Summary
This study evaluates the use of HMG-CoA reducatase inhibitors, or statins, as an adjunctive to BRAF and MEK inhibition as a treatment in melanomas and other tumors with driver mutations in the MAPK pathway. Experiments used simvastatin in conjunction with vemurafenib and selumetinib in vitro and simvastatin with vemurafenib in vivo to demonstrated additional growth abrogation beyond MAPK blockade alone. Additional studies demonstrated that statin anti-tumor effects appeared to depend on inhibition of isoprenoid synthesis given rescue with add-back of downstream metabolites. Ultimately we concluded that statins represent a possible useful adjunctive therapy in MAPK-driven tumors when given with current approved targeted therapy.
Keywords: melanoma, statin, cholesterol, metabolism, hippo
Introduction
With an incidence that is increasing at approximately 3% per year, malignant melanoma is currently the fifth most common cancer in the US, causing an estimated 9,710 deaths in 2014, with an increasing incidence of approximately 3% per year(Yamada et al., 2005). Recent sequencing studies have shown that the MAPK pathway is hyperactivated in virtually all melanomas, with activating mutations in BRAF present in 50% of human tumors (Davies et al., 2002; Held et al., 2013; Krauthammer et al., 2012) and activating mutations in NRAS driving an additional 15–20%. Though targeted therapy for late-stage BRAF-mutant melanoma patients often produces dramatic initial results, virtually 100% of responders eventually develop drug-resistant disease, usually within 6–11 months(Chapman et al., 2011; Sosman et al., 2012). As acquisition of BRAF inhibitor resistance frequently results in reactivation of the MAPK pathway(Flaherty et al., 2012a), one of the earliest attempts to address recurrence was the addition of therapeutic MEK inhibitors, blocking activity of BRAF’s immediate downstream target. Though trials of combined BRAF/MEK inhibition have been found to prolong progression-free survival in BRAF-mutant melanoma by 2–4 months (Flaherty et al., 2012a; Flaherty et al., 2012b; Long et al., 2014), overall survival in stage IV melanoma patients remains essentially the same. As a result, significant effort is currently being focused on developing adjunct targeted therapies to augment combined BRAF/MEK inhibition.
Serving as both a structural component and a synthetic precursor, cholesterol functions as an important biological substrate in both benign and malignant biology(Nielsen et al., 2012). Unsurprisingly deregulation of the mevalonate pathway, upstream of cholesterol synthesis, has been noted to be important for growth in cancers such as prostate, breast, acute myeloid leukemia, and melanoma(Kang et al., 2015; Santos and Schulze, 2012). Much of this is thought to be due to the aberrantly increased production of farnesyl and geranylgeranyl groups: two types of post-translational isoprenoid moieties that affect the ability of proteins to localize to lipid rich regions such as the interior plasma membrane and the Golgi apparatus.
Members of the RAS family are known to be regulated through prenylation, greatly increasing their ability to activate downstream substrates such as BRAF(Berndt et al., 2011; Omerovic et al., 2007). In addition to prenylation, cholesterol synthesis also directly promotes activity of oncogenes such as RAS through aggregation of cholesterol, glycosphingolipids, and other small lipids into “lipid rafts(Simons and Toomre, 2000)”, which further enhance co-localization of lipophilic proteins to the membrane for activation. Apart from MAPK signaling, prenylation also plays a role in activation of growth factors feeding into the PI3K/AKT/mTOR pathway, with inhibition leading to loss of AKT phosphorylation and growth inhibition in melanoma(Berndt et al., 2011; Jiang et al., 2000). One of these factors is NRAS itself, which is known to colocalize with and activate PI3K in parallel to RAF(Fedorenko et al., 2013; Wu et al., 2003). Consistent with this model of dual MAPK and PI3K activation by NRAS, significantly fewer RAS-driven melanomas are known to harbor activating mutations in AKT or inactivating mutations in phosphatase and tensin homolog (PTEN), a negative regulator of PI3K/AKT signaling(Davies et al., 2009; Davies et al., 2008; Tsao et al., 2004). Given the known importance of PI3K/AKT activation in BRAF inhibitor resistance(Deng et al., 2012; Held et al., 2013; Silva et al., 2014), the importance of RAS activation of PI3K in tumors with concurrent BRAF and NRAS mutations represents a potentially attractive target for therapeutic exploitation.
Given the breadth of studies demonstrating the importance of cholesterol and its precursors to melanoma growth, we sought to investigate the utility of HMG-CoA reductase inhibitors as mono- or adjunct therapy in melanomas with acquired resistance to BRAF and MEK inhibition. HMG-CoA reductase inhibitors, also known as statins, are the current FDA-approved first-line therapy for hypercholesterolemia, and work by blocking the rate-limiting step of cholesterol synthesis: conversion of HMG-CoA to mevalonate. Here, we provide evidence that statins show unique synergy with dual BRAF and MEK inhibition by preventing activating RAS isoprenylation, inhibiting RAS-dependent AKT and Hippo pathway activation and greatly limiting growth in in vitro and in vivo pre-clinical models.
Materials and Methods
Cell Culture and Generation of Acquired Resistant Lines
Specimens were collected with patients’ informed consent in accordance with the Health Insurance Portability and Accountability Act (HIPAA) under a Human Investigations Committee protocol. Expression profiling and Sanger sequencing were used to screen for mutations as described previously(Held et al., 2013), with additional rule-out of resistance-generating NRAS mutations confirmed by Taqman-based PCR. YUMAC, YURIF, and their resistant counterparts were grown in OPTI-MEM (Invitrogen, Carlsbad, CA, USA). The BRAF-mutant colorectal cancer line RKO and the NSCLC line H1395 were grown in RPMI (Thermo Fisher Scientific,Waltham, MA, USA). Both were supplemented with 1% penicillin-streptomycin and 10% fetal bovine serum and maintained in a 37°C incubator maintained at 5% CO2. Surgical melanoma resections were used to generate YUMAC and YURIF and were provided by Dr. Ruth Halaban (Yale University, New Haven, CT, USA)(Halaban et al., 2010). Parental YUMAC and YURIF lines were continuously grown in vemurafenib (up to 5 μM) and AZD-6244 (up to 150nM) until they exhibited resistance to growth inhibition and were designated YUMACdr and YURIFdr. Acquired resistance was previously verified over a 72 hour time period and quantified using the CyQUANT® NF Cell Proliferation Assay Kit (Life Technologies, Carlsbad, CA, USA).
Single and Multi-Agent Screening
Cells were plated in optical-bottom 96-well plates (Thermo Scientific, Waltham, MA, USA) at a density of 5×103 cells per well. Cells were grown in basal media for 48 hours before 150ml of fresh media was added. Media with maximal drug concentration (simvastatin, lovastatin, GGTI 298, LB 42708, YM-53601) or mevalonate pathway rescue substrates (mevalonolactone, FPP, GGPP) was used to perform serial dilution across wells using a multichannel pipette (Eppendorf, Hamburg, Germany). After 72 hours of treatment, cell numbers were counted by Cell Titer Glo assay (Promega, Madison, WI, USA). Dose-response curves were generated using Graphpad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Simvastatin, lovastatin, GGTI 298, LB 42708, verteporfin, and MK-2206 were purchased from Tocris Bioscience, Bristol, UK. YM-53601 was purchased from Cayman Chemical, Ann Arbor, MI, USA.
