Abstract
Objectives
This study aimed to extend an epidemiological model (SEIHFR) to analyze epidemic trends, and evaluate intervention efficacy.
Methods
SEIHFR was modified to examine disease transmission dynamics after vaccination for the Ebola outbreak. Using existing data from Liberia, sensitivity analysis of various epidemic scenarios was used to inform the model structure, estimate the basic reproduction number ℜ0 and investigate how the vaccination could effectively change the course of the epidemic.
Results
If a randomized mass vaccination strategy was adopted, vaccines would be administered prophylactically or as early as possible (depending on the availability of vaccines). An effective vaccination rate threshold for Liberia was estimated as 48.74% among susceptible individuals. If a ring vaccination strategy was adopted to control the spread of the Ebola virus, vaccines would be given to reduce the transmission rate improving the tracing rate of the contact persons of an infected individual.
Conclusion
The extended SEIHFR model predicted the total number of infected cases, number of deaths, number of recoveries, and duration of outbreaks among others with different levels of interventions such as vaccination rate. This model may be used to better understand the spread of Ebola and develop strategies that may achieve a disease-free state.
Keywords: basic reproduction number, ebolavirus, Liberia, vaccination, West Africa
Introduction
Ebola virus disease (EVD) is an extremely infectious disease and can be fatal to humans [1,2]. The virus spreads through direct contact with an infected person or their bodily fluids (through broken skin, mucus, fluids, and contaminated objects such as syringes or needles). The Ebola vaccination is expected to be the most promising treatment for preventing an Ebola outbreak.
Ebola vaccine candidates have been developed in the last decade, but none have yet been approved for clinical use. During 2014–2015, trials of candidate vaccines for EVD were fast-tracked in response to the unprecedented EVD epidemic in West Africa. Interim results from the Guinea Ebola ring vaccination trial suggested that the vaccine could have a high level of efficacy [3–5]. Rings, i.e., closest contacts of newly infected cases, defined as the contacts, and contacts of contacts, of those confirmed EVD cases, gave a cluster of contacts. A modeling study [6] suggested that implementation of a ring vaccination strategy could supplement case isolation and contact tracing, to reduce transmission. However, if there were many cases not in the known transmission chains, as in West Africa in early 2014, the results of a stochastic model [7] using individual-level transmission data suggest that ring vaccination might be insufficient to contain the outbreak, and mass vaccination or hybrid strategies involving mass and ring vaccination, might need to be considered. It has been reported that using a spatially explicit agent-based model of EVD transmission (in a region of Sierra Leone), numerical estimates for the effectiveness of ring vaccination by varying the ring definition (contacts, contacts of contacts, and spatial components), as well as the performance of ring vaccination assuming different levels of reproduction number and contact tracing [8]. However, the efficacy of this, and the likely impact on a large, Ebola epidemic such as in West Africa, remains unclear. Simulation and forecasting of the impact of different vaccination strategies may provide important information in developing public health strategies.
In this paper, the SEIHFR epidemic model [9,10] was modified to study the spread of Ebola by intervention (vaccination) and incorporating contact tracing [11,12]. Different from the ring vaccination model [6], the effectiveness of vaccination is modeled to directly reduce the numbers of susceptible people in a population. a thorough mathematical analysis of the SEIHFR model was conducted and the transmission rates due to contact in the community, hospitals, and funerals, as well as the intervention time to best fit the data of the 2014 Ebola outbreak in Liberia were estimated. Based on the fitted model, a sensitive analysis on the scenarios of different levels of vaccination to Ebola in Liberia 2014 was performed while keeping other parameters. The threshold of the percentage of the initial vaccinated population to cease the Ebola outbreak was determined. From numerical simulation, it was calculated that if more than 48.74% of the population in Liberia were immunized, the effective reproduction number would be less than 1 and the outbreak would be under control. Some comments are made to improve the vaccination effectiveness for the randomized mass vaccination strategy and ring vaccination strategy. Although it is well known that the time to intervention is an important parameter, the scenario analysis on intervention time provides some quantified information for controlling the size of an epidemic.
Mathematical Models and Stability Analysis
There are many studies on Ebola outbreaks, with some using mathematical modeling [12–19]. Through mathematical modeling of the epidemiological distribution and transmission pattern of EVD during the African outbreak, many useful insights can be obtained to determine how these intervention measures affect the total number of infected individuals. This would lead to the development of more effective control and prevention measures for future outbreaks. Different mathematical models have been studied to provide valuable information for better understanding of EVD outbreaks, for example the SEIR model (Susceptible, Exposed, Infective, Removed) [20], the SEIHFR model (Susceptible, Exposed, Infective, Hospitalized, Funeral, Removed) [9,10,21] and many others [12,13,17–19,22]. These models use Ebola data to identify the parameters involved in transmission rates, to estimate the average number of secondary infections generated by a typical infected case, in its entire period of infectiousness, in a completely susceptible population, and this quantity is called the basic reproduction number which is denoted ℜ0. These constructed models were well fitted and were demonstrated to be effective for predictions. However, the different transmission patterns of infectious diseases could be due to the unique settings of transmission in the community, in the hospital, during burial ceremonies, and due to the availability of licensed Ebola virus vaccines as well as pre- and post-exposure treatments.
Fisman et al [16] used a simple, 2 parameter mathematical model of epidemic growth and control to estimate ℜ0 which was between 1.6 and 2.0 at the early stages of the 2014 Ebola outbreak in Liberia. Althaus [13] applied a SEIR model with time-dependency of the reproduction number, to capture the effects of control interventions by using the data of each country in West Africa from March 2014 to August 2014. ℜ0 was estimated to be 1.51 for Guinea, 2.53 for Sierra Leone and 1.59 for Liberia. Gomes et al [17] used the Global Epidemic and Mobility Model to evaluate the risk of international spread, and the basic reproduction number ranged from 1.5 to 2.0.
Moreover, Rivers et al [21] used World Health Organization (WHO) data from Liberia and Sierra Leone between March to October 2014, to parameterize a SEIHFR model, following the same model by Legrand et al [10]. They used this model to forecast the progression of the epidemic up to December 2014, as well as forecasting the efficacy of several interventions including increased contact tracing, improved infection control practices, and the use of a hypothetical pharmaceutical intervention to improve survival in hospitalized patients. There have been studies [2,14,23] and a review [24] that have presented epidemiological parameter estimates of the 2014 Ebola outbreak in West Africa.
