Abstract
The neurohypophyseal hormones vasopressin and oxytocin are produced and released within the mammalian brain, where they act via multiple receptor subtypes. The neural distributions of these receptors, for example, V1a and oxytocin receptors, have been well described in many mammals. In birds, the distribution of binding sites for the homologous neuropeptides, vasotocin (VT) and mesotocin, has been studied in several species by using synthetic radioligands designed to bind to mammalian receptors. Such binding studies, however, may not reveal the specific distributions of each receptor subtype. To identify and map the receptors likely to bind VT and mesotocin, we generated partial cDNA sequences for four VT receptor subtypes, VT1, VT2 (V1b), VT3 (oxytocin-like), and VT4 (V1a), in white-throated sparrow (Zonotrichia albicollis) and zebra finch (Taeniopygia guttata). These genes shared high sequence identity with the homologous avian and mammalian neurohypophyseal peptide receptors, and we found evidence for VT1, VT3, and VT4 receptor mRNA expression throughout the brains of both species. As has been described in rodents, there was striking interspecific and intraspecific variation in the densities and distribution of these receptors. For example, whereas the VT1 receptor mRNA was more widespread in zebra finch brain, the VT3 (oxytocin-like) receptor mRNA was more prevalent in the sparrow brain. Although VT2 (V1b) receptor mRNA was abundant in the pituitary, it was not found in the brain. Because of their association with brain regions implicated in social behavior, the VT1, VT3, and VT4 receptors are all likely candidates for mediating the behavioral effects of VT.
Most vertebrates possess two distinct forms of neurohypophyseal hormones. Vasopressin and oxytocin (OT) are commonly expressed in mammals, whereas their homologues vasotocin (VT) and mesotocin (MT) are found in birds. These peptides are perhaps best understood as hormones manufactured in the paraventricular and supraoptic nuclei of the hypothalamus and released by the neurohypophysis into the general circulation. They are also found in other neuroanatomical locations; for example, vasopressin and VT are synthesized by neurons that project from the extended amygdala widely throughout the brain (1–3). They thus have neural functions independent of their role as neurohypophyseal hormones and are strongly implicated, for example, in social behavior (4, 5).
Each of these neuropeptides interacts with constituents of a subfamily of G protein-coupled receptors, most of which mediate turnover of phosphatidylinositol and mobilization of calcium ions (6). In the vertebrates studied to date, multiple neurohypophyseal peptide receptors have been identified with overlapping but unique distributions in the brain (7–11). Studies in mammals have suggested that the anatomical location of a receptor can be an important determinant of its role in behavior. For example, the neural distribution of V1a receptor differs between monogamous prairie voles (Microtus ochrogaster) and nonmonogamous montane voles (Microtus montanus) (12), and manipulation of local V1a expression can alter pair bond formation (13). Thus, interspecies differences in the distribution of neurohypophyseal peptide receptors may explain interspecific variation in behaviors that depend on these peptides. In birds, the behavioral responses to exogenously administered VT and MT vary according to species and even according to social system and behavioral context (5). It is therefore informative to study in some detail the neural distributions of neurohypophyseal peptide receptors in birds and to compare these distributions across as well as within species.
Four avian VT receptors have been identified to date, all of them originally identified in chickens. They have been named VT1, VT2, VT3, and VT4 and likely correspond to the mammalian V2, V1b, OT receptor (OTR), and V1a receptors, respectively. Their nomenclature (6) reflects the chronological order in which they were described, and some authors are now shifting to a more straightforward, homology-based nomenclature (e.g. Ref. 11). Here, we have used the standard avian nomenclature but have also included the mammalian names alongside the avian ones at each major mention, for example, in headings and figure captions.
VT1, the first neurohypophyseal peptide receptor to be characterized in birds, is expressed in brain and in shell gland (14). Although it was first proposed as a homolog to the mammalian V1a (15), the VT1 shares less sequence identity with the V1a than does the more recently sequenced VT4 (see below). Functional characterization revealed that the VT1 receptor binds VT-like hormones (VT and vasopressin) with greater potency than OT-like hormones (14); the endogenous ligand in birds is therefore thought to be VT. Phylogenetic analysis indicates that this receptor clusters with the mammalian V2 receptors and may be closely related to them (11, 14).
VT2, the second avian neurohypophyseal peptide receptor to be identified, is thought to be homologous to the mammalian V1b receptor (16). Like the VT1, the VT2 receptor binds VT with greater potency than it does MT (16). Like the V1b, it is expressed at high levels in pituitary corticotrophs (17) where it forms a heterodimer with CRH receptor (18). Whereas the V1b receptor has been detected in the hippocampus, hypothalamus, and amygdala in rodents (19), the VT2 receptor has not been detected in the avian brain either by Northern blot analysis (16) or immunohistochemistry (17). Its primary function is thought to be to mediate the effects of VT on the secretion of adrenocorticotropic hormone during stress (16).
VT3 shares high sequence identity with the mammalian OTR and is therefore sometimes referred to as “OT- like” (20). It is important to note, however, that its similarity to OTR does not necessarily mean that MT is the only endogenous ligand of this receptor. In rough-skinned newts, VT is thought to act as a partial ligand at the MT receptor (MTR) (21). The function of avian MT is unclear, insofar as VT plays both vasopressin-like and OT-like roles in the regulation of blood pressure (22), contractility of the shell gland (23) and behavior (24). Some authors have argued that VT is more likely the endogenous ligand of the avian VT3 receptor both inside and outside the brain (6, 20). Srivastava et al. (25–27) have characterized the regulation of VT3 mRNA by photoperiod, estrogen, and age in shell gland, but the expression and regulation of this receptor have not been investigated in avian brain.
A fourth receptor, VT4, shares a high degree of sequence identity with the mammalian V1a receptor (Genbank ACCN abv24997). Analysis of the predicted amino acid sequence reveals V1a-like residues at critical locations that confer specificity to vasopressin-like rather than OT-like ligands (29).
The neural distribution of neurohypophyseal peptide binding has been investigated in songbird species by using radiolabeled vasopressin and synthetic ligands (30–33). The results of such studies suggest that receptor distribution varies according to species. For example, binding of the V1a antagonist [125I]-linear vasopressin antagonist was widespread throughout the brain in white-throated sparrows (Zonotrichia albicollis) (33), whereas it was limited to the lateral septum (LS) in zebra finches (Taeniopygia guttata) (32, 33). Binding of the OT antagonist [125I]-ornithine VT analog (OVTA) was robust in the LS and arcopallium of white-throated sparrows (33), whereas in canaries, it was limited to the arcopallium (31). The complement of receptor subtypes may also vary according to species; in the LS of white-throated sparrows, [125I]-linear vasopressin antagonist was better displaced by MT than by VT (33), whereas in the zebra finch LS, it was displaced better by VT than by MT (32). In the current study, we obtained partial sequence for all four known VT receptors in both white-throated sparrow and zebra finch and then used in situ hybridization (ISH) to map the neural distribution of each receptor type in both species. We chose zebra finches because they have been a popular model system in neuroscience for decades (34) and, with the complete sequencing of the zebra finch genome, have more recently become important for studies in behavioral genetics and genomics (35). Zonotrichia sparrows such as the white-throated sparrow have long been model systems for studying photoperiodicity and field endocrinology and have recently become a model for the genetics of social behavior (36, 37).
Materials and Methods
Animals
All research was conducted in accordance with National Institutes of Health principles of animal care, federal and state laws, and university guidelines. All animal protocols were approved by the Emory Institutional Animal Care and Use Committee. White-throated sparrows were captured in mist nets during fall migration in Atlanta, GA and sex was determined by PCR analysis (38). They were housed at Emory University on a short photoperiod (10-h light, 14-h dark) initially in mixed-sex aviaries and then in individual cages in small rooms housing four birds (two males and two females) per room. Zebra finches were obtained from a breeding colony at Georgia State University (Atlanta, GA), where they had been housed in mixed-sex groups on long photoperiod (14-h light, 10-h dark) for at least 6 months. The sex of the zebra finches was determined by plumage characteristics.
Behavioral observations
Each of the sparrows was acclimated to individual housing (see above) for 30 d prior to behavioral observation. On the day before tissue collection, each bird was moved with one other bird to an identical room containing a microphone and video camera. At lights-on the following morning the behavior of the two birds in each room was recorded for 120 min. The recordings were later scored for vocalizations, which consisted entirely of contact calls (39), feeding, defined as the number of seeds picked up and hulled, drinking, defined as the number of bill dips into the water dish, bouts of preening, defined as a period of preening without a break longer than 30 sec, and beak wiping, or wiping the beak against the perch. The behavior of the zebra finches was not observed.
VT receptor sequencing and alignment
mRNA was extracted from brain tissue that included the telencephalon, diencephalon, and midbrain from two male white-throated sparrows, one female white-throated sparrow, and four male zebra finches. The mRNA was then reverse transcribed into single stranded cDNA using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA). The primers used for amplification are listed in Table 1. Amplification was performed at 94–95 C melting for 30 sec, 55–59 C annealing for 30 sec, and 68–72 C extension for 5 min, for 40 cycles. The resulting fragments were cloned into pCRII vectors using a TOPO TA Cloning kit (Invitrogen) and sequenced by Retrogen (San Diego, CA). To confirm their identity, nucleotide sequences were aligned with the published sequences from chicken (VT1, AF147743; VT2, NM_001031498; VT3, NM_001031569; and VT4, EU124684.1) and other species using ClustalW (40). The sequences were then translated into amino acid sequences and the correct reading frame identified by the presence of highly conserved motifs, for example, the CRXXVKYLQXXGMFASXY motif in transmembrane domain (TMD) III. Protein sequences were then aligned with the published protein sequences of neurohypophyseal peptide receptors in other species (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) using ClustalW. The locations of each TMD and extracellular loop (ECL) were estimated with reference to Acharjee et al. (7), Cotte et al. (41), and Tahtaoui et al. (42).
Table 1.
Primer sequences, insert lengths in base pairs, and accession nos. for the neurohypophyseal peptide receptor mRNA sequences used to transcribe riboprobes
| mRNA | Primer sequences | White-throated sparrow |
Zebra finch |
||
|---|---|---|---|---|---|
| Length | ACCN | Length | ACCN | ||
| VT1 | F ATGTGTTCATGCTTCACCTCAG | 863 | JN594024 | 813 | JN594025 |
| R ATCCTCAGGGTCTCTGTCTACG | |||||
| VT2 (V1b) | F CAGCTCCTCTGGAAGGTCAC | 635 | JN594026 | 646 | JN594027 |
| R GGTCCCACACTGACCACATC | |||||
| VT3 (OT-like) | F ACATCACCTTCAGGTTCTACGG | 680 | JN594028 | 676 | JN594029 |
| R ATGTAGATCCACGGGTTACAGC | |||||
| VT4 (V1a) | F GCTCGTACGGGATGATCG | 477 | JN594030 | ||
| R1 CGGGTTACAGCAACTGTTCA | |||||
| F GCTCGTACGGGATGATCG | 534 | JN594031 | 534 | JN594032 | |
| R2 GGGAAGCTCTGTAGGCAGTC | |||||
ACCN, accession nos.; F, forward; R, reverse.
Riboprobe preparation
To map the expression of each receptor in brain tissue, we generated 35S-UTP-labeled riboprobes for ISH. Using 35S-labeled probes allowed us to generate film images that would be directly comparable with the images from our previous study of radioligand binding (33) and to perform a semiquantitative analysis. Plasmids containing the inserts were introduced into One Shot Mach 1-T1 chemically competent Escherichia coli bacteria (Invitrogen), isolated with the QIAprep Spin Miniprep kit (QIAGEN, Valencia, CA), and linearized using either BamHI or NotI to generate antisense or sense riboprobes. Riboprobes were synthesized using a T7/SP6 Riboprobe In Vitro Transcription kit (Promega, Madison, WI) with 35S-UTP (PerkinElmer, Waltham, MA) and purified using Illustra ProbeQuant G-50 Micro Columns (GE Healthcare, Piscataway, NJ).