Clonogenic Assays
For 2-D clonogenic assays, cells were plated at 5 × 102 cells per well in 6-well tissue culture–treated plates in triplicate and grown for 48 hours in 3 mL basal medium. Drug treatments were carried out at 48 hours and then every 4th day for a total of 3 treatments. Each time 4mL’s fresh media was added. Colonies were fixed in ice-cold 100% methanol for 15 minutes and stained for 20 minutes with 0.05% crystal violet, followed by destaining with water. Plates were scanned with a VersaDoc Model 3000 imager (Bio-Rad, Hercules, CA, USA) and Quantity One software.
Xenografts
Six-week-old, male, NCr nude mice (Taconic) were fed chow containing 417 mg/kg PLX-4720 (Research Diets, Inc., provided by Plexxikon) or control chow without inhibitor. After two days, the mice were injected in both rear flanks and upper shoulder with 106 YUMACr cells per site. Small tumors were established in 15 days. The mice were then divided into four cohorts with five mice per cohort. Treatment groups consisted of control chow, no simvastatin; control chow, simvastatin; PLX-4720 chow, no simvastatin; and PLX-4720 chow, simvastatin. Simvastatin-treated mice were given 30mg/kg simvastatin by IP injection for the first 4 days of treatment followed by 30mg/kg every other day until endpoint was reached. Endpoint was defined as the time at which the first tumors reached 1cm3. Mice not treated with simvastatin were given sterile DMSO by IP injection. Mice were treated for 12 days total, and tumors were measured with a digital caliper. Tumor volume was calculated using V = 0.5233 × length × width × height. All animal studies were conducted under the supervision of the Yale Institutional Animal Care and Use Committee, protocol number 2017–11212, IRB Protocol ID #1501015235.
The Cancer Genome Atlas Analysis
The Cancer Genome Atlas melanoma (SKCM) RNA-seq Level 3 dataset was downloaded from the Cancer Genomics Hub (San Diego, CA). Cohorts were ranked by RSEM normalized count of gene expression and divided at the 75th and 25th percentile into high and low expression subgroups, respectively. Kaplan-Meier curves and survival analysis, using the Log-rank test, was performed in the R environment for statistical computing (Vienna, Austria) using the ‘Survival’ library. Raw data available publicly at https://tcgadata.nci.nih.gov/tcgafiles/ftp_auth/distro_ftpusers/anonymous/tumor/skcm/cgcc/unc.edu/illuminahiseq_rnaseqv2/rnaseqv2/
Results
Statins synergize with MAPK inhibitors to inhibit growth in treatment-resistant melanomas
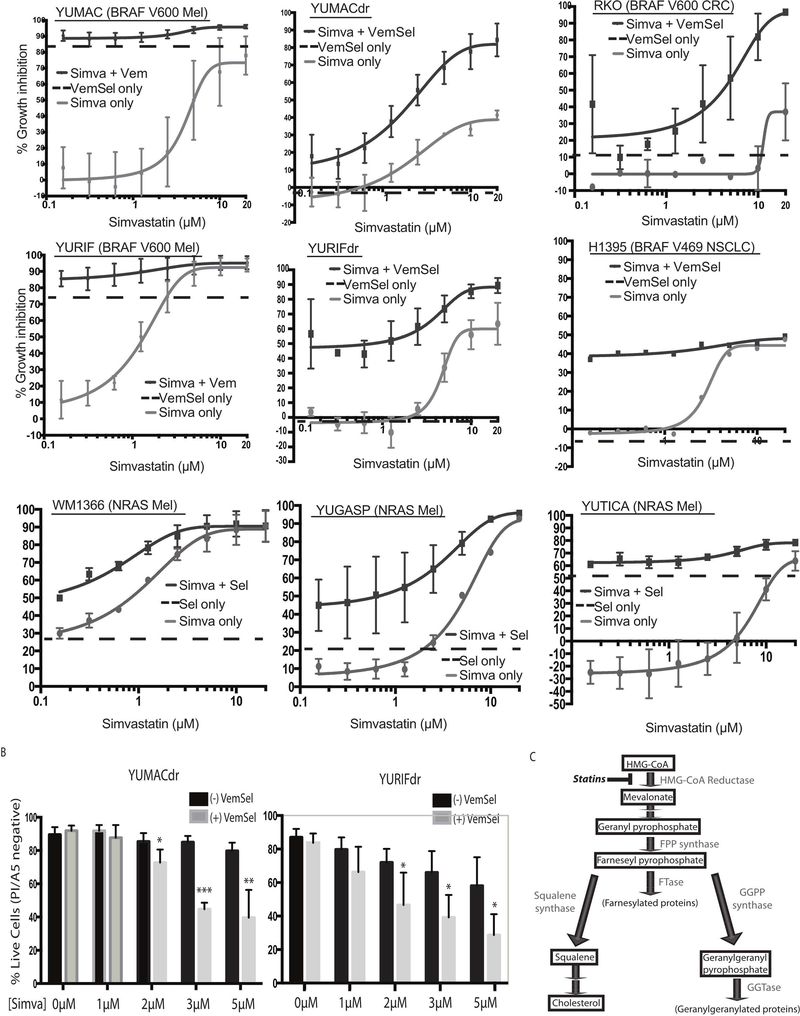
We initially screened a large number of existing targeted cancer therapies for synergy with vemurafenib against BRAF-mutant melanoma. One of the classes of compounds that showed promising efficacy in the setting of dual therapy was HMG-CoA reductase inhibitors, also known as statins. In keeping with previous research showing improved anti-cancer efficacy with lipophilic statins(Campbell et al., 2006; Clendening and Penn, 2012), we noted that simvastatin showed the most significant growth inhibition among compounds tested among a panel of eight BRAF-mutant melanoma cell lines derived from treatment-naïve patients (Supplementary Figure 1A). We then went on to rigorously evaluate the interaction between statins and standard-of-care dual BRAF/MEK inhibition by testing simvastatin in combination with the BRAF inhibitor vemurafenib and the MEK inhibitor selumetinib in cell lines previously unexposed to both MAPK inhibitors. We treated the drug-naïve BRAF-mutant melanoma cell lines YUMAC and YURIF with both simvastatin monotherapy and 5 μM vemurafenib, 150 nM selumetinib, and simvastatin triple therapy, noting that while effective as a monotherapy in high doses, simvastatin added little benefit to combined BRAF and MEK inhibition (Figure 1). More interestingly, when tested on subclones of these two lines that had been selected for either vemurafenib resistance (YUMACr and YURIFr) or dual vemurafenib and selumetinib resistance (YUMACdr and YURIFdr), we found that triple therapy significantly increased the reduction in growth proliferation at low concentrations of simvastatin despite little to no efficacy of vemurafenib and selumetinib alone (Figure 1, Supplementary Figure 1B). A similar pattern was observed with lovastatin, another lipophilic member of the statin drug class (Supplementary Figure 2). Finally, to determine whether this synergistic effect between MAPK pathway inhibitors and statins extended to BRAF-mutant cancer of other types, we tested simvastatin mono- vs triple therapy in a V600-mutant BRAF colorectal cancer cell line, RKO, and a G469-mutant NSCLC cell line, H1395. Interestingly, despite minimal growth inhibition by either vemurafenib/selumetnib dual therapy or simvastatin monotherapy alone, the combination of all three agents significantly reduced growth in both lines (Figure 1). This suggested that simvastatin efficacy was greatly enhanced by MAPK inhibition in BRAF-mutant tumors across cell types. Similar effects were also seen with MEK inhibition in NRAS-mutant melanoma cell lines. Furthermore, simvastatin showed significant efficacy in both the mono- and triple therapy settings in long-term colony formation over a 14-day assay. (Supplementary Figure 1C).
