Abstract
Background & Aims:
Hepatitis C virus (HCV) treatment for patients with hepatocellular carcinoma (HCC) was uncommon before direct-acting antiviral (DAA) medications. Real-world effectiveness of DAAs for HCV in patients with HCC is unclear. We describe rates of sustained virologic response (SVR) with DAA regimens by HCV genotype in patients with a history of HCC.
Methods:
We identified patients who initiated antiviral treatment between January 1, 2014 and June 30, 2015 in the national Veterans Affairs health care system. Regimens included sofosobu-vir, ledipasvir/sofosbuvir, and paritaprevir/ritonavir/ombitasvir and dasabuvir with or without ribavirin. HCC patients were divided into those who were treated with liver transplantation after HCC diagnosis (“HCC/LT” group) and those treated with other modalities prior to antiviral therapy (“HCC” group).
Results:
Of 17,487 HCV treatment recipients, 624 (3.6%) had prior HCC, including 142 with HCC/LT and 482 with HCC. Overall SVR was 91.1% in non-HCC, 74.4% in HCC, and 94.0% in HCC/LT. Among HCC patients, genotype 1 had the highest SVR overall (79.1% in HCC and 96.4% in HCC/LT), and genotype 3 the lowest (47.0% in HCC and 88.9% in HCC/LT). After adjustment for con-founders, the presence of HCC was associated with lower likelihood of SVR overall (AOR 0.38 [95% Cl 0.29, 0.48], p <0.001).
Conclusion:
HCV can be cured with DAAs in the majority of patients with prior HCC, and in virtually all HCC patients post-liver transplant. Deferral of HCV treatment until the post-transplant setting may be considered among HCC patients listed for transplantation.
Lay summary:
Over three-quarters of patients with hepatocellular carcinoma who have hepatitis C can achieve viral cure with direct-acting antiviral drugs. Among patients with hepatocellular carcinoma who subsequently received liver transplantation, over 90% of patients can achieve viral cure.
Keywords: Ledipasvir/sofosbuvir, Paritaprevir/ritonavir/ombitasvir and dasabuvir, Liver transplant, Sustained virologic response
Introduction
Hepatocellular carcinoma (HCC) mortality increased 72% in the United States (US) between 2003–2012, making it the fastest-growing cause of cancer-related mortality.1 HCC incidence also rose during this time, with a delay-adjusted average annual percentage change of 3.7%, second only to thyroid cancer nationally.1 HCV is implicated in the majority of HCC cases in the US.2–4 Until the widespread use of direct-acting antivirals (DAAs), antiviral treatment for patients with HCC and HCV was hampered by the poor efficacy and tolerability of interferon. Very few HCC patients were eligible for treatment due to interferon contraindications, such as advanced cirrhosis. HCV is now largely curable using DAAs, even in populations once considered difficult to treat.5–7 However, only one clinical trial for a DAA-based regimen has specifically targeted patients with HCC (n = 61), and this trial involved patients already listed for liver transplantation.8
The role of DAAs in patients with a history of HCC remains uncertain. Patients with advanced HCC may not be offered antiviral treatment due to high cancer-related mortality. Those with severely decompensated cirrhosis may not be offered antiviral treatment due to high cirrhosis-related mortality, particularly if they are not candidates for transplantation. Finally, HCC patients tend to be older than other patients with HCV, which is associated with extrahepatic comorbidities that might limit possible benefits from antiviral treatment.
There are also many reasons for considering HCV treatment in selected patients with a history of HCC. First, HCV treatment in liver transplant recipients is a cornerstone of care, including in those with prior HCC.9 Patients with decompensated cirrhosis may benefit from regression or stabilization of their cirrhosis and improvement in liver function as a result of HCV eradication, even allowing for transplant delisting in some cases.10,11 Finally, in the interferon era, HCV treatment was suggested to reduce the future risk of HCC recurrence,12–16 though conflicting reports have arisen surrounding risks of HCC recurrence after DAA therapy.11,17–20
The real-world effectiveness of DAA treatment in patients with a history of HCC is unknown. The Department of Veterans Affairs (VA) health care system encompasses the largest cohorts of HCV patients and HCC patients in the US. The objective of this study was to describe the characteristics of HCC patients who receive DAA-based antiviral treatment and to report the rates and predictors of SVR.
Materials and methods
Data source
All data were obtained from the VA Corporate Data Warehouse, a comprehensive repository of data from the VA’s universal electronic medical record system.21 Data extracted included all patient pharmacy prescriptions, demographics, inpatient and outpatient visits, problem lists, procedures, vital signs, diagnostic tests, and laboratory tests.
Human subjects
All study procedures were approved by the VA Puget Sound Institutional Review Board. All procedures conform to the ethical guidelines of the 1975 Declaration of Helsinki. A waiver of informed patient consent was obtained prior to project initiation.
Study population
The national VA system includes 167 medical centers and 875 ambulatory clinics, and served a total of 174,889 patients with chronic HCV diagnosis in 2014.22 We identified 24,089 HCV regimens initiated between 1st January, 2014–30th June, 2015 and completed before 1st October, 2015. We identified patients who initiated antiviral treatment including sofosbuvir (SOF), ledipasvir/sofosbuvir (LDV/ SOF) and paritaprevir/ritonavir/ombitasvir and dasabuvir (PrOD) during the 18- month period from 1st January, 2014 (when SOF was approved by the US Food and Drug Administration) to 30th June, 2015 (n = 24,089). We excluded 6,193 regimens that were no longer recommended in the VA by the time we conducted this study, such as SOF + simeprevir ± ribavirin (n = 3,669), SOF + pegylated- interferon (PeglFN) ± ribavirin for genotype 1 HCV (n= 1,766), SOF + ribavirin for genotype 1 HCV (n = 418) or other regimens (n = 340). We also excluded 409 duplicate regimens that were very short in duration (≤14 days) and were therefore considered erroneous or postponed prescriptions, leaving 17,487 patients in the current analysis.
Data extended forward until 10th April, 2016 to allow completion of treatment and ascertainment of SVR. Duration of antiviral therapy was calculated using the total duration of the DAA prescriptions filled. A course was considered terminated if medications were not refilled within 45 days after the final prescription was exhausted. Data extended backwards to 10th January, 1999 in order to allow determination of previous antiviral treatments and past medical history.
