Abstract
Background:
Oxidative stress plays an important role in the development of atherosclerosis. An association exists between the alterations of liver markers and the risk of coronary heart disease (CHD). This study was designed to investigate the status of oxidative stress and liver markers in patients with CHD.
Methods:
This study included 50 CHD patients and 50 healthy volunteers. Serum activities of glutathione peroxidase (GPX), catalase (CAT), alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and glutathione (GSH), malondialdehyde (MDA), nitric oxide (NO), and fasting blood sugar (FBS) concentrations were measured. The Unpaired Student’s t-test was used to analyze the data.
Results:
Serum GSH level and CAT and GPX activities were significantly greater in healthy controls than in CHD patients. Serum MDA, NO, and FBS levels and GGT, ALT, ALP activities were significantly greater in CHD patients than in healthy controls. Serum AST activity was greater in CHD patients than in controls, but the difference was not statistically significant.
Conclusion:
Our results indicate that CHD is related to oxidative stress, lipid peroxidation, inflammation, and elevated liver enzyme activity. CHD is a deadly disease that requires appropriate medical care. Antioxidant treatment might inhibit disease progression.
Coronary heart disease: Inflammation, Liver markers, Oxidative stress
Introduction
Coronary heart disease (CHD), also called coronary artery disease (CAD), is a leading cause of death worldwide. Although therapeutic strategies to reduce CHD have been developed, deaths caused by CHD continue. CHD is anticipated to rise 30–60% from 1990 to 2020 in developed countries, while in developing countries, it is predicted to increase by 120% in women and 137% in men during the same period (1-3).
It is well known that oxidative stress and reactive oxygen species (ROS) play important roles in the development of atherosclerosis (4). Oxidative stress promotes the alteration of low-density lipoprotein (LDL) to oxidized LDL (ox-LDL). Ox-LDL is absorbed by macrophage scavenger receptors leading to foam cell formation, cholesterol agglomeration in the cells, fatty streak formation, and subsequent atherosclerosis (5, 6).
It has also been demonstrated that antioxidant enzyme activities are reduced in CHD, and this is linked to the increased disease risk (7). Activity levels of the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX), and non-enzymatic antioxidants, are considered as predictors of CHD (8). Malondialdehyde (MDA), another oxidative stress indicator, is produced by long-chain fatty acid peroxidation. Many studies have reported that MDA, which has a role in the modification of lipoproteins that contribute to atherosclerotic plaque development (9), is elevated in CHD (10, 11); therefore, serum MDA is widely utilized to measure oxidative stress in CHD patients (10, 12). Monitoring oxidative stress biomarkers and consuming antioxidants are important in the management of CHD (8).
It has been suggested that Ox-LDL and ROS promote inflammatory processes. In the inflammatory condition, NO is overproduced by the vascular system (13). This NO overproduction has been proposed as a major underlying mechanism for cell dysfunction and CHD pathogenesis (13, 14). Overproduction of NO causes lipid peroxidation and ROS generation; hence, NO is a good marker of endothelial function, oxidative stress, and inflammation (13, 14).
Liver enzymes are widely used to evaluate liver function. Several studies have indicated that liver enzyme activities are altered in CHD and this alteration is associated with CHD risk (15, 16). Studies have also shown that liver enzymes, including alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), and aspartate aminotransferase (AST) are independent risk factors for CHD. They might also be considered as prognosticators for atherosclerosis (15, 17, 18). ALT activity has been shown to rise in CAD and is linked to CAD severity (19). Therefore, liver enzyme activity monitoring may be important for CHD management.
Due to the role of oxidative stress in CHD pathogenesis and liver enzyme activities, this study was designed to investigate the status of oxidative stress and liver markers in CHD patients.