Figure 1: MAPK inhibition potentiates cancer growth inhibition with statins.
(A). Dose-response curves show growth inhibition over 72 hours across eight serial statin dilutions. Wells in the combination setting were treated with either 5μmol/L vemurafenib, 150nmol/L selumetinib, or both in together with simvastatin as noted. Wells were assayed in triplicate. Simvastatin-free condition plotted for reference. Error bars represent SEM, n=3. (B). Combination MAPK inhibition and simvastatin treatment significantly increased the fraction of apoptotic cells over 72 hour treatment course as assessed by propidium iodide and annexin V flow cytometry. Live cells are defined as events negative for both PI and A5. Error bars represent SEM, n=3 (C). A diagram of the mevalonate pathway.
To elucidate the relative contribution of growth arrest versus induction of apoptosis from statin therapy, we conducted combined propidium iodide/annexin V apoptosis detection flow. Much of these growth inhibitory effects appear to be mediated by induction of apoptosis, with a significant portion of cells positive for either or both of these markers by 72 hours (Figure 1B). Consistent with previous experiments, simvastatin induced significantly higher rates of apoptosis in conjunction with vemurafenib and selumetinib despite nonsignificant apoptosis induction by MAPK inhibition alone. With this phenotype fully characterized, we set out to elucidate which specific species downstream of HMG-CoA reductase (Figure 1C) were vital for melanoma growth.
Growth-inhibitory activity of statins are mediated by their effect on protein isoprenylation
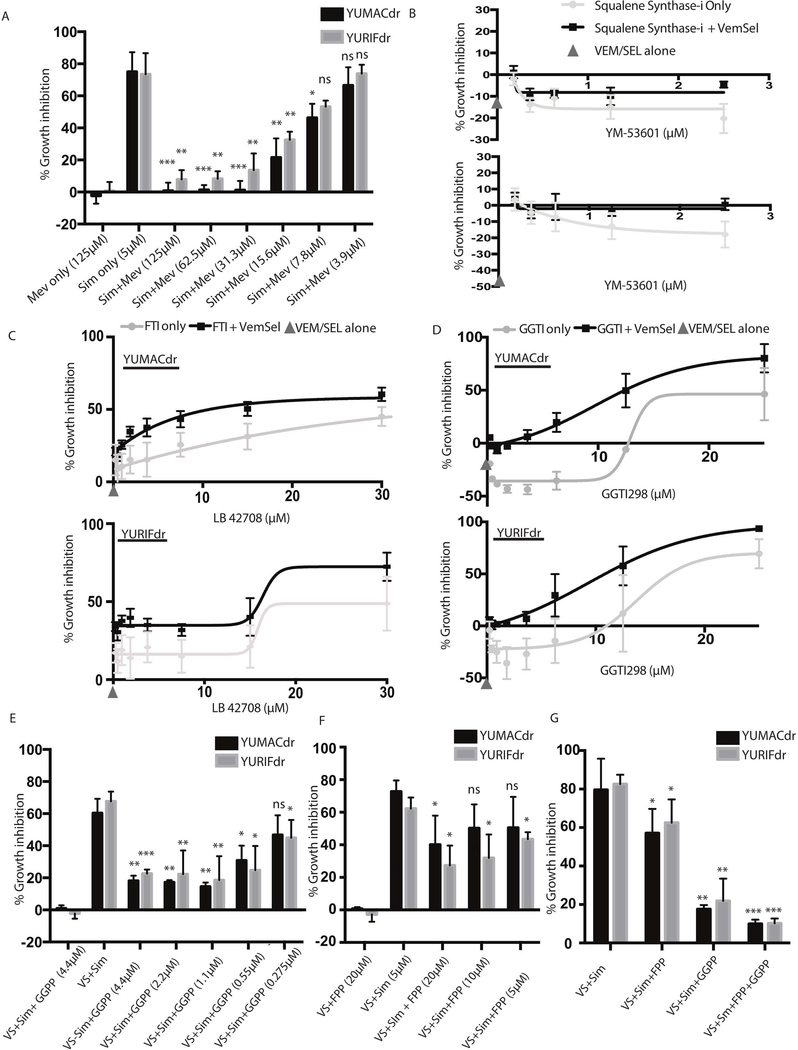
To verify that these observed effects are mediated by the HMG-CoA reductase inhibitory activity of statins as opposed to an off-target effect, we co-treated YUMACdr and YURIFdr cells with simvastatin triple therapy and mevalonolactone. Mevalonolactone is the cell-permeable end product of HMG-CoA reduction, and has been previously validated as rescuing the effects of statins in a number of settings, including cancer(Gruenbacher et al., 2010; Jiang et al., 2014). At concentrations as low as single-digit micromolar, mevalonolactone was able to significantly rescue statin-induced growth inhibition, with near-complete rescue down to doses as low as 30 μM (Figure 2A). After being converted to farnesyl pyrophosphate (FPP), mevalonate pathway intermediates may be converted to any of several different end products (Figure 1C). Squalene synthase catalyzes conversion of FPP to squalene: the first step down toward synthesis of cholesterol. Inhibition of squalene synthase with YM-53601(Montero et al., 2008), a small molecule therapeutic, showed minimal effect on melanoma proliferation in single or triple therapy (Figure 2B). This suggested while direct loss of cholesterol synthesis may have some effect on growth inhibition, other branches of the mevalonate pathway likely play a larger role in driving growth in BRAF-mutant tumors.
Figure 2: Statin-mediated growth inhibition is a result of loss of isoprenoid synthesis due to mevalonate pathway blockade.