1. SEIHFR model
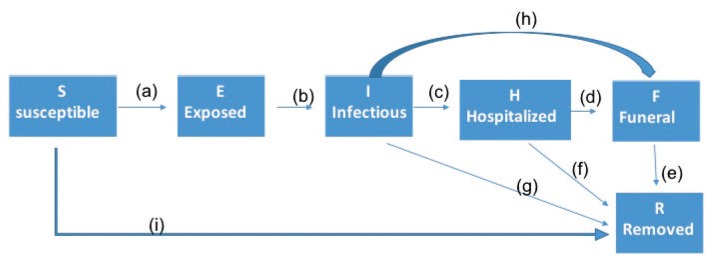
The SEIHFR model has been widely cited [10]. Its underlying assumptions are made regarding the relationship between the overall waiting time in the infectious state, and the waiting times from onset to hospitalization, recovery without hospitalization, and death without hospitalization. These assumptions have been reviewed in detail [9,11] and a simpler model, which is equivalent to the Legrand model is proposed [9]. The SEIHFR model consists of the following compartments of populations in time t (Figure 1).
Figure 1.
Schematic diagram of the model compartments and variables.
S(t): susceptible individuals who can be infected by Ebola virus following contact with infectious cases (Figure 1a), or who can be immunized (Figure 1i).
E(t): exposed individuals who have been infected by Ebola virus but are not yet infectious or symptomatic (during incubation period).
I(t): symptomatic and infectious individuals in the community.
H(t): hospitalized Ebola cases who are infectious.
F(t): dead Ebola cases who may transmit the disease before safe burial.
R(t): individuals removed from the chain of transmission [immunized or isolated without causing a new case (Figure 1i), cured (Figure 1f, 1g) or dead (Figure 1e)].
The “ring vaccination” strategy involves vaccinating anyone who has come into contact with a person infected with Ebola, as well as contacts of theirs. This strategy was used in an experimental Ebola vaccination in Guinea in March 2016 and was found to be highly effective in preventing Ebola infection [7,8,25]. Vaccination was incorporated into the SEIHFR model framework by deriving a link between vaccination and contact tracing. The Contact tracing process for Ebola consisted of 3 basic elements; contact identification, contact listing and contact follow-up [11]. In order to model this process, the following assumptions were made in addition to the base SEIHFR model. Reported cases from infectious, hospitalized or contaminated deceased cases, triggered contact tracing and ring vaccination. In addition, the reported cases that triggered ring vaccination were not removed from their corresponding cases, but ring vaccination was applied to contacts and contacts of contacts. The probability ζ accounted for unreported cases and reported cases that did not trigger contact tracing and ring vaccination. The probability (1− ζ) accounted for the reported cases that triggered ring vaccinations and no secondary cases that were caused by them.
The number of susceptible individuals removed due to ring vaccination at time t has the following formula:
φ1 is the average number of contacts traced per reported case that triggered contact tracing.
φ2is the rate of vaccination and/or isolation of the traced contacts.
ν = φ1 × φ2 ×(1− ζ) is the average number of removal susceptible individuals who were successfully traced/vaccinated/isolated per reported case. The total removal number is in the mass-action form to avoid negative compartments.
In this context, a paradigm for the process of ring vaccination was considered: 1) identification of contacts and contacts of contacts of reported cases that triggered a ring vaccination; 2) vaccination of contacts and contacts of contacts where no secondary cases were caused by these contacts. This requires a high standard in public health work for contact tracing, isolation, community engagement and availability of effective vaccines [7]. Although ν and ζ are not independent of each other, ν can have different values for same ζ due to the variables φ1 and φ2; scenarios analysis can be conducted based on the values ν and ζ to find out the impact of vaccination.
The only way a person can leave the susceptible group is to become infected or immunized. The only way a person can leave the infected group is to be hospitalized/recovered/removed from the disease. Once a person has recovered, the person has immunity. The SEIHFR model is as follows:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
N is the size of the population. βI is the transmission rate in the community. βH is the transmission rate after hospitalization. βF is the transmission rate during traditional burial. The 1/α is the mean duration of incubation period. The 1/γ is the average overall time in the I compartment, and 1/ω is the average overall time in the H compartment, which can be computed as follows:
The 1/γh is the mean duration between onset of symptoms and hospitalization. The 1/1/γi is the mean duration of the infectious period for patients who survived their illness. The 1/1/γd is the mean duration of the infectious period for patients who died. The 1/1/γf is the mean duration of the infectious period between death and burial. θ1 is computed in order that θ% of infectious cases are hospitalized. δ1 and δ2 are computed to obtain a case fatality ratio at δ. and . Table 1 shows more information about the parameters and more details of derivation of these differential equations have been previously described explaining distribution of people across different paths [9,21].
Table 1.
Epidemiological parameters for Ebola virus disease used in this study.
| Parameter | Description | All countries | Guinea | Liberia | Sierra Leone | Ref. |
|---|---|---|---|---|---|---|
| 1/α days | incubation period | 11.4 | 10.9 | 11.7 | 10.8 | [2] |
| 1/γh days | symptom onset to hospitalization | 5 | 5.3 | 4.9 | 4.6 | [2] |
| 1/γi days | symptom onset to hospital discharge | 16.4 | 16.3 | 15.4 | 17.2 | [2] |
| 1/γd days | symptom onset to death | 7.5 | 6.4 | 7.9 | 8.6 | [2] |
| 1/γf days | infectious period between death and burial | 2 | 2 | 2 | 2 | [10] |
| θ % | Proportion of infectious cases hospitalized | 54 | 52 | 51 | 58 | [21, 23] |
| δ % | Ratio of case-fatality | 70:8 | 70.7 | 72.3 | 69.0 | [2, 17] |
| 1/γih days | Time from hospitalization to recovery | 11.4 | 11 | 10.5 | 12.6 | Calculation |
| 1/γdh days | Time from hospitalization to death | 2.5 | 1.1 | 3 | 4 | Calculation |
| θ1 % | Fraction of infected hospitalization | 36.8 | 38.2 | 33.9 | 36.1 | Calculation |
| δ1 % | Case fatality rate, unhospitalized | 52.6 | 48.7 | 57.3 | 52.7 | Calculation |
| δ2 % | Case fatality rate, hospitalized | 34.7 | 19.4 | 42.7 | 41.4 | Calculation |
The “randomized mass vaccination” strategy can be used before an Ebola outbreak. By vaccinating a percentage of whole population randomly (have the same interaction with the vaccinated and non-vaccinated population), the initial susceptible population decreases and it reduces the force of infection (rate at which a susceptible person becomes infected). Let (1−μ) be the percentage of whole population that was initially vaccinated randomly before an Ebola outbreak. Scenarios analysis was conducted based on the percentages of the initial vaccinated population. This parameter does not occur in the differential equations but does in the initial values. A threshold for the parameter was deduced to make the basic reproduction number less than 1.
Here 2 recording variables were introduced Ca (t) and Cd (t) which are not epidemiological states, but they keep track of the cumulative number of all Ebola cases, and the cumulative number of deaths of Ebola cases respectively from the time of onset of symptoms. These 2 cumulative data were reported by the WHO. The differential equations are:
| (7) |
| (8) |
To measure the number of vaccines needed in the ring vaccination, another recording variable Cv (t) was used to keep track of the cumulative number of vaccinations.
| (9) |
Note that ring vaccination is a spatial phenomenon. A spatially explicit agent-based model [8] is calibrated to reproduce the most important features of the EVD outbreak in Pujehun district, Sierra Leone. The process of ring vaccination requires the identification of contacts and contacts of contacts of a reported case. The modified SEIHFR model cannot possibly be faithful to the process as understood by field epidemiologists. However, it can provide a numerical estimate of the effectiveness of vaccination strategies for the EVD outbreak in Liberia or other countries.
