Abstract
Background: Quinolones are a family of synthetic antimicrobial agents with a broad antibacterial activity commonly used as a suitable therapy in patients with urinary tract infection (UTI). In the present study, we aimed to evaluate the prevalence of quinolones resistance and the presence of plasmid-mediated quinolone resistance (PMQR) genes among Escherichia coli isolates.
Methods
This study was performed on a collection of 121 E. coli isolates derived from patients with UTI. Antimicrobial susceptibility to nalidixic acid, ciprofloxacin, levofloxacin, norfloxacin, and ofloxacin was specified by the disk diffusion method. The presence of PMQR genes was determined by PCR method.
Results
Antibiotic susceptibility results showed that the highest and lowest resistance rates were against nalidixic acid (71.9%) and norfloxacin (44.6%), respectively. The molecular results showed that 40 (33.1%) and 15 (12.4%) of the isolates were positive for qnrS and qnrB genes, respectively. Meanwhile, 5 (4.1%) of the isolates were found positive for both genes, while none were found to be positive for qnrA gene. There was no significant association between the presence of qnr genes and higher antibiotic resistance.
Conclusion
We found high levels of quinolones resistance (more than 40%) among E. coli strains isolated from patients with UTIs in the south of Iran. We further report the prevalence of PMQR genes among uropathogenic E. coli; however, it seems that these genes are not the main components of quinolone resistance in our region.
Keywords: urinary tract infection, Escherichia coli, quinolone, antibiotic resistance
Introduction
Among the wide range of uropathogens conducing to the development of urinary tract infections (UTIs), uropathogenic Escherichia coli (UPEC) strains are considered as the main causative agents.1 UPEC account for the preponderance of both community- and hospital-acquired UTIs.1 Several risk factors including renal diseases increase the risk of UTI; however, the treatment of infection more often do not require antimicrobial therapy.2,3 Over the recent years, antibiotic therapy of UTI has become problematic due to the misuse and irregular consumption of antibiotics entailing the emergence of resistant strains.4 E. coli may be resistant to various types of antibiotics and act in different ways to transfer antibiotic resistance genes to other strains and bacteria such as transposon, bacteriophage, and plasmid.5
Quinolones are a family of synthetic antimicrobial agents with a broad antibacterial activity commonly used as a suitable therapy in patients with UTI.6,7 This family has been classified into four generations based on their antimicrobial activity. The most well-known quinolone antibiotics are nalidixic acid, ciprofloxacin, and levofloxacin as members of the first, second, and third generations, respectively.6 Quinolones prevent bacterial DNA synthesis through inhibiting DNA gyrase and topoisomerase IV enzymes leading to cell death.8 Quinolone resistance is caused by various mechanisms, particularly plasmid-mediated quinolone resistance (PMQR) which contains the pentapeptide repeat family Qnr proteins (QnrA, QnrB, QnrS, QnrC, and QnrD). These proteins confer quinolone resistance by physically protecting DNA gyrase and topoisomerase IV from quinolone acts.8 This condition may provide a selective advantage for the development of quinolone resistance which could result in therapeutic failure.9
Due to the high horizontal gene transferring capability of E. coli, it is necessary to estimate the burden and control the spread of quinolone-resistant strains in hospitals. In this study, we aimed to evaluate the prevalence of quinolones resistance and the presence of PMQR genes in E. coli strains isolated from Iranian patients with UTI, in order to categorize the genes with a more significant role in the development of resistance to quinolones in our region.
Materials and methods
Study design and Escherichia coli samples
This study was performed on a collection of 121 non-duplicated E. coli isolates (one per patient) derived from patients with UTI in our previous work.10 Samples were collected from November 2016 to May 2017 from inpatients who presented with symptomatic UTI at a tertiary care hospital (Nemazee) in Shiraz, the south of Iran. The patients had not received antibiotics at least one week before the sample collection. The mixed growth of bacteria was considered as contamination and was excluded. The study design was in accordance with the declaration of Helsinki and ethical permission was sought previously from the institutional Ethics Committee of Shiraz University of Medical Sciences (Approval No. IR.SUMS.REC.1395.S747). However, because only leftovers from clinical specimens were used, the local ethics committee waived the need for informed consent. E. coli isolates were identified by standard microbiological tests and API 20E strip (API-bioMérieux, France). Confirmed E. coli isolates were preserved at −80ºC for further works.
