Abstract
Alpinia oxyphylla Miq. (A. oxyphylla) is an important edible and traditional herbal medicine. In this study, the complete chloroplast genome of A. oxyphylla was sequenced, analysed, and compared to five species in the Zingiberaceae family. The size of the A. oxyphylla chloroplast genome was 161351 bp, which consisted of a large single-copy (LSC, 87248 bp) and small single-copy (SSC, 16175 bp) region separated by a pair of inverted repeats (IRa and IRb, 28964 bp each). The genome encoded 132 unique genes, including 87 protein-coding genes, 37 tRNAs and four rRNAs. The GC content of the genome was 36.17%. A total of 53 simple sequence repeats (SSRs) and 80 long repeats were identified in the A. oxyphylla chloroplast genome. The chloroplast genome of A. oxyphylla shared the highest sequence similarity of >90% with the chloroplast genome of A. zerumbet, and six chloroplast genomes in the Zingiberaceae family were compared by using CGView Comparison Tool (CCT). According to the phylogenetic tree, the Zingiberaceae family is divided into two categories, which coincide with the classification of the characteristics of sun-like and shade-like in plants. Our results reveal the phototrophic component of NADH-dehydrogenase (ndhB and ndhC), photosystem II (psbZ) and ATP synthase (atpE, atpF) exhibit adaptive evolution under different environments, and the strength of light is an important trigger for the adaptations at the chloroplast level.
Introduction
Alpinia oxyphylla Miq. (A. oxyphylla) is an important edible and traditional Chinese herbal medicine that originates in Hainan and is widely cultivated in southern China [1]. The fruits of A. oxyphylla have been used as valuable medicines that have a long clinical history and are often used as condiment food in China [2,3]. Numerous studies have reported that A. oxyphylla is rich in flavonoids, sesquiterpenes, diterpenes, and diarylheptanoids, which have many pharmacological effects, such as improved memory, anti-oxidation, anti-inflammatory, neuroprotective and anticancer[4–8].
Chloroplasts are small organelles inside the cells of plants that contain photosynthetic machinery and produce essential energy for plants [9]. Chloroplasts have their own genetic systems, which consist of a closed circular DNA molecule [9,10]. In recent years, chloroplast genomes have been commonly used for the identification and phyletic evolution analysis of species because of their conserved gene sequences and important role in plants [11]. With the development of high-throughput DNA sequencing technologies, there has been an explosion in the number of available chloroplast genome sequences. However, the chloroplast genome sequences of medicinal plants still require further study. To date, five species of the Zingiberaceae family plant chloroplast genome have been reported, namely, Alpinia zerumbet, Amomum kravanh, Curcuma roscoeana, Curcuma flaviflora and Zingiber spectabile [12–15]. A. oxyphylla chloroplast genome sequences have not yet been reported, which has seriously hindered the development of genetic diversity and breeding of Alpinia plants. Therefore, it is highly important and essential to study the phylogeny and evolution of Zingiberaceae plants [16].
In this study, we reported the complete chloroplast genome sequence of A. oxyphylla, including a description of its general features, IR contraction and expansion, codon usage and analysis of SSRs and long repeats. In addition, six chloroplast genome sequences in the Zingiberaceae family were compared by using CGView Comparison Tool (CCT). Moreover, we constructed a phylogenetic tree of the Zingiberales, which provides basic genetic information on the genetic diversity and breeding of Alpinia plants.
Materials and methods
Ethical statement
No specific permits were required for the collection of specimens for this study.
Plant material, DNA extraction, and sequencing
Fresh A. oxyphylla leaves were collected from cultivated fields in Hainan Province, China. Total genomic DNA was extracted from 100 mg of fresh leaves using the Plant Genomic DNA Kit with a standard protocol (Tiangen, Beijing, China). Purified genomic DNA was quantified with a Nanodrop 2000 spectrometer (Thermo Fisher Scientific, Wilmington, USA). Normalized genomic DNA was used to generate a 500 bp (insert size) paired-end library, following the Illumina HiSeq4000 standard protocol. Approximately 2 G raw data were generated with read lengths of 150 bp, and the chloroplast genome sequencing depth was nearly 60×.
Chloroplast genome assembly and annotation
First, Illumina paired-end reads were filtered on the basis of quality values, and the low-quality reads were trimmed. The remaining clean reads were used for assembly with SOAPdenovo2 (http://soap.genomics.org.cn/soapdenovo.html) on the basis of overlapping and paired-end relationships. Next, all clean reads were mapped onto the assembled contigs to obtain a complete chloroplast genome sequence. Genome confirmation was indispensable to perform after assembly. Finally, the paired-end clean reads were mapped onto the assembled genome with 100% coverage, and the insert-size matched the information of the sequenced library.