Diagnosis of HCC was ascertained using previously validated methods requiring at least 2 instances of International Classification of Diseases, 9th Revision (ICD-9) codes for HCC (155.0) on separate days.23–28 We identified 624 cases with a diagnosis of HCC prior to first DAA prescription, including 142 with HCC patients who received liver transplantation (HCC/LT) prior to antiviral treatment. HCC/LT patients before HCV treatment were analysed separately, as we predicted that this group would have very different SVR rates. HCC patients who received liver transplant after HCV treatment (n = 8) were included in the HCC group.
Treatments for HCC were classified as local ablation (radiofrequency ablation, cryotherapy, ethanol injection, or unspecified ablation), surgical resection, trans-arterial chemoembolization (TACE), Yttrium-90, or sorafenib. ICD-9 and Current Procedural Terminology codes for HCC-related procedures were identified by two board-certified interventional radiologists (MJK, SKA) and through review of previously published work (Table S1 ).29 VA facilities that do not offer certain HCC treatments may outsource them to non-VA facilities through the “fee services” mechanism. Therefore, in addition to searching for HCC treatments provided at VA facilities, we also searched ‘‘fee services” data.
Baseline characteristics
We ascertained age, race, ethnicity, gender, and prior antiviral treatment since 1999 (“treatment experienced”). We used ICD-9 codes to define diagnosis of cirrhosis, diabetes, liver transplantation, depression, post-traumatic stress disorder, anxiety or panic disorder, schizophrenia, alcohol use disorders, and substance use disorders (Table S2). ICD-9 codes used in defining comorbidities have been widely used and validated in VA medical records.26,30–34 All comorbid diagnosis variables were ascertained using ICD-9 codes recorded at least twice, on separate days, before the initiation of antiviral treatment. All patients with HCC/LT were considered to be non-cirrhotic, given that it would be impossible to determine whether ICD-9 codes for cirrhosis reflected their current condition as opposed to their pre-transplant condition.
We extracted all laboratory tests shown in Table 1 and recorded the value of each test closest to the treatment start date within the preceding 6 months. We calculated the FIB-4 score35 (FIB-4 = [age × AST]/[platelets × ALT1/2]), which is associated with advanced fibrosis and cirrhosis. Of 17,487 regimens, 678 had missing genotype. These patients were treated either with PrOD ± ribavirin (n = 130) or with LDV/SOF monotherapy (n = 548) and were assigned genotype 1 since these regimens are used almost exclusively for genotype 1 HCV infection. Among patients with known genotype treated with these regimens 11,761 out of 11,871 (99%) had genotype 1 HCV infection.
Table 1.
Baseline characteristics of patients with HCC who initiated HCV antiviral treatment in the national VA healthcare system from January 2014 to June 2015 (N = 17,487).
| No HCC (n = 16,863) |
HCC (n = 482) |
HCC/LT (n = 142) |
|
|---|---|---|---|
| Age, years mean, (SD) | 60.7 (6.6) | 63.0 (4.9) | 62.7 (4.7) |
| Male (%) | 96.8 | 98.1 | 97.2 |
| Race/ethnicity (%) | |||
| White, non-Hispanic | 52.1 | 52.5 | 66.9 |
| Black, non-Hispanic | 29.0 | 28.4 | 16.9 |
| Hispanic | 5.2 | 6.0 | 7.8 |
| Asian, Pacific Islander, American Indian | 1.6 | 2.3 | 2.1 |
| Missing | 12.2 | 10.8 | 6.3 |
| HCV treatment experienced (%) | 28.7 | 43.9 | 40.1 |
| HCV RNA viral load >6 million IU/ml (%) | 19.7 | 12.2 | 22.1 |
| HIV co-infection (%) | 4.2 | 2.1 | 1.4 |
| Cirrhosis (%) | 28.7 | 85.1 | n/a |
| Decompensated cirrhosis (%) | 7.7 | 31.5 | n/a |
| Diabetes (%) | 28.7 | 36.9 | 61.3 |
| Alcohol use disorder (%) | 43.8 | 53.7 | 56.3 |
| Substance use disorder (%) | 37.1 | 35.5 | 33.8 |
| Depression (%) | 47.3 | 47.9 | 59.2 |
| PTSD (%) | 26.8 | 29.3 | 23.9 |
| Anxiety/panic disorder (%) | 34.1 | 32.6 | 35.2 |
| Schizophrenia (%) | 5.5 | 3.3 | 1.4 |
| Genotype (%) | |||
| 1 | 79.9 | 80.9 | 83.8 |
| 2 | 12.4 | 6.9 | 2.8 |
| 3 | 7.0 | 11.6 | 13.4 |
| 4 | 0.8 | 0.6 | 0.0 |
| Laboratory results (%) | |||
| Anemia§ | 13.9 | 30.3 | 34.5 |
| Platelet count <100,000/μl | 12.8 | 39.4 | 16.2 |
| Creatinine >1.1 mg/dl | 19.6 | 16.1 | 53.6 |
| Bilirubin >1.1 g/dl | 13.4 | 33.1 | 21.8 |
| Albumin <3.6 g/dl | 19.4 | 51.2 | 26.8 |
| INR >1.1 | 21.1 | 47.9 | 21.4 |
| FIB-4 score† >3.25 | 34.7 | 71.4 | 45.0 |
HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LT, liver transplantation; PTSD, post-traumatic stress syndrome; INR, international normalized ratio.
FIB-4 score35 = [age × AST]/[platelets × ALT1/2].
Defined as a hemoglobin concentration <13 g/dl in men or <12 g/dl in women.
Sustained virologic response
SVR was defined by a viral load below the limit of quantification performed >12 weeks after the end of treatment.36 Prior work has demonstrated that SVR ascertained based on viral load 4 weeks after the end of treatment has 98% concordance with SVR based on viral load > 12 weeks after the end of treatment in SOF-treated patients.36 Therefore, if no viral load test was available >12 weeks after the end of treatment, then we defined SVR by a viral load performed 4–12 weeks post-treatment (n = 996).
