Abstract
Background:
Renal ischemia-reperfusion injury (RIR) occurs when there is a temporary restriction of blood flow to the kidneys followed by an influx of blood, re-oxygenating the tissues. This occurs as a severe complication of major surgery. This process causes significant damage to the tissues and is responsible for the development of acute kidney injury (AKI), a life-threatening condition with high mortality rates. Here, we evaluated the potential protective effects of the antioxidant, gallic acid (GA), on RIR in an in vivo rat model.
Methods:
Adult male Sprague Dawley rats were randomly divided into three groups: group 1 (control, n = 8), group 2 (Ischemia-reperfusion (IR) with no-treatment, n = 7), and group 3 (IR + daily GA 100 mg/kg i.p, n = 7). The abdomens of the rats in the control group were opened during the surgical procedure, then sutured closed. GA pretreatment began daily 15 days prior to inducing RIR. To induce RIR, the umbilical arteries were obstructed on both sides and clamped with mild pressure for 45 min. Following the 45 min ischemia, the clamps were removed to allow for the induction of reperfusion. The reperfusion phase was 24 hours.
Results:
Following IR, the serum levels of urea and creatinine significantly increased compared to the controls. Pretreatment with GA was observed to reduce urea and creatinine levels following IR. However, this decrease was not statistically significant. The serum and renal levels of malondialdehyde (MDA) in the IR group was significantly elevated compared to the control group. Conversely, glutathione (GSH) levels and the activity of glutathione peroxidase (GPX) significantly decreased in the IR group compared to controls. Our findings show GA pretreatment to significantly improve the levels of renal MDA, serum GSH, and GPX activity following RIR.
Conclusion:
Our findings highlight the protective role for GA in mitigating the damage caused by RIR and its applications as a potential treatment.
Antioxidant enzymes: Gallic acid, Renal functional markers, Renal ischemia-reperfusion
Introduction
Renal ischemia-reperfusion (RIR) occurs when there is a transient restriction of blood flow to the kidneys followed by an influx of blood flow, re-oxygenating the kidneys. This process contributes to the development of acute kidney injury (AKI), which can lead to a rapid decline in kidney function and high rates of mortality. This injury can occur following major surgeries, renal transplantation, cardiovascular stroke, heminephrectomy, and septicemia (1-3). Several factors contribute to the pathophysiology of RIR including, the activation of neutrophils, the production of reactive oxygen species (ROS), and inflammatory mediators. In the ischemia phase, the change of metabolism from the aerobic to the anaerobic induces intracellular acidosis and reduces ATP production. These events subsequently cause excess calcium to accumulate within the cell, and the generation of ROS. In the reperfusion phase, there is a restoration of oxygen and increase in pH within the tissue. However, this process of restoring to normal physiological levels is damaging to the tissues following ischemia. Reperfusion causes further calcium overload and ROS production, and a decline in the activity of the antioxidant enzyme system (2, 4). Reactive oxygen species are heavily involved in the pathophysiology of several renal injuries through their ability to cause oxidative damage to biomolecules such as lipids, proteins, and DNA, ultimately leading to cellular apoptosis (5). To treat this, efforts have focused on decreasing the presence of ROS and enhancing the function of the antioxidant system.
Gallic acid (GA or 3, 4, 5- trihydroxybenzoic acid) is a compound found in several plants including, tea leaves, grapes, pineapple, and coriander. GA has been shown to have antioxidant, anti-inflammatory, antifungal, antiviral, anti-cancer, and anti-apoptotic properties. The protective function of GA is due to its ability to inhibit ROS induced cellular damage, induce apoptosis of cancerous cells, upregulate glutathione peroxidase (GPX) expression, and mitigate the presence of free radicals (6, 7).
In the present study, we examined the effect of GA pretreatment on kidney function and oxidative stress in an in vivo rat model of RIR.
Materials and Methods
Animals and study design
Twenty-two adult male Sprague Dawley rats (weighing 180-200g) provided by the Tehran Pasteur Institute, were housed in a room with a stable temperature of 22 °C and a humidity of 50 ± 10 %. The rats were randomly divided into three groups: group 1 (control, n = 8), group 2 (Ischemia-reperfusion (IR) with no-treatment, n = 7), and group 3 (IR + daily GA 100mg/kg i.p, n = 7). The abdomens of the rats in the control group were opened during the surgical procedure, then sutured closed (8). The pretreatment of GA began 15 days prior to inducing RIR and was received daily.