Tissue collection and preparation
Thirteen zebra finches (seven male and six female) and 15 white-throated sparrows (seven male and eight female) were killed by rapid decapitation under isoflurane anesthesia. Brains were rapidly dissected from the skulls, frozen in powdered dry ice, and stored at −80 C until sectioning on a cryostat. From each brain, seven series of 20-μm coronal sections were thaw mounted on Superfrost Plus microscope slides (Fisher, Pittsburgh, PA) and stored at −80 C until the day of the ISH assay. Our ISH protocol was adapted from Wiemann et al. (43) and Burmeister et al. (44). Slides were defrosted, dried with air from a conventional blow dryer, fixed in 4% paraformaldehyde (pH 7.4) at room temperature for 5 min, and rinsed twice for 5 min in 1× PB or PBS. After a dip in water, they were washed in 0.1 m triethanolamine with 0.25% acetic anhydride for 10 min, rinsed in 2× sodium saline citrate (SSC) for 3 min, then dehydrated for 3 min in 70% ethanol (EtOH), 3 min in 95% EtOH, and 3 min in 100% EtOH, delipidated for 5 min in chloroform, and rehydrated for 3 min each in 100% and 95% EtOH.
In situ hybridization
Pilot ISH assays were conducted for each receptor subtype using antisense and sense probes on brain sections from three male zebra finches and two female and two male white-throated sparrows. ISH assays were then run separately for each receptor subtype using antisense probes. For the sparrows, each assay contained one series of sections each of the seven male and eight female birds, with the exception of the VT2 assay, which was run on series from three males and two females. For the finches, each assay contained one series from both males and females as follows: VT1, five males and five females; VT2, three males and two females; and VT3 and VT4, six males and six females. All riboprobes were transcribed from cDNA isolated from the species in which they were used. Riboprobes were heat denatured at 80 C for 3 min, placed on ice for 5 min, and diluted to a final concentration of 1 × 107 cpm/ml in hybridization buffer consisting of 50% deionized formamide, 10% dextran sulfate, 300 mm NaCl, 10 mm Tris, 1 mm EDTA, 10 mm dithiothreitol (DTT), 0.05 mg/ml transfer RNA, and 1× Denhardt's solution. Hybridization buffer (100 μl) containing the probe was applied to each slide, and the slides were then incubated overnight at a temperature (54–71 C) determined by the length and GC content of the probe (45).
After overnight hybridization, the slides were washed twice for 15 min in 4× SSC with 2 mm DTT, then incubated in ribonuclease (RNase) A (0.03 mg/ml; Roche Applied Science, Indianapolis, IN) diluted in RNase buffer (0.5 m NaCl, 10 mm Tris-HCl, and 1 mm EDTA) for 30 min at 37 C, and washed in RNase buffer with 1 mm DTT for 30 min at 37 C. Slides were then washed for 30 min at room temperature in 2× SSC with 1 mm DTT, followed by a 30-min wash in 0.1× SSC with 1 mm DTT in a shaking water bath at a temperature (53–70 C) determined by the length and GC content of the probe (45). After a final 3-min wash in 0.1× SSC without DTT, the sections were dehydrated in 50% EtOH with 300 mm ammonium acetate, 85% EtOH with ammonium acetate, 100% EtOH, and allowed to dry. Slides were then placed against Kodak BioMax maximum-resolution film (Kodak, Rochester, NY) and protected from light for 4–9 d. An autoradiographic [14C] microscale [3.7 3700 Bq/g (0.1 100 nCi/g); Amersham BioSciences UK Ltd., Little Chalfont, UK] was included in each cassette. The film was then developed in a Konica SRX101-A developer and scanned at 1200 or 2400 dpi using a digital scanner (Canoscan 8400F or Epson V700).
Identification of labeled cell groups
After film exposure, at least one set of sections from each brain was Nissl-stained with toluidine blue (Fisher) using a protocol adapted from Charlier et al. (46). This procedure allowed visualization of landmarks such as ventricles, fiber tracts, lamina, and major cell groups. Because the RNase step in the ISH destroys RNA, which is necessary for good cytoplasmic Nissl staining (47), the sets of tissue that underwent ISH could not be used to delineate every cell group of interest. We therefore fixed and stained alternate sets of sections for eight of the sparrows (six complete sets and two partial sets). Alternate sets were not available for the zebra finches, so in addition to Nissl staining the slides that underwent ISH, we fixed and stained a set from an individual not in the study. All together, our Nissl-stained material allowed us to identify the labeled cell groups in all individuals in both species. At least one set of stained sections from each brain was scanned at 1600 dpi using an Olympus Nanozoomer System (Olympus, Center Valley, PA). When borders between cell groups needed to be discerned more precisely, we inspected the slides on a Zeiss Axioskop microscope at ×40 (using a 4× objective) magnification (Zeiss, Oberkochen, Germany).
Analysis of individual variation
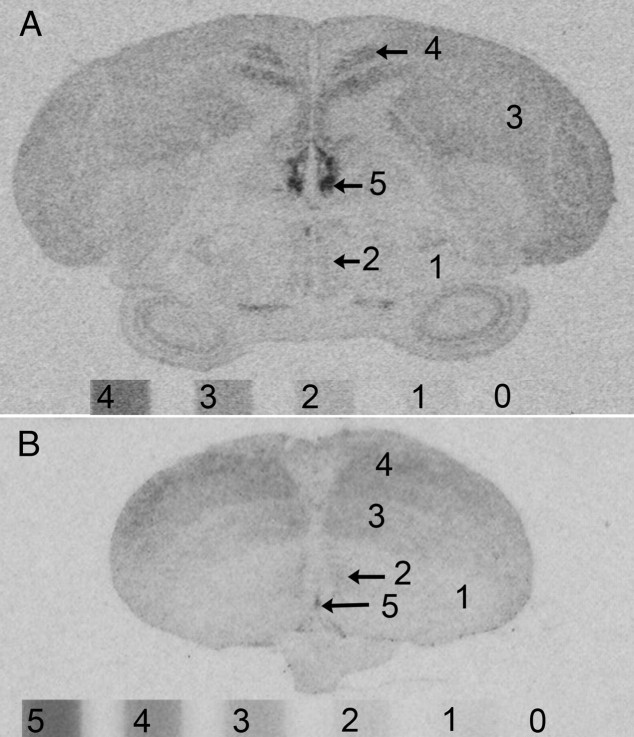
Our method to estimate signal intensities and individual variation therein is described in detail in Supplemental Materials and Methods. It was not our intention to quantify the absolute levels of signal but rather to compare the relative intensities among individuals and brain regions. The signal in each labeled region was therefore estimated by comparing its optical density directly to that produced by a [14C] microscale included in each film cassette (Fig. 1). Each labeled region in each brain could be unambiguously matched to a whole number, between 1 and 5, corresponding to the values on the microscale. After determining that individual variation was not explained by overall lighter or darker values throughout the brains of some individuals (see Supplemental Materials and Methods), we inspected the ranges of signal intensities in each brain region for each mRNA to identify regions with notable individual variation. Ranges of one unit or less (e.g. 2–3 or 3–4) were not considered notable and those cases were not investigated further. For each mRNA, the values for every region for which the range spanned two or more units (e.g. 2–4) were 1) plotted in a histogram to show the variation, 2) subjected to a Mann-Whitney U test to determine whether the variation was explained by sex differences, and 3) for the sparrows, correlated with the five behavior measures (contact calls, feeding, drinking, preening, and beak wiping) using a Spearman correlation test. All statistical analyses were done in PASW 18.0 for Macintosh.
Fig. 1.
The signal intensity in each labeled brain region in sparrows (A) and finches (B) was estimated by comparing its optical density on the film to that produced by the 14C microscales included in each film cassette (see Supplemental Materials and Methods). Signal intensities higher than 4 on the sparrow microscale (A) were assigned a value of 5. There was no signal clearly higher than 5 on the zebra finch films (B).
Results
Sequence alignment
We generated partial sequences for zebra finch and sparrow VT1, VT2 (V1b), VT3 (OT-like), and VT4 (V1a) receptors and aligned them with published sequences from other species (Supplemental Table 1). Our sequences spanned from TMDII (VT1), ECL1 (VT2 and VT3), or TMDIV (VT4) to ECL3 (VT2) or beyond TMDVII (VT1, VT3, and VT4). The zebra finch and sparrow VT1 protein sequences shared 91 and 90% sequence identity with the chicken VT1 and 95% sequence identity with each other. The VT2 sequences for zebra finch and sparrow shared 78 and 75% sequence identity with chicken VT2, respectively, and 94% with each other. For VT3, the zebra finch and sparrow sequences were 94 and 95% identical to the chicken VT3 and 99% identical to each other. For VT4, the zebra finch and sparrow sequences shared 99% identity with each other and 95 and 91% with chicken VT4 (V1a), respectively. Each of the sequences shared identity with the other neurohypophyseal peptide receptors, particularly at residues in the binding pocket (see Supplemental Table 1). At the residues thought to confer ligand specificity, the sparrow and zebra finch VT3 sequences were typical of OTR and MTR sequences, for example, showing a nonpolar phenylalanine (F) residue at a critical location in ECL1 (29) and a polar tyrosine (Y) residue at a critical location in TMDV (48). In contrast, the VT2 and VT4 sequence for both sparrow and zebra finch showed V1a-typical residues at critical locations. Note however that the most important site for conferring V1a specificity, a tyrosine residue numbered 115 in the rat and human proteins, was outside the area we sequenced for VT4 (Supplemental Table 1). The songbird VT2 and VT3 receptors contained a V1a-like residue and an OT-like residue, respectively, at this location. The VT1 receptors contained the same residue as chicken VT1, aspartic acid (D), which is known to confer specificity to vasopressin in the mammalian V2 receptor (29). The VT1 receptors were similarly V2-like at other critical locations (see Supplemental Table 1), suggesting that the avian VT1 may share high homology with the mammalian V2 (11). A maximum parsimony phylogenetic tree, constructed using the close-neighbor-interchange algorithm (49) in MEGA4 (50), showed the expected relationships among the vertebrate neurohypophyseal peptide receptors with the songbird sequences closely related to the chicken sequences (Fig. 2).
Fig. 2.
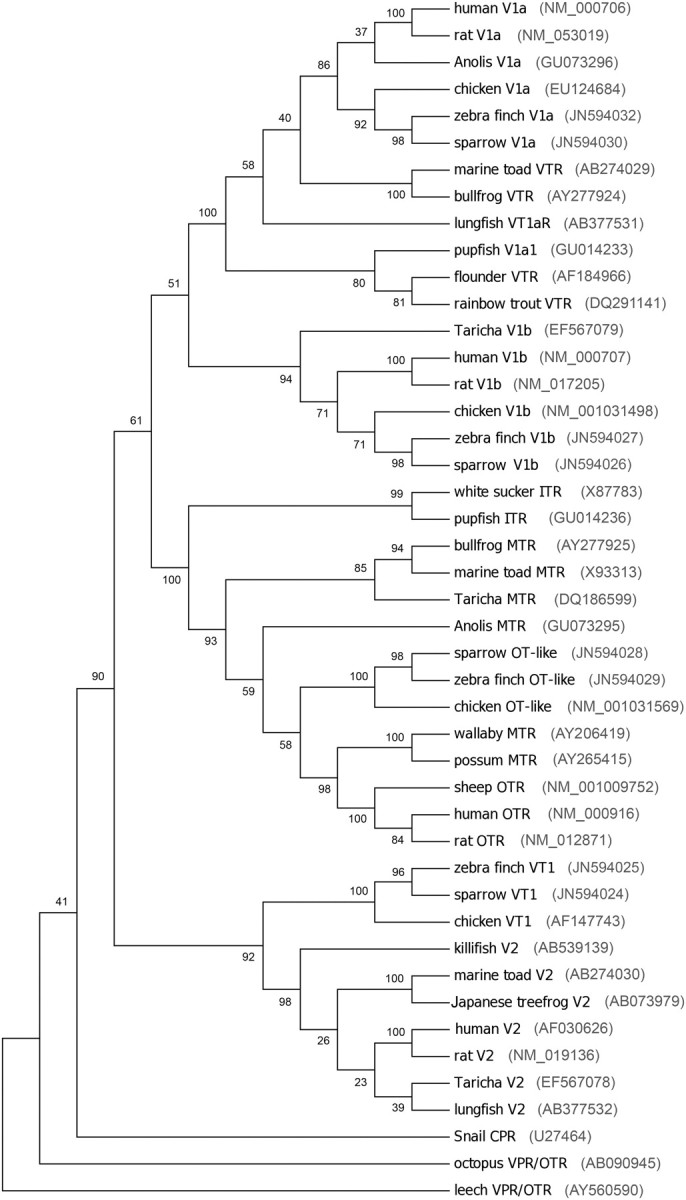
Evolutionary relationships among neurohypophyseal peptide receptors in 45 taxa. The evolutionary history was inferred using the maximum parsimony method (51). Tree 1 out of the three most parsimonious trees (length, 3612) is shown. The percentage of replicate trees, in which the associated taxa clustered together in the bootstrap test (100 replicates), is shown next to the branches (52). The MP tree was obtained using the close-neighbor-interchange algorithm (49) with search level 3 (49, 52), in which the initial trees were obtained with the random addition of sequences (10 replicates). All alignment gaps were treated as missing data. There was a total of 568 positions in the final dataset, of which 367 were parsimony informative. Phylogenetic analyses were conducted in MEGA4 (50). CPR, Conopressin receptor. Sequence-based nomenclature is used for the avian sequences: V1b, VT2; OT-like, VT3; and V1a, VT4.