Statistical analysis
Descriptive statistics were used to characterize study participants overall and by HCC status. We determined unadjusted SVR and 95% confidence intervals by HCC status, regimen, genotype and clinically significant subgroups. We developed a multivariable logistic regression model to identify predictors of SVR. Covariates were selected a priori based on putative relationships between SVR and HCC. These included age, gender, race/ethnicity, genotype, subgenotype, baseline viral load, prior antiviral treatment, diabetes, alcohol use disorder, cirrhosis, platelets <100,000, serum bilirubin >1.1 g/dl, serum albumin <3.6 g/dl, and FIB-4 score >3.25.
Some patients had no post-treatment viral load data >4 weeks after the conclusion of DAA therapy, precluding ascertainment of SVR. Missing SVR occurred more frequently in HCC (11.6%) compared to HCC/LT (6.3%) or no HCC (9.1%). To evaluate the impact of missing data, we compared patients with and without SVR data with respect to baseline characteristics and treatment duration. We multiply imputed missing SVR using a logistic regression model with the 23 baseline patient characteristics shown in Table 1, along with duration of treatment. The number of imputations varied from 10 to 200 resulting in identical estimates up to four significant digits. The model was determined stable and m = 20 imputations were used. The assumption of randomly missing data was found to be reasonable using the observed data.
Analyses were performed using STATA MP v14 (StataCorp, College Station,TX).
For further details regarding the materials used, please refer to the CTAT table.
Results
Population characteristics
Patients with HCC (n =482) or HCC/LT (n = 142) tended to be of an older age, white, and treatment experienced compared to non-HCC (n = 16,863, Table 1). The majority (85.1%) of non-transplanted HCC patients had documented cirrhosis; 31.5% had decompensated cirrhosis. Diabetes and depression were more common in HCC/LT compared to HCC (61.3% vs. 36.9%, and 59.2% vs. 47.9%).
Genotype 3 HCV was more common in HCC (11.6%) and HCC/ LT (13.4%) compared to non-HCC (6.9%). Patients with HCC/LT tended to have higher rates of elevated creatinine and anemia compared to others, consistent with the use of anti-rejection drugs. HCC patients without transplant tended to have higher rates of thrombocytopenia, elevated bilirubin, and low albumin, consistent with the higher prevalence of cirrhosis in this group.
Prior HCC treatments in patients without liver transplantation
Of 482 non-transplanted HCC patients, we identified 368 (76.4%) who received HCC treatment through the VA. Of these, 50.6% received at least one instance of TACE, 37.3% local ablation, 17.0% surgical resection, and 14.3% sorafenib. HCC patients were included in multiple treatment categories if they received >1 modality.
Antiviral treatment regimens by genotype and HCC status
Distribution of antiviral treatment regimens by genotype and HCC status is shown in Table 2. More genotype 1-infected patients with HCC and HCC/LT (88.1% and 99.1%) received a LDV/SOF-based regimen compared to 77.2% in the non-HCC group. Compared to patients who received LDV/SOF regimens, those receiving PrOD regimens were less likely to have elevated FIB-4 score (47.7% vs. 73.1%), thrombocytopenia (23.1% vs. 40.2%), or elevated bilirubin (21.6% vs. 35.9%).
Table 2.
SVR among clinically relevant subgroups by HCC treatment class and antiviral regimen.*
| n | No HCC, % (95% CI) | n | HCC % (95% CI) | n | HCC/LT % (95% CI) | |
|---|---|---|---|---|---|---|
| All patients | 15,325 | 91.1 (90.6, 91.5) | 426 | 74.4(70.0, 78.3) | 133 | 94.0 (88.3, 97.0) |
| Genotype 1 | 12,289 | 93.1 (92.6, 93.5) | 344 | 79.1 (74.4, 83.1) | 111 | 96.4(90.1,98.7) |
| LDV/SOF | 7,224 | 93.1 (92.5, 93.7) | 152 | 76.3 (68.8, 82.5) | 36 | 97.2 (81.2, 99.6) |
| LDV/SOF + ribavirin | 2,275 | 92.9 (91.8, 93.9) | 151 | 76.8 (69.3, 82.9) | 74 | 96.0 (87.9, 98.7) |
| PrOD | 702 | 94.9 (93.0, 96.2) | 2 | 100.0 - | 0 | - |
| PrOD + ribavirin | 2,088 | 92.3 (91.1, 93.4) | 39 | 97.4 (82.6, 99.7) | 1 | 100.0 - |
| Genotype 2 | ||||||
| SOF + ribavirin | 1,877 | 86.5 (84.9, 88.0) | 29 | 68.9 (49.0, 83.7) | 4 | 50.0 (2.5, 97.5) |
| Genotype 3 | 1,036 | 75.9 (73.3,78.5) | 51 | 47.0 (33.5, 61.1) | 18 | 88.9 (61.0, 97.6) |
| LDV/SOF + ribavirin | 319 | 78.9 (74.1,83.1) | 18 | 50.0 (26.4, 73.6) | 7 | 100.0 - |
| SOF + PeglFN + ribavirin | 125 | 88.8 (81.9,93.3) | 6 | 50.0 (9.1, 90.9) | 0 | - |
| SOF + ribavirin | 592 | 71.6(67.8, 75.1) | 27 | 44.4 (26.2, 64.3) | 11 | 81.8 (42.0, 96.5) |
| Genotype 4 | ||||||
| LDV/SOF ± ribavirin or PrOD ± ribavirin | 123 | 90.2 (83.5, 94.4) | 2 | 50.0- | 0 | - |
| No cirrhosis | 10,915 | 92.4(91.8, 92.8) | 63 | 82.5 (70.8, 90.2) | 133 | 94.0 (88.3, 97.0) |
| Cirrhosis | 4,410 | 87.9 (86.1, 88.8) | 363 | 73.0 (68.2, 77.3) | 0 | - |
| Treatment naïve | 10,840 | 91.4(90.9,91.9) | 238 | 76.8 (71.1, 81.8) | 81 | 92.6 (84.2, 96.7) |
| Treatment experienced | 4,485 | 90.3 (89.4, 91.1) | 188 | 71.3 (64.3, 77.3) | 52 | 96.1 (85.2, 99.1) |
HCC, hepatocellular carcinoma; HCC/LT, HCC with previous liver transplantation; SOF, sofosbuvir; LDV/SOF, ledipasvir/sofosbuvir; PrOD, paritaprevir/ritonavir/ombitasvir and dasabuvir.