Annotation was performed using the online program Dual Organellar GenoMe Annotator (DOGMA) [17]. To prove the correctness of gene and exon boundaries, putative gene and protein sequences were BLAST searched in the Nt and Nr databases. The tRNA genes of A. oxyphylla were further verified using the online tRNAscan-SE and tRNADB-CE search servers [18–20]. The map of the circular A. oxyphylla chloroplast genome was drawn through Organellar Genome DRAW (OGDRAW v1.2) [21].
Genome structure analyses and genome comparison
The distribution of codon usage was detected by the software CodonW (University of Texas, Houston, TX, USA) with the relative synonymous codon usage (RSCU) ratio [22]. The mVISTA program in Shuffle-LAGAN mode was applied to compare the A. oxyphylla chloroplast genome with five other chloroplast genomes. The boundaries between the IR and SC regions of A. oxyphylla and five other Zingiberaceae species were compared and analysed. The visualization of codon usage in the form of heatmaps of 17 species of Zingiberales and a histogram were conducted with R language with an RSCU value.
Repeat sequence analyses
Repeat sequences in chloroplast genomes were detected by the REPuter program [23], including forward, reverse, palindrome, and complement sequences in the chloroplast genome of A. oxyphylla. The length and identity of the repeats were limited to ≥ 30 bp and >90%, respectively [24]. The SSRs were searched using MISA [25], with the following repeat threshold settings: 10 repeats for mono-nucleotide, 5 repeats for di-, 4 repeats for tri-nucleotide, and 3 repeats for tetra- and penta-nucleotide SSRs [26].
CCT map
The A. oxyphylla chloroplast genome was compared with other available chloroplast genomes of Zingiberales by using CCT [27]. Genes were assigned by Clusters of Orthologous Groups, and BLAST was used to align other genomes to that of A. oxyphylla. The visualization of the circular map was conducted by CCT. AT distributions were measured on the basis of AT skewed using the equation: AT-skew = (A−T)/(A+T).
Phylogenetic analysis
Concatenated alignments of 17 chloroplast genome sequences were performed using MUSCLE v.3.8.31. The phylogenetic analysis was carried out using the ML method with RAxML8.1, and the trees were visualized and annotated using the tree viewer of MEGA6 [28]. Statistical supports were assessed with 1000 bootstrap pseudo-replicates.
Positive selection analysis of protein sequence
To investigate the evolutionary process of light adaptation of Zingiberaceae plants, we calculated the Nonsynonymous (Ka), Synonymous (Ks) and Ka/Ks ratios of protein coding genes associated with the photosystem using KaKs_Calculator 2.0 [29].
Results and discussion
General features of the A. Oxyphylla chloroplast genome
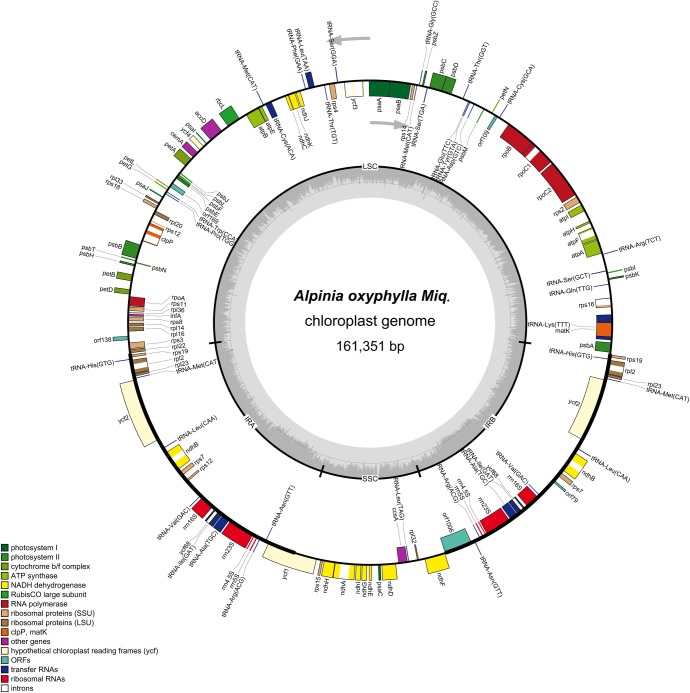