(A). Mevalonolactone rescues 72 hour 5μmol/L simvastatin and MAPK inhibitor growth inhibition. (B). The squalene synthase inhibitor YM-53601 does not recapitulate statin-induced growth inhibition as either mono- or combination therapy. (C,D). 72 hour treatment with both the farnesyl transferase inhibitor LB 42708 or the geranylgeranyl transferase inhibitor GGTI298 shows a similar pattern of growth inhibition to simvastatin. (E,F) Rescue with either farnesyl pyrophosphate (FPP), geranylgeranyl pyrophosphate (GGPP), or the combination significantly abrogates statin-induced growth inhibition over 72 hours, with the combination (G) providing significantly greater rescue than either group alone. All 72 hour assays were performed in technical triplicate or sextuplicate. Error bars represent SEM, n=3.
In addition to being used directly to post-translationally modify proteins, FPP may also be converted to geranylgeranyl pyrophosphate (GGPP), a separate isoprenoid post-translational protein-modifying subunit. The ability of both FPP and GGPP to regulate protein activity by increasing lipophilicity is well-documented(Berndt et al., 2011; Jiang et al., 2000; Omerovic et al., 2007), generally through re-localization to lipid-rich regions such as the golgi, the endoplasmic reticulum, or the inner surface of the plasma membrane. Several important oncogenes are known to undergo FPP and GGPP isoprenylation, including RAS (Avruch et al., 1994; Downward, 2003), RAC(Berndt et al., 2011; Joyce and Cox, 2003), and RHO(Berndt et al., 2011). To evaluate the importance of FPP and GGPP isoprenylation to the growth inhibitor effects of simvastatin, we measured growth inhibition with the farnesyl transferase inhibitor LB 42708 and the geranylgeranyl transferase inhibitor GGTI 298 both alone and in combination with vemurafenib and selumetinib (Figure 2C and 2D). Results showed a similar pattern to simvastatin treatment, with MAPK inhibition potentiating the effects of both transferase inhibitors.
To verify this dependency, we performed rescue experiments with both FPP and GGPP alone (Figure 2E, 2F) and together (Figure 2G) in the presence of 5μ vemurafenib, 150 nM selumetinib, and 5 μM simvastatin. While both FPP and GGPP showed significant rescue on their own, the combination was sufficient to nearly completely restore melanoma proliferation in 72-hour culture, strongly suggesting that inhibition of protein isoprenylation is the primary mechanism by which statins prevent tumor growth.
Loss of farnesyl and geranylgeranyl post-translational isoprenylation inhibits AKT and Hippo signaling
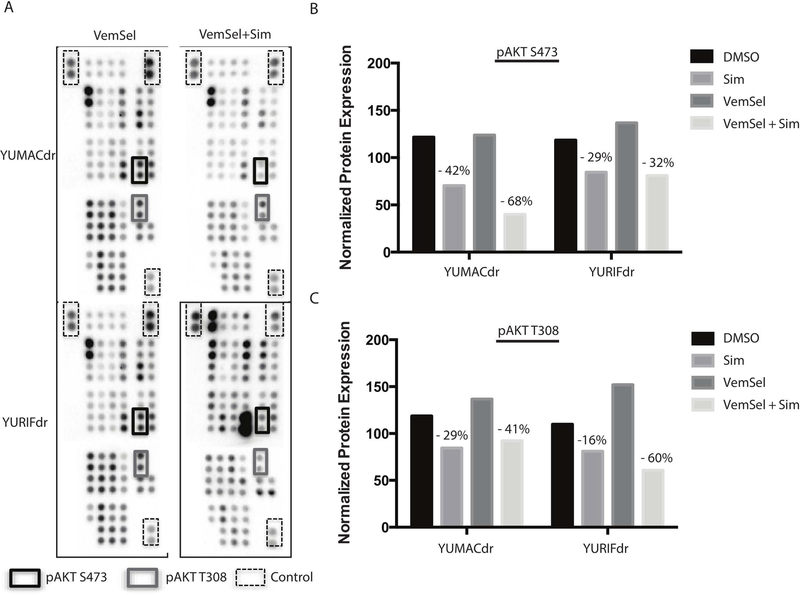
To determine the effect of isoprenylation loss on major growth pathways, we performed broad proteome profiling using human phospho-kinase antibody arrays (R&D Systems, Minneapolis, MN) with lysates of YUMACdr and YURIFdr melanoma cells treated for 24 hours with either vehicle, vemurafenib and selumetinib dual therapy, simvastatin, or all three drugs in combination (Figure 3A, Figure 3B, Supplementary Figure 3). Across a panel of 43 kinases known to be important for growth and proliferation, we observed significant downregulation primarily in phospho-AKT at both S473 and T308, suggesting that AKT signaling shows a strong dependence on isoprenylation in these cell lines.
Figure 3: Major effects of statins are restricted to a limited number of pathways.
(A). Human phospho-kinase antibody array profiling of lysates from resistant lines YUMACdr and YURIFdr treated with 5 μmol/L vemurafenib and 150nmol/L selumetinib with or without 5μmol/L simvastatin for 24 hours showing significant downregulation of AKT activation. (B), (C). Densitometric changes in AKT phosphorylation as measured by antibody array. Percent changes depicted with simvastatin treatment are relative to relevant control (DMSO only for Sim and VemSel only for VemSel+Sim).
