Abstract
Background:
Data on rates of heart failure (HF) hospitalizations, recurrent hospitalizations and outcomes related to HF hospitalizations in CKD are limited.
Objective:
We examined rates of HF hospitalizations and re-hospitalizations within a large, CKD population and evaluated the burden of HF hospitalizations with risk of subsequent CKD progression and death.
Methods:
Evaluating the prospective Chronic Renal Insufficiency Cohort (CRIC) Study, we estimated glomerular filtration rate (eGFR) and urine albumin-to-creatinine ratio (ACR) were measured at baseline. We calculated crude rates and rate ratios of HF hospitalizations and 30-day HF re-hospitalizations using Poisson regression models. Cox regression was used to assess the association of the frequency of HF hospitalizations within the first 2 years of follow-up with risk of subsequent CKD progression and death.
Results:
Among 3,791 participants, the crude rate of HF admissions was 5.8 per 100 person-years (with higher rates of preserved vs. reduced ejection fraction HF). The adjusted rate of HF was higher with lower eGFR (vs. eGFR >45 ml/min/1.73m2): rate ratio 1.7 and 2.2 for eGFR 30-44 and <30 ml/min/1.73m2 (vs. >45 ml/min/1.73 m2), respectively. Similarly, the adjusted rates of HF hospitalization were significantly higher in those with higher urine ACR (vs. urine ACR <30 mg/g): rate ratios of 1.9 and 2.6 for urine ACR 30-299, and ≥300 mg/g, respectively. Overall, 20.6% of participants had a subsequent HF re-admission within 30 days. HF hospitalization within 2 years of study entry was associated with greater adjusted risks for CKD progression (1 hospitalization: HR 1.93, 95%CI: 1.40, 2.67; 2+ hospitalizations: HR 2.14, 95%CI: 1.30, 3.54) and all-cause death (1 hospitalization: HR 2.20 (1.71, 2.84; 2+ hospitalizations: HR 3.06, 95%CI: 2.23, 4.18).
Conclusions:
Within a large U.S. CKD population, the rates of HF hospitalizations and rehospitalization were very high, with even higher rates across categories of lower eGFR and higher urine ACR. CKD patients hospitalized with HF had greater risks of CKD progression and death. HF prevention and treatment should be a public health priority to improve CKD outcomes.
CONDENSED ABSTRACT:
This study examined rates of heart failure hospitalizations within a large, U.S. population of chronic kidney disease participants. The study found that the rates of heart failure hospitalizations and 30-day heart failure re-hospitalizations were high in this population and the rates increased across categories of lower kidney function and higher urinary protein. Furthermore, chronic kidney disease participants hospitalized with heart failure had greater risks of kidney disease progression and death. Prevention and treatment of heart failure should be a public health priority to improve outcomes in chronic kidney disease.
Introduction
Heart failure (HF) is one of the leading causes of morbidity and mortality in the U.S. HF is the primary diagnosis in >1 million hospitalizations annually and the rates of hospitalizations for HF continue to increase (1,2). The total cost of HF care in the U.S. exceeds $30 billion annually (1). Hospitalization for HF represents a sentinel prognostic event in the course of many patients, with a high risk for recurrent hospitalization (e.g., 50% at 6 months), and a 1-year mortality rate of approximately 30% (3,4).
The risk of HF is even greater in patients with chronic kidney disease (CKD) (5-10). Prior studies have estimated that patients with CKD have threefold increased risk of incident HF (6). It is also well known that patients with CKD, in particular those with end-stage-renal-disease (ESRD) requiring dialysis, have higher rates of hospitalizations. Data from the U.S. Renal Data System (USRDS) indicate that, on average, ESRD patients are hospitalized twice a year and about 30% have an unplanned re-hospitalization within 30 days (11). However, there are limited published data on rates of HF hospitalizations, recurrent hospitalizations and outcomes related to HF hospitalizations specifically in patients with CKD, which may help prioritize research and health care policy initiatives. Therefore, we examined rates of HF hospitalizations and rehospitalizations within a large, multi-center CKD population, as well as evaluated the association between burden of HF hospitalizations and subsequent progression of CKD and all-cause death.
Methods
Study population
We studied 3,939 individuals with mild to severe CKD enrolled in the Chronic Renal Insufficiency Cohort (CRIC) study. The CRIC study recruited participants with mild to severe CKD, defined as an eGFR 20-70 ml/min/1.73m2, between June 2003 and August 2008 at 7 clinical centers across the U.S. (Ann Arbor/Detroit, MI; Baltimore, MD; Chicago, IL; Cleveland, OH; New Orleans, LA; Philadelphia, PA; and Oakland, CA) (12,13). All study participants provided written informed consent, and the study protocol was approved by institutional review boards at each of the participating sites. Detailed inclusion and exclusion criteria have been previously described (12). Participants on maintenance dialysis or with a kidney transplant were not included at cohort entry. CRIC also excluded participants with advanced HF, defined as New York Heart Association Class III or IV, on cohort entry. All participants enrolled in the study had annual in-person study visits where detailed interviews were conducted, brief physical examination performed, laboratory measures done, and cardiovascular testing performed. In addition to the annual study visits, all CRIC participants were contacted every 6 months to obtain updated information on medication use and interim updates to medical history/hospitalizations.
For the present study, we excluded 148 participants who were missing urine albumin to creatinine ratio at the baseline study visit, leaving us with a final analytical cohort of 3,791 participants.