2. Mathematical analysis of the SEIHFR model
Prior to the use of the SEIHFR model to study the 2014 Ebola outbreak in West Africa, a mathematical analysis was conducted on the model. It proved that there always exists a non-negative bounded solution of the differential equation model for any non-negative initial condition. The mathematical formula for basic reproduction number ℜ0 was dependent on the effectiveness of vaccination ζ and μ, and it can be decomposed into 3 parts due to transmission rate in the community, after hospitalization, and during traditional burial.
| (10) |
By Routh-Hurwitz Criteria, a disease-free equilibrium is linearly stable when the basic reproduction number ℜ0 is less than 1 Appendix I).
Results on 2014 Ebola outbreak in Liberia
Ebola was first detected in 1976 when there were 2 simultaneous outbreaks in Nzara, Sudan and Yambuku, Democratic Republic of the Congo. It later occurred in a village near the Ebola River which gave its name to Ebola. Ebola has caused at least 21 confirmed outbreaks between 1976 and 2016 [1]. The 2014 Ebola epidemic in West Africa was the largest ever recorded. Ten countries in 3 continents (Africa, Europe and North America) had confirmed cases. According to the WHO situation report (Data up to March 27, 2016), 28,646 confirmed, probable, and suspected cases were reported, primarily in Guinea, Liberia, and Sierra Leone, with 11,323 deaths since the onset of the Ebola outbreak [26]. The majority of these cases and deaths were reported between August and December 2014, after which case incidence began to decline as a result of the rapid scale-up of treatment, isolation, and safe burial capacity in Guinea, Liberia, and Sierra Leone. While previous outbreaks in small villages have come to an end due to local depletion of susceptible individuals, this epidemic spread across entire countries, and thus could only be curtailed by interventions aimed at reducing new infections across all locations [14].
Organizations such as the World Health Organization (WHO) and local governments implemented intervention measures to try to control the outbreak and bring it to an end as soon as possible. While some of these interventions were simple, such as the deployment of health care workers and personal protective equipment, other interventions required much more coordination and strategy, such as establishing cross-border coalitions for the dissemination of intelligence regarding the outbreak. Among these intervention measures, the “70-70-60” plan [27,28] which aimed to isolate and treat 70% of infected individuals, and safely bury 70% of deceased individuals, within 60 days, affected a major reduction in the spread of the EVD epidemic.
In this study the SEIHFR model was used to analyze the 2014 Ebola outbreak in Liberia. Although immunization (in the context of an outbreak or epidemic) has the attractive property of reducing the force of infection, and several candidate Ebola vaccines had been produced, no vaccines had been approved for clinical use during the 2014 outbreak. However, the potential impact of a vaccination for Ebola needs to be studied to provide insights for future planning [4–8]. Fisman et al [15] studied the projected impact of vaccination timing and dose availability, and they predicted that an effective vaccination rate could be projected to prevent tens of thousands of deaths, if vaccination could be used before the epidemic peaked. This estimation of the effective vaccination rate was also discussed in another study [29]. It is reasonable to assume ν = 0, ζ = 1 and μ = 1 to use the SEIHFR model to study the 2014 Ebola outbreak in West Africa. The least-squares can be used to fit the data from the WHO to estimate the epidemic parameters. The fitted model can then be used to estimate the basic reproductive number ℜ0 and to evaluate the impact of intervention measures on the transmission rate of the disease. In this study the fitted model was used to simulate the impact of vaccination.
1. The transmission rate and the impact of interventions
Various intervention strategies [27,28,30] including surveillance, quarantine, education and rapid burial have been employed to control an outbreak and bring it to an end as soon as possible. The impact of the intervention plan such as “70-70-60” plan is not instantaneous [20]. The net effect of intervention strategies, is to reduce the transmission rate β (including βI, βH, βF, in our model) from β0, to β1< β0. Between the time of onset of the intervention, to the time of full compliance, the transmission rate is assumed to decrease gradually from β0 to β1 according to:
where T0 is the time at which interventions start, and q controls the rate of transition from β0 to β1. The larger q, the faster β0 decays to β1.
2. Model parameters
The parameters in the model were put into 2 categories, epidemiological and model. Epidemiological parameters are those which characterize the Ebola virus, such as incubation period and case-fatality ratio. They are consistent across the modeling literature for different outbreaks [10,17] and are listed in Table 1. These epidemiological parameters followed the WHO Ebola Response Team study [2,23]. The case-fatality ratio in Liberia was 0.7234 before intervention and 0.3844 after intervention.
The model parameters shown in Table 2 were fitted to the 2014 Ebola outbreak in Liberia by least-squares fit to the cumulative number of cases Ca (t, Ψ) in equation (7). The nonlinear least-squares solver “lsqnonlin” in Matlab 2016b was used and appropriate initial conditions for the differential equations were chosen to feed the optimization process so that the “best fit” can be determined. The optimization process was repeated by feeding different initial conditions before the “best fit” was chosen. The confidence interval was computed following the method described by Chowella et al [20].
Table 2.
Ebola epidemic parameters and our fitted values.
| Parameter | Description | Fitted value Liberia |
|---|---|---|
| βI0 | Transmission rate in the community before intervention | 1.2596 |
| βI1 | Transmission rate in the community after intervention | 0.3988 |
| βH0 | Transmission rate after hospitalization before intervention | 1.1338 |
| βH1 | Transmission rate after hospitalization after intervention | 0.4418 |
| βF0 | Transmission rate during traditional burial before intervention | 0.9794 |
| βF1 | Transmission rate during traditional burial after intervention | 0.4566 |
| T0 | Duration from beginning of epidemic to invention taken place | 14.8057 |
| q | Measure the speed of transition from β0 to β1 | 36.8342 |
| ℜ0 | Basic reproduction number | 1.9508 |
The asymptotic variance-covariance AV(Ψ) of the least-squares estimate was
which was estimated by
where n is the total number of observations, and ∇̂C are numerical derivatives of C. To compute the confidence intervals, F-distribution for small samples was used. Formally, the confidence intervals were of the form:
where yai is the observed cumulative number of cases, and Aα is the 1−α quantile of an F-distribution with appropriate degrees of freedom. To have a better approximation to both the cumulative number of cases and the cumulative number of deaths, least-squares could be applied to the 2 cumulative numbers, that is, where ydi was the observed cumulative number of deaths.
Confidence intervals were computed by fixing all other parameters and parameters estimates are given in Table 2. There were many different parameter combinations which fitted; thus, confidence intervals may not be relevant for these purposes. For example, the 95% confidence interval (6.1513,∞) for q was not “sharp” because it covered an infinity range. This was due to the exponential decay (e−q(t−T0) for t>T0) of time and its value would not change much when q is large.