Surgical procedure
Prior to the surgery, animals were fasted for 8 hours, with the exception of water no food was allowed. Rats were anesthetized via intraperitoneal injection of ketamine (75 mg/kg, i.p) and xylazine (8 mg/kg,i.p). All procedures were performed with sterilized instruments. The abdomen was cut opened, the kidneys and renal pedicles were located. For groups 2 and 3, the umbilical arteries of the kidneys were closed on both sides clamped with mild pressure for 45 min. This resulted in the kidneys to turn white. After 45 minutes of ischemia, the clamps were removed. The color of the kidneys began to change to brown. During 45 minutes of ischemia, the viscera and kidneys were preserved with a humid and hot gauze. After confirming blood flow had returned to the kidneys, the abdomen was sutured closed. Following 24 hours after induced RIR, the animals were anesthetized for blood collection. Blood samples were collected from the right ventricle of the heart. The right kidney was frozen for tissue enzymatic studies. Twenty minutes following blood collection, the clotted blood was centrifuged and the serum was isolated and frozen.
Biochemical analysis
Measuring renal functional markers
The serum levels of renal functional markers such as, urea and creatinine (Cr), were measured via biochemical auto analyzer (Olympus AU-600, Tokyo, Japan).
Determining serum and renal lipid peroxidation (LPO) levels
Malondialdehyde (MDA) levels, as a marker of LPO, in the serum and kidneys were measured as previously described (8).
Determining the serum and renal glutathione (GSH) levels
The measurement of serum and renal GSH levels were analyzed via Ellman’s spectrophotometric method as previously described (9).
Assay for analyzing serum and renal glutathione peroxidase (GPX) activity
Serum and renal GPX activity was determined as previously described (10).
Statistical analysis
Results were presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) and LSD test was used to compare differences between the groups. The statistical software, SPSS version 20, was used for statistical analysis (SPSS Inc., Chicago, IL). P values < 0.05 were considered statistically significant.
Results
The effects of GA pretreatment on the serum levels of urea and creatinine (Cr)
The serum levels of urea and Cr are indicated in Table 1. In the IR group, urea and Cr levels were significantly increased (1.28-Fold, 1.61-Fold, respectively) compared to the control group. Pretreatment with GA prior to IR was able to reduce the increase in urea and Cr serum levels that followed after surgery (non-treated group 2 = 15.68%, GA treated group 3 = 4.52%). However, this decrease in urea and Cr levels was not statistically significant (P = 0.62, P = 0.059, respectively).
Table 1.
The effects of Gallic acid (GA) on the serum levels of urea and creatinine (Cr) in RIR. The three groups consisted of the control, ischemia-reperfusion (IR), and pretreated (IR + GA) groups
| Experimental groups | Urea (mg/dl) | Cr (mg/ dl) |
|---|---|---|
| Control | 49.00 ± 3.02 | 0.63 ± .02 |
| IR | 63.14 ± 4.46* | 1.02 ± 0.09* |
| IR + GA | 60.28 ± 4.32 | 0.86 ± 0.04 |
Values are expressed as a mean ± standard deviation
Shows statistical significance compared to the control group (P<0.05). # Shows significance of data compared to the IR group (P<0.05).
The effects of GA pretreatment on serum and renal oxidative stress biomarkers
The serum and renal levels of MDA are shown in Figure 1 (Fig. 1). Serum and renal MDA levels in the IR group were significantly elevated (1.89-Fold, 2.22-Fold, respectively) compared to the control group. Pretreatment with GA (20.11%, 42.11%, respectively) resulted in a reduction in the serum and renal MDA levels compared to the IR group. However, the decrease in serum MDA levels was not statistically significant.
Fig. 1.
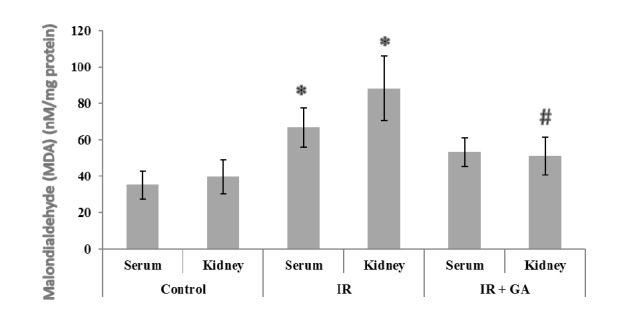
The impacts of GA pretreatment on serum and renal MDA levels in the control, IR, and IR + GA groups. * P < 0.05 as compared with the control group. # P < 0.05 as compared with the IR group.