Materials and Methods
Subjects
This study included 50 CHD patients and 50 healthy volunteers at Shahid Madani Hospital, Lorestan University of Medical Sciences, Khorramabad, Iran. Our study was in accordance with the Institutional Ethics Committee. Study participants signed the informed consent form as a registration requirement. This was an observational and cross-sectional study. Subjects were selected using the following criteria:
Inclusion Criteria
1. The subjects were between the ages of 35 and 65.
2. The CHD group included patients whose disease was diagnosed based on angiography results.
Exclusion Criteria:
1. Patients affected by any synchronic disease such as cancer, diabetes, lung disease, gastro-intestinal disease, chronic liver disease, renal disease, or thyroid disease.
2. Patients taking drugs such as steroids, diuretics, or sedatives.
Biochemical Analysis
Venous blood was collected from the participants between 7:00 and 8:00 a.m. after an overnight fast of at least eight hours. About 6 ml of blood was collected by venapuncture and transferred to test tubes. Serum was isolated by centrifugation for 15 min at 3000 × g. The serum samples were stored at -70 °C until analyses.
Determination of Serum Fasting Blood Sugar (FBS) Level and Liver Enzyme Activities:
Fasting blood sugar (FBS) and serum activities of liver enzymes, including gamma-glutamyltransferase (GGT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) were assayed on a biochemical analyzer based on the protocols of commercial kits (Olympus AU-600, Tokyo, Japan).
Measurement of Serum Malondialdehyde (MDA), Glutathione (GSH), and Nitric Oxide (NO) concentrations, and Glutathione Peroxidase (GPX) and Catalase (CAT) activities
Serum MDA, GSH, and NO concentrations, and GPX and CAT activities were determined as reported in our previous studies (20, 21, 14. 22).
Statistical Analyses
The Unpaired Student’s t-test was used to analyze the data. The software package for statistical analysis version 13 (SPSS 13) for Windows was used to analyze the statistics. The significant difference between groups was set at P < 0.05.
Results
The FBS concentration was significantly greater in CHD patients than in healthy volunteers (1.22-fold), as were the activities of the liver enzymes GGT, ALT, and ALP (1.75-fold, 1.51-fold, and 1.45-fold, respectively). Serum AST activity was 1.33-fold greater in CHD patients than in controls, but this difference was not statistically significant (Table 1).
Table 1.
Serum concentrations of FBS and serum GGT, AST, ALT, and ALP activities in healthy controls and coronary heart disease (CHD) patients. Values are represented as means ± SEs.
| Parameter | Control | CHD | P Value |
|---|---|---|---|
| FBS (mg/dl) | 86.33±2.39 | 105.50±4.36 | 0.001 |
| GGT (Unit/L) | 20.32±4.66 | 35.62±4.66 | 0.014 |
| AST (Unit/L) | 18.25±1.21 | 24.29 ±3.37 | 0.098 |
| ALT (Unit/L) | 9.83±0.75 | 14.92 ±2.07 | 0.022 |
| ALP (Unit/L) | 140.12±6.34 | 203.33±24.67 | 0.034 |
Sera were collected from all subjects and GGT, AST, ALT, and ALP were assayed on a biochemical analyzer based on the protocols of commercial kits.
Serum MDA and NO concentrations were significantly greater in CHD patients than in healthy controls (2.56 and 1.06-fold, Fig. 1 and Fig. 5, respectively), while GSH, CAT, and GPX were significantly less in CHD patients than in healthy controls (1.30, 1.69, and 1.78-fold, Fig. 2, Fig. 3, and Fig. 4, respectively).
Fig. 1.
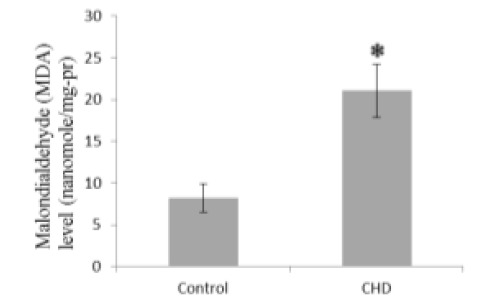
Serum malondialdehyde (MDA) concentrations in coronary heart disease (CHD) patients and controls. Sera were collected from all subjects and MDA concentrations were measured by a colorimetrical assay. * P < 0.05
Fig. 5.