N’s differ from Table 1 because Table 2 is limited to patients with known SVR status.
All genotype 2-infected patients received a combination of SOF and ribavirin. The majority of genotype 3-infected patients with HCC and HCC/LT received SOF + ribavirin (52.3% and 61.1%). A significant portion of HCC and HCC/LT patients received LDV/SOF plus ribavirin (35.3% and 38.9%) with the remainder of HCC patients (25.8%) receiving SOF/PeglFN + ribavirin. Among genotype 3-infected patients without HCC, 57.1% received SOF plus ribavirin, 30.8% received LDV/SOF, and 12.1% received PeglFN/SOF + ribavirin.
Observed SVR results
SVR outcomes were available for 15,884 patients who received HCV antiviral treatment (90.8% of those who initiated treatment) (Table 2). Overall SVR rates were substantially higher in the non-HCC group (91.1% [95% CI 90.6, 91.5]) and the HCC/LT group (94.0% [95% Cl 88.3, 97.0]) than in the HCC group, (74.4% [95% Cl 70.0, 78.3]) (Fig. 1). A pattern of higher SVR rates in the non-HCC and HCC/LT groups compared to the HCC group persisted across all genotypes and DAA regimens, and across subgroups defined by cirrhosis status or prior antiviral treatment (Table 2). Patients infected with genotypes 1 and 4 HCV had the highest rates of SVR within each HCC category and genotype 3 the lowest. Those with HCC/LT tended to have the highest SVR rates for each genotype. Among patients with HCC, those with cirrhosis had substantially lower SVR rates than those without (73.0% [95% CI 68.2, 77.3] vs. 82.5% [95% CI 70.8, 90.2]). Non-HCC patients with cirrhosis had lower SVR rates compared to the non-cirrhotic group (87.9% [95% CI 86.1, 88.8] vs. 92.4% [95% CI 91.8, 92.8]).
Fig. 1. SVR rates among patients with HCC, HCC/LT, and no HCC.
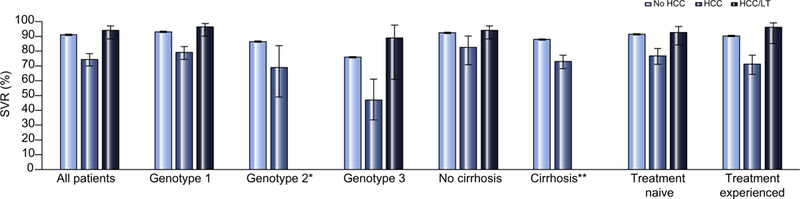
SVR, sustained virologic response; HCC, hepatocellular carcinoma; HCC/LT, HCC with previous liver transplantation. *Bar not shown due to small subgroup <5 patients; **Bar not shown because all patients with HCC/LT were considered to be non-cirrhotic.
For genotype 1-infected patients, SVR rates were 79.1% (95% CI 74.4, 83.1) for patients with HCC, 96.4% (95% CI 90.1, 98.7) for HCC/LT, and 93.1% (95% CI 92.6, 93.5) for non-HCC. PrOD regimens (n = 41) had the highest SVR in genotype 1-infected patients with HCC. Among patients who received PrOD with ribavirin (n = 39), SVR was 97.4% (95% CI 82.6, 99.7) vs. 76.3 (95% CI 68.8, 82.5) for LDV/SOF (n = 152) and 76.8% (95% CI 69.3, 82.9) for LDV/SOF with ribavirin (n = 151). Only one patient with HCC/LT received a PrOD regimen. SVR in genotype 1-infected patients with HCC/LT was 97.2% (95% CI 81, 99.6) for LDV/SOF and 96.0% (95% CI 87.9, 98.7) for LDV/SOF with ribavirin.
For genotype 2-infected patients, the rate of SVR was 68.9% (95% CI 49.0, 83.7) for HCC, and 86.5% (95% CI 84.9, 88.0) for patients without HCC. A very small number of genotype 2-infected patients with HCC/LT were identified (n = 4), with an SVR rate of 50.0% (95% CI 2.5, 97.5). Among genotype 3-infected patients, the rate of SVR was 47.0% (95% CI 33.5, 61.1) for HCC, 88.9% (95% CI 61.0, 97.6) for HCC/LT and 75.9% (95% CI 73.3, 78.5) for patients without HCC. In HCC genotype 3-infected patients, treatment success was highest among those receiving LDV/SOF + ribavirin (100%) compared with other regimens, though the sample size was small (n = 7).
We examined SVR rates by type of HCC treatment modality (Table S3). Patients with surgical resection had the highest SVR rate (78.9% [95% CI 68.1,86.8]), followed by local ablation (70.1% [95% CI 62.6,76.6]), TACE (70.0% [95% CI 63.6,75.7]), and sorafenib (59.0% [95% CI 46.0, 70.9]). The SVR rate for patients without documented HCC treatment data was 78.0% (95% CI 68.2, 85.4), similar to the rate of 73.4% (95% CI 68.4, 77.9) for patients with HCC treatment documented within VA.
Early treatment discontinuation, treatment duration and HCC status
Among all patients (n = 17,487), early discontinuation of treatment before 8 weeks was slightly more common in patients without HCC (6.3%) compared to HCC (4.6%) or HCC/LT (4.9%) (Table S4). Mean duration of treatment was shorter in non-HCC patients (84.9 days [SD 32.3]) than in HCC (102.2 [SD 42.4]) or HCC/LT patients (102.3[SD 40.4]). This was driven by two factors: first, non-HCC patients more commonly completed 8-week regimens (14.4%) than HCC or HCC/LT patients (4.6% and 1.4%), as expected since patients with cirrhosis or LT are ineligible for 8-week LED/SOF regimens; second, patients with HCC or HCC/LT more commonly completed 24 week regimens (18.7% and 22.5%, respectively) than patients without HCC (8.0%).