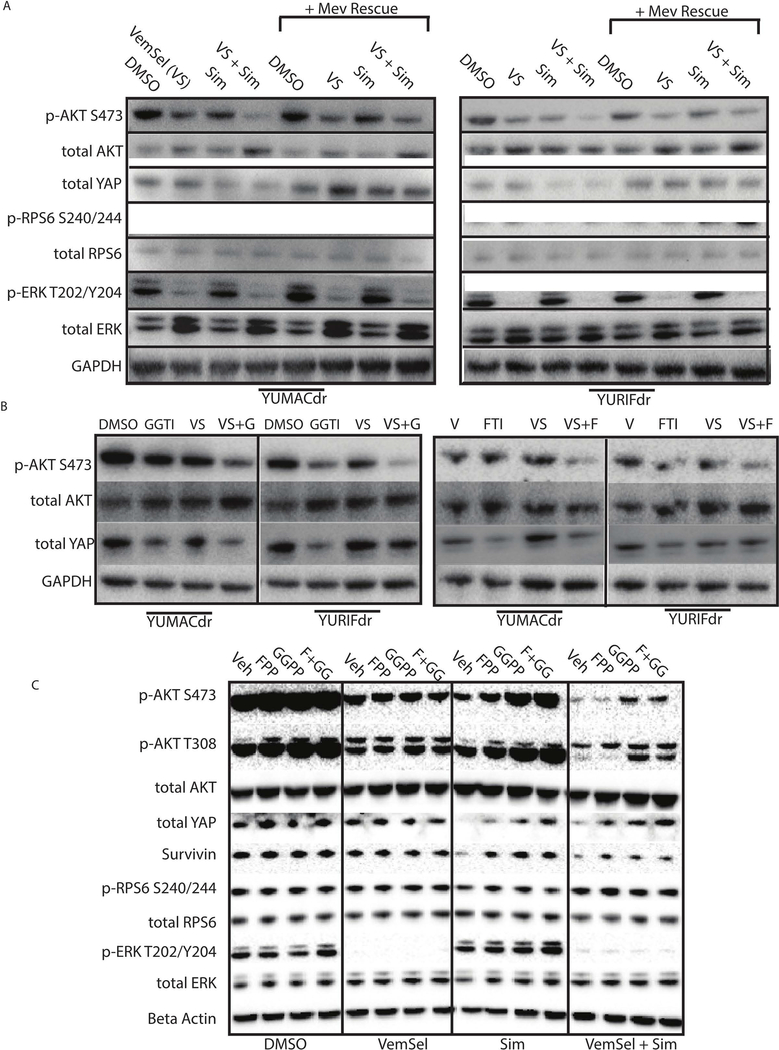
To verify on-target drug effects, we paired drug-treated samples with identical conditions additionally treated with 125μM mevalonate rescue (Figure 4A). Notably, we noticed no additional decrease in ERK phosphorylation in the setting of statin therapy, indicating that MAPK inhibition was likely not the major mechanism of action of the drug. Consistent with kinase array results, we observed significant loss of S473 AKT phosphorylation with no discernible decrease in S240/244 phospho-RPS6, suggesting a possible mTOR-independent AKT effect. Given the decreased AKT signaling observed by kinase array and the lack of significant effect on mTOR and MAPK, we wished to identify additional targets of AKT known to be important isoprenylation-dependent growth pathways in cancer. The Hippo pathway, with YAP as functioning as its effector transcription factor, is known to be regulated by the small GTP-ase RAS through inhibition of the negative YAP regulators LATS1 and LATS2(Park and Guan, 2013; Sorrentino et al., 2014). Several previous studies of BRAF-mutant melanoma have noted the importance of the Hippo pathway to robust melanoma growth and invasion, as well as resistance to targeted therapy (Lin et al., 2015; Nallet-Staub et al., 2014). Nevertheless, no studies to date have investigated the effect of statins on hippo pathway activity in the setting of BRAF-mutant cancers. In keeping with previous studies, we noticed a significant decrease in YAP that was rescued with reintroduction of mevalonate. These findings were recapitulated with direct inhibition of farnesyl transferase and geranylgeranyl transferase for 24 hours with our previously-validated small molecule inhibitors (Figure 4B). We also observed downregulation of survivin, an anti-apoptotic factor under transcriptional control of YAP (Huang et al., 2013), in line with previous data showing significant apoptosis rates with simvastatin treatment. Notably, both FTI and GGTI inhibition independent of one another, when combined with vemurafenib and selumetinib, were sufficient to decrease phospho-AKT and YAP to levels comparable to simvastatin, suggesting that both isoprenoid species are required for proper functioning of these pathways. To elucidate relative contributions of isoprenoid species to changes in signaling, we went on to treat YURIFdr with simvastatin and FPP, GGPP, or a combination of the two (Figure 4C). Consistent with our growth inhibitory data, we found that while FPP and GGPP were able to rescue AKT phosphorylation and YAP levels alone, the combination of the two provided near total rescue to non-simvastatin levels.
Figure 4: Statins inhibits AKT and Hippo signaling.
(A). 24 hour treatment with 5μmol/L simvastatin with or without 5 μmol/L vemurafenib and 150nmol/L selumetinib significantly downregulates phospho-AKT S473 and YAP, with rescue of the effect by mevalonolactone. (B). Treatment with both farnesyl and geranylgeranyl transferase inhibitors with or without 5 μmol/L vemurafenib and 150nmol/L selumetinib show comparable effects on AKT signaling over 24 hours, with greatest effect in the combination setting. (C). Downregulation of AKT and YAP signaling from 24 hour treatment with vemurafenib, selumetinib, and simvastatin is rescued with repletion of farnesyl- or geranylgeranyl-pyrophophate, with greatest rescue observed with repletion of both. n = 3 for all blots.
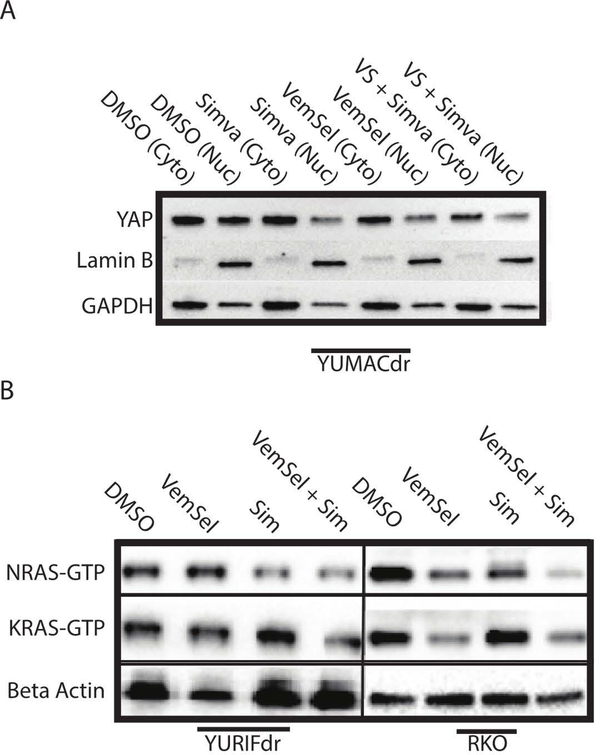
Given the dependence on YAP nuclear localization for its activity as a transcription factor, we performed cellular fractionation experiments to quantify changes in the relative distribution of YAP between the cytosol and the nucleus (Figure 5A). Treatment of YUMACdr cells with 5μM simvastatin with and without dual vemurafenib and selumetinib therapy for 24 hours was sufficient to significantly relocalize YAP out of the nucleus in both simvastatin mono- and triple therapy conditions, where it is subsequently degraded(Zhao et al., 2010), with the strongest decrease across both compartments observed with triple therapy. The downregulation and delocalization away from the nucleus was also confirmed by confocal microscopy (Supplementary Figure 4).
Figure 5: Relocalization of YAP and RAS species are tied to isoprenoid loss.
(A). Nuclear fractionation after 24 hour simvastatin treatment in the combination therapy setting significantly reduces active YAP in the nucleus. n=3. (B). Immunoprecipitation of GTP-bound active RAS species after 24 hour treatment with 5 μmol/L vemurafenib and 150nmol/L selumetinib, 5 μmol/L simvastatin, or the combination shows a significant decrease in RAS activity, with the greatest pan-RAS decrease observed with the triple combination. n=3.