3. Ebola outbreak in Liberia
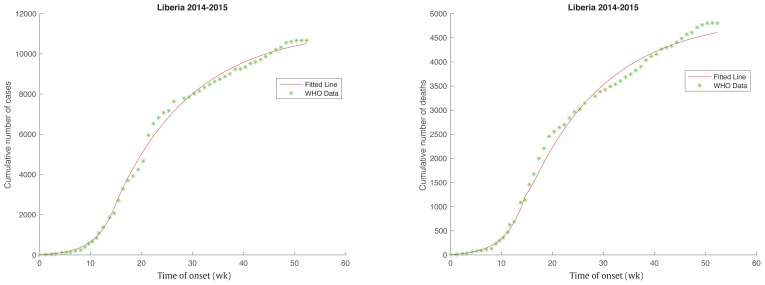
Data were retrieved from the WHO website, and was based on the cumulative total numbers of total cases and total deaths. The data were recorded by days and used to fit the model chosen in an interval around a whole week. The total population size N = S + E + I + H + F + R was assumed to be 4,345,000 in Liberia in 2014. Note that the exact population size does not need to be known to estimate the model parameters as long as the number of total cases was relatively small. In fact, the true mass-action assumption was used which made the model parameters independent of N. In Liberia, the cases that were reported on June 2, 2014 were the first new cases since April 7, 2014. To fit the model for Liberia, the starting date was June 2, 2014 with 19 confirmed total cases and 9 confirmed total deaths, which were used as the initial values Ca (0) and Cd (0). By using the optimization tool “lsqnonlin” in Matlab R2016b, parameters were estimated (Table 2). The deterministic model fitted well for Liberia (Figure 2).
Figure 2.
Fitted line with World Health Organization data for Ebola Epidemic in Liberia. The solid red line represents the deterministic model fit of the epidemic in Liberia. The green star represents the actual data from World Health Organization. The start date was June 2, 2014.
4. A scenario analysis on the vaccination
In the fitted base model in this study, ν (the average number of removals [susceptible individuals away due to vaccination] per infected case) was assumed to be 0, (1−ζ) (the reducing rate of infected cases due to vaccination) was assumed to be 0 (ζ =1), and μ was assumed to be 1 (no randomized vaccination before Ebola outbreak). The potential impact of vaccination against Ebola was evaluated under different assumptions around the values ν, ζ and initial percentage (1−μ) of the vaccinated population, while keeping the other estimated parameters the same.
5. Randomized mass vaccination before Ebola outbreak
Formula 17 (Appendix I) for the basic reproduction number ℜ0:
which indicates that if sufficient fractions of population are immunized, the Ebola epidemic should not happen. In the appendix, it was shown that the disease epidemic was predicted to decline when ℜ0 <1, and the disease spread would increase when ℜ0 >1. By corollary 5.4, when random and continuous vaccination strategies for susceptible population were undertaken, the minimum effective vaccination rate and the Ebola epidemic, would be under control if the initial vaccinated rate of the total population was greater than rν.
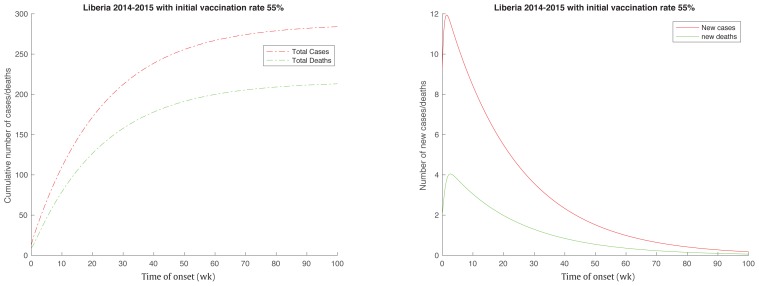
According the estimated value in this study (Table 2), if 1−μ > 48.74% or μ < 51.25%, the number of new cases would decline from the very beginning (Figure 3 right) and the Ebola outbreak in Liberia would be under control (Figure 3 left). Based on the estimates of the basic reproduction number in [2], it was estimated that the minimum effective vaccination rates and the Ebola outbreak would be under control if > 41.52% and > 50.50% of the total population were vaccinated in Guinea and Sierra Leone, respectively.
Figure 3.
Ebola outbreak in Liberia would be under control, and would stop the spread more quickly if more than 48.74% of the population were vaccinated even if there were no other interventions (Intervention time T0 is set to be infinity).
These predictions provide scientific evidence of the need for vaccine production and application. For purposes of simplicity, the assumption was made that the vaccine was 100% efficacious with a single dose required for immunity. Roughly speaking, nearly 2.1 million highly effective vaccines are needed to prevent an Ebola outbreak. Real-world vaccination would require more doses of vaccines if the efficacy of the vaccine was less than 100%. Mass vaccination may impose lots of challenges to the countries, cities and families affected. Financial challenges and ethical challenges would be associated with the implementation of wide-scale vaccination with a new Ebola vaccine that had been fast tracked with limited evaluations for safety and efficacy, however a benefit risk assessment would be made.
The impact of a small percentage of the population being vaccinated initially was explored. It was observed that this can also substantially reduce the final size of the Ebola epidemic if current interventions were maintained (Figure 4). The initial vaccinated population that reduced the initial susceptible population by 1%, 5% or 10% resulted in a corresponding reduction of the final total case by 8.8%, 36.5%, 59.1%, and a reduction of the final total deaths by 8.2%, 34.2%, and 56.2% respectively. The epidemic would also end sooner with larger fractions of the population immunized. The significance of a reduction of the final epidemic size would make vaccination a highly attractive intervention to help control this epidemic.
Figure 4.
Ebola outbreak in Liberia with different level of initial vaccinated population (randomized vaccination strategy). 1%, 5% or 10% of initial vaccinated population result in a corresponding reduction of the final total case by 8.8%, 36.5%, 59.1%, and a reduction of the final total deaths by 8.2%, 34.2%, 56.2% respectively.
6. Continuous Ring Vaccination
The ring vaccination strategy (that involves vaccinating anyone who has come into contact with a person infected with Ebola, as well as contacts of theirs) was modeled. This strategy was used in an experimental Ebola vaccine in Guinea in March 2016 and was found to be highly effective in preventing Ebola infection. Some evaluations and analysis on the efficacy of the vaccination have been studied [4–8,25]. The parameter ν models the average required number for vaccinating contact persons per infected person, and the parameter ζ models the direct impact of ongoing vaccination/isolating on the transmission rate. In this study the impact on the epidemic due to these 2 parameters while keeping all other parameters/interventions the same was investigated.