Measures of kidney function and definition of CKD progression
We evaluated two measures of kidney function: estimated glomerular filtration rate (eGFR) and urine albumin-to-creatinine ratio (urine ACR), which were measured via standardized methods at annual research study visits Serum creatinine was measured using an enzymatic method on an Ortho Vitros 950 at the CRIC central laboratory and standardized to isotope dilution mass spectrometry-traceable values (14,15). Estimated GFR was calculated using serum creatinine and the CKD-EPI equation (16) and categorized as ≥45, 30-44, and <30 ml/min/1.73 m2 (17). Urine ACR was quantified from 24-hour urine samples and categorized as <30, 30-299 or >300 mg/g (17).
CKD progression was defined as either 50% decline in eGFR or progression to ESRD. All eGFR measures were calculated from serum creatinine obtained at annual study visits. ESRD was defined as receipt of chronic dialysis or a kidney transplant and was identified through participant self-report, medical records review and data from the USRDS.
Heart failure hospitalizations
Hospitalizations for HF were adjudicated from study entry through May 2014. Hospitalizations for HF were identified by asking study participants semi-annually if they were hospitalized and electronic health records from selected hospitals or health care systems were also queried for qualifying encounters. The first 30 discharge codes were identified for all hospitalizations, and codes relevant to HF resulted in retrieval of medical records by study personnel for centralized adjudicated review. At least 2 study physicians reviewed all possible HF events and deaths using medical records and adjudicated for clinical HF based on clinical symptoms, radiographic evidence of pulmonary congestion, physical examination of the heart and lungs and, when available, central venous hemodynamic monitoring data, and echocardiographic imaging. HF was confirmed when both reviewers agreed upon a “probable” or “definite” occurrence of HF based on modified clinical Framingham criteria.(18)
In addition to quantifying the number of HF hospitalizations, we also evaluated the number of HF readmissions within 30 days during all available CRIC follow-up. Additionally, we evaluated the total number of HF hospitalization days during CRIC follow-up. This was calculated from length of stay data available for each HF hospitalization.
In secondary analyses, we stratified the outcome of HF by preserved and reduced ejection fraction HF (HFpEF and HFrEF). HFpEF was defined as ejection fraction ≥40% and HFrEF was defined as ejection fraction <40%. Ejection fraction was ascertained from echocardiograms performed during the index hospitalization for clinical purposes. If an echocardiogram was not performed during the index hospitalization, we utilized the ejection fraction quantified from an ambulatory CRIC research echocardiogram ≤1 year before or after the index HF hospitalization. Research echocardiograms in CRIC were performed at multiple time-points including years 1, 4, 7, as well as when the participant progressed to eGFR<20 ml/min/1.73m2 Our previous work has shown that ejection fraction in CRIC is generally stable over time.(19,20) Among a total of 1774 first (during CRIC follow-up) or recurrent HF hospitalizations, 1127 (64%) had ejection fraction available through either a clinical echocardiogram during the index hospitalization or a CRIC research echocardiogram.
All-cause mortality
Death status was ascertained from study entry through May 2014. Deaths were identified from report from next of kin, retrieval of death certificates or obituaries, review of hospital or outpatient records, and searching Social Security Death vital status and state death certificate files, if available.
Covariates
At the baseline study visit, participants provided information on their sociodemographic characteristics, medical history, medication usage, and lifestyle behaviors. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Hispanic, and other. History of cardiovascular disease was determined by self-report and included history of HF, myocardial infarction, coronary revascularization or stroke. Anthropometric measurements and blood pressure (BP) were assessed using standard protocols.(21) Body mass index (BMI) was calculated as weight in kg divided by height in meters squared. Diabetes mellitus was defined as a fasting glucose >126 mg/dL, a non-fasting glucose >200 mg/dL, or use of insulin or other antidiabetic medication. Cardiovascular medications including diuretics, angiotensin converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs), and β-blockers and were ascertained by detailed review with participants at baseline.
Statistical analyses
All analyses were performed using R 3.4.0 (R Foundation for Computing, Vienna, Austria). We first described characteristics of study participants, across categories based on number of HF hospitalizations per year. We then calculated the crude rates (per 100 person-years) of HF hospitalizations and 30-day HF re-hospitalizations, overall and by categories of eGFR and urine ACR individually and collectively; 95% confidence intervals were obtained using bootstrap methods. Participants were censored at death, loss to follow-up, study withdrawal or end of study follow-up (through May 2014). Rate ratios for HF hospitalizations and 30-day re-hospitalizations were calculated using Poisson regression models with Huber-White robust standard errors. We serially adjusted for age, sex, race/ethnicity in Model 1; additionally adjusted for diabetes, history of cardiovascular disease, use of statins, current smoking, systolic blood pressure and BMI in Model 2; and additionally adjusted for use of cardiovascular medications (diuretics, ACEi/ARBs and β-blockers) in the final Model 3. In secondary analyses, we repeated these analyses examining rates and rate ratios for HFrEF and HFpEF among participants who had available data on ejection fraction.
Cox regression was used to model the association of the number of HF hospitalizations within the first 2 years of study follow-up with subsequent risk of CKD progression (defined as eGFR decline by 50% or progression to ESRD) and all-cause mortality. Participants were censored at death, loss to follow-up, study withdrawal or at the end of study follow-up (through May 2014), whichever came first. For these analyses, time to follow-up started at year 2 and only included participants who did not experience the event within the first two years of the study or were not censored during this time (N = 2978 for CKD progression, N = 3582 for mortality). These models were adjusted for the same covariates as described above.
In a secondary analysis, we examined the total number of HF hospital days as a predictor. We calculated crude rates and rate ratios of HF hospital days across eGFR and urine ACR categories (similar to the primary analysis). We then used Cox models to examine the association of number of HF hospital days within the first 2 years of study follow-up with subsequent risks of CKD progression and all-cause mortality.