Impact of missing SVR data and imputation for missing SVR
SVR data was missing in 1,603 (9%) of 17,487 patients who initiated DAAs. We compared patients with and without missing SVR and found no clinically meaningful differences in HCC status, age, genotype, cirrhosis, and most other baseline characteristics (Table S5). However, patients with missing SVR were more likely to be Hispanic, treatment naive, and to have prior depression, or alcohol or substance use disorders. Duration of treatment was 71 days (SD 38) among patients with available SVR data and 87 days (SD 31) among patients with missing data. Among patients without SVR data, 25% discontinued therapy between week 0–8 compared with 4.4% with available SVR, suggesting a possible relationship between early discontinuation and missing SVR. We performed several analyses to impute missing SVR using a logistic regression model including duration of treatment together with baseline patient characteristics shown in Table 1. After including imputed results, SVR rates were slightly lower compared to observed SVR results (1–2% difference across HCC subgroups) (Table S6).
Predictors of antiviral treatment outcome
In multivariable analyses in all genotypes combined, HCC patients were less likely to achieve SVR than non-HCC patients (AOR 0.38 [95% CI 0.26, 0.45], p <0.001) after adjusting for genotype, cirrhosis, and other characteristics (Table 3). HCC negatively predicted SVR in patients infected with genotypes 1 (AOR 0.34 [95% CI 0.26, 0.45], p <0.001) and 3 (AOR 0.41 [95% CI 0.22, 0.76], p = 0.005), with a non-significant trend observed in genotype 2 (AOR 0.59 [95% CI 0.25,1.38], p = 0.22). A non-significant trend towards higher SVR was observed in patients with HCC/ LT overall, and within genotypes 1, 2, and 3.
Table 3.
Association between HCC and SVR in multivariable logistic regression models.
| All patients (n = 15,573) |
Genotype 1 (n = 12,493) |
Genotype 2 (n = 1,868) |
Genotype 3 (n = 1,084) |
|||||
|---|---|---|---|---|---|---|---|---|
| AOR (95% CI)† | p value | AOR (95% CI)† | p value | AOR (95% CI)† | p value | AOR (95% CI)† | p value | |
| No HCC | 1 | - | 1 | - | 1 | - | 1 | - |
| HCC | 0.38 (0.29, 0.48) | <0.001 | 0.34 (0.26, 0.45) | <0.001 | 0.59 (0.25,1.38) | 0.224 | 0.41 (0.22, 0.76) | 0.005 |
| HCC/LT | 1.57 (0.75,3.28) | 0.23 | 1.89 (0.68,5.20) | 0.21 | 0.10(0.01, 0.82) | 0.03 | 2.7 (0.58, 12.6) | 0.20 |
AOR: Adjusted odds ratio, by multivariable logistic regression modeling including age, gender, race/ethnicity, alcohol use disorders, genotype, subgenotype, HCV regimen, baseline HCV viral load, diabetes, treatment naive/experienced, cirrhosis, decompensated cirrhosis, platelet count, bilirubin, and albumin. HCC, hepatocellular carcinoma; HCC/LT, HCC with previous liver transplantation.
In multivariate analysis of non-transplanted HCC patients, genotype 3 was the only independent predictor of failure to achieve SVR (AOR 0.19 [95% CI 0.10, 0.41], p <0.001) (Table 4). Among HCC patients with genotype 1 HCV, multivariate analysis including drug regimen revealed that black race was associated with failure to achieve SVR (AOR 0.49 [95% CI 0.24, 0.97], p = 0.04) while receipt of PrOD + ribavirin was associated with SVR relative to LDV/SOF (AOR 10.07 [95% CI 1.28, 79.3], p = 0.028). Hispanic race closely approached statistical signify-cance as a negative predictor of SVR (AOR 0.30 [95% CI 0.09, 1.00], p = 0.05) (Table 4).
Table 4.
Predictors of SVR among patients with history of HCC and without prior liver transplant.
| HCC, all genotypes (n = 426) |
HCC, genotype 1 HCV (n = 344) |
|||||
|---|---|---|---|---|---|---|
| AOR† | 95% (CI) | p value | AOR† | 95% (CI) | p value | |
| Age | 0.98 | 0.92, 1.03 | 0.42 | 0.96 | 0.90, 1.02 | 0.24 |
| Race/Ethnicity | ||||||
| White, non-Hispanic | 1 | 1 | 1 | 1 | 1 | 1 |
| Black, non-Hispanic | 0.66 | 0.35, 1.23 | 0.19 | 0.49 | 0.24, 0.97 | 0.04 |
| Hispanic | 0.51 | 0.18, 1.43 | 0.20 | 0.30 | 0.09, 1.00 | 0.05 |
| Asian, Pacific Islander, American Indian | 0.90 | 0.17, 4.66 | 0.90 | 0.59 | 0.11, 3.30 | 0.55 |
| Missing | 1.41 | 0.53, 3.78 | 0.49 | 1.41 | 0.42, 4.73 | 0.57 |
| Alcohol use disorder | 0.66 | 0.39, 1.11 | 0.12 | 0.69 | 0.38, 1.26 | 0.23 |
| Diabetes | 0.64 | 0.38, 1.08 | 0.09 | 0.64 | 0.35, 1.18 | 0.16 |
| Genotype | ||||||
| 1 | 1 | 1 | 1 | n.a. | n.a. | n.a. |
| 2 | 0.43 | 0.16, 1.15 | 0.09 | n.a. | n.a. | n.a. |
| 3 | 0.19 | 0.10, 0.41 | < 0.001 | n.a. | n.a. | n.a. |
| 4 | 0.26 | 0.01, 5.01 | 0.37 | n.a. | n.a. | n.a. |
| Antiviral regimen | ||||||
| LDV/SOF | n.a. | n.a. | n.a. | 1 | 1 | 1 |
| LDV/SOF + ribavirin | n.a. | n.a. | n.a. | 1.07 | 0.58, 1.95 | 0.83 |
| PrOD | n.a. | n.a. | n.a. | - | - | - |
| PrOD + ribavirin | n.a. | n.a. | n.a. | 10.07 | 1.28, 79.3 | 0.028 |
| Baseline HCV RNA >6 million IU/ml | 0.55 | 0.24, 1.24 | 0.15 | 0.55 | 0.24, 1.24 | 0.15 |
| FIB-4 >3.25 | 0.59 | 0.30, 1.16 | 0.12 | 0.76 | 0.34, 1.67 | 0.49 |
| Platelet count <100,000/μl | 0.85 | 0.47, 1.55 | 0.60 | 0.51 | 0.24, 0.97 | 0.07 |
| Bilirubin >1.1 g/dl | 0.70 | 0.39, 1.25 | 0.23 | 0.82 | 0.42, 1.59 | 0.56 |
| Albumin <3.6 g/dl | 1.41 | 0.80, 2.48 | 0.23 | 1.62 | 0.81, 3.23 | 0.17 |
| Cirrhosis | 0.68 | 0.31, 1.48 | 0.33 | 0.84 | 0.35, 2.07 | 0.71 |
| Treatment experienced | 0.79 | 0.48, 1.31 | 0.36 | 0.81 | 0.45, 1.47 | 0.49 |
AOR: Adjusted odds ratio, by multivariable logistic regression modeling including all variables shown in the table.