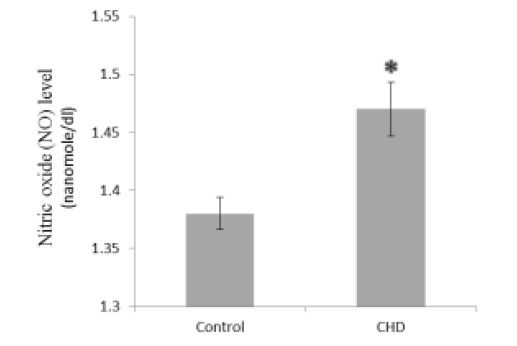
Serum nitric oxide (NO) concentrations in healthy controls and coronary heart disease (CHD) patients. Sera were collected from all subjects and NO concentrations were assayed by a colorimetrical assay. * P < 0.05
Fig. 2.
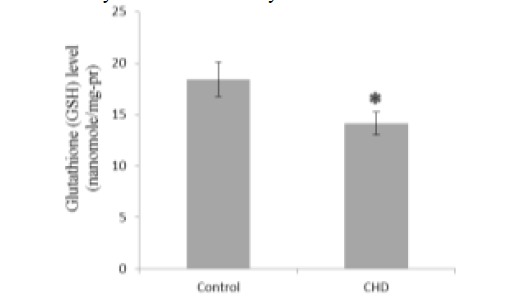
Serum glutathione (GSH) concentrations in healthy controls and coronary heart disease (CHD) patients. Sera were collected from all subjects and GSH concentrations were determined by a colorimetrical assay. * P < 0.05
Fig. 3.
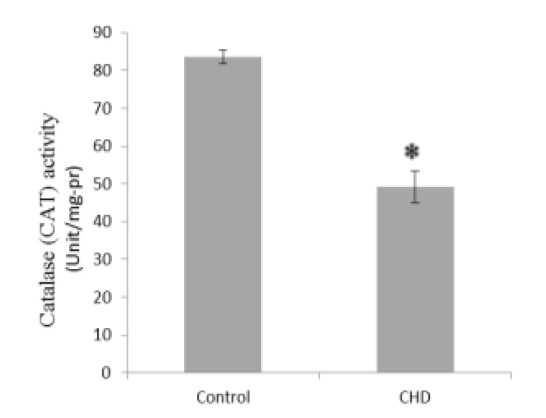
Serum catalase (CAT) activities in healthy controls and coronary heart disease (CHD) patients. Sera were collected from all subjects and CAT activities were evaluated by a colorimetrical assay. * P < 0.05
Fig. 4.
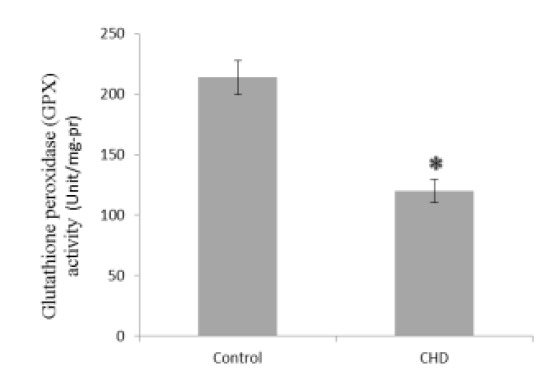
Serum glutathione peroxidase (GPX) activities in healthy controls and coronary heart disease (CHD) patients. Sera were collected from all subjects and GPX activities were measured by a colorimetrical assay. * P < 0.05
Discussion
In this study, serum MDA in was significantly greater in CHD patients than in healthy controls, while GSH concentration and the antioxidant enzymes CAT and GPX activities were significantly less in CHD patients than in controls. Many studies have indicated that free radical generation increases and antioxidant defenses decrease in response to increased oxidative stress in CHD patients (4). Various sources of ROS, including NADPH-oxidase, mitochondria, xanthine oxidoreductase, cytochrome P450, NO-synthases, peroxidases, cyclooxygenase and lipoxygenases, and hemoglobin and red blood cells have been suggested to participate in the development of atherosclerosis (4). Furthermore, it has been reported that low antioxidant enzyme activity is linked to increased CHD risk (7). In a previous study, Priya et al. showed decreased erythrocyte CAT activity and GSH concentration in CAD patients (23). Mogadam et al. also reported that serum MDA was abnormally high in stable CHD patients (24). Serdar et al. demonstrated that erythrocyte GPX and serum CAT activities were decreased in patients with angiographically-proven CAD (25). In our previous study, we found that the paraoxonase 1 activity was significantly less in CHD patients than in healthy controls (26). The present study is similar to the previous ones, for it indicates a decrease in antioxidant enzyme activity and an increase in lipid peroxidation in CHD patients. These alterations might be reflected by the enhancement of oxidative stress and free radical production in CHD patients as previously reported (7). Many researchers have shown that free radicals play a role in CHD pathogenesis by LDL oxidation and endothelial dysfunction (4). Many studies have also indicated that using antioxidants could strengthen antioxidant defense and reduce the disease risk in CHD patients (8). Hence, monitoring oxidative stress biomarkers and consuming antioxidants may aid in the management of CHD.