To confirm our hypothesis regarding impaired RAS activity in the setting of statin therapy, we conducted a number of experiments aimed at verifying the effects of isoprenylation inhibition on RAS species. We first performed an immunoprecipitation for GTP-bound activated RAS species, demonstrating decreases in levels of both active NRAS and KRAS in statin-treated cells (Figure 5B). In line with previous findings, the most significant decrease in active RAS species occurred in the triple therapy setting across all lines tested. While the effects of cholesterol synthesis inhibition on AKT and the Hippo pathway appear to be greater based on data presented in Figure 4, these GTP binding data also suggest that there is some direct inhibition of MAPK activity by statin treatment as well. To confirm mislocalization, we performed confocal microscopy for NRAS in one of our resistant melanoma lines, which showed clear delocalization of NRAS away from the membrane, where it’s known to be most active, in the setting of HMG-CoA reductase inhibition (Supplementary Figure 5).
Mevalonate pathway activity plays an important role in melanoma growth in vivo
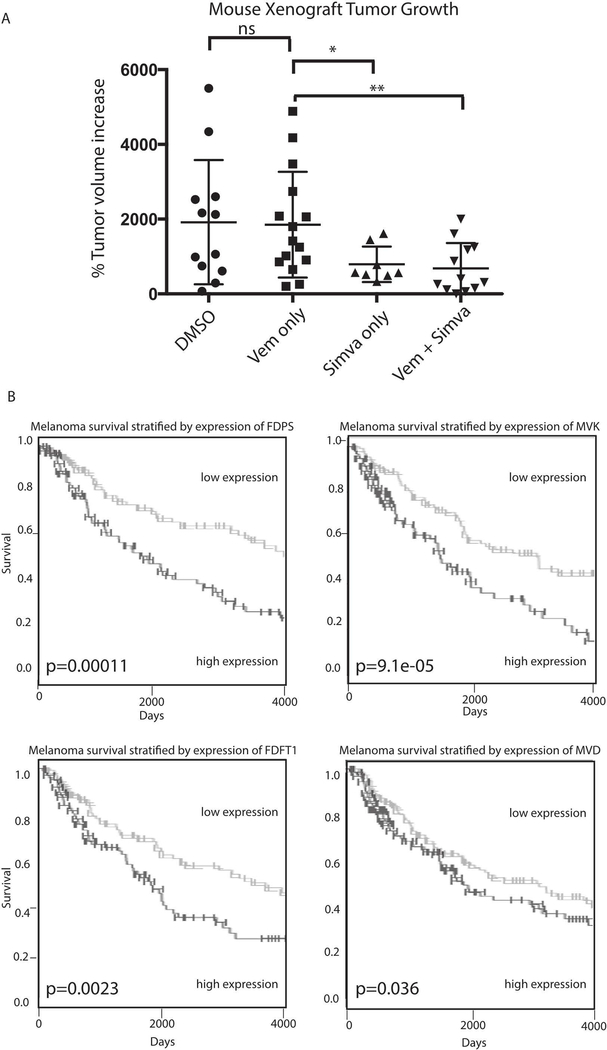
To verify the in vivo efficacy of simvastatin therapy, we xenografted vemurafenib-resistant YUMACr cells into NCr immunocompromised nude mice. Each mouse was grafted in the left and right flanks as well as the base of the neck. Cohorts were treated with either vehicle, vemurafenib chow, 30 mg/kg intraperitoneal simvastatin every other day, or combination vemurafenib chow and simvastatin. Mice treated with simvastatin either alone or in combination with vemurafenib showed significantly decreased tumor growth when endpoint of 1 cm3 was reached in the first mice versus vehicle or vemurafenib-treated mice (p=0.05 simvastatin only, p=0.01 combination) (Figure 6A, Supplementary Figure 6). There was no statistically significant difference in growth between vehicle and vemurafenib-only treated mice at the experimental endpoint.
Figure 6: Mevalonate pathway activity plays a significant role in melanoma activity in vivo.
(A). Four cohorts of five mice each were grafted with YUMACr vemurafenib-resistant human melanoma cells. Treatment was begun once the first mice showed tumors of at least 1mm3. Mice receiving vemurafenib were fed vemurafenib-containing chow, while mice receiving simvastatin received 30mg/kg simvastatin injection in DMSO every other day after an initial loading phase of 4 daily doses. The experiment was concluded when the first mouse reached tumor size endpoint of 1cm3. (B). Query of The Cancer Genome Atlas showed that levels of multiple mevalonate pathway members significantly predicted overall survival in melanoma patients.
To determine the significance of mevalonate pathway activity to disease prognosis in human melanoma patients, we used data from the Cancer Genome Atlas to independently analyze correlation of pathway member levels with overall survival. We found that upregulation of farnesyl pyrophosphate synthase (FDPS), Farnesyl-Diphosphate Farnesyltransferase (FDFT1), mevalonate kinase (MVK), and mevalonate dehydrogenase (MVD) were all significantly associated with decreased overall survival (Figure 6B), highlighting the importance of this pathway to human cancer biology and prognosis.
Discussion
Despite promising in vitro data, previous investigations of statins as potential cancer therapeutics have been limited by their significant toxicity at high doses(Graaf et al., 2004; Kiortsis et al., 2007). Nevertheless, our previous high-throughput drug screens provided preliminary evidence of synergistic efficacy between MAPK inhibition and simvastatin in melanoma(Held et al., 2013). Expanding on these initial findings, we show that MAPK inhibition in tumors in which the pathway is hyperactivated synergizes with statin therapy across not only melanoma, but multiple cancer types. Interestingly, dual MAPK and HMG-CoA reductase inhibition synergy was also observed among a wide range of mutation types, including BRAF V600 mutations, non-V600 BRAF mutations, and activating mutations in NRAS. This novel finding suggests that dependence on mevalonate pathway products is a general feature of MAPK dependence irrespective of where in the pathway the activating mutation is located. By showing a decrease in the GI50 of statins in combination therapy, our findings suggest a way to utilize the observed anti-cancer effects of statins while bypassing the documented toxicity observed at the high doses previously thought to be required for full effect.
While previous research has demonstrated the importance of protein prenylation for cancer growth and progression(Berndt et al., 2011; Collisson et al., 2003), a number of different mechanistic explanations have been asserted in different contexts to account for this dependence. Our findings suggest an increased dependence of RAF and MEK inhibitor-resistant MAPK-driven tumors on RAS-dependent AKT signaling, as well as dependence on the Hippo pathway, which is known to be at least partially dependent on AKT(Basu et al., 2003). Given the previous research identifying both AKT(Silva et al., 2014) and YAP (Lin et al., 2015) activity as key resistance mechanisms to MAPK inhibition, the ability to inhibit both targets simultaneous appears to be advantageous. Our combinatorial farnesyl and geranylgeranyl supplementation experiments suggest that continued activity of both AKT and Hippo are dependent to some degree on both farnesylation and geranylgeranylation independently, with greatest rescue of proliferation with reintroduction of both groups. The relatively greater rescue with geranylgeranyl supplementation alone versus farnesyl supplementation may provide a partial explanation for the poor clinical performance of farnesyl transferase inhibitors in previous clinical trials(Appels et al., 2005), as might their evaluation before the advent of targeted BRAF or MEK inhibitors.