Quinolones susceptibility testing
The susceptibility of isolates toward five quinolone antibiotics including nalidixic acid, ciprofloxacin, levofloxacin, norfloxacin, and ofloxacin was investigated by standard disk diffusion technique on Muller-Hinton agar (Merck, Germany) plates according to the clinical and laboratory standards institute (CLSI) guidelines.11 All antibiotic disks were provided from Mast Co., UK. The plates were then incubated at 37°C for 16–18 hrs. E. coli ATCC 25922 was used as a quality control strain for antibiotic susceptibility testing. Based on antibiotic susceptibility results, fluoroquinolones resistant and nalidixic acid non-susceptible (resistant or intermediate) isolates were classified as high-level quinolone-resistant bacteria.12 Extended spectrum β-lactamases (ESBLs) production was tested using the double-disk synergy test elsewhere.10
Detection of Qnr encoding genes
The bacterial whole genome was extracted by the boiling method as previously described.13 Polymerase chain reaction (PCR) assays were performed for the detection of qnr resistance genes including qnrA, qnrB, and qnrS. The primers used to detect qnr genes were selected from previously described sequences by Cattoirin and colleagues.14 PCR was done in a total volume of 25 μL containing 3 μL DNA template, 2.5 μL PCR buffer (1X), 1 μL deoxyribonucleotide triphosphates solution (dNTPs, 200 μM), 1.5 μL MgCl2 (1.5 mM), 0.25 μL Taq DNA polymerase (1 Unit), and 1 μL each specific primers (1 μM). PCR amplifications for the studied genes were carried out on a T100™ thermal cycler (Bio-Rad, Hercules, CA, USA). The cycling conditions were set up as follows: 95°C for 5 mins (step 1), 95°C for 1 min (step 2), annealing for 45 sec (step 3), 72°C for 1 min (step 4), and 72°C for 5 mins (step 5); steps 2–4 were repeated for 30 cycles. The amplifications were separated on 1.5% agarose gel prepared in 1X TAE (Tris/Acetate/EDTA) buffer and visualized using ultraviolet light after staining with safe stain load dye (CinnaGen Co., Iran).
Statistical analysis
The analysis was performed through the use of SPSSTM software, version 21.0 (IBM Corp., USA). The results are presented as descriptive statistics in terms of relative frequency. Categorical variables were expressed as counts and percentages. The Chi-square (χ2) or Fisher’s exact tests were performed to analyze significant differences. AP -value <0.05 was considered as statistically significant.
Results
Analysis of antibiotic susceptibility tests showed that the highest and lowest resistance rates were against nalidixic acid (71.9%) and norfloxacin (44.6%), respectively. The full results of antibiotic susceptibility assay for E. coli isolates are shown in Table 1. Moreover, 60 (49.6%) of E. coli isolates were classified as high-level quinolone-resistant.
Table 1.
The antibiotic susceptibility testing results of 121 E. coli isolates
| Antibiotic | Resistant No. (%) | Intermediate-resistant No. (%) | Susceptible No. (%) |
|---|---|---|---|
| Nalidixic acid | 87 (71.9) | 11 (9.1) | 23 (19) |
| Ciprofloxacin | 59 (48.4) | 12 (9.9) | 50 (41.3) |
| Levofloxacin | 58 (47.9) | 3 (2.5) | 60 (49.6) |
| Ofloxacin | 56 (46.3) | 4 (3.3) | 61 (50.4) |
| Norfloxacin | 54 (44.6) | 5 (4.1) | 62 (51.2) |
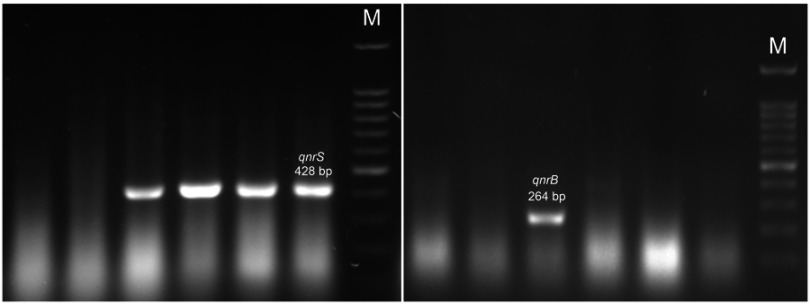
The molecular results showed that 40 (33.1%) and 15 (12.4%) of the isolates were positive for qnrS and qnrB genes, respectively (Figure 1). Meanwhile, 5 (4.1%) of the isolates were found positive for both genes, while none were found to be positive for qnrA gene. In addition, 50 (41.3%) of the isolates were qnr positive. The distribution of qnr-positive isolates in different hospital units was almost the same; however, the positivity rate in the transplant unit was high (Data not shown).