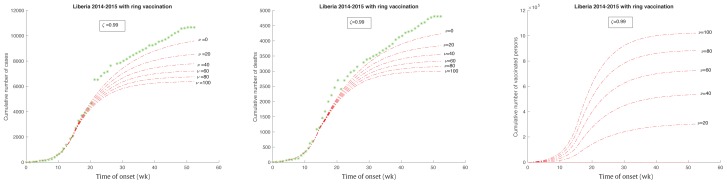
In this study it was assumed that there was a small direct reduction on the transmission rate (ζ = 0.99). The cumulative number of infected cases was reduced as the parameter ν increased from ν = 0,20,40,60,80 to 100 (Figure 5). Although the SEIHFR model did not randomize mass vaccination during the course of the Ebola outbreak, the vaccination strategy in this case could be treated as a randomized mass vaccination strategy rather than a ring vaccination strategy. However, the impact on the reduction of the total number of cases was not significant, compared with the total number of vaccinations needed in the case of a randomized mass vaccination that was used before the Ebola outbreak. In this case, about 200 vaccines would be needed to decrease 1 infected case. When ν = 100, about 1.02 million (23% of total population) vaccines would be used, but this could only reduce the number of infected cases by approximately 3,219 (34% of the total infected cases). This is far below the ratio where 10% of the initially vaccinated population would reduce 59.1% of potentially infected cases. This implies that vaccination should be given as soon as possible if a randomized strategy is used.
Figure 5.
Ebola outbreak in Liberia with different level of vaccinated population with a reducing rate of 1% (ζ= 0.99).
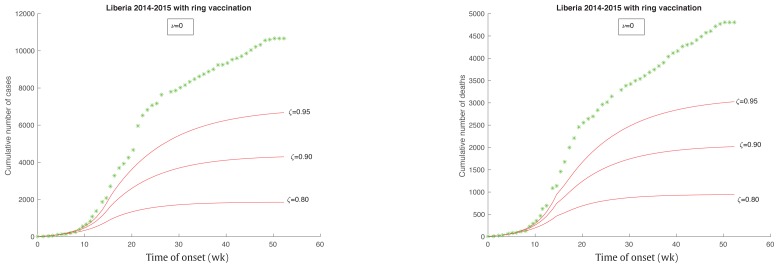
As shown in an experimental Ebola vaccination in Guinea in March 2016, the ring vaccination strategy would be effective at preventing Ebola infection. To see how the reduction rate changes the epidemic case, ν was assumed to be zero in this numerical simulation. Figure 6 shows that the reduction rate that decreased by 5% (ζ = 0.95), 10% (ζ = 0.90), 20% (ζ = 0.80) would result in a corresponding reduction of infected cases by 36.5%, 59.1%, and 82.4%, and a reduction of total deaths by 33.1%, 56.2%, and 80.0%, respectively. The higher the reduction rate, the smaller the cumulative number of infected cases was, and the quicker the epidemic would end.
Figure 6.
Ebola outbreak in Liberia with a different level reducing rate of 5% (ζ= 0.95), 10% (ζ= 0.90), 20% (ζ= 0.80).
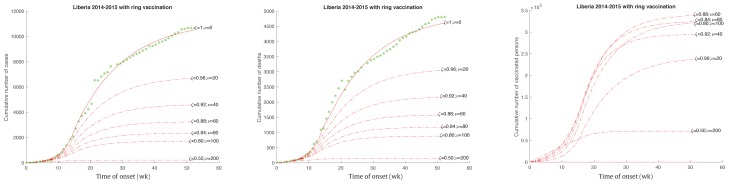
By carefully tracing the contact persons of an infected person the average number of vaccinations given per cluster of infected persons would increase and it is assumed that the infected rate would be reduced. However, this data was not available to fit the model in this study to determine how to achieve a reduced infected rate. In this study, combination effects (Figure 7) of (ζ,ν) at the different levels (1,0), (0.96,20), (0.92, 40), (0.88, 60), (0.84, 80), (0.80, 100), (0.50, 200) were proposed. In general, the total number of vaccines would increase while ν increased. However, when the ring vaccination strategy becomes more effective (small ζ), the total number of vaccines could decrease while the average number of vaccines per infected person ν increases (see right in Figure 7). With an average of 200 vaccines per infected person and a reduced rate of infection at 50%, only about 71,000 vaccines (about 1.6% of the total population) would be used, and there would be about 214 total infected cases and 145 total deaths. The outbreak would also end within 20 weeks (about October 14, 2014). This observation indicated that reducing the infection rate could be more effective in mitigating an epidemic. Therefore, the ring vaccination strategy could be a good choice if the infection rate could be reduced significantly. It depends on the level of effectiveness of contact tracing which is highly dependent on public health resources [11,12].
Figure 7.
Ebola outbreak in Liberia with different efficiency level of ring vaccinated population, where (ζ,ν) = (1,0), (0.96,20), (0.92, 40), (0.88, 60), (0.84, 80), (0.80, 100), (0.50, 200).
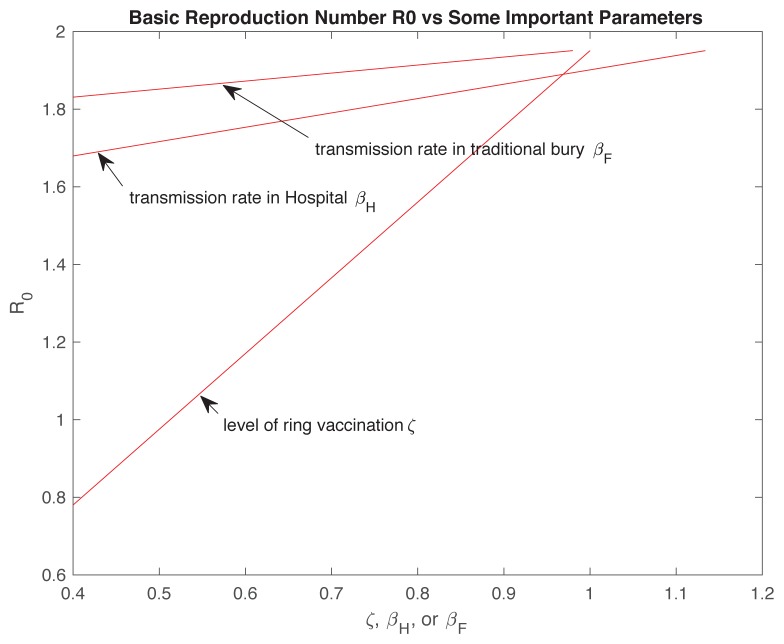
By the formula of the basic reproduction number ℜ0 given in equation 17 (Appendix), it was obvious that ℜ0 linearly dependeds on the level of ring vaccination (ζ), the transmission rate after hospitalization (βH) and the transmission rate during traditional burial (βF). In Figure 8, it shows that the basic reproduction number ℜ0 was decreasing when the corresponding parameter decreased and all other parameters were fixed. For example, the estimated basic reproduction number for the 2014 Liberia Ebola outbreak decreased from 1.9508 to 1.6795, if the transmission rate after hospitalization decreased from 1.1338 to 0.4, ℜ0 decreased from 1.9508 to 1.8310, transmission rate during traditional burial decreased from 0.9794 to 0.4 and ℜ0 decreased from 1.9508 to 0.7803, the level of not triggering a ring vaccination would decrease from 1 to 0.4. Although decreasing each parameter can reduce the basic reproduction number, only the level of ring vaccination will affect the basic reproduction number in all aspects and it can reduce the basic reproduction number to < 1, which will terminate the epidemic of Ebola. On the other hand, only focusing on decreasing the transmission rate after hospitalization or during traditional burial, cannot reduce the basic reproduction number to < 1, which means the epidemic can continue. To effectively slow/stop the spread of Ebola, combined actions have been taken to reduce the transmission rate in the community, after hospitalization and during traditional burial (prohibited during the 2014 outbreak of Ebola in Liberia).