Results
Study population
A total of 1,774 HF hospitalizations during the follow-up period were observed among 3791 participants (range 0-17 per participant per year), of which 702 episodes were incident HF and 1072 were among participants with known HF. Among study participants, 607 participants had an annual HF hospitalization rate of >0 – 1 HF hospitalizations per year while 69 had an annual HF hospitalization rate of > 1 HF hospitalizations per year. Compared with participants who remained HF free during CRIC follow-up (N=3115), those who had a HF hospitalization were more likely to be older, men, black, have lower baseline eGFR and higher proteinuria, more likely to have diabetes, prevalent HF and a history of CVD (including atrial fibrillation), have higher blood pressure, and were more likely to be taking cardiovascular medications (Table 1).
Table 1:
Baseline characteristics, by annual heart failure hospitalization rate among participants with chronic kidney disease.
| No HF events/year | >0 – 1 event/year | > 1 event/year | |
|---|---|---|---|
| N | 3115 | 607 | 69 |
| Mean (SD) age, years | 57.2 (11.1) | 60.2 (9.5) | 61.6 (9.2) |
| Women | 1425 (46) | 265 (44) | 25 (36) |
| Race/ethnicity | |||
| Non-Hispanic white | 1383 (44) | 207 (34) | 14 (20) |
| Non-Hispanic black | 1230 (39) | 305 (50) | 45 (65) |
| Hispanic | 374 (12) | 78 (13) | 8 (12) |
| Other | 128 (4) | 17 (3) | 2 (3) |
| Mean (SD) eGFR, mL/min/1.73m2 | 45.6 (15.1) | 38.4 (13.0) | 38.4 (13.4) |
| Median (interquartile range) urine albumin to creatinine ratio (μg/mg), | 37.3 (7.3-335.4) | 309.6 (33.4-1405.1) | 210.6 (42.6-944.9) |
| Diabetes mellitus | 1348 (43) | 434 (71) | 48 (70) |
| Prior cardiovascular disease | 872 (28) | 346 (57) | 51 (74) |
| Prior heart failure | 191 (6) | 140 (23) | 38 (55) |
| Prior atrial fibrillation | 433 (14) | 177 (29) | 32 (46) |
| Current smoker | 399 (13) | 83 (14) | 13 (19) |
| Mean (SD) body mass index, kg/m2 | 31.6 (7.6) | 34.3 (8.4) | 32.9 (7.1) |
| Mean (SD) systolic blood pressure, mmHg | 126.7 (21.4) | 136.0 (23.3) | 133.7 (24.4) |
| Mean (SD) diastolic blood pressure, mmHg | 71.7 (12.4) | 70.0 (13.8) | 69.8 (15.6) |
| Mean (SD) hemoglobin, g/dL | 12.8 (1.7) | 12.0 (1.8) | 11.9 (1.7) |
| Mean (SD) LDL cholesterol, mg/dL | 103.4 (34.8) | 98.7 (36.5) | 96.2 (40.3) |
| Mean (SD) HDL cholesterol, mg/dL | 48.0 (15.7) | 45.4 (14.3) | 44.2 (13.2) |
| ACEi/ARBs | 2076 (67) | 463 (76) | 49 (71) |
| Diuretics | 1710 (55) | 478 (79) | 57 (83) |
| Loop diuretics | 1001 (32) | 374 (62) | 55 (80) |
| Thiazide diuretics | 905 (29) | 155 (26) | 8 (12) |
| Beta blockers | 1387 (45) | 412 (68) | 52 (75) |
| Calcium channel blockers | 1173 (38) | 320 (53) | 33 (48) |
| Aldosterone receptor antagonists | 105 (3) | 33 (5) | 16 (23) |
| Statins | 1621 (52) | 412 (68) | 47 (68) |
| Median (interquartile range) FGF-23 (RU/mL) | 134.2 (90.9-212.6) | 207.7 (133.9-346.5) | 289.6 (148.4-456.2) |
| Mean (SD) serum phosphorus, mg/dL | 3.7 (0.6) | 3.9 (0.8) | 3.9 (0.6) |
| Median (interquartile range) total parathyroid hormone, pg/mL | 50.1 (33.0-82.6) | 72.9 (45.8-120.0) | 87.7 (47.5-120.5) |
| Mean (SD) left ventricular ejection fraction, % | 55.2 (7.5) | 50.2 (11.0) | 43.9 (14.2) |
| Mean (SD) left ventricular mass index, g/m2 | 49.0 (12.5) | 61.4 (15.0) | 68.9 (16.7) |
| Median (interquartile range) NTpro-BNP, (pg/mL | 109.1 (39.3-285.7) | 432.2 (175.5-972.8) | 808.1 (428.9-2675.6) |
| Median (interquartile range) high sensitive troponin T, pg/mL | 13.1 (8.0-22.7) | 25.4 (15.7-41.4) | 35.0 (18.1-50.2) |