The serum and renal GSH levels are indicated in Figure 2(Fig. 2). The induction of RIR caused a significant decrease in the levels of renal and serum GSH in the IR group compared to the control group (2.06-Fold, 1.52-Fold, respectively). GA pretreatment significantly (57.73%) increased serum levels of GSH compared to the non-treated IR group. However, the increase in GSH levels (30.38%) was not significant between the pretreated and the non-treated IR groups.
Fig. 2.
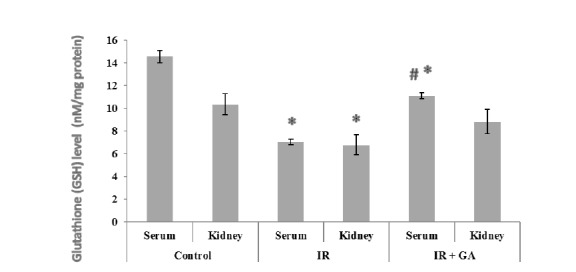
The impacts of GA pretreatment on serum and renal GSH levels in the control, IR, and IR + GA groups. * P < 0.05 as compared with the control group. # P < 0.05 as compared with the IR group.
The serum and renal GPX activity is shown in Figure 3(Fig. 3). In the IR group, the serum and renal GPX activity was significantly reduced in comparison to the control group (1.88- Fold, 1.17- Fold, respectively). In the pretreated IR group, only serum GPX activity was significantly (77.42%) increased compared to the IR group.
Fig. 3.
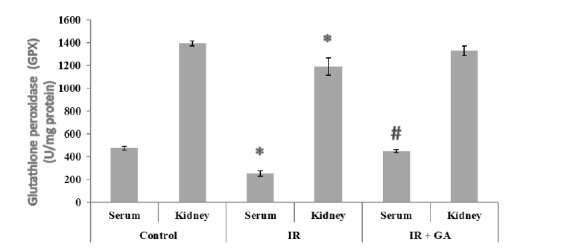
The impacts of GA pretreatment on serum and renal GPX activities in the control, IR, and IR + GA groups. * P < 0.05 as compared with the control group. # P < 0.05 as compared with the IR group.
Discussion
Renal ischemia-reperfusion injury (RIR) is characterized as a pathophysiological condition that occurs as a result of the temporary restriction of blood to the kidneys, followed by the restoration of blood flow and re-oxygenation of the tissues. This contributes to the development of acute kidney injury (AKI) which leads to a decline in kidney function and mortality (11). Our findings indicate that pretreatment with the antioxidant, GA, prior to RIR results in a decrease in the levels of serum and renal MDA and an increase GSH levels and GPX activity. Additionally, GA pretreatment reduced the levels of urea and Cr. The main factor contributing to kidney damage during IR is the production of reactive oxygen species (ROS) or reactive nitrogen species (RNS) (11). ROS are highly involved in the pathophysiology of IR through promoting apoptosis, lipid peroxidation, and activating cellular stress signaling pathways (12). Antioxidants such as SOD, CAT, GPX, and GSH are responsible forprotecting cells from free radicals and oxidative stress. Therefore, the use of antioxidants to control and treat various disorders including RIR has attracted considerable attention (13). Recently, several studies have shown that antioxidant substances reduce the functional and inflammatory complications related to RIR associated damage (14). The well-known antioxidant, GA, is a polyhydroxy phenolic compound that is able to scavenge ROS during IR (15). Our findings showed that in rats who have undergone IR, without any treatment, develop elevated levels of MDA and decreased levels of GSH and a decrease in GPX activity. The significant reduction in renal and serum GSH levels and GPX activity reported in the IR group, compared to controls, corroborates previous work. The oxidative stress caused during IR leads to the damage of macromolecules due to lipid peroxidation, DNA oxidation, protein oxidation, the inactivation of enzymes, and the malfunction of various membranes. GA mitigates these damaging events through enhancing the enzymatic activity of antioxidants, such as GSH and GPX (16).