Distribution of VT receptor mRNA
The neuroanatomical distributions of the signals produced by the antisense riboprobes were largely consistent with studies of VT-like binding in songbirds (30–33). Examples of signal are shown in Figs. 3–10. For most of the sense control probes (Supplemental Fig. 1), we observed either no signal or a weak signal only in the cerebellum (Cb). A weak signal with a distribution similar to that seen with the antisense probe was observed for VT4 mRNA in sparrow (Supplemental Fig. 1D); we therefore repeated the ISH for that mRNA using a newly generated antisense riboprobe. The second riboprobe, which was directed against a region of the VT4 cDNA that overlapped partially with the first riboprobe, contained unique sequence within a highly variable region in exon 1. This sequence (∼80 bp) was not present in the zebra finch sequence but overlapped with the published chicken sequence (see Supplemental Table 1, residues 95–115 of sparrow VT4 sequence). The sequence also appeared in a third sparrow VT4 fragment amplified independently using different primers. Because we have amplified multiple fragments both with and without this sequence, it may represent alternative splicing or an indel polymorphism. The signal obtained using the second VT4 antisense probe had a distribution identical to that obtained with the first (compare Supplemental Fig. 1C with Fig. 5I) and was unique compared with all other mRNA we have detected in sparrow or finch brain. We are therefore confident that our VT4 signal is specific.
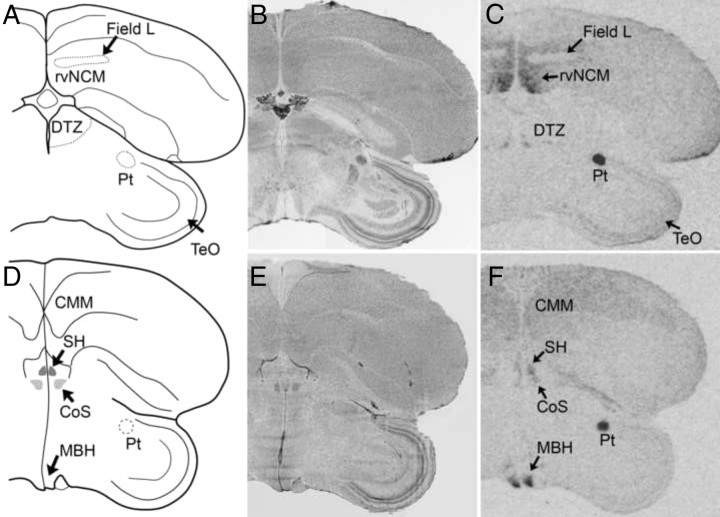
Fig. 3.
Examples of signal corresponding to VT1 receptor mRNA in coronal sections from white-throated sparrow (A–C) and zebra finch (D–F). Film images are shown in C and F. B and E depict alternate, Nissl-stained sections from the same individuals shown in C and F, respectively. The Nissl-stained section from the zebra finch (E) underwent ISH with RNase, and some cell groups are not clearly visible. It is included to show major anatomical landmarks. Abbreviations, see Table 2.
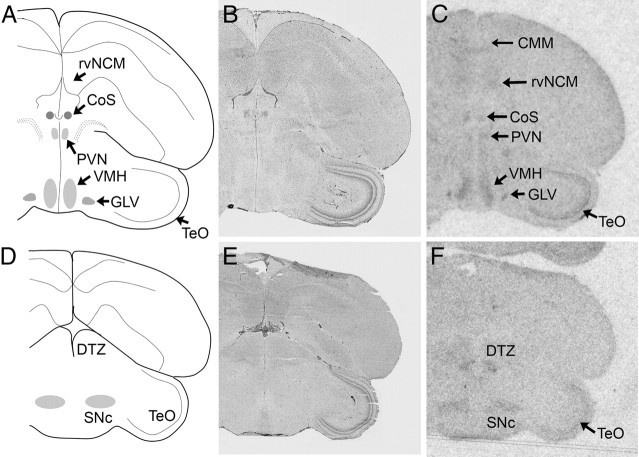
Fig. 4.
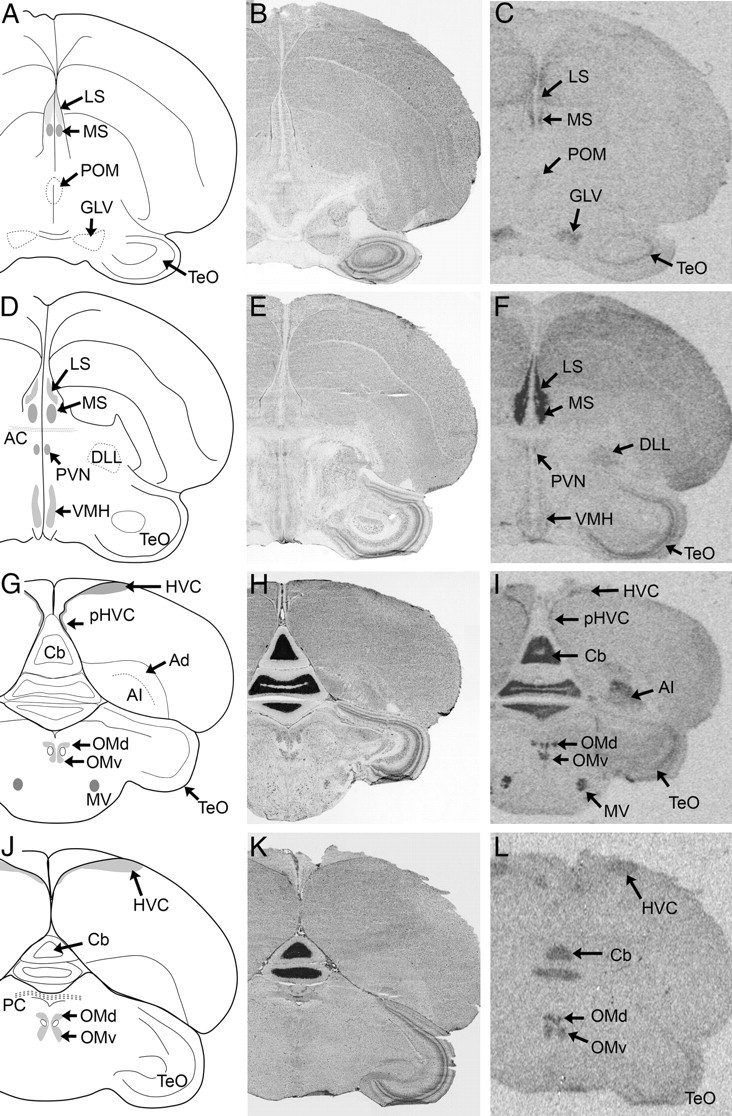
Examples of signal corresponding to VT3 (OT-like) receptor mRNA in coronal sections from white-throated sparrow (A–I) and zebra finch (J–L). Film images are shown in C, F, I, and L. Alternate, Nissl-stained sections from the same individuals are shown in B, E, H, and K. The Nissl-stained section from the zebra finch (K) underwent ISH with RNase, and some cell groups are not clearly visible. It is included to show major anatomical landmarks. AC, anterior commissure; PC, Posterior commissure. Abbreviations, see Table 2.
Fig. 5.
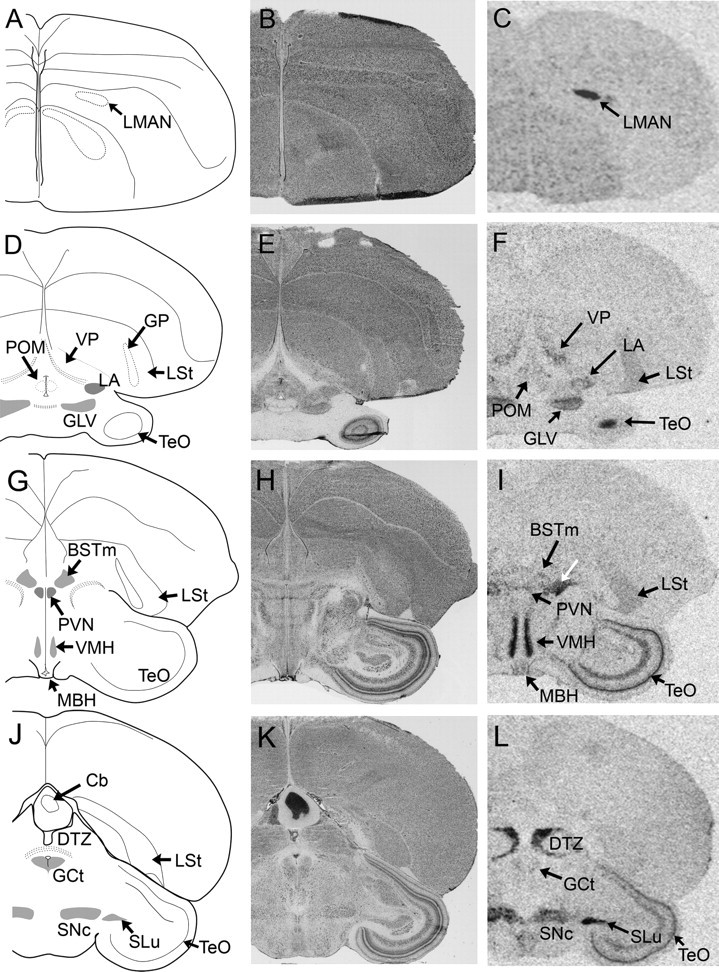
Examples of VT4 (V1a) receptor mRNA signal in coronal sections from white-throated sparrow. Film images are shown in C, F, I, and L. Nissl-stained, alternate sections from the same individuals shown in B, E, H, and K, respectively. The area indicated by the white arrow in I lies dorsomedial to the occipitomesencephalic tract and was labeled intensely in all sparrows. It corresponds to an area rich in VT-immunoreactive neurons and fibers in this species (Maney, D. L., unpublished observation) and other birds (2, 53, 54). It may be a rostral extension of the medial portion of the bed nucleus of the stria terminalis (BSTm) (55). The area of signal labeled SNc in L overlaps medially with the caudalmost portion of VTA (56, 57). Abbreviations, see Table 2.
Fig. 6.
Examples of VT4 (V1a) receptor mRNA signal in coronal sections from zebra finch. Exposed films are shown in C and F. Nissl-stained sections from the same individuals shown in B and E, respectively. The area of signal labeled SNc in F overlaps medially with the caudalmost portion of VTA (56, 57). Abbreviations, see Table 2.