Rates of HF admissions overall and by eGFR and urine ACR categories
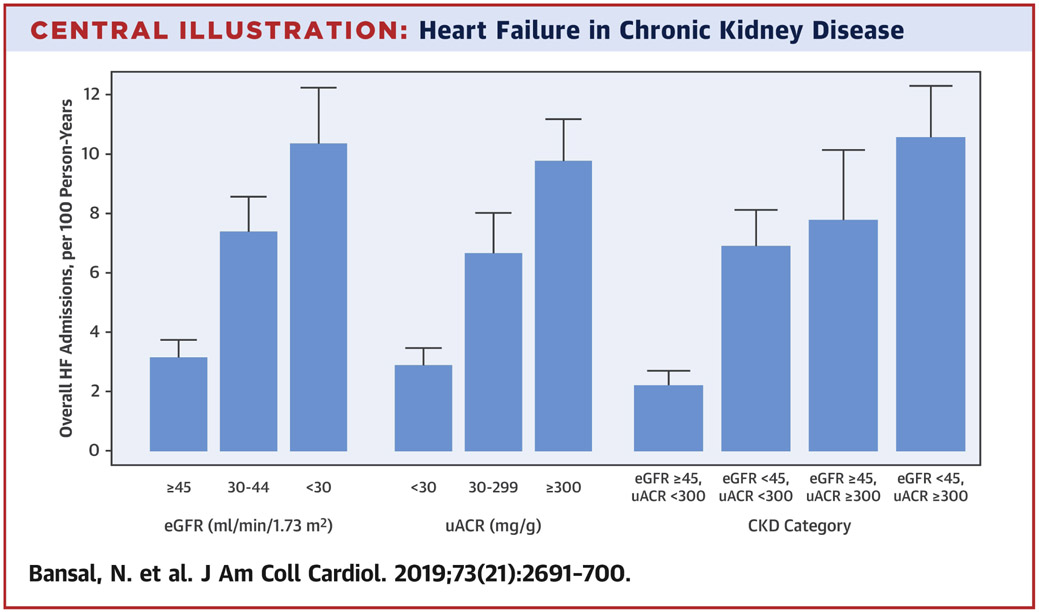
Median follow-up for participants who experience at least one HF hospitalization was 7.8 [Interquartile Range (IQR) 5.6, 9.2] years. The overall crude rate of HF admissions was 5.8 (95% CI:5.2-6.4) per 100 person-years, with higher rates with lower baseline eGFR and higher urine ACR (Central Illustration). After adjustment for demographic characteristics, the rate of HF was 2.1 and 2.9-fold higher for participants with eGFR 30-44 and <30 ml/min/1.73m2, respectively (Table 2). These associations remained robust with adjustment for cardiovascular risk factors and medication use: rate ratio 1.7 (95% CI 1.3, 2.2) and 2.2 (95% CI: 1.7, 2.9) for participants with eGFR 30-44 and <30 ml/min/1.73m2 (vs. >45 ml/min/1.73 m2), respectively.
Central Illustration. Heart Failure in Chronic Kidney Disease.
Unadjusted rates of heart failure admissions across by level of kidney function among participants with chronic kidney disease
Table 2.
Rates and rate ratios of heart failure admissions across levels of kidney function among participants with chronic kidney disease
| Rate Ratio (95% CI) | |||||
|---|---|---|---|---|---|
| Number at risk (Total number of events) |
Crude rate, per 100 pys (95% bootstrapped CIs) |
Model 1 | Model 2 | Model 3 | |
| Overall | 3791 (1706) | 5.8 (5.2, 6.4) | |||
| eGFR stage | |||||
| eGFR ≥ 45 | 1714 (437) | 3.1 (2.5, 3.7) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| eGFR 30-44 | 1379 (766) | 7.4 (6.2, 8.6) | 2.1 (1.7, 2.7) | 1.9 (1.5, 2.4) | 1.7 (1.3, 2.2) |
| eGFR < 30 | 698 (503) | 10.3 (8.4, 12.2) | 2.9 (2.2, 3.8) | 2.5 (1.9, 3.3) | 2.2 (1.7, 2.9) |
| Albuminuria | |||||
| uACR < 30 | 1629 (383) | 2.9 (2.3, 3.5) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| uACR 30 - < 300 | 1001 (510) | 6.6 (5.3, 8.0) | 2.3 (1.8, 3.1) | 1.9 (1.4, 2.6) | 1.9 (1.5, 2.6) |
| uACR ≥ 300 | 1161 (813) | 9.7 (8.3, 11.2) | 3.8 (3.0, 4.9) | 2.6 (2.0, 3.5) | 2.6 (1.9, 3.5) |
| CKD category | |||||
| eGFR ≥ 45, uACR < 300 | 1401 (253) | 2.2 (1.7, 2.7) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| eGFR < 45, uACR < 300 | 1229 (640) | 6.9 (5.7, 8.1) | 2.7 (2.0, 3.6) | 2.5 (1.9, 3.4) | 2.3 (1.7, 3.0) |
| eGFR ≥ 45, uACR > 300 | 313 (184) | 7.7 (5.4, 10.1) | 4.1 (2.8, 6.1) | 2.9 (1.9, 4.4) | 2.9 (1.9, 4.4) |
| eGFR < 45, uACR > 300 | 848 (629) | 10.6 (8.8, 12.3) | 4.6 (3.5, 6.2) | 3.2 (2.3, 4.4) | 3.0 (2.2, 4.1) |
All rate ratios with p-value<0.05
eGFR= estimated glomerular filtration rate; uACR= urine albumin-to-creatinine-ratio
Model 1: age, sex, and race/ethnicity
Model 2: model 1 + diabetes, history of cardiovascular disease, use of lipid-lowering medications, smoking, systolic blood pressure, and body mass index
Model 3: model 2 + use of diuretics, angiotensin-converting-enzyme inhibitors/aldosterone receptor blockers, β-blockers
The crude rate of HF hospitalizations was also higher across categories of higher urine ACR with a rate of 9.7 (95% CI: 8.3, 11.2) among participants with a urine ACR≥300 mg/g (Figure 1). In models adjusted for demographic characteristics, the rate of HF hospitalizations was significantly higher among participants with urine ACR 30-299 or ≥300 mg/g vs. urine ACR <30 mg/g (Table 2). Even after additional adjustment for cardiovascular risk factors and medication use, the rates of HF hospitalization remained significantly higher in those with higher baseline urine ACR (vs. urine ACR<30 mg/g): rate ratios of 1.9 (95% CI: 1.5, 2.6) and 2.6 (95% CI: 1.9, 3.5) for urine ACR 30-299 and ≥300 mg/g, respectively.