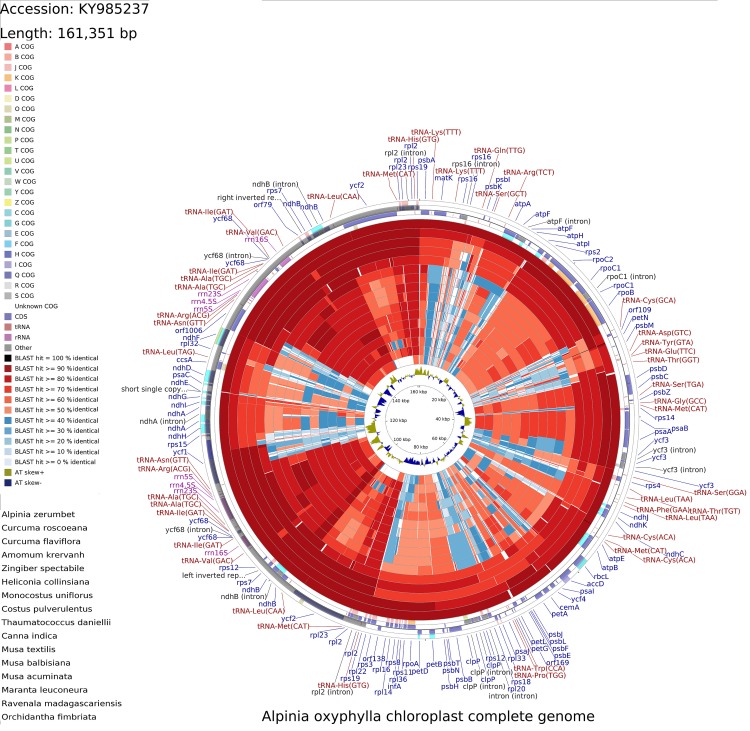
The complete chloroplast genome of A. oxyphylla (GenBank Accession Number: KY985237) has a typical quadripartite structure and is a circular molecule 161,351 bp in size (Fig 1 and Table 1). The genome contains a small single-copy (SSC) region of 16175 bp and a large single-copy (LSC) region of 87248 bp, separated by a pair of inverted repeats (IRa and IRb) of 28964 bp each (Fig 1 and Table 1). The GC content of the A. oxyphylla chloroplast genome is 36.17%, which is similar to other chloroplast genomes previously reported [14,15,30]. The genome consists of 132 genes, including 87 distinct protein-coding genes, four distinct rRNA genes and 37 distinct tRNA genes, 21 of which were duplicated in the IR regions, 12 in the SSC region and 84 in the LSC region (Table 1).
Fig 1. Gene map of the complete chloroplast genome of A. oxyphylla.
Genes on the outside of the circle are transcribed clockwise, while those inside are counterclockwise. Genes belonging to different functional groups are colour-coded. The darker and lighter grey in the inner circle correspond to GC and AT content, respectively.
Table 1. Gene contents in the complete chloroplast genome of A. oxyphylla.
| Category of genes | Group of gene | Name of gene |
|---|---|---|
| Self-replication | Small subunit of ribosome (SSU) | rps2, rps3, rps4, rps7*, rps8, rps11, rps12, rps14, rps15, rps16, rps18, rps19* |
| Large subunit of ribosome (LSU) | rpl2*, rpl14, rpl16, rpl20, rpl22, rpl23*, rpl32, rpl33, rpl36 | |
| DNA-dependent RNA polymerase | rpoA, rpoB, rpoC1, rpoC2 | |
| Ribosomal RNA genes (rRNA) | rrn16S*, rrn5S*, rrn23S*, rrn4.5S* | |
| Translation initiation factor | infA | |
| Transfer RNA genes (tRNA) | trnA-TGC*, trnC-GCA, trnC-ACA, trnD-GTC, trnE-TTC, trnF-GAA, trnG-GCC, trnH-GTG*, trnI-GAT*, trnK-TTT, trnL-TAA, trnL-CAA*, trnL-TAG, trnM-CAT*, trnN-GTT*, trnP-TGG, trnQ-TTG, trnR-TCT, trnR-ACG*, trnS-GCT*, trnS-TGA, trnS-GGA, trnT-GGT, trnT-TGT, trnV-GAC*, trnW-CCA, trnY-GTA | |
| Genes for photosynthesis | Subunits of NADH dehydrogenase | ndhA, ndhB*, ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK |
| Large subunit of Rubisco | rbcL | |
| Subunits of photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ | |
| Subunits of photosystem I | psaA, psaB, psaC, psaI, psaJ | |
| Subunits of ATP synthase | atpA, atpB, atpE, atpF, atpH, atpI | |
| Subunits of cytochrome | petA, petB, petD, petG, petL, petN | |
| Photosystem I assembly | ycf3, ycf4 | |
| Other genes | Envelope membrane protein | cemA |
| C-type cytochrome synthesis gene | ccsA | |
| Subunit of acetyl-CoA | accD | |
| Protease | clpP | |
| Maturase | matK | |
| Unknown | Conserved open reading frames | orf109, orf138, orf169 |
| Pseudogenes | ycf1, ycf2, ycf68*, orf79, orf1006 |
Note
* indicates a duplicated gene.