Figure 8.
Dependence of the basic reproduction number ℜ0 and some important parameters: ζ, βH, βF.
7. A scenario analysis on the time to intervention T0
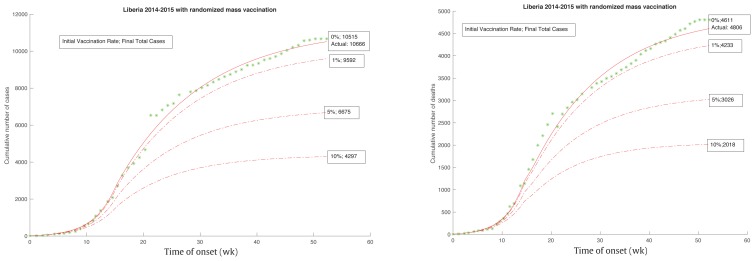
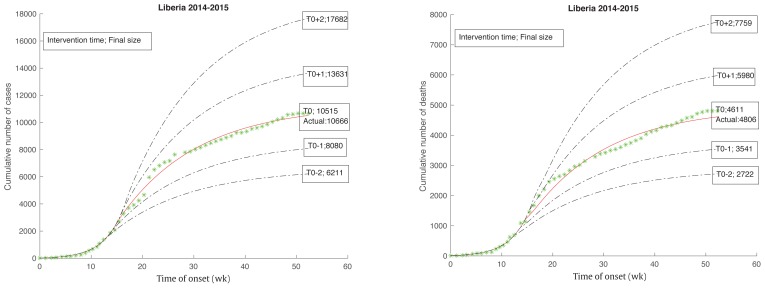
From the start of the epidemic on June 2, 2014, the intervention measures would take place for 14.8 weeks, until September 12, 2014. By that time the cumulative number of cases would have already reached 2,081 total deaths, with 1,137 cases showing no signs of slowing down. After the start of the intervention, the transmission rates would start to decline and the epidemic would eventually end. This intervention time was consistent with an intervention measure implemented for Liberia by a joint declaration of governments, and it was also consistent with the “70-70-60” plan which was implemented on October 1, 2014. Figure 9 shows how a change in the intervention time affected the final size of the epidemic.
Figure 9.
Epidemic size changes as intervention time changes.
The earlier intervention, with the smaller final size (Figure 9), resulted in the intervention time starting 1–2 weeks earlier and resulted in a corresponding reduction of final total cases from 10,515 to 8,080, and 6,211, and final total deaths from 4,611 to 3,541, and 2,722 respectively. The intervention time that began 1–2 weeks later resulted in a corresponding increase in total cases from 10,515 to 13,631, 17,682, and final total deaths from 4,611 to 5,980, 7,759 respectively. Roughly speaking, 1 week change in intervention time resulted in about 23% to 30% change in its final size.
Conclusion
Many mathematical models such as the SEIR model and its variants, the stochastic model, and the spatially explicit agent-based model have been developed to study the EVD transmission under different interventions. Of the interventions modeled, the most frequently suggested intervention was a combined strategy of isolating infected individuals from the general population, and providing sufficient supplies to treat them. In this study the SEIHFR model of Ebola epidemics was extended to evaluate the potential impact of Ebola vaccination.
The data was fitted to the model in this study without vaccination (μ = 1, ζ = 1, ν = 0). According to the simulation study it fitted very well in Figure 2. The basic reproduction number decreased from 1.9058 before intervention, to 0.8456 after intervention.
A detailed analysis was carried to understand the mathematical dynamics of the disease. The system had a disease-free equilibrium (S, R, E, I, H, F) = (μ N,(1−μ)N, 0, 0, 0, 0) and it was stable in its infection subsystem if the basic reproduction number ℜ0 was < 1. In turn, this generated a threshold for the effective vaccination rate to arrest or mitigate an epidemic.
The simulation study in Figure 3 showed that if the initial vaccination rate was larger than the threshold, then the spread of the epidemic was under control and would gradually stop. If a 100% single dose efficacious vaccine was available for immunity, nearly 2.1 million highly effective vaccines would be needed to substantially prevent an Ebola outbreak in Liberia, and nearly 49% of the total population in Liberia would need to be vaccinated before or at the beginning of the outbreak.
Simulation studies in Figures 4 and 5 showed that vaccination should be implemented as soon as possible if a randomized mass vaccination strategy was used, which is consistent with observations reported by Fisman et al [15]. The simulation study in Figure 9 showed that a delay in intervention by 2 weeks would result in a total case increase of about 68%. Early intervention and early vaccination should be implemented side-by-side to achieve the greatest effectiveness.
Reducing the infection rate was the key impact factor on vaccine administration. The model allowed quantification of the parameters corresponding to the number of vaccines (ν) and a reduced infection rate (1−ζ) for evaluating the impact of the vaccine policy. Simulation studies in Figures 6 and 7 showed that the ring vaccination strategy could be a good choice if the infection rate could be reduced significantly. Potentially, the most effective way may be a combined strategy of intensifying contact tracing to remove infected individuals from general populations, and vaccinating contact persons of an infected individual as well as their contacts. Even if the ring vaccination with 200 vaccines per infected person could reduce the infection rate by 50% in Liberia, the simulation study in Figure 7 showed that the outbreak would end within 20 weeks, and there would be about 214 total infected cases and 145 total deaths, which was equivalent to 2% and 3% of the current total infected cases and deaths, respectively.
Mathematical modeling is a valuable tool to understand the dynamics of infectious diseases. However, there are also limitations in applying mathematical models for Ebola outbreaks due to the complexity of real world events. It cannot be implied that a vaccine with 100% effectiveness against the Ebola virus can automatically stop an Ebola outbreak. Other intervention strategies (including accurate and timely identification and quarantine, sufficient medical care, safe burials, tracing the contact persons of an infected individual as well as their contacts), need to be implemented collaboratively.
Acknowledgments
This research was partially supported by W. Wright and the Annie Rea Cross Endowment at the University of Southern Mississippi. The author would also like to thank the anonymous referees and English editors for their valuable suggestion and comments to improve the appearance and quality of the paper.
Appendix I.