Additionally, serum and renal MDA levels were observed to decrease in the IR + GA group compared to the non-treated IR group, however the decrease in serum MDA levels was not significant. MDA is a biomarker of oxidative stress for free radicals produced in the body. Elevated levels of MDA indicate an increase in cell membrane damage. In fact, free radicals increase lipid peroxidation of unsaturated fatty acids, which ultimately leads to apoptosis. One of the reasons for increased MDA levels can be due to the increase in the production of free radicals in the electron transport chain. Furthermore, increased serum levels of MDA can be the cause of many diseases associated with oxidative stress, such as diabetes (17).
A study examining the effect of antioxidants against cisplatin-induced nephrotoxicity in rats found that both GA and tannic acid significantly increased SOD, CAT, GPX, and GSH enzymatic activity and decreased renal MDA levels (18). A separate study by Yurdakul et al. showed that pretreatment with a combination of the antioxidants, α-tocopherol and erdosteine, resulted in a significant decrease in MDA levels. Furthermore, they provided protection against the damaging oxidative process in an in vivo RIR model in rats (19). It has also been reported that tannins and anthocyanins present in pomegranate extract can reduce the oxidative damage due occurring as a result of RIR by decreasing serum MDA levels, total oxidant status (TOS) levels, and the oxidative stress index (OSI) (20). Furthermore, other antioxidants such as rutin, caffeic acid phenethyl ester, and naringin mitigate the oxidative damage in renal tissue (21-23). Therefore, the use of GA and other antioxidants may be effective in preventing the damage caused by oxidative stress in RIR.
Different factors are associated with the progression of acute renal failure and damage to renal tubules that can lead to increased Cr clearance, blood urea nitrogen (BUN) and serum uric acid, and tissue damage. Therefore, damage to the tissues of the kidney causes changes in serum biochemical factors (24). The results of our study showed that urea and Cr levels were significantly increased following the induction of IR compared to control group, and GA could decrease these increased levels of urea and Cr that follow after IR. Turgut et al (2008) found that silymarin (a polyphenolic compound) increased the levels of cystatin C, serum Cr, and urea, while it reduced serum and tissue MDA levels (25). Interestingly, although we observed a decrease in the levels of urea and Cr in the GA pretreated IR group compared to the non-treated IR group, this decrease was not statistically significant. It seems that GA had a positive effect on renal function without significantly reducing the levels of urea and Cr. Similar to these results, previous research has shown that the levels of urea and Cr increase significantly following IR. However, there were no significant differences in levels of urea and Cr between the IR group and the IR group that received pretreatment with the antioxidants, melatonin and vitamin E (26). Examining the levels of another marker, in addition to urea and Cr, may provide a more accurate description of the effect of GA on renal function. It is suggested that the presence of a sensitive marker called, glomerular filtration rate (GFR), may be a better indicator of renal function. In this current study, collecting enough urine samples to evaluate GFR was one of the limitations.
Our findings highlight the role for GA as an antioxidant capable of mitigating the tissue damage caused by oxidative stress and improving renal function during following IR through reducing the levels of MDA, urea, and Cr and increasing the levels of GSH and the enzymatic activity of GPX. Given these protective functions of GA, this compound may have great potential as a treatment for renal failure due to oxidative stress.
Acknowledgements
We would like to thank the Razi Herbal Research Center of Lorestan Medical University, Lorestan, Iran.
References
- 1.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. . Compr Physiol. 2012;2:1303–53. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev. 2015;4:20–7. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccoli GB, Alrukhaimi M, Liu ZH, Zakharova E, Levin A. Women and kidney disease: reflections on World Kidney Day 2018. Kidney Int. 2018;14:67–70. doi: 10.1016/j.nephro.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Salvadori M, Rosso G, Bertoni E. Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World J Transplant. 2015;5(2):52–67. doi: 10.5500/wjt.v5.i2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigo R, Bosco C. Oxidative stress and protective effects of polyphenols: comparative studies in human and rodent kidney. Comp Biochem Physiol C Toxicol Pharmacol. 2006;142:317, 27. doi: 10.1016/j.cbpc.2005.11.002. A review. [DOI] [PubMed] [Google Scholar]