Many studies indicate that atherosclerosis is an inflammatory disorder (7). The roles of ROS and Ox-LDL in the promotion of the inflammatory process have been defined. It has been suggested that NO overproduction occurs in the vascular system in response to inflammation. Although the physiological production of NO is necessary for vascular system functions, the overproduction of NO is proposed as a major underlying mechanism for CHD pathogenesis. NO, at high levels, reacts with ROS and produces peroxynitrite. Peroxynitrite, a cytotoxic agent, causes lipid and protein peroxidation and DNA modification (13). Our results showed that serum NO is significantly greater in CHD patients than in healthy people. This finding agrees with that of Shaikh et al. (27), who reported a similar result. Moreover, other studies reported that plasma NOx, a stable end product of NO, was also significantly greater in CAD patients than in healthy subjects (28). This increased serum NO in CHD patients is associated with increased iNOS activity, as was previously reported (29).
This study showed that the activities of the liver enzymes ALT, ALP, and GGT were significantly greater in CHD patients than in healthy controls. AST activity was also greater in CHD patients than in controls, but this result was not statistically significant. Many studies have indicated that liver enzyme activities including those of AST, ALT, ALP, and GGT were greater in CHD patients than in healthy subjects (15, 16). A strong association between alterations of liver enzyme activities and CHD risk has been demonstrated (30). It has also been suggested that AST, ALT, and GGT are independent risk factors for CHD (15, 17). Epidemiological studies have also showed the association between increased GGT activity and CHD mortality (16). Also, some researchers have indicated that ALT and AST can be considered as independent predictors of CHD (31). Our results are in accordance with all the reports indicating that liver enzyme activities are higher in CHD patients than in healthy subjects. Therefore, the evaluation of liver markers can be important for the management of CHD. Few pathophysiological mechanisms are proposed for the association of liver markers and CHD. Some authors have asserted that the increase in ALT activity is related to insulin resistance as a main risk factor for atherosclerosis (16). Alterations in AST and ALT are also associated with metabolic syndrome, a category of disorders associated with elevated risk for cardiovascular disease-mediated T2DM atherosclerosis (31). Transaminases, including AST and ALT, might be markers of lipoprotein metabolism disorders that cause an increase in triglyceride-containing lipoproteins in blood (31).
Our findings indicate that oxidative stress and inflammation contribute to CHD development; hence, we recommend the use of antioxidants in CHD patients to inhibit disease progression. Moreover, our study demonstrated liver marker changes in CHD patients. Therefore, the liver enzyme tests can be important for CHD management. The detailed pathophysiologic mechanism(s) concerning the association between liver markers and CHD was not fully addressed in our study. More studies are required to explain this matter.