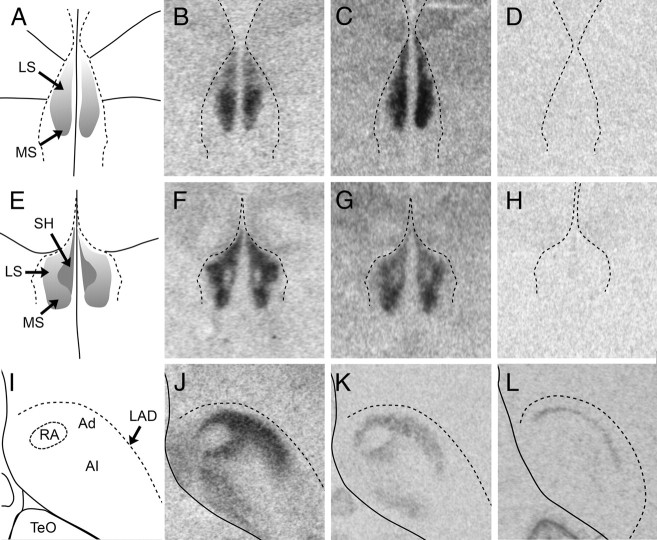
Fig. 7.
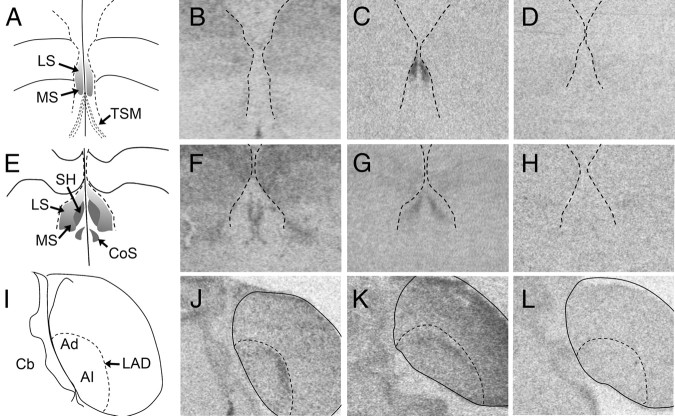
Comparison of the binding distribution of radiolabeled ornithine VT analog ([125I]-OVTA) (B, F, and J) with the distribution VT3 (OT-like) receptor mRNA (C, G, and K) and either VT1 receptor mRNA (D and H) or VT4 (V1a) receptor mRNA (L) at two coronal levels of the septum (A–H) and in the arcopallium (I–L) of white-throated sparrow. A, E, and I correspond to A2.4, A1.8, and P1.2 in Stokes et al. (58), respectively. The position of the lateral ventricles (B–D and F–H) and the dorsal arcopallial lamina (LAD) (J–L) was determined by superimposing the images of the Nissl-stained sections onto the film images. Note the striking similarity between [125I]-OVTA binding and VT3 receptor mRNA expression. We detected no specific signal for VT1 receptor in the rostral septum (D) and only very faint signal at the more caudal level (H). No VT4 receptor expression was detected in the septal area in any of the birds (see Fig. 5I for an example of VT4 receptor mRNA expression in a comparable section containing the septum), but it was present in Ad (L). Images of [125I]-OVTA binding were acquired using material from Leung et al. (33). In A–H, the midline is in the center; in I–L, the midline is to the left. Abbreviations, see Table 2.
Fig. 8.
VT receptor mRNA expression at three rostro-caudal levels in zebra finch. A–D, Rostral LS. E–H, Caudal LS. I–L, Caudal telencephalon. VT1 receptor mRNA expression is shown in B, F, and J. VT3 (OT-like) receptor mRNA expression is shown in C, G, and K. VT4 (V1a) receptor expression is shown in D, H, and L. The position of the lateral ventricles (B–D and F–H) and the LAD (J–L) was determined by superimposing images of Nissl-stained alternate sections onto the film images. In A–H, the midline is in the center; in I–L, the midline is to the left. LAD, dorsal arcopallial lamina; TSM, septomesencephalic tract. Other abbreviations, see Table 2.
Fig. 9.
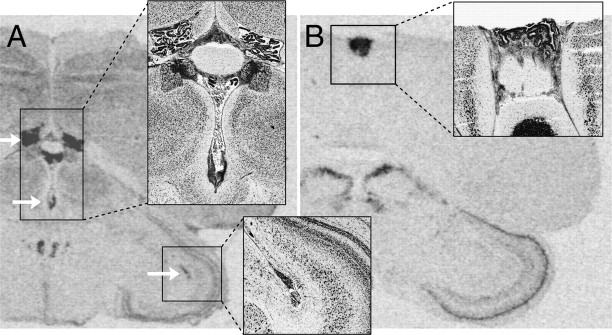
VT3 and VT4 receptor mRNA expression in nonneural areas in white-throated sparrow. Insets show Nissl-stained alternate sections from the same individuals. VT3 (OT-like) receptor mRNA was detected in the choroid plexus of the lateral, third, and tectal ventricles (arrows in A). In zebra finch, choroid plexus contained mRNA for VT1 (not depicted in figure). In sparrow, VT4 (V1a) receptor mRNA produced a strong signal in the pineal gland (box in B).
Fig. 10.
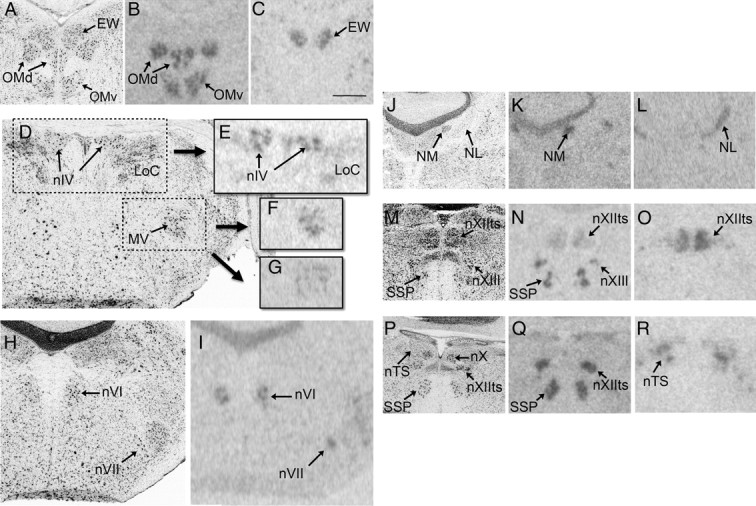
VT3 (OT-like) and VT4 (V1a) mRNA signal in nuclei associated with cranial nerves. All images are from white-throated sparrows. The images on the left in all rows (A, D, H, J, M, and P) show Nissl-stained sections, and the corresponding film images on each row show alternate sections from the same individuals. A–C, Whereas the oculomotor nucleus (OMd and OMv) contained VT3 receptor mRNA (B), the EW contained VT4 receptor mRNA (C). D–G, VT3 receptor mRNA signal was present in nIV (E). MV contained mRNA for both VT3 (F) and VT4 (G) receptors. H and I, The abducens nucleus (nVI) and the motor nucleus of the facial nerve (nVII) both expressed VT3 receptor mRNA. J–L, Two auditory nuclei, the nucleus magnocellularis (NM) and the nucleus laminaris (NL), expressed VT3 (K) and VT4 (L) mRNA, respectively. M–O, VT3 receptor mRNA was present in two subdivisions of the hypoglossal nucleus (nXIIts and nXIIl) and in the motor nucleus of the eleventh cranial nerve (SSP). Of these regions, only nXIIts contained VT4 receptor mRNA (O). P–R, More caudally, nXIIts contained only VT3 receptor mRNA. In R, VT4 receptor mRNA signal can be seen in the nucleus of the solitary tract (nTS). Scale bar in C, 500 μm, applicable to all images. Other abbreviations, see Table 2.
A complete list of brain regions in which VT receptor mRNA was detected is presented in Table 2. For many regions, we found remarkable individual variation that was not explained by sex or the recorded behaviors (see Supplemental Figs. 2–5 for histograms and the P values generated by the Mann-Whitney U and Spearman correlation tests). The highlights of our findings are described in the text below.
Table 2.
Neural distribution and comparative abundance of VT mRNA in the white-throated sparrow and zebra finch
| Region | VT1 |
VT3 (OT-like) |
VT4 (V1a) |
References | |||
|---|---|---|---|---|---|---|---|
| WTSP | ZF | WTSP | ZF | WTSP | ZF | ||
| Pallial structures | |||||||
| Ad | − | ++* | +++ | ++ | ++* | − | 59 |
| AI | − | − | +++ | − | − | − | 56 |
| CMM | + | ++ | ++* | − | − | ++* | 34, 60 |
| HA | − | − | +* | − | − | − | 56 |
| Hp-D | − | ++ | ++* | − | − | − | 61 |
| Hp-VM | − | ++* | ++* | − | − | − | 61 |
| HVC | − | − | ++* | −* | − | − | 62, 63 |
| LMAN | ++ | − | − | − | +++* | − | 62, 63 |
| M | − | ++ | ++* | − | − | − | 56 |
| MMAN | ++ | − | − | − | +++* | − | 62, 63 |
| N | + | − | + | ++* | − | − | 56 |
| pHVC | ++* | ++* | ++* | ++* | − | − | 64 |
| RA | − | ++ | ++* | − | − | − | 62, 63 |
| rdNCM | ++* | ++ | ++ | ++ | − | ++ | 34, 65 |
| rvNCM | ++* | ++ | ++* | ++ | − | ++* | 34, 65 |
| TnA | − | ++ | + | − | − | − | 56, 66 |
| Subpallial structures | |||||||
| BSTl | − | − | − | − | + | − | 55 |
| BSTm | − | − | + | − | + | − | 55 |
| CoS | − | ++ | − | − | − | ++* | 67 |
| INP | + | ++ | − | − | − | − | 56 |
| LS | − | − | +++ | +++ | − | − | 67 |
| LSt | − | − | +* | − | + | − | 56 |
| MS | − | − | +++* | + | − | − | 67 |
| MSt | − | ++* | − | − | + | + | 56 |
| SH | +* | ++ | ++ | + | − | − | 67 |
| VP | − | − | − | − | ++ | − | 56 |
| Diencephalic structures | |||||||
| DLL | − | − | ++ | − | − | − | 68 |
| DTZ | − | +++ | ++* | ++ | +++ | ++* | 69, 70 |
| GLV | − | − | ++* | − | ++* | ++ | 58 |
| Hb | − | +++ | ++* | ++ | − | ++ | 67, 69 |
| LA | ++ | − | − | − | ++ | − | 58 |
| MBH | − | +++ | ++ | − | − | ++* | 72, 73 |
| POM | − | ++* | +* | − | +* | ++ | 74, 75 |
| Pt | +++ | +++ | +* | − | − | − | 30, 58 |
| PVN | − | − | ++ | − | + | ++ | 76 |
| VMH | − | ++ | ++ | − | +++ | ++ | 76, 77 |
| Brainstem and cerebellum | |||||||
| Cb | − | − | +++* | ++ | − | ++ | 58 |
| CS | − | − | ++* | ++ | ++* | − | 58, 78 |
| GCt | +* | − | +* | − | +* | ++ | 56, 57, 79 |
| ICo | − | − | +* | − | ++* | − | 58 |
| IO | − | − | ++* | − | − | − | 80 |
| LoC | − | − | + | ++ | ++ | ++ | 56, 57, 79 |
| SLu | − | − | ++ | − | +++ | − | 80 |
| SNc | +* | ++ | − | − | ++ | ++ | 56, 57, 79 |
| TeO | + | ++ | ++ | ++ | +++ | ++ | 58 |
| VTA | + | ++* | +* | − | ++ | ++* | 56, 57, 79 |
| EW | − | − | − | − | ++* | − | 82, 83 |
| OMd/OMv | − | − | +++ | +++ | − | − | 81, 82 |
| nIV | − | − | +++ | +++ | − | − | 81, 83 |
| MV | − | − | +++ | +++ | ++* | − | 84 |
| NL | − | − | +* | 83 | |||
| NM | − | ++* | − | 83 | |||
| nVI | − | − | +++ | +++ | − | 84 | |
| nVII | − | − | +++ | +++ | − | 84 | |
| nTS | − | − | +++ | 85 | |||
| SSP | − | +++ | − | 56, 85 | |||
| nXIIts | ++* | +++ | ++* | +++ | 85–87 | ||
| nXIIl | − | +++ | +++ | ++ | 85 | ||
The regions are listed first according to major subdivision of the brain, then alphabetically except under Brainstem and cerebellum, where the nuclei associated with the cranial nerves are listed in order at the end. Plus signs indicate relative intensity of signal for each mRNA (+ denotes a median value of 1–2, ++ a median value of 3–4, and +++ a median value of 5 or above) (see Fig. 1 and Materials and Methods). Minus signs indicate the absence of obvious signal. Asterisks indicate notable individual variation (see text and Supplemental Figs 1–4). When the available tissue was inadequate to assess the signal, no symbol is given. Ad, Dorsal arcopallium; AI, intermediate arcopallium; BSTl, lateral bed nucleus of the stria terminalis; BSTm, medial bed nucleus of the stria terminalis; Cb, cerebellum; CMM, caudomedial mesopallium; CoS, commissural septal nucleus; CS, superior central nucleus; DLL, dorsal portion of the dorsolateral nucleus of the anterior thalamus; DTZ, dorsal thalamic zone; EW, nucleus of Edinger Westphal; GCt, midbrain central gray; GLV, lateral geniculate nucleus; HA, apical hyperpallium; Hb, habenula; Hp-D, dorsal region of the hippocampus; Hp-VM, ventromedial region of the hippocampus; HVC, used as a proper name; ICo, intercollicular nucleus; INP, intrapeduncular nucleus; IO, isthmo-optic nucleus; LA, lateral anterior nucleus of the thalamus; LMAN, lateral magnocellular nucleus of the anterior nidopallium; LoC, locus coeruleus; LS, lateral septum; LSt, lateral striatum; M, mesopallium; MBH, mediobasal hypothalamus; MMAN, medial magnocellular nucleus of the anterior nidopallium; MS, medial septum; MSt, medial striatum; MV, trigeminal motor nucleus; N, nidopallium; nIV, trochlear nucleus; NL, nucleus laminaris; NM, nucleus magnocellularis; nVI, abducens nucleus; nVII, motor nucleus of the facial nerve; nTS, nucleus of the solitary tract; nXIIl, linqual portion of the hypoglossal nucleus; nXIIts, tracheosyringeal portion of the hypoglossal nucleus; OMd, dorsal portion of the oculomotor nucleus; OMv, ventral portion of the oculomotor nucleus; pHVC, para HVC; POM, medial preoptic area; Pt, nucleus pretectalis; PVN, paraventricular nucleus; RA, robust nucleus of the arcopallium; rdNCM, rostrodorsal domain of the caudomedial nidopallium; rvNCM, rostroventral domain of the caudomedial nidopallium; SH, septohippocampal septum; SLu, semilunar nucleus; SNc, substantia nigra, pars compacta; SSP, supraspinal nucleus; TeO, optic tectum; TnA, nucleus taeniea; VMH, ventromedial hypothalmus; VP, ventral pallidum; VTA, ventral tegmental area.