- 6.Malinda K, Sutanto H, Darmawan A. Characterization and antioxidant activity of gallic acid derivative. AIP Conference Proceedings. 2017;1904:020030. [Google Scholar]
- 7.Yoon CH, Chung SJ, Lee SW, Park YB, Lee SK, Park MC. allic acid, a natural polyphenolic acid, induces apoptosis and inhibits proinflammatory gene expressions in rheumatoid arthritis fibroblast-like synoviocytes. Joint Bone Spine. 2013;80(274):9. doi: 10.1016/j.jbspin.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Ahmadvand H, Babaeenezhad E, Hadipour-Moradi F, Cheraghi-Venool A. Effect of gallic acid on liver oxidative stress markers in renal ischemiareperfusion injury in rats. Ann Res Antioxid. 2017;2:e03. [Google Scholar]
- 9.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 10.Ahmadvand H, Ghasemi Dehnoo M, Cheraghi R, Rasoulian B, Ezatpour B, Azadpour M, et al. Amelioration of altered serum, liver, and kidney antioxidant enzymes activities by sodium selenite in alloxan-induced diabetic rats. Rep Biochem Mol Biol. 2014;3:14–20. [PMC free article] [PubMed] [Google Scholar]
- 11.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–20. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 12.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–90. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 13.Murphy MP. Antioxidants as therapies: can we improve on nature? Free Radic Biol Med. 2014;66:20–3. doi: 10.1016/j.freeradbiomed.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Chen G, Fu Y, Wu X. Protective effect of Salvia miltiorrhiza extract against renal ischemia-reperfusion-induced injury in rats. Molecules. 2012;17:1191–202. doi: 10.3390/molecules17021191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jadon A, Bhadauria M, Shukla S. Protective effect of Terminalia belerica Roxb. and gallic acid against carbon tetrachloride induced damage in albino rats. J Ethnopharmacol. 2007;109:214–8. doi: 10.1016/j.jep.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014;224:164–75. doi: 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Kumari S, Panda S, Mangaraj M, Mandal M, Mahapatra P. Plasma MDA and antioxidant vitamins in diabetic retinopathy. Indian J Clin Biochem. 2008;23:158–162. doi: 10.1007/s12291-008-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akomolafe SF, Akinyemi AJ, Anadozie SO. Phenolic Acids (Gallic and Tannic Acids) modulate antioxidant status and cisplatin induced nephrotoxicity in rats. Int Sch Res Notices. 2014;2014:984709. doi: 10.1155/2014/984709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yurdakul T, Kulaksizoglu H, Pişkin MM, Avunduk MC, Ertemli E, Gokçe G, et al. Combination antioxidant effect of α-tocoferol and erdosteine in ischemia-reperfusion injury in rat model. Int Urol Nephrol. 2010;42:647–55. doi: 10.1007/s11255-009-9641-y. [DOI] [PubMed] [Google Scholar]
- 20.Sancaktutar AA, Bodakci MN, Hatipoglu NK, Soylemez H, Basarılı K, Turkcu G. The protective effects of pomegranate extracts against renal ischemia-reperfusion injury in male rats. Urol Ann. 2014;6:64–50. doi: 10.4103/0974-7796.127029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korkmaz A, Kolankaya D. Protective effect of rutin on the ischemia/reperfusion induced damage in rat kidney. J Surg Res. 2010;164:309–15. doi: 10.1016/j.jss.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Tolba MF, Omar HA, Azab SS, Khalifa AE, Abdel-Naim AB, Abdel-Rahman SZ. Caffeic acid phenethyl ester: a review of its antioxidant activity, protective effects against ischemia-reperfusion injury and drug adverse reactions. Crit Rev Food Sci Nutr. 2016;56:2183–90. doi: 10.1080/10408398.2013.821967. [DOI] [PubMed] [Google Scholar]
- 23.Singh D, Chopra K. The effect of naringin, a bioflavonoid on ischemia reperfusion induced renal injury in rats. Pharmacol Res. 2004;50:187–93. doi: 10.1016/j.phrs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Palant CE, Amdur RL, Chawla LS. Long-term consequences of acute kidney injury in the perioperative setting. Curr Opin Anaesthesiol. 2017;30:100–104. doi: 10.1097/ACO.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 25.Turgut F, Bayrak O, Catal F, Bayrak R, Atmaca AF, Koc A, et al. Antioxidant and protective effects of silymarin on ischemia and reperfusion injury in the kidney tissues of rats. Int Urol Nephrol. 2008;40:453–60. doi: 10.1007/s11255-008-9365-4. [DOI] [PubMed] [Google Scholar]
- 26.Aktoz T, Aydogdu N, Alagol B, Yalcin O, Huseyinova G, Atakan IH. The protective effects of melatonin and vitamin E against renal ischemia-reperfusion injury in rats. Ren Fail. 2007;29:535–42. doi: 10.1080/08860220701391738. [DOI] [PubMed] [Google Scholar]