Figure 1.
Agarose gel electrophoresis of PCR products for qnrB and qnrS genes. M: 100 bp DNA size marker.
There was no significant association between qnr genes and higher antibiotic resistance. However, qnrS gene was found to be relatively higher than qnrB gene among antibiotic-resistant isolates (Table 2). Meanwhile, it seems that qnr genes are more likely to occur in nalidixic acid resistant rather than fluoroquinolones-resistant isolates.
Table 2.
Distribution of qnr genes in relation with quinolone resistance
| Antibiotic | Pattern | qnrS-positive No. (%) | qnrS-negative No. (%) | P-value | qnrB-positive No. (%) | qnrB-negative No. (%) | P-value |
|---|---|---|---|---|---|---|---|
| Nalidixic acid | Ra (n=98) | 34 (34.7) | 64 (65.3) | 0.430 | 10 (10.2) | 88 (89.8) | 0.131 |
| S (n=23) | 6 (26.1) | 17 (73.9) | 5 (21.7) | 18 (78.3) | |||
| Ciprofloxacin | R (n=71) | 21 (29.6) | 50 (70.4) | 0.332 | 2 (2.8) | 69 (97.2) | <0.001 |
| S (n=50) | 19 (38) | 31 (62) | 13 (26.2) | 37 (74) | |||
| Levofloxacin | R (n=61) | 16 (26.2) | 45 (73.8) | 0.107 | 2 (3.3) | 59 (96.7) | 0.002 |
| S (n=60) | 24 (40) | 36 (60) | 13 (21.7) | 47 (78.3) | |||
| Ofloxacin | R (n=60) | 15 (25) | 45 (75) | 0.062 | 3 (5) | 57 (95) | 0.014 |
| S (n=61) | 25 (41) | 36 (59) | 12 (19.7) | 49 (80.3) | |||
| Norfloxacin | R (n=98) | 14 (23.7) | 45 (76.3) | 0.033 | 2 (3.4) | 57 (96.6) | 0.003 |
| S (n=23) | 26 (41.9) | 36 (58.1) | 13 (21) | 49 (79) |
Notes: aIncluding resistant and intermediate-resistant isolates.
Abbreviations: R, Resistant; S, Susceptible.
Of totally 51 ESBLs producing isolates, 14 (25.7%) and 6 (11.8%) isolates contained qnrS and qnrB genes, respectively. Distribution of qnr genes among ESBLs-producing isolates in relation to quinolones susceptibility is presented in Table 3. The proportion of qnrS gene among ESBLs-producing quinolone-resistant isolates compared to susceptible isolates was relatively high, but the differences were not statistically significant. The proportion of qnrB gene was more prevalent among ESBLs-producing quinolone-susceptible isolates.
Table 3.
Distribution of qnr genes among 51 ESBLs producing isolates
| Antibiotic | Pattern | qnrS-positive No. (%) | qnrS-negative No. (%) | P-value | qnrB-positive No. (%) | qnrB-negative No. (%) | P-value |
|---|---|---|---|---|---|---|---|
| Nalidixic acid | Ra (n=45) | 13 (28.9) | 32 (71.1) | 0.468 | 4 (8.9) | 41 (91.1) | 0.141 |
| S (n=6) | 1 (16.7) | 5 (83.3) | 2 (33.3) | 4 (66.7) | |||
| Ciprofloxacin | R (n=38) | 11 (28.9) | 27 (71.1) | 0.492 | 2 (5.3) | 36 (94.7) | 0.031 |
| S (n=13) | 3 (23.1) | 10 (76.9) | 4 (30.8) | 9 (69.2) | |||
| Levofloxacin | R (n=34) | 10 (29.4) | 24 (70.6) | 0.463 | 2 (5.9) | 32 (94.1) | 0.087 |
| S (n=17) | 4 (23.5) | 13 (76.5) | 4 (23.5) | 13 (76.5) | |||
| Ofloxacin | R (n=33) | 9 (27.3) | 24 (72.7) | 0.608 | 2 (6.1) | 31 (93.9) | 0.106 |
| S (n=18) | 5 (27.8) | 13 (72.2) | 4 (22.2) | 14 (77.8) | |||
| Norfloxacin | R (n=31) | 7 (22.6) | 24 (77.4) | 0.257 | 2 (6.5) | 29 (93.5) | 0.154 |
| S (n=20) | 7 (35) | 13 (65) | 4 (20) | 16 (80) |
Notes: aIncluding resistant and intermediate-resistant isolates.
