;385(9982):2057‐2066. [DOI] [PubMed] [Google Scholar]
- 31. Dungan KM, Povedano ST, Forst T, et al. Once‐weekly dulaglutide veruis once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomized, open‐label, phase 3, non‐inferiorioty trial. Lancet. 2014;384(9951):1349‐1357. [DOI] [PubMed] [Google Scholar]
- 32. Dungan KM, Weitgasser R, Manghi FP, et al. A 24‐week study to evaluate the efficacy and safety of once‐weekly dulaglutide added on to glimepiride in type 2 diabetes (AWARD‐8). Diabetes Obes Metab. 2016;18:475‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giorgino F, Benroubi M, Sun JH, Zimmerman AG, Pechtner V. Efficacy and safety of once‐weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD‐2). Diabetes Care. 2015;38(12):2241‐2249. [DOI] [PubMed] [Google Scholar]
- 34. Ludvik B, Frias JP, Tinahones FJ, et al. Dulaglutide as add‐on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD‐10): a 24‐week, randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2018;6(5):370‐381. [DOI] [PubMed] [Google Scholar]
- 35. Pozzilli P, Norwood P, Jodar E, et al. Placebo‐controlled, randomized trial of the addition of once‐weekly glucagon‐like peptide‐1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD‐9). Diabetes Obes Metab. 2017;19(7):1024‐1031. [DOI] [PubMed] [Google Scholar]
- 36. Skrivanek Z, Gaydos BL, Chien JY, et al. Dose‐finding results in an adaptive, seamless, randomized trial of once‐weekly dulaglutide combined with metformin in type 2 diabetes patients (AWARD‐5). Diabetes Obes Metab. 2014;16(8):748‐756. [DOI] [PubMed] [Google Scholar]
- 37. Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate‐to‐severe chronic kidney disease (AWARD‐7): a multicentre, open‐label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605‐617. [DOI] [PubMed] [Google Scholar]
- 38. Umpierrez G, Tofe Povedano S, Perez Martin F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD‐3). Diabetes Care. 2014;37(8):2168‐2176. [DOI] [PubMed] [Google Scholar]
- 39. Wysham CH, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitizone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care. 2014;37(8):2159‐2167. [DOI] [PubMed] [Google Scholar]
- 40. Kennedy‐Martin T, Boye KS, Peng X. Cost of medication adherence and persistence in type 2 diabetes mellitus: a literature review. Patient Prefer Adherence. 2017;11:1103‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Prevalence and economic consequences of medication adherence in diabetes: a systematic literature review. Manage Care Interface. 2006;19(7):31‐41. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.