We performed an exploratory multivariable analysis of the impact of ribavirin in genotype 1 patients with HCC, limited to LDV/SOF since virtually all HCC patients receiving PrOD patients also received ribavirin. The adjusted odds ratio for LDV/SOF compared to LDV/SOF + ribavirin was 1.09 (95% CI 0.60, 2.0), indicating no statistically significant effect of adding ribavirin.
Discussion
DAAs have increased the efficacy of HCV therapy for many patients once considered difficult to treat, including those with prior HCC, with or without liver transplantation. Among non-transplanted HCC patients from the VA who received DAA therapy between 1st January, 2014 and 30th June, 2015 (n = 426), SVR was achieved in 74.4% (95% CI 70.0, 78.3). Among 133 additional HCC patients who underwent liver transplantation following HCC diagnosis, the SVR rate was much higher (94.0% [95% CI 88.3, 97.0]) and similar to that of the general HCV population (91.1% [95% CI 90.6, 91.5]). While the odds of SVR in non-transplanted HCC patients was significantly lower compared to non-HCC (AOR 0.34 [95% CI 0.26, 0.45], p <0.001), treatment success remains within reach for most HCC patients.
As in the non-HCC population, SVR results vary by genotype in patients with HCC. HCC patients with genotype 1 infection achieved an SVR rate of 79.1% [95% CI 74.4, 83.1], while those with HCC/LT achieved an SVR rate of 96.4% [95% CI 90.1, 98.7] which compares favorably to the general genotype 1-infected population (93.1% [95% CI 92.6, 93.5]). The overall rate of SVR in HCC genotype 2 and 3-infected patients was lower than for genotype 1 at 68.9% (95% CI 49.0, 83.7) and 47.0% (95% CI 33.5, 61.1), though findings are limited by small sample sizes. We did not detect an independent relationship between prior treatment and SVR.
It is unclear why patients with HCC had such lower SVR rates compared to patients without HCC. The association between HCC and treatment failure persisted after adjustment for cirrhosis, markers of liver dysfunction, and genotype (Table 3). Therefore, these factors cannot explain the lower SVR in patients with HCC, and lead us to suspect that HCC, itself, could be causally linked to antiviral treatment failure. For example, HCV within tumor cells could be relatively inaccessible to DAAs due to differences in blood supply or other reasons. Additionally, HCC arises in the setting of chronic inflammation, distorted liver architecture, and alterations in the cytokine and chemokine environment.37,38 We speculate that altered hepatic immune processes may predispose both to HCC and to poorer antiviral treatment outcomes. Finally, it is possible that viral factors such as resistance associated substitutions and quasispecies may differ in patients with HCC and contribute to treatment failure.
When limiting to genotype 1 patients, black race was an independent predictor of SVR (AOR 0.49 [95% CI 0.24, 0.97], p = 0.04) (Table 5). A recent pooled analysis showed similar rates of SVR12 in black (95%) vs. non-black patients (97%) after 12 weeks of LDV/SOF ± ribavirin.39 While DAA regimens may have greatly mitigated the association between black race and treatment failure seen in the interferon era, additional study is warranted to explore the finding of lower SVR outcomes in black patients with HCC.
Among genotype 1-infected HCV patients with HCC, only a minority (15.3%, n = 52) were initiated on PrOD compared to LDF/SOF regimens (84.6%, n = 338). The rate of SVR was 97.4% (95% CI 82.6, 99.7) for PrOD + ribavirin compared to 76.8% (95% CI 69.3, 82.9) for LDV/SOF + ribavirin. We investigated whether genotype 1-infected patients who received LDV/SOF were more likely to have selected adverse factors related to poor liver function, given contraindications to PrOD in decompensated cirrho-sis.40 Compared to patients who received LDV/SOF regimens, those receiving PrOD regimens were less likely to have markers of cirrhosis such as elevated FIB-4 (47.7% vs. 73.1%), thrombocytopenia (23.1% vs. 40.2%), or elevated bilirubin (21.6% vs. 35.9%). However, after adjustment for race, bilirubin, platelet count, albumin, FIB-4, viral load, alcohol use, diabetes, and treatment experience, we continued to find an association between PrOD regimens and SVR compared to LDV/SOF (AOR 10.07 [95% CI 1.28, 79.3], p = 0.028). In the general HCV population, PrOD regimens were previously reported to have similar SVR compared to LDV/SOF in genotype 1-infected patients.41 Given our small population of genotype 1-infected HCC patients who received PrOD, our finding requires confirmation in other populations with HCC.