Distribution of VT1 receptor mRNA
By far the most striking VT1 signal was obtained in the pretectal nucleus (Pt), which was labeled intensely in both species (Fig. 3, C and F). In the auditory forebrain, the distribution of signal was similar to the reported distribution of sound-induced immediate early gene responses (34). The caudomedial mesopallium (CMM), para-HVC (pHVC), and the rostrodorsal and rostroventral domains of the caudomedial nidopallium (rdNCM and rvNCM) were intensely labeled, whereas Field L2 stood out as unlabeled (Fig. 3C). Both species showed expression also in the intrapeduncular nucleus (INP) and the tracheosyringeal portion of the hypoglossal nucleus (nXIIts). The distribution of VT1 signal was more widespread in the zebra finches than the sparrows, with expression in several telencephalic and diencephalic areas such as the hippocampus, medial striatum, robust nucleus of the arcopallium (RA), nucleus taeniae of the amygdala (TnA), dorsal thalamic zone (DTZ), habenula (Hb), and ventromedial hypothalamus (VMH), which were not labeled in the sparrows. Although both species showed some expression in septal areas, in sparrows, the signal was limited to the septohippocampal septum (SH) (Fig. 7H) and was highly variable. The zebra finches showed more consistent signal in SH (Fig. 8F) as well as in the commissural septal nucleus (CoS). A few regions, such as the anterior forebrain nuclei of the song system, the lateral and medial magnocellular nuclei of the anterior nidopallium (LMAN and MMAN), were labeled in the sparrows but not the finches. The distribution of mRNA in the brain stem was similar in the two species (Table 2).
Distribution of VT2 (V1b) receptor mRNA
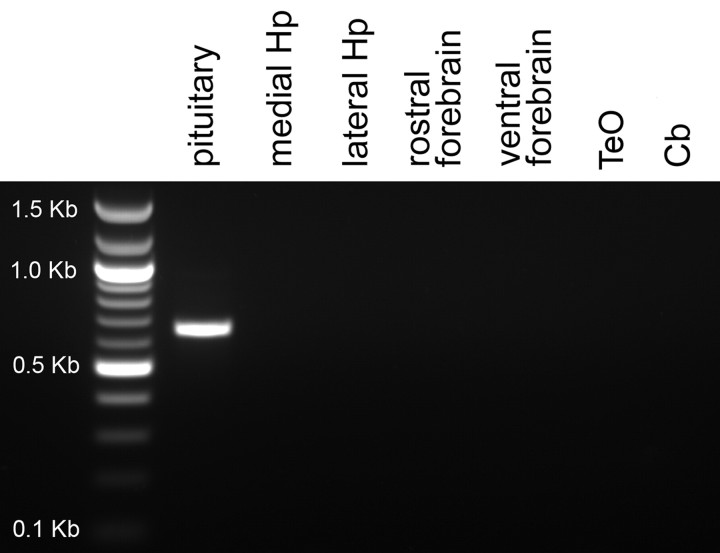
We did not detect a specific mRNA signal for VT2 in brain sections of either sparrow or zebra finch. In both species, signal was observed only in Cb and was similar in intensity using sense and antisense probes. This pattern was identical to what we commonly see using sense probes for other mRNAs (Leung, C. H., and D. L. Maney, unpublished observations). PCR amplification in sparrow showed that this mRNA was abundant in the pituitary but absent from hippocampus, rostral forebrain, ventral diencephalon, optic tectum (TeO), and Cb (Fig. 11).
Fig. 11.
PCR analysis of VT2 (V1b) receptor in white-throated sparrow. A fragment corresponding to VT2 receptor could be amplified using cDNA from pituitary but not hippocampus (Hp), rostral or ventral forebrain, optic tectum (TeO), or cerebellum (Cb).
Distribution of VT3 (OT-like) receptor mRNA
VT3 receptor mRNA was found in more regions in the white-throated sparrow than in the zebra finch. In both species, robust signal was detected in the dorsal arcopallium (Ad), rdNCM, rvNCM, pHVC, LS (Figs. 4, 7, and 8), DTZ, Hb, Cb, and superior central nucleus (CS). In the sparrow arcopallium, Ad was labeled intensely, whereas the rostral portion of RA stood out as unlabeled. This pattern, as well as that observed in LS, was strikingly similar to the pattern of OVTA binding reported by Leung et al. (33) (Fig. 7). In both species, VT3 mRNA was detected in most of the motor nuclei of the cranial nerves (Fig. 10), including the motor nucleus of the trigeminal nerve (MV), trochlear nucleus (nIV), and the dorsal and ventral portions of the oculomotor nucleus (OMd and OMv). White-throated sparrows exhibited high levels of VT3 mRNA also in the choroid plexus of the ventricular system (Fig. 9A) but the zebra finches did not. Other regions showing evidence for VT3 mRNA in sparrow but not finch included the hippocampus, RA, lateral geniculate nucleus, paraventricular nucleus (PVN), and VMH. In two of the male zebra finches, HVC was clearly labeled (Fig. 4L); in the others, however, it was not. In the sparrows, the signal in HVC was diffuse compared with the obvious signal in pHVC (Fig. 4I).
Distribution of VT4 (V1a) receptor mRNA
We obtained robust signal for VT4 mRNA in both species, and whereas this mRNA was expressed in a similar pattern in zebra finches and white-throated sparrows in the diencephalon and brain stem, in the telencephalon, the distributions were nonoverlapping. The anterior forebrain song nuclei LMAN and MMAN (Fig. 5C), the ventral pallidum (Fig. 5F) and the caudal Ad (Fig. 7L) were labeled in the sparrows, whereas the auditory forebrain (CMM, rdNCM, and rvNCM) was labeled in the finches (Fig. 6C and Table 2). The only septal region labeled in either species was CoS, which is located caudally in the septal complex, in finches (Fig. 6C). We noted signal in both species in the DTZ, VMH, locus coeruleus (LoC), substantia nigra pars compacta (SNc), TeO, ventral tegmental area (VTA), and the lingual portion of the hypoglossal nucleus (nXIIl). For several brain stem regions, including the CS, nucleus of Edinger-Westphal (EW), MV, and semilunar nucleus, expression was detected in the sparrow but not the finch. The most intense VT4 signal was seen in the pineal body of sparrows (Fig. 9B), but such expression was absent from finches.
Discussion
In this study, we mapped the neural distribution of VT receptor mRNA in two model species of songbird, the white-throated sparrow and the zebra finch. The VT3 and VT4 receptors, which are homologous to the OT and V1a receptors in mammals, respectively, were widely expressed in the brains of both species. Expression of the VT1 receptor, which may be homologous to the mammalian V2 receptor (11) (see also Supplemental Table 1 and Fig. 2), had a somewhat more limited distribution in the sparrow but was highly concentrated in Pt of both species (Fig. 3). The striking expression in Pt is consistent with a previous report of a highly localized concentration of 3H-vasopressin binding in that area (30). Overall, VT receptor mRNA was expressed at higher levels and in more brain regions in the sparrows than in the zebra finches. This finding is consistent with those of Leung et al. (33), who described the distribution and density of radioligand binding sites in the same two species. Like Cornett et al. (16) and Jurkevich et al. (17), we found no evidence of VT2 (V1b) receptor expression in the brain (Fig. 11).
Our analysis of the predicted amino acid sequences for the four VT receptors revealed the expected relationships (Supplemental Table 1 and Fig. 2). At critical locations that determine binding specificity (29, 41, 42, 48), the VT1 receptor is V2-like, the VT2 is V1b-like, VT3 receptor is OTR-like, and the VT4 is V1a-like. We might predict, therefore, that whereas the VT1, VT2, and VT4 receptors may bind VT preferentially, the VT3 receptor may bind the avian homolog of OT, MT, with a higher specificity than the other receptors. MT is present in several of the regions that contain VT3 receptor mRNA, including the LS, arcopallium, and TeO (88). We cannot assume, however, that MT, rather than VT, is the more likely endogenous ligand. VT binds to mammalian OTR with even greater affinity than OT itself (29), so the sequence identity between OTR and the VT3 receptor does not indicate that VT is a poor ligand at the VT3 receptor. VT is more effective than MT at stimulating contractions of uterine muscle in domestic hens (23), and both VT and MT contain a residue at positions 2 and 3 that likely interact favorably with residues 209 and 284 on the chicken VT3 receptor (89). Although the amphibian MTRs bind MT with higher affinity than they do VT (7, 21), VT may act as a partial agonist at the MTR (21). The relative affinity of the avian VT3 receptor is currently unknown. Cell transfection and binding assays will be necessary to determine the specificity and affinity for any of the receptors we describe here (4).