Rates of HFrEF and HFpEF hospitalizations overall and by categories of eGFR and urine ACR
There were 1,127 participants for whom HF subtype could be determined. The rate of the first HF hospitalization during CRIC follow-up was 8.3 (95%CI: 7.1, 9.5) per 1,000 person years (213 events) for HFrEF and 11.2 (95%CI: 9.9, 12.5) per 1,000 person years for HFpEF (288 events). The rates of all (first and recurrent) HFrEF hospitalization was 19.4 (95%CI: 16.3, 22.5) per 1,000 person years (568 events) and for HFpEF was 17.8 (95%CI: 15.6, 20) per 1,000 person years (522 events).
After accounting for differences in demographic characteristics, cardiovascular risk factors and medication use, there were graded, higher adjusted rates of both HFrEF and HFpEF hospitalizations with lower eGFR and higher urine ACR, with similar rate ratios to the primary analysis (Online Tables 1 and 2).
30-day readmissions for HF
Among 702 participants with incident HF during CRIC follow-up, 67 (9.5%) were readmitted for HF within 30 days, compared with 179 (16.7%) of 1072 participants with prevalent HF. Rates of recurrent HF hospitalizations increased across categories of lower eGFR and higher urine ACR (Online Figure 1, Table 3). In models that adjusted for demographic characteristics, cardiovascular risk factors and medication use, the rate ratio for re-hospitalization within 30 days was 2-3 fold in participants with the lowest eGFR and highest urine ACR: rate ratio 2.6 (95% CI: 1.4, 4.7) for eGFR<30 ml/min/1.73m2 and 3.6 (95%CI: 1.8, 7.3) for urine ACR ≥300 mg/g.
Table 3.
Rates of total number of 30-day heart failure re-hospitalizations across level of kidney function among participants with chronic kidney disease
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| Number of participants |
Number of HF hospitalizations |
Total number of 30-day HF rehospitalizations |
Crude rate, per 100 pys (95% CIs) |
||||
| Overall | 3791 | 1706 | 238 | 0.8 (0.6, 1.0) | |||
| eGFR stage | |||||||
| eGFR ≥ 45 | 1714 | 437 | 53 | 0.4 (0.2, 0.5) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| eGFR 30-44 | 1379 | 766 | 111 | 1.1 (0.7, 1.4) | 2.5 (1.5, 4.1) | 2.2 (1.3, 3.6) | 1.9 (1.2, 3.1) |
| eGFR < 30 | 698 | 503 | 74 | 1.5 (0.8, 2.2) | 3.4 (1.9, 6.2) | 3.0 (1.6, 5.6) | 2.6 (1.4, 4.7) |
| Albuminuria | |||||||
| uACR < 30 | 1629 | 383 | 43 | 0.3 (0.2, 0.4) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| uACR 30 - < 300 | 1001 | 510 | 80 | 1.0 (0.6, 1.5) | 3.2 (1.8, 5.5) | 2.7 (1.5, 4.7) | 2.7 (1.5, 4.8) |
| uACR ≥ 300 | 1161 | 813 | 115 | 1.4 (0.9, 1.9) | 4.6 (2.7, 7.7) | 3.6 (1.8, 7.1) | 3.6 (1.8, 7.3) |
| CKD category | |||||||
| eGFR ≥ 45, uACR < 300 | 1401 | 253 | 29 | 0.2 (0.1, 0.4) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| eGFR < 45, uACR < 300 | 1229 | 640 | 94 | 1.0 (0.7, 1.4) | 3.4 (1.9, 6.1) | 3.2 (1.8, 5.7) | 2.8 (1.6, 4.9) |
| eGFR ≥ 45, uACR > 300 | 313 | 184 | 24 | 1.0 (0.4, 1.6) | 4.5 (2.1, 9.7) | 3.6 (1.6, 8.2) | 3.8 (1.6, 8.7) |
| eGFR < 45, uACR > 300 | 848 | 629 | 91 | 1.5 (0.9, 2.2) | 5.5 (3.0, 10.2) | 4.2 (2.0, 8.8) | 3.8 (1.8, 8.1) |
All rate ratios with p-value<0.05
eGFR= estimated glomerular filtration rate; uACR= urine albumin-to-creatinine-ratio
Model 1: age, sex, and race/ethnicity
Model 2: model 1 + diabetes, history of cardiovascular disease, use of lipid-lowering medications, smoking, systolic blood pressure, and body mass index
Model 3: model 2 + use of diuretics, angiotensin-converting-enzyme inhibitors/aldosterone receptor blockers, β-blockers
Number of HF hospitalizations within 2 years and subsequent CKD progression
There were 884 participants who experienced a 50% decline in eGFR or developed ESRD during the follow-up period. In fully-adjusted models, participants who experienced a HF hospitalization within the first two years of follow-up had higher adjusted rates of subsequent CKD progression (1 hospitalization: HR 1.93, 95%CI:1.40-2.67; 2 or more hospitalizations: HR 2.14, 95%CI:1.30-3.54).
Number of HF hospitalizations within 2 years and subsequent all-cause mortality
A total of 819 participants died during follow-up. After adjustment for potential confounders, participants who experienced a HF hospitalization during the first 2 years of follow-up had a graded, higher risk of subsequent all-cause death. Participants with one HF hospitalization had a >2-fold increased adjusted rate of death (HR 2.20, 95%CI:1.71-2.84 and those with two or more HF hospitalizations had a threefold higher adjusted rate (HR 3.06, 95%CI:2.23-4.18).