Abbreviations: R, Resistant; S, Susceptible.
Discussion
The extensive use of quinolone antibiotics in poultry production and human medicine is associated with the increasing emergence of quinolone-resistant strains.8 Here, we investigated the quinolones resistance among a collection of UPEC in one of the largest tertiary care hospitals in the south of Iran in order to inform physicians as to the regional antibiotic resistance rates and further conduce to the international data to ameliorate antimicrobial stewardship programs. In the present study, the rate of quinolones resistance exceeded 40%, which discourages the empirical use of quinolones in our region since the risk of treatment failure increases when resistance rates exceed 10% to 20%.15,16 However, the evidence is insufficient to make a recommendation against using quinolones since no alternative oral antimicrobial options are available for the treatment of pyelonephritis.17 Currently, the incidence of trimethoprim-sulfamethoxazole and amoxicillin-clavulanate resistant E. coli approach or exceed from quinolones-resistant rate depending on the geographical region.18 Other oral agents such as cephalosporins, nitrofurantoin, and fosfomycin are not recommended for treatment of pyelonephritis.19 Also, a high drug concentration of quinolones in urine can affect clinical outcomes of patients with UTIs even those infected by resistant strains.17 So, quinolones remain an important treatment option for empirical therapy of complicated urinary tract infections (cUTIs), particularly after stratification of patients based on predicted risk of antimicrobial resistance.20
The literature review indicates a high prevalence of quinolones resistance in Iran. In 2014, Pouladfar et al showed that 38.5% and 75% of E. coli isolates causing UTI in children (in Shiraz) were resistant to ciprofloxacin and nalidixic acid, respectively, which is very close to the findings of the present research.21 Shenagari et al (2017) showed 45.3% resistance to norfloxacin, 48.9% to ofloxacin, 50.2% to ciprofloxacin, and 61.9% to nalidixic acid among their UPEC in Rasht (North of Iran).22 Rezazadeh et al (2014–2015) found 55.5% resistance to norfloxacin, 56% to ciprofloxacin, 56% to levofloxacin, 58% to gatifloxacin, and 66.5% to nalidixic acid among E. coli strains isolated from UTI patients in Qazvin (North of Iran) and Zanjan (Northwest of Iran).23 Damavandi et al (2013 to 2014) observed 45% resistance to norfloxacin, 48% to ciprofloxacin, and 62% to nalidixic acid in the E. coli strains isolated from the inpatients of Shahrekord (central Iran).24 In contrast to our findings, Sedighi et al (2010 to 2011) found 14.2% resistance to ofloxacin, 15% to norfloxacin, 15% to ciprofloxacin, and 40.9% to nalidixic acid among their UPEC strains in Hamadan (central Iran).25 Moreover, a recent systematic review and meta-analysis study has shown that ciprofloxacin-resistant E. coli in UTI is a global problem, where Asia has the highest pooled resistance (50%).26
Such a high prevalence of quinolone resistance may be linked to dissemination of multidrug-resistant (MDR) extraintestinal pathogenic E. coli (ExPEC) isolates.27 Recent data from Iran suggest that E. coli sequence type (ST) 131 has emerged as an important public health concern.28–30 This pandemic clone which was recognized since 2000 is strongly associated with ESBLs, fluoroquinolone, trimethoprim-sulfamethoxazole, and third-generation cephalosporins resistance.27,31
In the present study, 41.3% of the isolates contained qnr encoding genes, mainly qnrS. However, no significant association was observed between the presence of qnr genes and quinolone resistance. It seems that the Qnr family is not the main mechanism of quinolone resistance in E. coli in our region, and other mechanisms such as point mutations in the gyrA and parC genes may play a role.8 However, it is supposed that PMQR genes can be clinically important as they facilitate the selection of higher levels of quinolone resistance.32 In this regard, there are several comparable reports pointing to the prevalence of qnr genes among E. coli isolates. Yousefi et al found a high prevalence of qnrB (71.3%) and qnrS (62.8%) genes with a significant association with quinolone resistance among UPEC strains in the north of Iran.33 Sedighi et al found qnrB in 6.7% and qnrS in 5% of UPEC strains and a significant association between the quinolone resistance and presence of qnr genes.25 Rezazadeh et al observed a low level of qnrS1 gene among quinolone-resistant E. coli isolates, while qnrA and qnrB genes were not found in any of the isolates.23 In yet another study, Abbasi et al showed the prevalence of qnrS (36%) and qnrB (25%) as the only detected qnr genes among E. coli strains isolated from UTIs in Tehran.34 Despite the relative agreement on the higher prevalence of qnrS and qnrB genes among UPEC, the distribution of predominate genes is varied over different regions. These variations in results are consistent with observations from other Asian countries such as Iraq,35 Pakistan,36 Saudi Arabia,37 Korea,38 Taiwan,39 and China.40
The present study encountered certain limitations such as lack of investigation on other quinolone resistance mechanisms. Furthermore, due to the lack of a molecular typing method, there was no mention of the genetic relatedness of the quinolone-resistant strains.