Using the radioligand [125I]-OVTA, Voorhuis et al. (31) mapped a population of receptors in the arcopallium that surrounded the song control nucleus RA in canaries. We obtained a similar binding pattern with the same ligand in white-throated sparrows (33). We show here that this pattern of binding in the sparrows is largely attributable to VT3 receptors (compare Fig. 7J with Fig. 7K). The pattern of VT3 receptor expression closely resembles that for OVTA binding also in the LS (Fig. 7, C and G). The striking anatomical match between the ligand binding and the mRNA hybridization signal suggests either that the receptors are expressed on or near the somata of VT receptor neurons or that the mRNA transcripts are transported to the areas where the receptors will be expressed. In general, we found little mismatch between the expression of receptor mRNA and binding sites. Exceptions included the PVN, which did not contain binding sites but did contain VT receptor mRNA, and TnA, which contained high levels of mRNA but not OVTA binding sites (33). The low level of mismatch is made even more remarkable by the fact that the birds in the two studies were not in the same reproductive condition; we reported OVTA binding in photostimulated animals, whereas those in the current study were housed on short days and had regressed gonads. The mismatch that we report here may therefore be related to hormonal state. Follow-up studies are currently underway to assess the effects of gonadal steroids on mRNA expression.
Central administration of VT, MT, or their antagonists has been shown to affect social behavior in songbirds; in most cases, the target of such manipulations has been the LS. Elegant work by Goodson and Adkins-Regan (90) and Goodson et al. (91, 92) has shown that VT and MT may play very different roles in this area in zebra finches. Septal infusions of VT increased competitive aggression in both males and females (90, 91). Vasopressin antagonist decreased aggression in males, bringing it down to a female-like level (91). In contrast, MT infused via the same route had no affect on aggression. Instead, MT has been demonstrated to affect social affiliation; for example, septal administration of MT increased the time spent with familiar conspecifics as well as the preferred group size and an OT antagonist had opposite effects (92). These effects were limited to females, suggesting a sex difference in receptor distribution or function. Together, the infusion studies suggest that the LS in zebra finches contains a population of VT receptors that preferentially binds VT and affects competitive aggression, and another that preferentially binds MT and affects affiliation. Although there is currently no definitive evidence in birds that MT binds to any of the known VT receptor subtypes with higher affinity than does VT, of the known subtypes, VT3 is most likely to bind MT (7, 21). We show here that in fact the LS expresses VT3 mRNA at relatively high levels (Figs. 4, 7, and 8). We did not, however, find evidence for a sex difference in either species. A quantitative analysis with a larger sample size will be necessary to investigate this question thoroughly.
The population of VT3 receptors in the LS is likely to bind both MT and VT. Evidence for a separate population of receptors that binds VT preferentially, however, is less clear. Whereas the expression of VT3 receptor mRNA was robust in the LS, expression of VT1 and VT4 receptor mRNA was weak. In both species, the LS stood out not because it contained signal but because of the absence of signal compared with the surrounding tissue (Figs. 5I, 7H, and 8, D and H). Our study thus provides no evidence that neurohypophyseal peptides infused into the LS act in that region via receptors other than VT3 receptors and adds little insight into the finding that VT and MT infusions into the LS have disparate effects on social behavior. Perhaps these ligands act on VT1 receptors in the adjacent SH and medial septum. It will be necessary to perform receptor knockdown studies or infuse agonists or antagonists specific for the receptor subtypes to resolve this issue.
Previous research has indicated effects of VT administration on singing behavior specifically (93–95). In the current study, we noted that several nuclei within the song control system contained high concentrations of VT receptor mRNA. This result was expected for RA and the intercollicular nucleus (ICo), which contain VT fibers (2, 33, 54, 96) and binding sites (30, 33). We found evidence for VT3 receptor mRNA in RA in both species, although the surrounding arcopallium was more densely labeled than RA proper (Figs. 7K and 8K). ICo contained low amounts of VT3 and VT4 mRNA in sparrows but not zebra finches. Outside of these areas, VT receptors or fibers have not been widely reported in the song system. HVC was previously shown to contain low levels of [125I]-OVTA binding but not VT fibers (30, 33). In the present study, some but not all of the male zebra finches showed clear expression of VT3 receptor mRNA in HVC (Fig. 4L). In the sparrows, VT3 expression was higher in pHVC than in HVC proper (Fig. 4I). Some of the most striking expression of any mRNA was observed in the song control nuclei of the anterior forebrain pathway in sparrows. MMAN and LMAN labeled intensely for VT4 receptor mRNA (Fig. 5C) and to a much lesser degree VT1 receptor mRNA (Table 2). Similar expression was not observed in any of the zebra finches. The nXIIts, which innervates the syrinx and is considered to be part of the song system (62), contained high levels of all three subtypes of VT receptor mRNA (Table 2 and Fig. 10). The lingual portion of that nucleus was also labeled. Stimulation of V1a receptors in hypoglossal nucleus of rats causes powerful excitation (97) as well as enhanced inhibitory synaptic transmission (98), and both vasopressin and OT are thought to serve as neuromodulators of circuits involved in tongue movements in that species (99). In the present study, expression in nuclei associated with cranial nerves was not limited to the hypoglossal, however. Intense signal was also observed in the motor nuclei of the third, fourth, fifth, sixth, seventh, and eleventh cranial nerves (Fig. 10). VT receptor mRNA in brain stem cell groups is therefore unlikely to be related specifically to song. In bullfrogs, which also use vocalizations for courtship and territorial defense, VT and MT receptor mRNA is present in nIV and facial (nVII) motor nuclei, but not the vagal motor nucleus (nX), which innervates the vocal organ (7, 100). Studies are currently underway to characterize more fully the expression and function of VT receptors in the song control system of passerines.
Some of the most intense signal in the brain was seen in the DTZ, which in birds is made up of a number of subregions, including the dorsomedial anterior and posterior nuclei and more laterally the dorsal intermediate posterior and lateral sub-habenular nuclei (70, 101). In sparrows, VT4 (V1a) receptor mRNA was concentrated along the dorsomedial edge of the DTZ (Fig. 5L) and overlapped the dorsomedial posterior, dorsal intermediate posterior, and lateral sub-habenular nuclei in a pattern similar to that reported for GABA immunoreactivity in pigeons (70). In the finches, the VT4 expression was concentrated along the ventromedial edge of the DTZ and was more diffuse dorsally (Fig. 6F). The DTZ of birds is homologous to the intralaminar, midline, and mediodorsal nuclear complex of mammals (69, 70, 101), which serves diverse cognitive and sensorimotor functions and is considered mainly limbic in nature (101). In rodents, nuclei within this complex have been demonstrated to bind vasopressin-like ligands (9, 102, 103) as well as to express V1a receptor mRNA (104).
One of the most interesting findings in the present study was that the distribution of each mRNA showed a great deal of intraspecific variation (Table 2). For example, VT3 mRNA was expressed at moderate levels in the zebra finch HVC (Fig. 4L) but only in two individuals. Similarly, whereas the expression of VT3 receptor mRNA was reliable in the sparrow Ad and MV, the expression of VT4 mRNA in those regions was variable. We also noted remarkable individual variation in the auditory forebrain, thalamus, and septal areas in both species. Striking intraspecific variation in the density and distribution of neurohypophyseal peptide receptors has been described in the brains of several rodent species, for example, prairie voles (103, 105, 106) and rats (107). Some researchers have suggested that individual differences in neurohypophyseal peptide receptor expression may explain variation in social behaviors, and significant correlations have been reported for alloparental and parental care, space use, social investigation, and pair bonding (106, 108–111). We did not find convincing correlations between receptor signal and behavior, but most of the behaviors we scored were not social in nature. We hope that our present findings, which suggest significant variation both between and within songbird species, will lead to more detailed studies linking VT mRNA receptor expression with social behavior.
Summary
We have shown that in two species of songbird, the brain contains at least three unique VT receptors with distinct distributions. These receptors are concentrated in many areas where neurohypophyseal peptides were already demonstrated to affect social behavior in birds and mammals, for example, the LS and the hypothalamus. The role of these regions and associated neuropeptides in behavioral processes is already regarded as extraordinarily complex and depends on patterns of neural connectivity and activation, sociality, and social context (28). Our demonstration of the diversity of VT receptors in these regions adds yet another layer of complexity. We have also identified large populations of VT receptors in the song control and auditory pathways, which were not previously known to be sensitive to neurohypophyseal peptides. Future research should focus on the role of VT and MT in song perception and learning.
Supplementary Material
Acknowledgments
We thank Sabrina Burmeister, Karin Lent, Kerry Ressler, Lisa Stanek, Robert Steiner, James Thomas, and Larry Young for technical advice and Jamie Davis, Lisa Matragrano, and Sara Sanford for technical assistance. Zebra finches were generously provided by Laura Carruth. Patricia Brennan, Laura Carruth, Kim Wallen, and Larry Young provided comments on a previous version of the manuscript. We also thank the Departments of Biology, Human Genetics, and Psychiatry at Emory and the Department of Biology at Georgia State University (Atlanta, GA) for the use of facilities.
Present address for C.H.L.: Department of Biology, Widener University, Chester, Pennsylvania 19013.
Present address for C.T.G.: Department of Psychology, Georgia State University, Atlanta, Georgia 30302.
This work was supported by National Science Foundation Grants IBN-0346984 and IOS-0723805 (to D.L.M.) and the Center for Behavioral Neuroscience Grant IBN-9876754.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- DTT
dithiothreitol
- ECL
extracellular loop
- EtOH
ethanol
- ISH
in situ hybridization
- MT
mesotocin
- MTR
MT receptor
- OT
oxytocin
- OTR
OT receptor
- OVTA
ornithine VT
- RNase
ribonuclease
- SSC
sodium saline citrate
- TMD
transmembrane domain
- VT
vasotocin.
- Other abbreviations
see Table 2.