Number of HF hospitalization days and clinical outcomes by eGFR and urine ACR
In secondary analyses, we evaluated burden of HF hospitalization (based on number of hospital days) and outcomes by level of kidney function and damage. The overall rate of HF hospital days was 36.9 (95%CI: 32.2, 41.7) per 100 person-years. Lower baseline eGFR and higher baseline urine ACR were associated with graded, increased rates of HF hospital days (Online Figure 2). The rate of HF hospital days was highest for participants with both low eGFR and higher urine ACR (60.6 per 100 person years, 95%CI:48.8-72.4). In models adjusted for demographics, there was a 2 to 4-fold increase in rate of HF hospital days across categories of lower eGFR and higher urine ACR (Online Table 3). These associations persisted after further adjustment for cardiovascular risk factors and medication use: rate ratio 1.7 (95%CI 1.2, 2.4) and 2.3 (95%CI: 1.6, 3.3) for participants with eGFR 30-44 and <30 ml/min/1.73m2 (vs. >45 ml/min/1.73 m2), respectively. Similarly, the rates of HF hospital days remained significantly higher in those with higher urine ACR (vs. urine ACR<30 mg/g): rate ratios of 1.9 (95%CI: 1.4, 2.6) and 2.5 (95%CI: 1.8, 3.5) for urine ACR 30-299 and ≥300 mg/g, respectively (Online Table 3).
When we examined the total burden of HF hospitalization days in the first two years of follow-up with clinical outcomes, we found a strong association of a higher number of HF hospital days with higher subsequent risk of CKD progression and all-cause mortality (Online Table 4). Compared to participants with no HF hospital days in the first two years of follow-up, participants who had eight or more HF hospital days had a 90% higher rate (HR 1.90, 95%CI: 1.15-3.15) and nearly threefold higher rate (HR 2.91, 95%CI:2.17-3.90) of CKD progression and death, respectively, in fully-adjusted models (Online Table 4).
Discussion
In this large, prospective cohort of adults with CKD, the overall rate of HF hospitalizations was quite high at 5.8 hospitalizations per 100 person-years of follow-up and even higher among participants with either eGFR<30 ml/min/1.73m2 or urine ACR ≥300 mg/g (Central Illustration). The rate of first hospitalization for HFpEF was higher than that of HFrEF but the rates of all hospitalizations were similar between HFpEF and HFrEF. Overall, >1 in 5 patients were readmitted for HF within 30 days. Greater burden of HF hospitalizations or HF hospital days in the first 2 years of follow-up was associated with a 2-to-3-fold increased risk of CKD progression and death. Collectively, these data provide unique and comprehensive evidence of the tremendous burden of HF in CKD and the independent association of HF with subsequent renal outcomes and survival.
In our study, participants with CKD experienced 3 to 10 HF hospitalizations per 100 person-years, depending on level of eGFR and urine ACR, which is substantially greater than seen in the general population. In the Nationwide Inpatient Sample, the rates of primary HF hospitalizations was 0.5 per 100 person-years and 1.5 per 100 person-years for secondary diagnosis of a HF hospitalization in 2009.(22) Among Medicare beneficiaries, the rate of HF hospitalizations were 0.4 per 100 person-years in 2004, which was a significant increase over the previous 25 years.(23) While data from USRDS reports that the rate of hospitalization for any cardiovascular cause was 46 per 100 person-years for ESRD patients,(11) limited data exist for HF-specific hospitalizations in those with CKD. Our study provides novel data on this important public health burden in CKD.
There are even fewer data on the rates of HF subtypes in patients with CKD. We found that the rates of first hospitalization during follow-up in CRIC participants was higher for HFpEF than HFrEF. However, the rates of all hospitalizations for HFpEF and HFrEF were similar, suggesting that while HFpEF may be more prevalent in CKD, HFrEF patients may be more likely to have recurrent HF hospitalizations. In the Framingham Heart Study of participants with and without CKD, microalbuminuria was only associated with HFrEF and low eGFR was associated with HF overall but not HFpEF or HFrEF (24). In a study of patients from multiple healthcare delivery systems participating in the Cardiovascular Research Network (CVRN), the association of eGFR with risk of a HF hospitalization or mortality was similar among patients with known HFpEF or HFrEF (25). Given the different pathophysiology of HFrEF vs. HFpEF and the lack of proven therapies for HFpEF, our findings support the need to identify more effective treatment strategies for both HFrEF and HFpEF in patients with CKD.
Rates of 30-day readmissions for HF were also high at 20.6% overall and 27% among participants with known HF. The observed rates seen in our CKD population were greater than those observed in the general population. In a study of the ARIC cohort, approximately 8% of participants with known HF were re-hospitalized for HF within 30 days.(26) Among Medicare fee-for-service beneficiaries aged 65 years or older, the median 30-day readmission rate for any cause (not specifically for HF as in our study) was 24.4% after HF admission.(27) Previous studies have tested strategies to decrease rates of recurrent hospitalizations such as earlier physician visits post discharge, target education about medication and diet adherence and support from an ambulatory HF care team (28-31). These strategies have not been specifically tested in patients with CKD and may be promising to reduce the excessive burden of recurrent HF hospitalizations in this high-risk population.
Our study also found that higher number of HF hospitalizations and HF hospital days was independently associated with a greater risk of CKD progression. Of participants hospitalized for HF, 52% experienced significant CKD progression. In a previous study of patients with CKD in Canada, rates of ESRD were 4-to-14-fold higher among patients with previous HF hospitalizations (32). In study of patients with normal kidney function, HF was associated with a 2-fold greater risk of incident CKD.(33) Our findings support and materially expand on these associations in a large U.S. population of patients with CKD. There are several possibilities to explain these findings. HF leads to hemodynamic changes, endothelial injury, inflammation and other processes which may further injure the kidneys (34-36). Thus, further studies are needed to explore therapies to help preserve kidney function after an acute HF hospitalization.