Conclusion
In summary, we found a high level of quinolones resistance (more than 40%) among E. coli strains isolated from patients with UTI in the south of Iran. Rational use of antimicrobial policy as well as stopping the unnecessary prescription and non-prescription sales in retail pharmacies can be performed as strategies to prevent the increase of quinolones resistance. As the first preliminary survey, we further report the prevalence of PMQR genes among UPEC, while it seems that these genes are not the main mechanism of quinolone resistance. However, further studies are required to investigate all other possible mechanisms in larger series.
Acknowledgment
The authors wish to thank Ms. N. Zahedian Nezhad for her invaluable assistance in samples collection.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Terlizzi ME, Gribaudo G, Maffei ME. UroPathogenic Escherichia coli (UPEC) infections: virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front Microbiol. 2017;8:1566. doi: 10.3389/fmicb.2017.01566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemmati H, Khosravi M, Heidarzadeh A, Hashkavaei P, Refahibakhsh N. Vascular access and survival in hemodialysis patients in Rasht, Iran. Iran J Kidney Dis. 2011;5(1):34–37. [PubMed] [Google Scholar]
- 3.John U, Kemper MJ. Urinary tract infections in children after renal transplantation. Pediatr Nephrol. 2009;24(6):1129–1136. doi: 10.1007/s00467-007-0690-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tenney J, Hudson N, Alnifaidy H, Li JTC, Fung KH. Risk factors for aquiring multidrug-resistant organisms in urinary tract infections: a systematic literature review. Saudi Pharm J. 2018;26(5):678–684. doi: 10.1016/j.jsps.2018.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munita JM, Arias CA. Mechanisms of antibiotic resistance. Microbiol Spectr. 2016;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King DE, Malone R, Lilley SH. New classification and update on the quinolone antibiotics. Am Fam Physician. 2000;61(9):2741–2748. [PubMed] [Google Scholar]
- 7.Tayebi Z, Heidari H, Kazemian H, Ghafoori SM, Boroumandi S, Houri H. Comparison of quinolone and beta-lactam resistance among Escherichia coli strains isolated from urinary tract infections. Infez Med. 2016;24(4):326–330. [PubMed] [Google Scholar]
- 8.Correia S, Poeta P, Hebraud M, Capelo JL, Igrejas G. Mechanisms of quinolone action and resistance: where do we stand? J Med Microbiol. 2017;66(5):551–559. doi: 10.1099/jmm.0.000475 [DOI] [PubMed] [Google Scholar]
- 9.Jacoby GA. Mechanisms of resistance to quinolones. Clin Infect Dis. 2005;41(Suppl 2):S120–126. doi: 10.1086/444499 [DOI] [PubMed] [Google Scholar]
- 10.Ebrahim-Saraie HS, Nezhad NZ, Heidari H, Motamedifar A, Motamedifar M. Detection of antimicrobial susceptibility and integrons among extended-spectrum beta-lactamase producing uropathogenic Escherichia coli isolates in Southwestern Iran. Oman Med J. 2018;33(3):218–223. doi: 10.5001/omj.2018.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CLSI. Performance Standards for Antimicrobial Susceptibility Testing; 28th Informational Supplement. CLSI Document M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2018:2018. [Google Scholar]
- 12.Oktem IM, Gulay Z, Bicmen M, Gur D. qnrA prevalence in extended-spectrum beta-lactamase-positive enterobacteriaceae isolates from Turkey. Jpn J Infect Dis. 2008;61(1):13–17. [PubMed] [Google Scholar]
- 13.Nobari S, Shahcheraghi F, Rahmati Ghezelgeh F, Valizadeh B. Molecular characterization of carbapenem-resistant strains of klebsiella pneumoniae isolated from Iranian patients: first identification of blaKPC gene in Iran. Microb Drug Resist. 2014;20(4):285–293. doi: 10.1089/mdr.2013.0074 [DOI] [PubMed] [Google Scholar]