Patients with a history of HCC and subsequent liver transplant had superior SVR (94.0% [95% CI 88.3, 97.0]) compared to non-transplanted patients with HCC (74.4% [95% CI 70.0, 78.3]). High rates of SVR in HCC/LT likely result from correction of liver dysfunction and removal of the underlying HCC. In addition, transplant patients typically receive expert follow-up care, usually from subspecialists at tertiary centers. Finally, transplant patients are selected for high levels of motivation and medical adherence, which likely predicts compliance with antiviral regimens. The high rates of SVR observed in post-transplant patients echo multiple HCV clinical trials performed in the post-transplant setting, though no published trials have focused solely on transplant patients with a history of HCC.6,8,42–44
Whether to offer HCV treatment pre-transplant versus deferring to the post-transplant setting remains an area of clinical uncertainty. Our findings suggest that HCC patients currently listed for transplantation, or those with a high probability of getting transplanted quickly, might benefit from postponing antiviral treatment until after transplant in order to maximize the chances of SVR. However, our findings also show that non-transplanted HCC patients, representing the majority of the HCC population, can still achieve SVR in most cases (74.4% [95% Cl 70.0, 78.3]). A small (n = 58), retrospective, non-randomized study recently reported an “unexpected high rate” of early tumor recurrence in patients with HCV-related HCC after DAA therapy.17 The investigators speculated that HCV eradication may facilitate HCC recurrence by altering host immune status. A second single-center observational report from the US (n = 112) similarly reported a trend towards higher risk of HCC recurrence in patients who received DAAs pre-transplant compared to untreated patients (27.8% vs. 9.5%, p = 0.06), though these results were not adjusted for length of follow-up time nor for predictors of HCC recurrence.20 On the converse, the largest retrospective cohort study to date (n = 314 HCC patients with transplant; n = 202 HCC patients without) did not find an increased risk of HCC recurrence after DAA therapy.19 These early findings needs to be confirmed by larger, prospective, controlled studies before they can be used to guide practice.
Our study benefited from a large cohort of HCC patients in a single-payer national system with near-complete capture of laboratory and pharmacy data obtained within VA. Our findings should be interpreted with several caveats. SVR outcome was missing in 11.6% of HCC patients and 6.3% of HCC patients treated with transplant. Most baseline patient characteristics were similar for those with and without known SVR status, but patients with missing SVR data appeared more likely to discontinue treatment before 8 weeks. Of note, patients with HCC and HCC/LT were less likely to discontinue therapy early (before 8 weeks) compared to those without HCC, suggesting that the lower SVR noted in the HCC population is unlikely due to premature discontinuation of therapy. After careful imputation of missing SVR results, including duration of therapy as a predictor, imputed SVR appeared negligibly lower than observed values (1–2% difference across HCC subgroups). A second limitation concerns the availability of HCC treatment data, which was missing in 23.6% of non-transplanted cases, even after we searched for HCC interventions outsourced to non-VA settings. Patients with a history of HCC are extremely likely to have received some form of HCC-specific treatment as a prerequisite before antiviral therapy, because it would be difficult to rationalize antiviral therapy in a patient with untreated liver cancer. It is possible that some HCC treatments were provided through non-VA health insurance, and therefore not captured by our methodology, or that the coding of HCC-related interventions is incomplete. It is also possible that some patients who were listed for liver transplantation received antiviral therapy pre-emptively, in the absence of HCC treatment. Further validation work is needed to clarify the specific types of HCC treatments received, responses to HCC therapy, and the time elapsed between HCC treatment and antiviral therapy. A third limitation concerns the ascertainment of HCC itself. Although we used well-validated ICD-9 codes to identify cases of HCC, misclassification of patients with HCC is always a risk. Finally, results from our overwhelmingly male study population may have limited generalizability to women.
Future study is needed to determine if overall and cancer-specific mortality is improved in HCC patients who achieve SVR as a result of HCV antiviral therapy relative to those who do not achieve SVR or relative to those who remain untreated. Our observational results suggest that HCV can be cured in the majority of patients with HCC, and in virtually all with a prior history of HCC and subsequent liver transplantation.
Supplementary Material
Acknowledgments
This material is the result of work supported by resources from the VA Puget Sound Health Care System (Seattle, Washington). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Financial support
The study was funded by Merit Review grants (I01CX000320 and I01CX001156), Clinical Science Research and Development, Office of Research and Development, Veterans Affairs (GNI).
Footnotes
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jhep.2017.02.027.
References
- [1].Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden ofcirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology 2015. [DOI] [PubMed] [Google Scholar]
- [3].El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med 2000;160:3227–3230. [DOI] [PubMed] [Google Scholar]
- [4].Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 2010;138:513–521, 521 e511–516. [DOI] [PubMed] [Google Scholar]
- [5].Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis 2016. [DOI] [PubMed] [Google Scholar]
- [6].Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS Jr, et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015;149:649–659. [DOI] [PubMed] [Google Scholar]
- [7].Foster GR, Irving WL, Cheung MC, Walker AJ, Hudson BE, Verma S, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol 2016;64:1224–1231. [DOI] [PubMed] [Google Scholar]
- [8].Curry MP, Forns X, Chung RT, Terrault NA, Brown R Jr, Fenkel JM, et al. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology 2015;148(100–107): e101. [DOI] [PubMed] [Google Scholar]
- [9].AASLD/IDSA/IAS-USA. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. [cited 10/9/2015]; Available from: http://www.hcvguidelines.org.