We found a graded, strong association between greater burden of HF with the risk of death. Among participants with 2 or more HF hospitalizations, the risk of all-cause death was 3-fold higher compared with participants who did not experience HF. Among participants in our study who experienced a HF hospitalization, 60% died during follow-up. A previous study using data from Medicare fee-for-service beneficiaries reported that all-cause mortality rates after a HF hospitalization was 11.7% overall (37). Another study from the same population found that reductions in rates of HF hospitalizations were associated with a reduction in post-discharge mortality (38). Given the even higher proportion of post-HF hospitalization deaths in patients with CKD, interventions to improve post-HF care should also be prioritized in this high-risk population.
These findings have important implications in the management of patients with CKD. Primary and secondary treatment of HF in CKD should be a public health priority. The high burden of HF hospitalizations in CKD likely contributes to the higher cost of care observed for the treatment of CKD and cardiovascular disease. Therefore, there is a need to improve treatment of HF in patients with known disease. Strategies would include better implementation of current therapies that may often be withheld in patients with CKD (e.g., RAAS inhibitors); development of novel therapies that may target CKD-specific HF risk factors; incentives to improve the transition between inpatient and outpatient HF care in CKD to reduce the risk of readmissions; and improve patient education around HF in CKD.
Our study had several strengths. We studied a large, multi-center, well-characterized, U.S.-based CKD population specifically designed to study cardiovascular complications with extensive longitudinal follow-up. All HF hospitalizations that occurred during CRIC follow-up were adjudicated using standardized criteria. We recognize a few limitations as well. We were not able to determine whether acute kidney injury occurred during the HF hospitalizations, which may have contributed to subsequent CKD progression. Ejection fraction data to determine HF subtype was not available in all participants. Kidney biopsy data on the cause of the CKD was not available in all participants; however, we did control for primary contributors of CKD, such as diabetes, in our statistical models. We did not have detailed data on whether certain medications (e.g. RAAS inhibitors or diuretics) were held or doses adjusted after the hospitalization since medication use was ascertained every six months per the CRIC study protocol. While the CRIC adjudication process is known to capture >90% of hospitalizations, it is possible that some HF hospitalizations were missed. This was a clinic-based population of research volunteers so it may not be generalizable to all CKD patients. In this observational study, we cannot determine causality and we cannot exclude reverse causality.
In conclusion, in a large U.S. CKD population, the rates of HF hospitalizations and 30-day re-hospitalization were very high, with even higher rates across categories of lower eGFR and higher urine ACR. HF hospitalizations were independently associated with 2-to-3-fold higher rates of CKD progression and death. Prevention and treatment of HF should be a major priority to improve clinical outcomes in patients with CKD.
Supplementary Material
Table 4:
Association of number of heart failure episodes in the first 2 years of follow-up with risk of chronic kidney disease progression and all-cause mortality among participants with chronic kidney disease
| Decline in eGFR by 50% or progression to ESRD | ||||||
|---|---|---|---|---|---|---|
| HR (95% CI) | ||||||
| Number of HF events, years 0 - 2 |
N at risk | N events | Unadjusted | Model 1 | Model 2 | Model 3 |
| 0 | 2868 | 827 | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| 1 | 80 | 41 | 3.27 (2.39, 4.48) | 2.61 (1.90, 3.59) | 1.98 (1.43, 2.73) | 1.93 (1.40, 2.67) |
| ≥ 2 | 30 | 16 | 3.77 (2.30, 6.19) | 3.41 (2.08, 5.60) | 2.32 (1.41, 3.83) | 2.14 (1.30, 3.54) |
| All-cause mortality | ||||||
| 0 | 3380 | 698 | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) |
| 1 | 135 | 74 | 3.48 (2.74, 4.43) | 3.41 (2.68, 4.35) | 2.35 (1.83, 3.03) | 2.20 (1.71, 2.84) |
| ≥ 2 | 67 | 47 | 6.03 (4.48, 8.11) | 5.81 (4.31, 7.83) | 3.38 (2.48, 4.61) | 3.06 (2.23, 4.18) |
All HR with p-value <0.05
Model 1: age, sex, and race/ethnicity
Model 2: model 1 + diabetes, history of cardiovascular disease, use of lipid-lowering medications, smoking, systolic blood pressure, and body mass index
Model 3: model 2 + use of diuretics, angiotensin-converting-enzyme inhibitors/aldosterone receptor blockers, β-blockers
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge:
Compared to patients with preserved renal function, those with chronic kidney disease (CKD; defined as eGFR<45 ml/min per 1.73m2 or microalbuminuria (uACR >30 mg/g) hospitalized with heart failure (HF) are at high risk of recurrent heart failure, re-admission for heart failure, and death within 2 years.
Translational Outlook:
Additional research is needed to improve outcomes of HF in patients with CKD.
Acknowledgements:
This work was supported by the following grants: NIDDK R01DK103612 (Bansal) and an unrestricted fund from the Northwest Kidney Centers. Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131.