References
- 1. Van Leeuwen F , Caff é R. 1983. Vasopressin-immunoreactive cell bodies in the bed nucleus of the stria terminalis of the rat. Cell Tissue Res 228:525–534 [DOI] [PubMed] [Google Scholar]
- 2. Kiss JZ , Voorhuis TA , van Eekelen JA , de Kloet ER , de Wied D. 1987. Organization of vasotocin-immunoreactive cells and fibers in the canary brain. J Comp Neurol 263:347–364 [DOI] [PubMed] [Google Scholar]
- 3. Rood BD , De Vries GJ. 2011. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J Comp Neurol 519:2434–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caldwell HK , Lee HJ , Macbeth AH , Young WS. 2008. Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol 84:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goodson JL. 2008. Nonapeptides and the evolutionary patterning of sociality. Prog Brain Res 170:3–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baeyens DA , Cornett LE. 2006. The cloned avian neurohypophysial hormone receptors. Comp Biochem Physiol B Biochem Mol Biol 143:12–19 [DOI] [PubMed] [Google Scholar]
- 7. Acharjee S , Do-Rego JL , Oh DY , Moon JS , Ahn RS , Lee K , Bai DG , Vaudry H , Kwon HB , Seong JY. 2004. Molecular cloning, pharmacological characterization, and histochemical distribution of frog vasotocin and mesotocin receptors. J Mol Endocrinol 33:293–313 [DOI] [PubMed] [Google Scholar]
- 8. Lim MM , Hammock EA , Young LJ. 2004. The role of vasopressin in the genetic and neural regulation of monogamy. J Neuroendocrinol 16:325–332 [DOI] [PubMed] [Google Scholar]
- 9. Campbell P , Ophir AG , Phelps SM. 2009. Central vasopressin and oxytocin receptor distributions in two species of singing mice. J Comp Neurol 516:321–333 [DOI] [PubMed] [Google Scholar]
- 10. Hasunuma I , Toyoda F , Kadono Y , Yamamoto K , Namiki H , Kikuyama S. 2010. Localization of three types of arginine vasotocin receptors in the brain and pituitary of the newt Cynops pyrrhogaster. Cell Tissue Res 342:437–457 [DOI] [PubMed] [Google Scholar]
- 11. Lema SC. 2010. Identification of multiple vasotocin receptor cDNAs in teleost fish: sequences, phylogenetic analysis, sites of expression, and regulation in the hypothalamus and gill in response to hyperosmotic challenge. Mol Cell Endocrinol 321:215–230 [DOI] [PubMed] [Google Scholar]
- 12. Young LJ , Winslow JT , Nilsen R , Insel TR. 1997. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: behavioral consequences. Behav Neurosci 111:599–605 [DOI] [PubMed] [Google Scholar]
- 13. Lim MM , Wang Z , Olazábal DE , Ren X , Terwilliger EF , Young LJ. 2004. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 429:754–757 [DOI] [PubMed] [Google Scholar]
- 14. Tan FL , Lolait SJ , Brownstein MJ , Saito N , MacLeod V , Baeyens DA , Mayeux PR , Jones SM , Cornett LE. 2000. Molecular cloning and functional characterization of a vasotocin receptor subtype that is expressed in the shell gland and brain of the domestic chicken. Biol Reprod 62:8–15 [DOI] [PubMed] [Google Scholar]
- 15. Scarbrough K , Kirby JD , Cornett LE , Okimoto R. 2003. Chromosomal assignment and mapping of the vasotocin receptor 1, homologue to the mammalian V1a and vasotocin receptor 2 homologue to the mammalian V1b receptors in the domestic fowl. Anim Genet 34:393. [DOI] [PubMed] [Google Scholar]
- 16. Cornett LE , Kirby JD , Vizcarra JA , Ellison JC , Thrash J , Mayeux PR , Crew MD , Jones SM , Ali N , Baeyens DA. 2003. Molecular cloning and functional characterization of a vasotocin receptor subtype expressed in the pituitary gland of the domestic chicken (Gallus domesticus): avian homolog of the mammalian V1b-vasopressin receptor. Regul Pept 110:231–239 [DOI] [PubMed] [Google Scholar]
- 17. Jurkevich A , Berghman LR , Cornett LE , Kuenzel WJ. 2005. Characterization and immunohistochemical visualization of the vasotocin VT2 receptor in the pituitary gland of the chicken, Gallus gallus. Gen Comp Endocrinol 143:82–91 [DOI] [PubMed] [Google Scholar]
- 18. Mikhailova MV , Mayeux PR , Jurkevich A , Kuenzel WJ , Madison F , Periasamy A , Chen Y , Cornett LE. 2007. Heterooligomerization between vasotocin and corticotropin-releasing hormone (CRH) receptors augments CRH-stimulated 3′,5′-cyclic adenosine monophosphate production. Mol Endocrinol 21:2178–2188 [DOI] [PubMed] [Google Scholar]
- 19. Young WS , Li J , Wersinger SR , Palkovits M. 2006. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience 143:1031–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gubrij KI , Chaturvedi CM , Ali N , Cornett LE , Kirby JD , Wilkerson J , Mikhailova M , Turner ML , Baeyens DA. 2005. Molecular cloning of an oxytocin-like receptor expressed in the chicken shell gland. Comp Biochem Physiol B Biochem Mol Biol 142:37–45 [DOI] [PubMed] [Google Scholar]
- 21. Searcy BT , Bradford CS , Thompson RR , Filtz TM , Moore FL. 2011. Identification and characterization of mesotocin and V1a-like vasotocin receptors in a urodele amphibian, Taricha granulosa. Gen Comp Endocrinol 170:131–143 [DOI] [PubMed] [Google Scholar]
- 22. Robinzon B , Koike TI , Marks PA. 1994. Oxytocin antagonist blocks the vasodepressor but not the vasopressor effect of neurohypophysial peptides in chickens. Peptides 15:1407–1413 [DOI] [PubMed] [Google Scholar]
- 23. Saito N , Koike TI. 1992. Alterations in uterine contractility during the oviposition cycle in domestic hens. Br Poult Sci 33:671–676 [DOI] [PubMed] [Google Scholar]
- 24. Goodson JL , Bass AH. 2001. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Rev 35:246–265 [DOI] [PubMed] [Google Scholar]
- 25. Srivastava R , Cornett LE , Chaturvedi CM. 2007. Effect of photoperiod and estrogen on expression of arginine vasotocin and its oxytocic-like receptor in the shell gland of the Japanese quail. Comp Biochem Physiol A Mol Integr Physiol 148:451–457 [DOI] [PubMed] [Google Scholar]
- 26. Srivastava R , Cornett LE , Chaturvedi CM. 2008. Effect of estrogen and its antagonist on the expression of arginine vasotocin (AVT) and its oxytocic-like receptor VT3 in the shell gland of Japanese quail, Coturnix coturnix japonica. Comp Biochem Physiol A Mol Integr Physiol 151:551–559 [DOI] [PubMed] [Google Scholar]
- 27. Srivastava R , Cornett LE , Chaturvedi CM. 2010. Age-dependent expression of AVT and its oxytocic-like receptor VT3 in the shell gland of Japanese quail, Coturnix coturnix japonica. Gen Comp Endocrinol 165:47–52 [DOI] [PubMed] [Google Scholar]
- 28. Goodson JL , Kabelik D. 2009. Dynamic limbic networks and social diversity in vertebrates: from neural context to neuromodulatory patterning. Front Neuroendocrinol 30:429–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chini B , Mouillac B , Ala Y , Balestre MN , Trumpp-Kallmeyer S , Hoflack J , Elands J , Hibert M , Manning M , Jard S , Barberis C. 1995. Tyr115 is the key residue for determining agonist selectivity in the V1a vasopressin receptor. EMBO J 14:2176–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Voorhuis TA , de Kloet ER , de Wied D. 1988. The distribution and plasticity of [3H]vasopressin-labelled specific binding sites in the canary brain. Brain Res 457:148–153 [DOI] [PubMed] [Google Scholar]
- 31. Voorhuis TA , Elands JP , Kloet ER. 1990. Vasotocin target sites in the capsular region surrounding the nucleus robustus archistriatalis of the canary brain. J Neuroendocrinol 2:653–657 [DOI] [PubMed] [Google Scholar]
- 32. Goodson JL , Evans AK , Wang Y. 2006. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm Behav 50:223–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leung CH , Goode CT , Young LJ , Maney DL. 2009. Distribution of vasotocin-like binding in two species of songbird. J Comp Neurol 513:197–208 [DOI] [PubMed] [Google Scholar]
- 34. Maney DL , Pinaud R. 10 December 2010. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Front Neuroendocrinol 10.1016/j.yfrne.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pinaud R. 2010. Genome of a songbird unveiled. J Biol 9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maney DL. 2008. Endocrine and genomic architecture of life history trade-offs in an avian model of social behavior. Gen Comp Endocrinol 157:275–282 [DOI] [PubMed] [Google Scholar]
- 37. Thomas JW , Cáceres M , Lowman JJ , Morehouse CB , Short ME , Baldwin EL , Maney DL , Martin CL. 2008. The chromosomal polymorphism linked to variation in social behavior in the white-throated sparrow (Zonotrichia albicollis) is a complex rearrangement that suppresses recombination. Genetics 179:1455–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Griffiths R , Double MC , Orr K , Dawson RJ. 1998. A DNA test to sex most birds. Mol Ecol 7:1071–1075 [DOI] [PubMed] [Google Scholar]
- 39. Falls JB , Kopachena JG. 2010. White-throated sparrow (Zonotrichia albicollis) In: , Poole A ed. The birds of North America online. Vol 128 Ithaca, NY: Cornell Lab of Ornithology; 10.2173/bna.128 [DOI] [Google Scholar]
- 40. Larkin MA , Blackshields G , Brown NP , Chenna R , McGettigan PA , McWilliam H , Valentin F , Wallace IM , Wilm A , Lopez R , Thompson JD , Gibson TJ , Higgins DG. 2007. Clustal W and clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 41. Cotte N , Balestre MN , Aumelas A , Mahé E , Phalipou S , Morin D , Hibert M , Manning M , Durroux T , Barberis C , Mouillac B. 2000. Conserved aromatic residues in the transmembrane region VI of the V1a vasopressin receptor differentiate agonist vs. antagonist ligand binding. Eur J Biochem 267:4253–4263 [DOI] [PubMed] [Google Scholar]
- 42. Tahtaoui C , Balestre MN , Klotz P , Rognan D , Barberis C , Mouillac B , Hibert M. , Brownstein MJ , Saito N , MacLeod V , Baeyens DA , Mayeux PR. 2003. Identification of the binding sites of the SR49059 nonapeptide antagonist into the V1a vasopressin receptor using sulfydryl-reactive ligands and cysteine mutants as chemical sensors. J Biol Chem 278:40010–40009 [DOI] [PubMed] [Google Scholar]
- 43. Wiemann JN , Clifton DK , Steiner RA. 1990. Gonadotropin-releasing hormone messenger ribonucleic acid levels are unaltered with changes in the gonadal hormone milieu of the adult male rat. Endocrinology 127:523–532 [DOI] [PubMed] [Google Scholar]
- 44. Burmeister SS , Mangiamele LA , Lebonville CL. 2008. Acoustic modulation of immediate early gene expression in the auditory midbrain of female túngara frogs. Brain Res 1190:105–114 [DOI] [PubMed] [Google Scholar]
- 45. Sambrook J , Russell DW. 2001. Molecular cloning: a laboratory manual Cold Spring Harbor, NY: Cold Spring Harbor Press [Google Scholar]
- 46. Charlier TD , Harada N , Ball GF , Balthazart J. 2006. Targeting steroid receptor coactivator-1 expression with locked nucleic acids antisense reveals different thresholds for the hormonal regulation of male sexual behavior in relation to aromatase activity and protein expression. Behav Brain Res 172:333–343 [DOI] [PubMed] [Google Scholar]
- 47. Kadar A , Wittmann G , Liposits Z , Fekete C. 2009. Improved method for combination of immunocytochemistry and Nissl staining. J Neurosci Meth 184:155–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chini B , Mouillac B , Balestre MN , Trumpp-Kallmeyer S , Hoflack J , Hibert M , Andriolo M , Pupier S , Jard S , Barberis C. 1996. Two aromatic residues regulate the response of the human oxytocin receptor to the partial agonist arginine vasopressin. FEBS Lett 397:201–206 [DOI] [PubMed] [Google Scholar]
- 49. Nei M , Kumar S. 2000. Molecular evolution and phylogenetics New York: Oxford University Press [Google Scholar]
- 50. Tamura K , Dudley J , Nei M , Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 51. Dayhoff MO. 1966. Atlas of protein sequence and structure Silver Springs, MD: Biomedical Research Foundation [Google Scholar]
- 52. Felsenstein J. 1985. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39:783–791 [DOI] [PubMed] [Google Scholar]
- 53. Fabris C , Ballarin C , Massa R , Granato A , Fabiani O , Panzica GC , Cozzi B. 2004. the vasotocinergic system in the hypothalamus and limbic region of the budgerigar (Melopsittacus undulatus). Eur J Histochem 48:367–372 [PubMed] [Google Scholar]