1. Mathematical analysis of the SEIHFR Model
A mathematical analysis on the model was conducted. It was shown that a non-negative bounded solution of the differential equation model for any non-negative initial condition always exists. The mathematical formula for basic reproduction number ℜ0 is generated and it can be decomposed into 3 parts due to transmission rate in the community, after hospitalization, and during traditional burial. By Routh-Hurwitz Criteria, a disease-free equilibrium is locally asymptotically stable in the infection subsystem when the basic reproduction number ℜ0 is less than 1.
1.1. Nonnegativity and boundedness
It is crucial to show that the solutions to the initial-value problem are non-negative and bounded and they retain the biological validity for all values of time.
Lemma 5.1. [Nonnegativity]
Let t0>0. If the initial conditions satisfy S(0)≥0, E(0)≥0, I(0)≥0, H(0)≥0, F(0)≥0, R(0)≥0, then S(t)≥0, E(t)≥0, I(t)≥0, H(t)≥0, F(t)≥0, R(t)≥0 for all t∈[0, t0].
Proof
Note that which implies that S+E+I+H+F+R=N, a constant size of population. It is easy to get the following lower bounds for any t∈[0, t0].
Then
Lemma 5.2. [Boundedness]
For any t∈ [0, t0], S(t), E(t), I, H(t), F(t), R(t) are less than N.
Proof
This is a direct consequence of that S+E+I+H+F+R=N and all S, E, I, H, F, R are nonnegative.
By the Fundamental Theorem of Existence and Uniqueness for differential equations, there exists a unique, positive, and bounded solution to the ordinary differential equations given in (1) to (6).
2. SEIHFR Model in matrix form
To better understand the transmission of an infectious disease, it is important to distinguish new infections from all other changes in population. It can be shown that SEIHFR model can be written as the system below and is defined on a forward invariant compact subset Ω of by Lemma 5.1 and 5.2.
where represents the states of different compartments of non-transmitting individuals (susceptible, recovered or immune, ……), represents the states of compartments of different transmitting individuals (infected, infectious, ……), and .
| (12) |
| (13) |
| (14) |
where
3. The basic reproduction number ℜ0
The basic reproduction number, ℜ0, is generally interpreted as the average number of secondary cases caused by a typical infected individual throughout its entire course of infection in a completely susceptible population and in the absence of control interventions [31–34]. It quantifies the potential growth for infectious disease transmission.
Note that for any μ∈[0,1], will be a disease free equilibrium for the SEIHFR model (11). (1−μ) can be regarded as the initial removal rate of the total population due to immunization/vaccination. Legrand et al [10] determined the formula for ℜ0 without considering the effect of control interventions such as antivirals and vaccines, where they assumed μ=1. Indeed, at the beginning of the 2014 Ebola outbreak in West Africa, susceptible individuals can be assumed to be all the population which means μ=1. Following the previously described methods [31–34], we determined the formula for ℜ0 here. The next generation matrix of the SEIHFR model (11) for (μN,(1−μ)N, 0, 0, 0, 0) is
| (15) |
The formula for ℜ0 is the spectral radius of the next generation matrix (the maximum value of its eigenvalues) and
| (16) |
Note, from the definition in Table 1, , one can obtain
Because in Table 1, we have which , is equivalent to:
By direct computation, we have:
Therefore, ℜ0 can be decomposed into 3 parts due to the transmission rate in the community, after hospitalization, and during traditional burial.
| (17) |
4. Equilibrium, stability and immunity
The SEIHFR model has equilibrium points which belong to the line segment
| (18) |
Note that the system only has disease free equilibria and it has no coexistence equilibrium.
Theorem 5.3
Let be a disease-free equilibrium, where μ∈[0,1]. Then P0∈L is a locally asymptotically stable disease-free equilibrium in the infection subsystem if the basic reproduction number ℜ0 for P0 is less than 1. Conversely, P0∈L is an unstable disease-free equilibrium if the basic reproduction number ℜ0 for P0 is greater than 1.
Proof
The Jacobian for the linearized system of the SEIHFR model along the equilibrium P0 is
| (19) |
and its characteristic polynomial is
where
The characteristic polynomial has eigenvalue zero and the equilibrium is not linearly stable for the whole system. But the characteristic polynomial of its infection subsystem is:
| (20) |
If ℜ0 >1, B0<0. By Descartes’ Rule of Signs, the characteristic polynomial has at least one positive root. So, the equilibrium P0 is linearly unstable. We will use the Routh-Hurwitz Criteria and the values of B0, B1, B2 and B3 to derive the stability of P0. We know that all of the parameters are positive. It is clear that B0>0, B1>0, B2>0, B3>0 if ℜ0<1. We use Maple 2016 to carry out the following calculations:
By Routh-Hurwitz Criteria, P0∈L is a locally asymptotically stable disease-free equilibrium in the infection subsystem when the basic reproduction number ℜ0 is less than 1. This ends the proof.
Therefore, if ℜ0 <1 then on average an infected individual produces less than one new infected individual over the course of its infectious period, and the infection can not generate a major epidemic. By contrast, if ℜ0 >1 then on average each infected individual produces more than 1 new infection, and an epidemic is likely to occur.
Corollary 5.4
Assume that an infectious disease has the basic reproduction number ℜ0 >1 without vaccination/immunization (μ =1 in Theorem 5.3). Let rν be the minimum effective vaccination rate which is given by:
| (21) |
Then the epidemic would be under control if the initial immunized/vaccinated rate (1−μ) of the total population is bigger than the minimum effective vaccination rate, that is, (1−μ)>rν.
Footnotes
Conflicts of Interest
No potential conflicts of interest to report that were relevant to this article.
References
- 1.CDC [Internet] Outbreaks Chronology: Ebola Virus Disease. [cited 2017 Jan 15]. Available from: https://www.cdc.gov/vhf/ebola/history/chronology.html#thirtyone.