ABBREVIATIONS
- HF
heart failure
- CKD
chronic kidney disease
- ESRD
end-stage-renal-disease
- CRIC
Chronic Renal Insufficiency Cohort
- eGFR
estimated glomerular filtration rate
- Urine ACR
urine albumin-to-creatinine-ratio
- EF
ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang PP, Wruck LM, Shahar E et al. Trends in Hospitalizations and Survival of Acute Decompensated Heart Failure in Four US Communities (2005-2014): ARIC Study Community Surveillance. Circulation 2018;138:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giamouzis G, Kalogeropoulos A, Georgiopoulou V et al. Hospitalization epidemic in patients with heart failure: risk factors, risk prediction, knowledge gaps, and future directions. J Card Fail 2011;17:54–75. [DOI] [PubMed] [Google Scholar]
- 4.Kociol RD, Hammill BG, Fonarow GC et al. Generalizability and longitudinal outcomes of a national heart failure clinical registry: Comparison of Acute Decompensated Heart Failure National Registry (ADHERE) and non-ADHERE Medicare beneficiaries. Am Heart J 2010;160:885–92. [DOI] [PubMed] [Google Scholar]
- 5.Bansal N, Katz R, Robinson-Cohen C et al. Absolute Rates of Heart Failure, Coronary Heart Disease, and Stroke in Chronic Kidney Disease: An Analysis of 3 Community-Based Cohort Studies. JAMA cardiology 2017;2:314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kottgen A, Russell SD, Loehr LR et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol 2007;18:1307–15. [DOI] [PubMed] [Google Scholar]
- 7.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305. [DOI] [PubMed] [Google Scholar]
- 8.Bansal N, Katz R, Robinson-Cohen C et al. Absolute Rates of Heart Failure, Coronary Heart Disease, and Stroke in Chronic Kidney Disease: An Analysis of 3 Community-Based Cohort Studies. JAMA cardiology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.2016 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. 2016. [Google Scholar]
- 10.Harel Z, Wald R, McArthur E et al. Rehospitalizations and Emergency Department Visits after Hospital Discharge in Patients Receiving Maintenance Hemodialysis. J Am Soc Nephrol 2015;26:3141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.http://www.usrds.org.
- 12.Feldman HI, Appel LJ, Chertow GM et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol 2003;14:S148–53. [DOI] [PubMed] [Google Scholar]
- 13.Lash JP, Go AS, Appel LJ et al. Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline Characteristics and Associations with Kidney Function. Clin J Am Soc Nephrol 2009;4:1302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joffe M, Hsu CY, Feldman HI et al. Variability of creatinine measurements in clinical laboratories: results from the CRIC study. Am J Nephrol 2010;31:426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Coresh J, Greene T et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007;53:766–72. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.http://www.kdigo.org/clinical_practice_guidelines/CKD.php.
- 18.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993;88:107–15. [DOI] [PubMed] [Google Scholar]
- 19.Bansal N, Roy J, Chen HY et al. Evolution of Echocardiographic Measures of Cardiac Disease From CKD to ESRD and Risk of All-Cause Mortality: Findings From the CRIC Study. Am J Kidney Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bansal N, Keane M, Delafontaine P et al. A longitudinal study of left ventricular function and structure from CKD to ESRD: the CRIC study. Clin J Am Soc Nephrol 2013;8:355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Anthropometry Procedures Manual. Centers for Disease Control and Prevention; [serial online] 2000. [Google Scholar]
- 22.Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart Failure Associated Hospitalizations in the United States. J Am Coll Cardiol 2013;61: 10.1016/j.jacc.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang J, Mensah GA, Croft JB, Keenan NL. Heart Failure-Related Hospitalization in the U.S., 1979 to 2004. Journal of the American College of Cardiology 2008;52:428–434. [DOI] [PubMed] [Google Scholar]
- 24.Nayor M, Larson MG, Wang N et al. The association of chronic kidney disease and microalbuminuria with heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail 2017;19:615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith DH, Thorp ML, Gurwitz JH et al. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE Study. Circulation Cardiovascular quality and outcomes 2013;6:333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caughey MC, Sueta CA, Stearns SC, Shah AM, Rosamond WD, Chang PP. Recurrent Acute Decompensated Heart Failure Admissions for Patients With Reduced Versus Preserved Ejection Fraction (from the Atherosclerosis Risk in Communities Study). Am J Cardiol 2018;122:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krumholz HM, Merrill AR, Schone EM et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circulation Cardiovascul qual outcomes 2009;2:407–13. [DOI] [PubMed] [Google Scholar]
- 28.Bilchick K, Moss T, Welch T et al. Improving Heart Failure Readmission Costs and Outcomes With a Hospital-to-Home Readmission Intervention Program. Am J med qual 2018:1062860618788436. [DOI] [PubMed] [Google Scholar]
- 29.Klassen SL, Miller RJ, Hao R et al. Implementation of a Multidisciplinary Inpatient Cardiology Service to Improve Heart Failure Outcomes in Guyana. J Card Fail 2018. [DOI] [PubMed] [Google Scholar]
- 30.Nouryan CN, Morahan S, Pecinka K et al. Home Telemonitoring of Community-Dwelling Heart Failure Patients After Home Care Discharge. Telemed J e-health. 2018. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA 2010; 303:1716–22. [DOI] [PubMed] [Google Scholar]
- 32.Sud M, Tangri N, Pintilie M, Levey AS, Naimark DM. ESRD and death after heart failure in CKD. J Am Soc Nephrol 2015;26:715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George LK, Koshy SKG, Molnar MZ et al. Heart Failure Increases the Risk of Adverse Renal Outcomes in Patients With Normal Kidney Function. Circ Heart Fail 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He J, Shlipak M, Anderson A et al. Risk Factors for Heart Failure in Patients With Chronic Kidney Disease: The CRIC (Chronic Renal Insufficiency Cohort) Study. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michowitz Y, Goldstein E, Wexler D, Sheps D, Keren G, George J. Circulating endothelial progenitor cells and clinical outcome in patients with congestive heart failure. Heart 2007;93:1046–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bansal N, Katz R, Dalrymple L et al. NT-proBNP and troponin T and risk of rapid kidney function decline and incident CKD in elderly adults. Clin J Am Soc Nephrol 2015;10:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krumholz HM, Lin Z, Keenan PS et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA 2013;309:587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dharmarajan K, Wang Y, Lin Z et al. Association of Changing Hospital Readmission Rates With Mortality Rates After Hospital Discharge. JAMA 2017;318:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.