- 14.Cattoir V, Poirel L, Rotimi V, Soussy CJ, Nordmann P. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J Antimicrob Chemother. 2007;60(2):394–397. doi: 10.1093/jac/dkm215 [DOI] [PubMed] [Google Scholar]
- 15.McQuiston Haslund J, Rosborg Dinesen M, Sternhagen Nielsen AB, Llor C, Bjerrum L. Different recommendations for empiric first-choice antibiotic treatment of uncomplicated urinary tract infections in Europe. Scand J Prim Health Care. 2013;31(4):235–240. doi: 10.3109/02813432.2013.844410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bidell MR, Palchak M, Mohr J, Lodise TP. Fluoroquinolone and third-generation-cephalosporin resistance among hospitalized patients with urinary tract infections due to Escherichia coli: do rates vary by hospital characteristics and geographic region? Antimicrob Agents Chemother. 2016;60(5):3170–3173. doi: 10.1128/AAC.02505-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim ES, Hooper DC. Clinical importance and epidemiology of quinolone resistance. Infect Chemother. 2014;46(4):226–238. doi: 10.3947/ic.2014.46.4.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryce A, Hay AD, Lane IF, Thornton HV, Wootton M, Costelloe C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysis. Bmj. 2016;352:i939. doi: 10.1136/bmj.i1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carson C, Naber KG. Role of fluoroquinolones in the treatment of serious bacterial urinary tract infections. Drugs. 2004;64(12):1359–1373. doi: 10.2165/00003495-200464120-00007 [DOI] [PubMed] [Google Scholar]
- 20.Shah A, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Application of fluoroquinolone resistance score in management of complicated urinary tract infections. Antimicrob Agents Chemother. 2017;61:5. doi: 10.1128/AAC.02313-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pouladfar G, Basiratnia M, Anvarinejad M, Abbasi P, Amirmoezi F, Zare S. The antibiotic susceptibility patterns of uropathogens among children with urinary tract infection in Shiraz. Medicine (Baltimore). 2017;96(37):e7834. doi: 10.1097/MD.0000000000007834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shenagari M, Bakhtiari M, Mojtahedi A, Atrkar Roushan Z. High frequency of mutations in gyrA gene associated with quinolones resistance in uropathogenic Escherichia coli isolates from the north of Iran. Iran J Basic Med Sci. 2018;21(12):1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rezazadeh M, Baghchesaraei H, Peymani A. Plasmid-mediated quinolone-resistance (qnr) genes in clinical isolates of Escherichia coli collected from several hospitals of Qazvin and Zanjan Provinces, Iran. Osong Public Health Res Perspect. 2016;7(5):307–312. doi: 10.1016/j.phrp.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damavandi MS, Gholipour A, Latif Pour M. Prevalence of class D carbapenemases among extended-spectrum beta-lactamases producing Escherichia coli isolates from educational hospitals in Shahrekord. J Clin Diagn Res. 2016;10(5):Dc01–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedighi I, Arabestani MR, Rahimbakhsh A, Karimitabar Z, Alikhani MY. Dissemination of extended-spectrum beta-lactamases and quinolone resistance genes among clinical isolates of uropathogenic Escherichia coli in children. Jundishapur J Microbiol. 2015;8(7):e19184. doi: 10.5812/jjm.19184v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fasugba O, Gardner A, Mitchell BG, Mnatzaganian G. Ciprofloxacin resistance in community- and hospital-acquired Escherichia coli urinary tract infections: a systematic review and meta-analysis of observational studies. BMC Infect Dis. 2015;15:545. doi: 10.1186/s12879-015-1282-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNally A, Kallonen T, Connor C, et al. Diversification of colonization factors in a multidrug-resistant Escherichia coli lineage evolving under negative frequency-dependent selection. MBio. 2019;10(2):e00644–00619. doi: 10.1128/mBio.00669-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hojabri Z, Mirmohammadkhani M, Kamali F, Ghassemi K, Taghavipour S, Pajand O. Molecular epidemiology of Escherichia coli sequence type 131 and its H30/H30-Rx subclones recovered from extra-intestinal infections: first report of OXA-48 producing ST131 clone from Iran. Eur J Clin Microbiol Infect Dis. 2017;36(10):1859–1866. doi: 10.1007/s10096-017-3021-9 [DOI] [PubMed] [Google Scholar]