- 54. Panzica GC , Plumari L , García-Ojeda E , Deviche P. 1999. Central vasotocin-immunoreactive system in a male passerine bird (Junco hyemalis). J Comp Neurol 409:105–117 [PubMed] [Google Scholar]
- 55. Aste N , Balthazart J , Absil P , Grossmann R , Mülhbauer E , Viglietti-Panzica C , Panzica GC. 1998. Anatomical and neurochemical definition of the nucleus of the stria terminalis in Japanese quail (Coturnix japonica). J Comp Neurol 396:141–157 [PubMed] [Google Scholar]
- 56. Reiner A , Perkel DJ , Bruce LL , Butler AB , Csillag A , Kuenzel W , Medina L , Paxinos G , Shimizu T , Striedter G , Wild M , Ball GF , Durand S , Güntürkün O , Lee DW , Mello CV , Powers A , White SA , Hough G , Kubikova L , Smulders TV , Wada K , Dugas-Ford J , Husband S , Yamamoto K , Yu J , Siang C , Jarvis ED , Gütürkün O. 2004. Avian brain nomenclature forum. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol 473:377–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reiner A , Karle EJ , Anderson KD , Medina L. 1994. Catecholaminergic perikarya and fibers in the avian nervous system In: , Smeets WFA , Reiner A eds. Phylogeny and development of catecholamine system in the CNS of vertebrates. Cambridge: Cambridge University Press; 135–181 [Google Scholar]
- 58. Stokes TM , Leonard CM , Nottebohm F. 1974. The telencephalon, diencephalons, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J Comp Neurol 156:337–374 [DOI] [PubMed] [Google Scholar]
- 59. Iyengar S , Viswanathan SS , Bottjer SW. 1999. Development of topography within song control circuitry of zebra finches during the sensitive period for song learning. J Neurosci 19:6037–6057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vates GE , Broome BM , Mello CV , Nottebohm F. 1996. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (Taeniopygia guttata). J Comp Neurol 366:613–642 [DOI] [PubMed] [Google Scholar]
- 61. Erichsen JT , Bingman VP , Krebs JR. 1991. The distribution of neuropeptides in the dorsomedial telencephalon of the pigeon (Columbia livia): a basis for regional subdivisions. J Comp Neurol 314:478–492 [DOI] [PubMed] [Google Scholar]
- 62. Nottebohm F , Stokes TM , Leonard CM. 1976. Central control of song in the canary, Serinus canarius. J Comp Neurol 165:457–486 [DOI] [PubMed] [Google Scholar]
- 63. Bottjer SW , Halsema KA , Brown SA , Miesner EA. 1989. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol 279:312–326 [DOI] [PubMed] [Google Scholar]
- 64. Foster EF , Bottjer SW. 1998. Axonal connections of the high vocal center and surrounding cortical regions in juvenile and adult male zebra finches. J Comp Neurol 397:118–138 [PubMed] [Google Scholar]
- 65. Sanford SE , Lange HS , Maney DL. 2010. Topography of estradiol-modulated genomic responses in the songbird auditory forebrain. Dev Neurobiol 70:73–86 [DOI] [PubMed] [Google Scholar]
- 66. Cheng M , Chaiken M , Zuo M , Miller H. 1999. Nucleus taenia of the amygdala of birds: Anatomical and functional studies in ring doves (Streptopelia risoria) and European starlings (Sturnus vulgaris). Brain Behav Evol 53:243–270 [DOI] [PubMed] [Google Scholar]
- 67. Goodson JL , Evans AK , Lindberg L. 2004. Chemoarchitectonic subdivisions of the songbird septum and a comparative overview of septum chemical anatomy in jawed vertebrates. J Comp Neurol 473:293–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nixdorf BE , Bischof HJ. 1982. Afferent connections of the ectostriatum and visual wulst in the zebra finch (Taeniopygia guttata castanotis Gould)—an HRP study. Brain Res 248:9–17 [DOI] [PubMed] [Google Scholar]
- 69. Reiner A , Yamamoto K , Karten HJ. 2005. Organization and evolution of the avian forebrain. Anat Rec A Discov Mol Cell Evol Biol 287:1080–10102 [DOI] [PubMed] [Google Scholar]
- 70. Veenman CL , Medina L , Reiner A. 1997. Avian homologues of mammalian intralaminar, mediodorsal and midline thalamic nuclei: immunohistochemical and hodological evidence. Brain Behav Evol 49:78–98 [DOI] [PubMed] [Google Scholar]
- 71. Foster EF , Mehta RP , Bottjer SW. 1997. Axonal connections of the medial magnocellular nucleus of the anterior neostriatum in zebra finches. J Comp Neurol 382:364–381 [DOI] [PubMed] [Google Scholar]
- 72. Maney DL , Goode CT , Lake JI , Lange HS , O'Brien S. 2007. Rapid neuroendocrine responses to auditory courtship signals. Endocrinology 148:5614–5623 [DOI] [PubMed] [Google Scholar]
- 73. Meddle SL , Follett BK. 1997. Photoperiodically driven changes in Fos expression within the basal tuberal hypothalamus and median eminence of Japanese quail. J Neurosci 17:8909–8918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Huber GC , Crosby EC. 1929. The nuclei and fiber paths of the avian diencephalon, with consideration of telencephalic and certain mesencephalic centers and connections. J Comp Neurol 48:1–225 [Google Scholar]
- 75. Berk ML , Butler AB. 1981. Efferent projections of the medial preoptic nucleus and medial hypothalamus in the pigeon. J Comp Neurol 203:379–399 [DOI] [PubMed] [Google Scholar]
- 76. Goodson JL , Evans AK , Lindberg L , Allen CD. 2005. Neuro-evolutionary patterning of sociality. Proc R Soc Lond B 272:227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kuenzel WJ , van Tienhoven A. 1982. Nomenclature and location of avian hypothalamic nuclei and associated circumventricular organs. J Comp Neurol 206:293–313 [DOI] [PubMed] [Google Scholar]
- 78. Casini G , Bingman VP , Bagnoli P. 1986. Connections of the pigeon dorsomedial forebrain studied with WGA-HRP and 3H-proline. J Comp Neurol 245:454–470 [DOI] [PubMed] [Google Scholar]
- 79. Appeltants D , Ball GF , Balthazart J. 2001. The distribution of tyrosine hydroxylase in the canary brain: demonstration of a specific and sexually dimorphic catecholaminergic innervation of the telencephalic song control nuclei. Cell Tissue Res 304:237–259 [DOI] [PubMed] [Google Scholar]
- 80. Holden AL , Powell TPS. 1972. The functional organization of the isthmo-optic nucleus in the pigeon. J Physiol 223:419–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wild JM , Li D , Eagleton C. 1997. Projections of the dorsomedial nucleus of the intercollicular complex (DM) in relation to respiratory-vocal nuclei in the brainstem of pigeon (Columbia livia) and zebra finch (Taeniopygia guttata). J Comp Neurol 377:392–413 [DOI] [PubMed] [Google Scholar]
- 82. Gamlin PD , Reiner A. 1991. The Edinger-Westphal nucleus: sources of input influencing accommodation, pupilloconstriction, and choroidal blood flow. J Comp Neurol 306:425–438 [DOI] [PubMed] [Google Scholar]
- 83. Karten HJ , Hodos W. 1967. A stereotaxic atlas of the brain of the pigeon (Columba livia) Baltimore: The Johns Hopkins University Press [Google Scholar]
- 84. Wild JM , Farabaugh SM. 1996. Organization of afferent and efferent projections of the nucleus basalis prosencephali in a passerine, Taeniopygia guttata. J Comp Neurol 365:306–328 [DOI] [PubMed] [Google Scholar]
- 85. Wild JM. 1993. Descending projections of the songbird nucleus robustus archistriatalis. J Comp Neurol 338:225–241 [DOI] [PubMed] [Google Scholar]
- 86. Bottjer SW , Arnold AP. 1982. Afferent neurons in the hypoglossal nerve of the zebra finch (Poephila guttata): localization with horseradish peroxidase. J Comp Neurol 210:190–197 [DOI] [PubMed] [Google Scholar]
- 87. DeVoogd TJ , Pyskaty DJ , Nottebohm F. 1991. Lateral asymmetries and testosterone-induced changes in the gross morphology of the hypoglossal nucleus in adult canaries. J Comp Neurol 307:65–76 [DOI] [PubMed] [Google Scholar]
- 88. Robinzon B , Koike TI , Neldon HL , Kinzler SL. 1988. Distribution of immunoreactive mesotocin and vasotocin in the brain and pituitary of chickens. Peptides 9:829–833 [DOI] [PubMed] [Google Scholar]
- 89. Zingg HH , Laporte SA. 2003. The oxytocin receptor. Trends Endocrinol Metab 14:222–227 [DOI] [PubMed] [Google Scholar]
- 90. Goodson JL , Adkins-Regan EA. 1999. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in male zebra finch (Taeniopygia guttata). J Neuroendocrinol 11:19–25 [DOI] [PubMed] [Google Scholar]
- 91. Goodson JL , Lindberg L , Johnson P. 2004. Effects of central vasotocin and mesotocin manipulations on social behavior in male and female zebra finches. Horm Behav 45:136–143 [DOI] [PubMed] [Google Scholar]
- 92. Goodson JL , Schrock SE , Klatt JD , Kabelik D , Kingsbury MA. 2009. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science 325:862–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Voorhuis TA , De Kloet ER , De Wied D. 1991. Effect of a vasotocin analog on singing behavior in the canary. Horm Behav 25:549–559 [DOI] [PubMed] [Google Scholar]
- 94. Maney DL , Goode CT , Wingfield JC. 1997. Intraventricular infusion of arginine vasotocin induces singing in a female songbird. J Neuroendocrinol 9:487–491 [DOI] [PubMed] [Google Scholar]
- 95. Goodson JL. 1998. Territorial aggression and dawn Song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla). Horm Behav 34:67–77 [DOI] [PubMed] [Google Scholar]
- 96. Voorhuis TA , de Kloet ER. 1992. Immunoreactive vasotocin in the zebra finch brain (Taeniopygia guttata). Brain Res Dev Brain Res 69:1–10 [DOI] [PubMed] [Google Scholar]
- 97. Palouzier-Paulignan B , Dubois-Dauphin M , Tribollet E , Dreifuss JJ , Raggenbass M. 1994. Action of vasopressin on hypoglossal motoneurones of the rat: presynaptic and postsynaptic effects. Brain Res 650:117–126 [DOI] [PubMed] [Google Scholar]
- 98. Reymond-Marron I , Raggenbass M , Zaninetti M. 2005. Vasopressin facilitates glycinergic and GABAergic synaptic transmission in developing hypoglossal motoneurons. Eur J Neurosci 21:1601–1609 [DOI] [PubMed] [Google Scholar]
- 99. Wrobel LJ , Reymond-Marron I , Dupré A , Raggenbass M. 2010. Oxytocin and vasopressin enhance synaptic transmission in the hypoglossal motor nucleus of young rats by acting on distinct receptor types. Neuroscience 165:723–735 [DOI] [PubMed] [Google Scholar]
- 100. Kelley DB , Bass AH. 2010. Neurobiology of vocal communication: mechanisms for sensorimotor integration and vocal patterning. Curr Opin Neurobiol 20:748–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Csillag A , Montagnese CM. 2005. Thalamotelencephalic organization in birds. Brain Res Bull 66:303–310 [DOI] [PubMed] [Google Scholar]
- 102. Insel TR , Shapiro LE. 1992. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci USA 89:5981–5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Insel TR , Wang ZX , Ferris CF. 1994. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci 14:5381–5392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ostrowski NL , Lolait SJ , Young WS. 1994. Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology 135:1511–1528 [DOI] [PubMed] [Google Scholar]
- 105. Phelps SM , Young LJ. 2003. Extraordinary diversity in vasopressin (V1a) receptor distributions among wild prairie voles (Microtus ochrogaster): Patterns of variation and covariation. J Comp Neurol 466:564–576 [DOI] [PubMed] [Google Scholar]
- 106. Ophir AG , Zheng DJ , Eans S , Phelps SM. 2009. Social investigation in a memory task relates to natural variation in septal expression of oxytocin receptor and vasopressin receptor 1a in prairie voles (Microtus ochrogaster). Behav Neurosci 123:979–991 [DOI] [PubMed] [Google Scholar]
- 107. Francis DD , Young LJ , Meaney MJ , Insel TR. 2002. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J Neuroendocrinol 14:349–353 [DOI] [PubMed] [Google Scholar]
- 108. Champagne F , Diorio J , Sharma S , Meaney MJ. 2001. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci USA 98:12736–12741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Olazábal DE , Young LJ. 2006. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and lateral septum. Horm Behav 49:681–687 [DOI] [PubMed] [Google Scholar]
- 110. Ophir AG , Wolff JO , Phelps SM. 2008. Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proc Natl Acad Sci USA 105:1249–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Young LJ , Nilsen R , Waymire KG , MacGregor GR , Insel TR. 1999. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature 400:766–768 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.