- [10].Belli LS, Berenguer M, Cortesi PA, Strazzabosco M, Rockenschaub SR, Martini S, et al. Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: A European study. J Hepatol 2016;65:524–531. [DOI] [PubMed] [Google Scholar]
- [11].Cheung MC, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol 2016;65:741–747. [DOI] [PubMed] [Google Scholar]
- [12].Kanogawa N, Ogasawara S, Chiba T, Saito T, Motoyama T, Suzuki E, et al. Sustained virologic response achieved after curative treatment of hepatitis C virus-related hepatocellular carcinoma as an independent prognostic factor. J Gastroenterol Hepatol 2015;30:1197–1204. [DOI] [PubMed] [Google Scholar]
- [13].Hsu YC, Ho HJ, Wu MS, Lin JT, Wu CY. Postoperative peg-interferon plus ribavirin is associated with reduced recurrence of hepatitis C virus-related hepatocellular carcinoma. Hepatology (Baltimore, MD) 2013;58:150–157. [DOI] [PubMed] [Google Scholar]
- [14].Harada N, Hiramatsu N, Oze T, Tatsumi T, Hayashi N, Takehara T. Efficacy of pegylated interferon and ribavirin combination therapy for patients with hepatitis C virus infection after curative resection or ablation for hepatocellular carcinoma—a retrospective multicenter study. J Med Virol 2015;87:1199–1206. [DOI] [PubMed] [Google Scholar]
- [15].Shirabe K, Sugimachi K, Harada N, Kayashima H, Maeda T, Tsujita E, et al. Favorable prognosis in patients with sustained virological response to antiviral therapy, including interferon, for chronic hepatitis C before hepatic resection for hepatocellular carcinoma. Anticancer Res 2015;35:6963–6969. [PubMed] [Google Scholar]
- [16].Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013;158:329–337. [DOI] [PubMed] [Google Scholar]
- [17].Reig M, Marino Z, Perello C, Inarrairaegui M, Ribeiro A, Lens S, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol 2016;65:719–726. [DOI] [PubMed] [Google Scholar]
- [18].Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016;65:727–733. [DOI] [PubMed] [Google Scholar]
- [19].Pol S Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol 2016;65:734–740. [DOI] [PubMed] [Google Scholar]
- [20].Yang JD, Aqel BA, Pungpapong S, Gores GJ, Roberts LR, Leise MD. Direct acting antiviral therapy and tumor recurrence after liver transplantation for hepatitis C-associated hepatocellular carcinoma. J Hepatol 2016;65:859–860. [DOI] [PubMed] [Google Scholar]
- [21].Corporate Data Warenouse (CDW). March 28, 2014. [cited Feb 2, 2016]; Available from: http://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm.
- [22].Beste LA, Ioannou GN. Prevalence and treatment of chronic hepatitis C virus infection in the US Department of Veterans Affairs. Epidemiol Rev 2015;37:131–143. [DOI] [PubMed] [Google Scholar]
- [23].Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Alimentary pharmacol Therapeut 2008;27:274–282. [DOI] [PubMed] [Google Scholar]
- [24].Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect ofHIV coinfection on the riskof cirrhosis and hepatocellular carcinoma in U.S. Veterans with hepatitis C. Am J Gastroenterol 2005;100:56–63. [DOI] [PubMed] [Google Scholar]
- [25].Ioannou GN, Splan MF, Weiss NS, McDonald GB, Beretta L, Lee SP. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2007;5:938–945, 945 e931–934. [DOI] [PubMed] [Google Scholar]
- [26].Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med 2011;154:85–93. [DOI] [PubMed] [Google Scholar]
- [27].Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, Boyko EJ. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology 2013;57:249–257. [DOI] [PubMed] [Google Scholar]
- [28].Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology 2015;149:1471–1482, e1475; quiz e1417–1478. [DOI] [PubMed] [Google Scholar]
- [29].Rajbhandari R, Simon RE, Chung RT, Ananthakrishnan AN. Racial disparities in inhospital outcomes for hepatocellular carcinoma in the United States. Mayo Clin Proc 2016;91:1173–1182. [DOI] [PubMed] [Google Scholar]
- [30].Kramer JR, Kanwal F, Richardson P, Giordano TP, Petersen LA, El-Serag HB. Importance of patient, provider, and facility predictors of hepatitis C virus treatment in veterans: a national study. Am J Gastroenterol 2011;106:483–491. [DOI] [PubMed] [Google Scholar]
- [31].Beste LA, Ioannou GN, Larson MS, Chapko M, DominitzJA. Predictors of early treatment discontinuation among patients with genotype 1 hepatitis C and implications for viral eradication. Clin Gastroenterol Hepatol 2010;8:972–978. [DOI] [PubMed] [Google Scholar]
- [32].Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology (Baltimore, MD) 2007;46:37–47. [DOI] [PubMed] [Google Scholar]
- [33].Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology 2011;140(1182–1188):e1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care 2004;27(Suppl 2):B10–21. [DOI] [PubMed] [Google Scholar]
- [35].Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007;46:32–36. [DOI] [PubMed] [Google Scholar]
- [36].Yoshida EM, Sulkowski MS, Gane EJ, Herring RW Jr, Ratziu V, Ding X, et al. Concordance of sustained virological response 4, 12, and 24 weeks posttreatment with sofosbuvir-containing regimens for hepatitis C virus. Hepa-tology 2015;61:41–45. [DOI] [PubMed] [Google Scholar]
- [37].Wirth TC, Manns MP. The impact of the revolution in hepatitis C treatment on hepatocellular carcinoma. Ann Oncol 2016;27:1467–1474. [DOI] [PubMed] [Google Scholar]
- [38].Hengst J, Falk CS, Schlaphoff V, Deterding K, Manns MP, Cornberg M, et al. DAA-induced HCV clearance does not completely restore the altered cytokine and chemokine milieu in patients with chronic hepatitis C. J Infect Dis 2016. [DOI] [PubMed] [Google Scholar]
- [39].Wilder JM, Jeffers LJ, Ravendhran N, Shiffman ML, Poulos J, Sulkowski MS, et al. Safety and efficacy of ledipasvir-sofosbuvir in black patients with hepatitis C virus infection: a retrospective analysis of phase 3 data. Hepatology 2016;63:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].VIEKIRA PAK [package insert]. North Chicago, IL: AbbVie Inc. [cited August 8, 2016]; Available from: https://www.viekirahcp.com/.
- [41].Ioannou GN, Beste LA, Chang MF, Green PK, Lowy E, Tsui JI, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology 2016;151(457–471):e455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Charlton M, Gane E, Manns MP, Brown RS Jr, Curry MP, Kwo PY, et al. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology 2015;148:108–117. [DOI] [PubMed] [Google Scholar]
- [43].Forns X, Charlton M, Denning J, McHutchison JG, Symonds WT, Brainard D, et al. Sofosbuvir compassionate use program for patients with severe recurrent hepatitis C after liver transplantation. Hepatology (Baltimore, MD) 2015;61:1485–1494. [DOI] [PubMed] [Google Scholar]
- [44].Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, Brown R Jr, et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med 2014;371:2375–2382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