Our encouraging murine and TCGA data lend further new credence to the potential efficacy of statins in cancer patients. While a single previous study demonstrated some benefit to statin therapy in reducing melanoma invasion and metastatic potential in a murine model(Kidera et al., 2010), there has been minimal previous data showing reduction in grafted tumor growth with statins in vivo. Moreover, the high prognostic significance of multiple key mevalonate pathway members with regards to overall survival strongly suggests the importance of this pathway in human cancer, further highlighting the need for more rigorous evaluation of the role of cholesterol metabolism in both tumor growth and therapeutic resistance. While it seems implausible that activity of mevalonate pathway enzymes alone would be rate-limiting for growth in melanoma, the extra substrates for macromolecular synthesis provided by overactivity of this pathway may, in concert with overactivity in other pathways, in aggregate allow certain subsets of tumors to grow more aggressively.
Given the current FDA approval of statins as treatment for hypercholesterolemia, our data provide support for evaluation of statins in combination targeted therapy in patients. Our results suggest that they may show clinical efficacy in the setting of not only BRAF and NRAS-mutant melanoma, but RAF-driven CRC and NSCLC as well, all of which together accounted for over 70,000 new cancer diagnoses in 2014 alone(Cremolini et al., 2015; Society, 2014; van Eijk et al., 2011). Furthermore, given the high monetary cost of current MAPK inhibitors, combination regimens which boost the efficacy of existing targeted therapies without significantly increasing cost represent an attractive approach for new pharmaceutical development. Vemurafenib prices at approximately $13,000 per month of treatment(Curl et al., 2014), while generic simvastatin by contrast can be obtained for less than $10 for the equivalent number of doses, providing significant economic incentive for anti-cancer statin trials. Given both the promising pre-clinical data described in this study, as well as the myriad regulatory and economic advantages represented by repurposing statins for cancer therapy, implementation of clinical trials could lead to improved responses to existing targeted agents with minimal new time and resource commitment.
Supplementary Material
Significance.
Given that the overwhelming majority of patients on RAF, MEK, and other MAPK pathway inhibitors ultimately relapse on therapy, the search for new adjunctive agents continues to be of clinical significance. Agents previously approved and broadly-prescribed for other indications are especially attractive given their ease of utilization. In this study, we identify a previously unevaluated niche for statins in combination with RAF and MEK inhibitors: a setting in which they show appear to show greatly enhanced anti-tumor activity.
Acknowledgments
We would like to acknowledge Ruth Halaban and Antonella Bacchiocchi for providing melanoma cell lines from the Yale melanoma SPORE tissue bank. We would also like to thank Matthew Held, Ramanaiah Mamillapalli, and Pinar Iyidogan for their help with the initial drug screening work that lead to this study. We would also like to acknowledge our grant support: NIH Yale SPORE in Skin Cancer NCI P50 CA121974 to MWB, Ruth L. Kirschstein NRSA 1F30CA180591–01 funded by the NCI to NT, American Skin Association Student Award to NT. CGL was supported by training Grant T32GM007324.
Footnotes
Conflict of Interest
Marcus Bosenberg is a consultant for Eli Lilly and Company. No other authors have conflicts to disclose.
References
- Appels NM, Beijnen JH, and Schellens JH (2005). Development of farnesyl transferase inhibitors: a review. The oncologist 10, 565–78. [DOI] [PubMed] [Google Scholar]
- Avruch J, Zhang XF, and Kyriakis JM (1994). Raf meets Ras: completing the framework of a signal transduction pathway. Trends in biochemical sciences 19, 279–83. [DOI] [PubMed] [Google Scholar]
- Basu S, Totty NF, Irwin MS, Sudol M, and Downward J (2003). Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14–3-3 and attenuation of p73-mediated apoptosis. Molecular cell 11, 11–23. [DOI] [PubMed] [Google Scholar]
- Berndt N, Hamilton AD, and Sebti SM (2011). Targeting protein prenylation for cancer therapy. Nature reviews. Cancer 11, 775–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MJ, Esserman LJ, Zhou Y, Shoemaker M, Lobo M, Borman E, Baehner F, Kumar AS, Adduci K, Marx C, et al. (2006). Breast cancer growth prevention by statins. Cancer research 66, 8707–14. [DOI] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. (2011). Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine 364, 2507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clendening JW, and Penn LZ (2012). Targeting tumor cell metabolism with statins. Oncogene 31, 4967–78. [DOI] [PubMed] [Google Scholar]
- Collisson EA, Kleer C, Wu M, De A, Gambhir SS, Merajver SD, and Kolodney MS (2003). Atorvastatin prevents RhoC isoprenylation, invasion, and metastasis in human melanoma cells. Molecular cancer therapeutics 2, 941–8. [PMC free article] [PubMed] [Google Scholar]
- Cremolini C, Di Maio M, Petrelli F, Berenato R, Loupakis F, and Pietrantonio F (2015). BRAF-mutated metastatic colorectal cancer between past and future. British journal of cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curl P, Vujic I, Van ‘T Veer LJ, Ortiz-Urda S, and Kahn JG (2014). Cost-effectiveness of treatment strategies for BRAF-mutated metastatic melanoma. PloS one 9, e107255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. (2002). Mutations of the BRAF gene in human cancer. Nature 417, 949–54. [DOI] [PubMed] [Google Scholar]
- Davies MA, Stemke-Hale K, Lin E, Tellez C, Deng W, Gopal YN, Woodman SE, Calderone TC, Ju Z, Lazar AJ, et al. (2009). Integrated Molecular and Clinical Analysis of AKT Activation in Metastatic Melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research 15, 7538–7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MA, Stemke-Hale K, Tellez C, Calderone TL, Deng W, Prieto VG, Lazar AJ, Gershenwald JE, and Mills GB (2008). A novel AKT3 mutation in melanoma tumours and cell lines. British journal of cancer 99, 1265–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Gopal YN, Scott A, Chen G, Woodman SE, and Davies MA (2012). Role and therapeutic potential of PI3K-mTOR signaling in de novo resistance to BRAF inhibition. Pigment cell & melanoma research 25, 248–58. [DOI] [PubMed] [Google Scholar]
- Downward J (2003). Targeting RAS signalling pathways in cancer therapy. Nature reviews. Cancer 3, 11–22. [DOI] [PubMed] [Google Scholar]
- Fedorenko IV, Gibney GT, and Smalley KS (2013). NRAS mutant melanoma: biological behavior and future strategies for therapeutic management. Oncogene 32, 3009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, et al. (2012a). Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. The New England journal of medicine 367, 1694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, Demidov LV, Hassel JC, Rutkowski P, Mohr P, et al. (2012b). Improved survival with MEK inhibition in BRAF-mutated melanoma. The New England journal of medicine 367, 107–14. [DOI] [PubMed] [Google Scholar]