Acknowledgements
All authors thank the Lorestan University of Medical Sciences officials for providing the grant that supported this study. The authors also thank the patients and healthy volunteers who participated in this study. The authors have no conflicts of interest.
References
- 1.Bogers RP, Bemelmans WJ, Hoogenveen RT, Boshuizen HC, Woodward M, Knekt P. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med. 2007;167(16):1720–8. doi: 10.1001/archinte.167.16.1720. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet. 1997;349(9064):1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 3.Imes CC, Austin MA. Low-density lipoprotein cholesterol, apolipoprotein B, and risk of coronary heart disease: from familial hyperlipidemia to genomics. Biol Res Nurs. 2013;15(3):292–308. doi: 10.1177/1099800412436967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.V Goncharov N, V Avdonin P, D Nadeev A, L Zharkikh I, O Jenkins R. Reactive oxygen species in pathogenesis of atherosclerosis. Curr Pharm Des. 2015;21(9):1134–46. doi: 10.2174/1381612820666141014142557. [DOI] [PubMed] [Google Scholar]
- 5.Leopold JA, Loscalzo J. Oxidative mechanisms and atherothrombotic cardiovascular disease. Drug Discov Today Ther Strateg. . 2008;5(1):5, 13. doi: 10.1016/j.ddstr.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ani M, Moshtaghie AA, Ahmadvand H. Comparative effects of copper, iron, vanadium and titanium on low density lipoprotein oxidation in vitro. Iran Biomed J. 2007;11(2):113–118. [PubMed] [Google Scholar]
- 7.Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. . N Engl J Med. 2003;349(17):1605–13. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 8.Shaikh AK, Suryakar AN. Oxidative stress and antioxidant status before and after supplementation of AZ anti-oxidant tablets in coronary artery disease. . Biomed Res. 2009;20(2):136–40. [Google Scholar]
- 9.Cavalca V, Cighetti G, Bamonti F, Loaldi A, Bortone L, Novembrino C, et al. Oxidative stress and homocysteine in coronary artery disease. Clin Chem. 2001;47(5):887–92. [PubMed] [Google Scholar]
- 10.Mutlu-Türkoðlu Ü, Akalýn Z, Ýlhan E, Yýlmaz E, Bilge A, Niþancý Y, et al. Increased plasma malondialdehyde and protein carbonyl levels and lymphocyte DNA damage in patients with angiographically defined coronary artery disease. Clin Biochem. 2005;38(12):1059–65. doi: 10.1016/j.clinbiochem.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Soydinç S, Çelik A, Demiryürek S, Davutoğlu V, Tarakçıoğlu M, Aksoy M. The relationship between oxidative stress, nitric oxide, and coronary artery disease. . Eur J Gen Med. 2007;4(2):62–6. [Google Scholar]
- 12.Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. . Nephrol Dial Transplant. 2003;18(7):1272–80. doi: 10.1093/ndt/gfg074. [DOI] [PubMed] [Google Scholar]
- 13.Lubos E, Handy DE, Loscalzo J. Role of oxidative stress and nitric oxide in atherothrombosis. Front Biosci. 2008;13:5323–44. doi: 10.2741/3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmadvand H, Shahsavari G, Tavafi M, Bagheri S, Moradkhani MR, Mohammadrezaei Khorramabadi R, et al. Protective effects of oleuropein against renal injury oxidative damage in alloxan-induced diabetic rats; a histological and biochemical study. J Nephropathol. 2017;6(3):204–209. doi: 10.15171/jnp.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason JE, Starke RD, Van Kirk JE. Gamma-Glutamyl transferase: a novel cardiovascular risk BioMarker. Prev Cardiol. 2010;13(1):36–41. doi: 10.1111/j.1751-7141.2009.00054.x. [DOI] [PubMed] [Google Scholar]