- 2.WHO Ebola Response Team. Ebola virus disease in West Africa- the first 9 months of epidemic and forward projections. N Engl J Med. 2014;371(16):1481–95. doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joshua B. Ebola vaccine gives 100% protection, study finds. CNN; 2016. Dec 22, [Internet] [cited 2016 Dec 27]. Available from: https://www.fiercepharma.com/vaccines/merck-to-miss-2017-filing-target-for-ebola-vaccine-spokesperson. [Google Scholar]
- 4.Ebola ça suffit ring vaccination trial consortium. The ring vaccination trial: A novel cluster randomised controlled trial design to evaluate vaccine efficacy and effectiveness during outbreaks, with special reference to Ebola. BMJ. 2015;351:h3740. doi: 10.1136/bmj.h3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henao-Restrepo A, Camacho A, Longini I, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial. Lancet. 2017;389(10068):505–18. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells C, Yamin D, Ndeffo-Mbah M, et al. Harnessing Case Isolation and Ring Vaccination to Control Ebola. PLoS Negl Trop Dis. 2015;9(5):e0003794. doi: 10.1371/journal.pntd.0003794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kucharski J, Eggo R, Watson C, et al. Effectiveness of Ring Vaccination as Control Strategy for Ebola Virus Disease. Emerg Infect Dis. 2016;22(1):105–8. doi: 10.3201/eid2201.151410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merler S, Ajelli M, Fumanelli L, et al. Containing Ebola at the Source with Ring Vaccination. PLoS Negl Trop Dis. 2016;10(11):e0005093. doi: 10.1371/journal.pntd.0005093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng Z, Zheng Y, Hernandez-Ceron N, et al. Mathematical models of Ebola-Consequences of underlying assumptions. Math Biosci. 2016;277:89–107. doi: 10.1016/j.mbs.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Legrand J, Grais RF, Boelle PY, et al. Understanding the dynamics of Ebola Epidemics. Epidemiol Infect. 2007;135(4):610–21. doi: 10.1017/S0950268806007217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browne C, Gulbudak H, Webb G. Modeling contact tracing in outbreaks with application to Ebola. J Theor Biol. 2015;384:33–49. doi: 10.1016/j.jtbi.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Webb G, Browne C, Huo X, et al. A Model of the 2014 Ebola Epidemic in West Africa with Contact Tracing. PLoS Curr. 2015;7 doi: 10.1371/currents.outbreaks.846b2a31ef37018b7d1126a9c8adf22a. ecurrents.outbreaks.846b2a31ef37018b7d1126a9c8adf22a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Althaus C. Estimating the Reproduction Number of Ebola Virus (EBOV) During the 2014 Outbreak in West Africa. PLoS Curr. 2014;6 doi: 10.1371/currents.outbreaks.91afb5e0f279e7f29e7056095255b288. ecurrents.outbreaks.91afb5e0f279e7f29e7056095255b288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowell G, Nishiura H. Transmission dynamics and control of Ebola virus disease (EVD): a review. BMC Med. 2014;12:196. doi: 10.1186/s12916-014-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisman D, Tuite A. Projected Impact of Vaccination Timing and Dose Availability on the Course of the 2014 West African Ebola Epidemic. PLoS Curr. 2014;6 doi: 10.1371/currents.outbreaks.06e00d0546ad426fed83ff24a1d4c4cc. ecurrents.outbreaks.06e00d0546ad426fed83ff24a1d4c4cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisman D, Khoo E, Tuite A. Early Epidemic Dynamics of the West African 2014 Ebola Outbreak: Estimates Derived with a Simple Two-Parameter Model. PLoS Curr. 2014;6 doi: 10.1371/currents.outbreaks.89c0d3783f36958d96ebbae97348d571. ecurrents.outbreaks.89c0d3783f36958d96ebbae97348d571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes M, Pastore Y, Piontti A, Rossi L, et al. Assessing the International Spreading Risk Associated with the 2014 West African Ebola Outbreak. PLoS Curr. 2014;6 doi: 10.1371/currents.outbreaks.cd818f63d40e24aef769dda7df9e0da5. ecurrents.outbreaks.cd818f63d40e24aef769dda7df9e0da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mamo D, Koya P. Mathematical Modeling and Simulation Study of SEIR disease and Data Fitting of Ebola Epidemic spreading in West Africa. J Multidiscip Eng Sci Technol. 2015;2(1):106–14. [Google Scholar]
- 19.Shaman J, Yang W, Kandula S. Inference and Forecast of the Current West African Ebola Outbreak in Guinea, Sierra Leone and Liberia. PLoS Curr. 2014;6 doi: 10.1371/currents.outbreaks.3408774290b1a0f2dd7cae877c8b8ff6. ecurrents.outbreaks.3408774290b1a0f2dd7cae877c8b8ff6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chowella G, Hengartner N, Castillo-Chaveza C, et al. The basic reproductive number of Ebola and the effects of public health measures: the cases of Congo and Uganda. J Theor Biol. 2004;229(1):119–26. doi: 10.1016/j.jtbi.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Rivers C, Lofgren E, Marathe M, et al. Modeling the Impact of Interventions on an Epidemic of Ebola in Sierra Leone and Liberia. PLoS Curr. 2014;6 doi: 10.1371/currents.outbreaks.fd38dd85078565450b0be3fcd78f5ccf. ecurrents.outbreaks.4d41fe5d6c05e9df30ddce33c66d084c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Do T, Lee Y. Modeling the Spread of Ebola. Osong Public Health Res Perspect. 2016;7(1):43–8. doi: 10.1016/j.phrp.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO Ebola Response Team. West African Ebola Epidemic after one year-slowing but not yet under control. N Engl J Med. 2015;372(6):584–7. doi: 10.1056/NEJMc1414992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerkhove M, Bento A, Mills H, et al. A review of epidemiological parameters from Ebola Outbreaks to inform early public health decision-making. Sci Data. 2015;2:150019. doi: 10.1038/sdata.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO [Internet] WHO coordinating vaccination of contacts to contain Ebola flare-up in Guinea. [cited 2017 Jan 15]. Available from: http://www.who.int/features/2016/ebola-contacts-vaccination/en/
- 26.WHO [Internet] Situation reports: Ebola response roadmap. [cited 2016 Dec 10]. Available from: http://apps.who.int/ebola/ebola-situation-reports.
- 27.Statement on the 1st meeting of the IHR Emergency Committee on the 2014 Ebola outbreak in West Africa [Intenret] [cited 2016 Dec 10] Available from: http://www.who.int/mediacentre/news/statements/2014/ebola-20140808/en/
- 28.UN News [Internet] Primary Focus of Response Must Be to Halt Spread of Ebola in West Africa –UN. 2014 Oct 23; [cited 2015 Dec 9]. Available from: https://news.un.org/en/story/2014/10/481802-primary-focus-response-must-be-halt-spread-ebola-west-africa-un.
- 29.Guo Z, Xiao D, Li D, et al. Predicting and Evaluating the Epidemic Trend of Ebola Virus Disease in the 2014–2015 Outbreak and the Effects of Intervention Measures. PLoS One. 2016;11(4):e0152438. doi: 10.1371/journal.pone.0152438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO [Internet] Virtual Press Conference on Ebola response. 2014 Oct 14; Available from: https://www.who.int/mediacentre/multimedia/vpc-14-october-2014.pdf?ua=1.
- 31.Anderson R, May R. Infectious Diseases of Humans. Oxford (UK): Oxford University; 1991. [Google Scholar]
- 32.Diekmann D, Heesterbeek J. Mathematical Epidemiology of Infectious Disease: Model Building, Analysis and Interpretation. Wiley; 2000. [Google Scholar]
- 33.Diekmann D, Heesterbeek J, Metz J. On the definition and the computation of the basic reproduction ratio ℜ0 in models for infectious diseases in heterogeneous populations. J Math Biol. 1990;28(4):365–82. doi: 10.1007/BF00178324. [DOI] [PubMed] [Google Scholar]
- 34.van den Driessche P, Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci. 2002;180(1–2):29–48. doi: 10.1016/S0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]