- Graaf MR, Richel DJ, Van Noorden CJ, and Guchelaar HJ (2004). Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer treatment reviews 30, 609–41. [DOI] [PubMed] [Google Scholar]
- Gruenbacher G, Gander H, Nussbaumer O, Nussbaumer W, Rahm A, and Thurnher M (2010). IL-2 costimulation enables statin-mediated activation of human NK cells, preferentially through a mechanism involving CD56+ dendritic cells. Cancer research 70, 9611–20. [DOI] [PubMed] [Google Scholar]
- Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S, Krauthammer M, Mccusker JP, Kluger Y, and Sznol M (2010). PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment cell & melanoma research 23, 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held MA, Langdon CG, Platt JT, Graham-Steed T, Liu Z, Chakraborty A, Bacchiocchi A, Koo A, Haskins JW, Bosenberg MW, et al. (2013). Genotype-selective combination therapies for melanoma identified by high-throughput drug screening. Cancer discovery 3, 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JM, Nagatomo I, Suzuki E, Mizuno T, Kumagai T, Berezov A, Zhang H, Karlan B, Greene MI, and Wang Q (2013). YAP modifies cancer cell sensitivity to EGFR and survivin inhibitors and is negatively regulated by the non-receptor type protein tyrosine phosphatase 14. Oncogene 32, 2220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Coppola D, Crespo NC, Nicosia SV, Hamilton AD, Sebti SM, and Cheng JQ (2000). The phosphoinositide 3-OH kinase/AKT2 pathway as a critical target for farnesyltransferase inhibitor-induced apoptosis. Molecular and cellular biology 20, 139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Mukthavaram R, Chao Y, Nomura N, Bharati IS, Fogal V, Pastorino S, Teng D, Cong X, Pingle SC, et al. (2014). In vitro and in vivo anticancer effects of mevalonate pathway modulation on human cancer cells. British journal of cancer 111, 1562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce PL, and Cox AD (2003). Rac1 and Rac3 are targets for geranylgeranyltransferase I inhibitor-mediated inhibition of signaling, transformation, and membrane ruffling. Cancer research 63, 7959–67. [PubMed] [Google Scholar]
- Kang HB, Fan J, Lin R, Elf S, Ji Q, Zhao L, Jin L, Seo JH, Shan C, Arbiser JL, et al. (2015). Metabolic Rewiring by Oncogenic BRAF V600E Links Ketogenesis Pathway to BRAF-MEK1 Signaling. Molecular cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidera Y, Tsubaki M, Yamazoe Y, Shoji K, Nakamura H, Ogaki M, Satou T, Itoh T, Isozaki M, Kaneko J, et al. (2010). Reduction of lung metastasis, cell invasion, and adhesion in mouse melanoma by statin-induced blockade of the Rho/Rho-associated coiled-coil-containing protein kinase pathway. Journal of experimental & clinical cancer research : CR 29, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiortsis DN, Filippatos TD, Mikhailidis DP, Elisaf MS, and Liberopoulos EN (2007). Statin-associated adverse effects beyond muscle and liver toxicity. Atherosclerosis 195, 7–16. [DOI] [PubMed] [Google Scholar]
- Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, Mccusker JP, Cheng E, Davis MJ, Goh G, Choi M, et al. (2012). Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nature genetics 44, 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, Asthana S, Neel D, Yan JJ, Lu X, et al. (2015). The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nature genetics 47, 250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GV, Stroyakovskiy D, Gogas H, Levchenko E, De Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, et al. (2014). Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. The New England journal of medicine 371, 1877–88. [DOI] [PubMed] [Google Scholar]
- Montero J, Morales A, Llacuna L, Lluis JM, Terrones O, Basanez G, Antonsson B, Prieto J, Garcia-Ruiz C, Colell A, et al. (2008). Mitochondrial cholesterol contributes to chemotherapy resistance in hepatocellular carcinoma. Cancer research 68, 5246–56. [DOI] [PubMed] [Google Scholar]
- Nallet-Staub F, Marsaud V, Li L, Gilbert C, Dodier S, Bataille V, Sudol M, Herlyn M, and Mauviel A (2014). Pro-invasive activity of the Hippo pathway effectors YAP and TAZ in cutaneous melanoma. The Journal of investigative dermatology 134, 123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SF, Nordestgaard BG, and Bojesen SE (2012). Statin use and reduced cancer-related mortality. The New England journal of medicine 367, 1792–802. [DOI] [PubMed] [Google Scholar]
- Omerovic J, Laude AJ, and Prior IA (2007). Ras proteins: paradigms for compartmentalised and isoform-specific signalling. Cellular and molecular life sciences : CMLS 64, 2575–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HW, and Guan KL (2013). Regulation of the Hippo pathway and implications for anticancer drug development. Trends in pharmacological sciences 34, 581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CR, and Schulze A (2012). Lipid metabolism in cancer. The FEBS journal 279, 2610–23. [DOI] [PubMed] [Google Scholar]
- Silva JM, Bulman C, and Mcmahon M (2014). BRAFV600E cooperates with PI3K signaling, independent of AKT, to regulate melanoma cell proliferation. Molecular cancer research : MCR 12, 447–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, and Toomre D (2000). Lipid rafts and signal transduction. Nature reviews. Molecular cell biology 1, 31–9. [DOI] [PubMed] [Google Scholar]
- Society AC (2014). Cancer Facts and Figures 2014. (Atlanta, GA. [Google Scholar]
- Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S, et al. (2014). Metabolic control of YAP and TAZ by the mevalonate pathway. Nature cell biology 16, 357–66. [DOI] [PubMed] [Google Scholar]
- Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, Mcarthur GA, Hutson TE, Moschos SJ, Flaherty KT, et al. (2012). Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. The New England journal of medicine 366, 707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao H, Goel V, Wu H, Yang G, and Haluska FG (2004). Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. The Journal of investigative dermatology 122, 337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eijk R, Licht J, Schrumpf M, Talebian Yazdi M, Ruano D, Forte GI, Nederlof PM, Veselic M, Rabe KF, Annema JT, et al. (2011). Rapid KRAS, EGFR, BRAF and PIK3CA mutation analysis of fine needle aspirates from non-small-cell lung cancer using allele-specific qPCR. PloS one 6, e17791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Goel V, and Haluska FG (2003). PTEN signaling pathways in melanoma. Oncogene 22, 3113–22. [DOI] [PubMed] [Google Scholar]
- Yamada K, Brink I, Bisse E, Epting T, and Engelhardt R (2005). Factors influencing [F-18] 2-fluoro-2-deoxy-D-glucose (F-18 FDG) uptake in melanoma cells: the role of proliferation rate, viability, glucose transporter expression and hexokinase activity. The Journal of dermatology 32, 316–34. [DOI] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, and Guan KL (2010). A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes & development 24, 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.