- 29.Moghanni M, Ghazvini K, Farsiani H, et al. High prevalence of sequence type 131 isolates producing CTX-M-15 among extended-spectrum beta-lactamase-producing Escherichia coli strains in northeast Iran. J Glob Antimicrob Resist. 2018;15:74–78. doi: 10.1016/j.jgar.2018.05.016 [DOI] [PubMed] [Google Scholar]
- 30.Namaei MH, Yousefi M, Ziaee M, et al. First Report of prevalence of CTX-M-15-producing Escherichia coli O25b/ST131 from Iran. Microb Drug Resist. 2017;23(7):879–884. doi: 10.1089/mdr.2016.0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badran EF, Qamer Din RA, Shehabi AA. Low intestinal colonization of Escherichia coli clone ST131 producing CTX-M-15 in Jordanian infants. J Med Microbiol. 2016;65(2):137–141. doi: 10.1099/jmm.0.000210 [DOI] [PubMed] [Google Scholar]
- 32.Jacoby GA, Strahilevitz J, Hooper DC. Plasmid-mediated quinolone resistance. Microbiol Spectr. 2014;2:5. doi: 10.1128/microbiolspec.PLAS-0006-2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yousefi S, Mojtahedi A, Shenagari MA. Survey of gyrA target-site mutation and qnr genes among clinical isolates of Escherichia coli in the North of Iran. Jundishapur J Microbiol. 2018;11(9):e67293. doi: 10.5812/jjm.67293 [DOI] [Google Scholar]
- 34.Abbasi H, Ranjbar R. The prevalence of quinolone resistance genes of A, B, S in Escherichia coli strains isolated from three major hospitals in Tehran, Iran. Cent European J Urol. 2018;71(1):129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Hasnawy HH, Jodi MR, Hamza HJ. Molecular characterization and sequence analysis of plasmid-mediated quinolone resistance genes in extended-spectrum beta-lactamases producing uropathogenic Escherichia coli in Babylon Province, Iraq. Rev Med Microbiol. 2018;29(3):129–135. doi: 10.1097/MRM.0000000000000136 [DOI] [Google Scholar]
- 36.Ali I, Rafaque Z, Ahmed S, Malik S, Dasti JI. Prevalence of multi-drug resistant uropathogenic Escherichia coli in Potohar region of Pakistan. Asian Pac J Trop Biomed. 2016;6(1):60–66. doi: 10.1016/j.apjtb.2015.09.022 [DOI] [Google Scholar]
- 37.Al-Agamy MH, Aljallal A, Radwan HH, Shibl AM. Characterization of carbapenemases, ESBLs, and plasmid-mediated quinolone determinants in carbapenem-insensitive Escherichia coli and Klebsiella pneumoniae in Riyadh hospitals. J Infect Public Health. 2018;11(1):64–68. doi: 10.1016/j.jiph.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 38.Shin JH, Jung HJ, Lee JY, Kim HR, Lee JN, Chang CL. High rates of plasmid-mediated quinolone resistance QnrB variants among ciprofloxacin-resistant Escherichia coli and Klebsiella pneumoniae from urinary tract infections in Korea. Microb Drug Resist. 2008;14(3):221–226. doi: 10.1089/mdr.2008.0834 [DOI] [PubMed] [Google Scholar]
- 39.Kao CY, Wu HM, Lin WH, et al. Plasmid-mediated quinolone resistance determinants in quinolone-resistant Escherichia coli isolated from patients with bacteremia in a university hospital in Taiwan, 2001–2015. Sci Rep. 2016;6:32281. doi: 10.1038/srep32281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao L, Zhang J, Zheng B, et al. Molecular epidemiology and genetic diversity of fluoroquinolone-resistant Escherichia coli isolates from patients with community-onset infections in 30 Chinese county hospitals. J Clin Microbiol. 2015;53(3):766–770. doi: 10.1128/JCM.02594-14 [DOI] [PMC free article] [PubMed] [Google Scholar]