- 16.Monami M, Bardini G, Lamanna C, Pala L, Cresci B, Francesconi P, et al. Liver enzymes and risk of diabetes and cardiovascular disease: results of the Firenze Bagno a Ripoli (FIBAR) study. Metabolism. 2008;57(3):387–92. doi: 10.1016/j.metabol.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer CD, Heine RJ, et al. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis. 2007;191(2):391–6. doi: 10.1016/j.atherosclerosis.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Ahmadvand H, Ghasemi-Dehnoo M. Antiatherogenic, hepatoprotective, and hypolipidemic effects of coenzyme Q10 in alloxan-induced type 1 diabetic rats. . ARYA Atheroscler. 2014;10(4):192–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Sahin I, Karabulut A, Gungor B, Avci II, Okuyan E, Kizkapan F, et al. Correlation between the serum alkaline phosphatase level and the severity of coronary artery disease. Coron Artery Dis. 2014;25(4):349–52. doi: 10.1097/MCA.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 20.Ahmadvand H, Bagheri S, Khosrobeigi A, Boshtam M, Abdolahpour F. Effects of olive leaves extract on LDL oxidation induced-CuSO(4) in vitro. Pak J Pharm Sci. 2012;25(3):571–5. [PubMed] [Google Scholar]
- 21.Khalatbary AR, Ahmadvand H, Ghabaee DNZ, Malekshah AK, Navazesh A. Virgin olive oil ameliorates deltamethrin-induced nephrotoxicity in mice: A biochemical and immunohistochemical assessment. Toxicol Rep. 2016;3:584–590. doi: 10.1016/j.toxrep.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pourkhodadad S, Alirezaei M, Moghaddasi M, Ahmadvand H, Karami M, Delfan B, et al. Neuroprotective effects of oleuropein against cognitive dysfunction induced by colchicine in hippocampal CA1 area in rats. J Physiol Sci. 2016;66(5):397–405. doi: 10.1007/s12576-016-0437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Priya VV, Surapaneni KM. Erythrocyte lipid peroxidation, glutathione, ascorbic acid, vitamin E, antioxidant enzymes and serum homocysteine levels in patients with coronary artery disease. J Clin Diagn Res. 2008;2:1180–5. [Google Scholar]
- 24.Mogadam RA-P, Nemati A, Baghi AN. Serum MDA as a diagnostics biomarker in stable coronary heart disease. Res J Biol Sci. 2008;3(2):206–10. [Google Scholar]
- 25.Serdar Z, Aslan K, Dirican M, Sarandöl E, Yeşilbursa D, Serdar A. Lipid and protein oxidation and antioxidant status in patients with angiographically proven coronary artery disease. Clin Biochem. 2006;39(8):794–803. doi: 10.1016/j.clinbiochem.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Cheraghi M, Shahsavari G, Maleki A, Ahmadvand H. Paraoxonase 1 activity, lipid profile, and atherogenic indexes status in coronary heart disease. . Rep Biochem Mol Biol. 2017;6(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Shaikh AK, Suryakar AN. Oxidative stress, endothelial dysfunction and status of L-arginine and nitric oxide in coronary artery disease. Biomed Res. 2008;19(3):211–4. [Google Scholar]
- 28.Leowattana w. Serum nitric oxide levels in patients with coronary artery disease. J Med Assoc Thai. 2001;84:S730–S9. [PubMed] [Google Scholar]
- 29.Besedina A. No-synthase activity in patients with coronary heart disease associated with hypertension of different age groups. J Med Biochem. 2016;35(1):43–9. doi: 10.1515/jomb-2015-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ioannou GN, Weiss NS, Boyko EJ, Mozaffarian D, Lee SP. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology. 2006;43(5):1145–51. doi: 10.1002/hep.21171. [DOI] [PubMed] [Google Scholar]
- 31.Doganer Y, Rohrer J, Aydogan U, Agerter D, Cayci T, Barcin C. Atherosclerosis and liver function tests in coronary angiography patients. West Indian Med J. 2015;64(4):333–7. doi: 10.7727/wimj.2014.192. [DOI] [PMC free article] [PubMed] [Google Scholar]