The size of the A. oxyphylla chloroplast genome was similar to those of five Zingiberaceae family species (Table 2). The size of the A. kravanh chloroplast genome (162766 bp) is the longest, and the Z. spectabile chloroplast genome (155890 bp) is the shortest. Interestingly, the SSC region (15,390 bp) of A. kravanh is the shortest, whereas the SSC region (18611 bp) of the Z. spectabile chloroplast genome is the longest. Five of the chloroplast genomes contain 132 genes, excluding only A. kravanh (135 genes). As shown in Table 2, the C. roscoeana chloroplast genome has the highest GC content (36.33%), while the A. kravanh chloroplast genome has the lowest GC content (31.3%). In addition, 87 protein genes were identified in A. oxyphylla, 80 were identified in A. kravanh, and 86 were identified in the other four species. Four conserved rRNAs were identified in every species. The A. oxyphylla chloroplast genome encodes 37 types of tRNAs, A. zerumbet, C. roscoeana, C. flaviflora and Z. spectabile encode 38, whereas A. kravanh encodes 30 (Table 2).
Table 2. Comparison of the general features of the six Zingiberaceae chloroplast genomes.
| Genome feature | A. oxyphylla | A. zerumbet | A. kravanh | C. roscoeana | C. flaviflora | Z. spectabile |
|---|---|---|---|---|---|---|
| GenBank | KY985237 | JX088668 | MF991963.1 | KF601574 | NC_028729.1 | NC_020363.1 |
| Size (bp) | 161351 | 159773 | 162766 | 159512 | 160478 | 155890 |
| LSC (bp) | 87248 | 87644 | 87728 | 87015 | 88008 | 85983 |
| SSC (bp) | 16175 | 18295 | 15390 | 18531 | 18570 | 18611 |
| IR (bp) | 28964 | 26917 | 29824 | 26983 | 26950 | 25554 |
| Total genes | 132 | 132 | 135 | 132 | 132 | 132 |
| Protein genes | 87 | 86 | 80 | 86 | 86 | 86 |
| tRNA genes | 37 | 38 | 30 | 38 | 38 | 38 |
| rRNA genes | 4 | 4 | 4 | 4 | 4 | 4 |
| GC (%) | 36.17% | 36.27% | 31.3% | 36.33% | 36.30% | 36.29% |
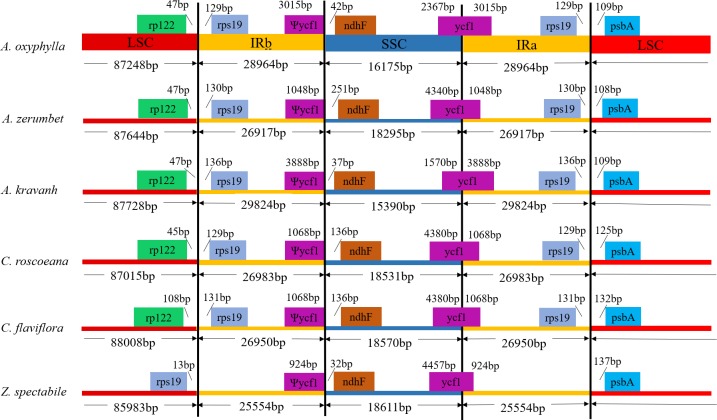
IR contraction and expansion
The contraction and expansion of the IR region are common evolutionary events and are considered the major reasons for size differences in different chloroplast genomes, which is best for studying the phylogeny and the chloroplast genome evolution history of early land plant lineages [31,32]. In the A. oxyphylla chloroplast genomes, the boundary of IR/LSC extended into the rps19 gene, and 129 bp of rps19 extended into the IR region; the boundary of IR/SSC extended into the ndhF gene, and 42 bp of ndhF extended into the IR region; the boundary of IRb/SSC extended into 3015 bp of ycf1; and the boundary of IRb/LSC and IRa/LSC extended into the rpl22 and psbA genes, respectively. In this study, a detailed comparison of the borders among the IR, LSC and SSC regions among the six Zingiberaceae chloroplast genomes is presented in Fig 2. The pseudogene ycf1 is often used to study genetic variation in the chloroplast genome in higher plants [32], and the length ranges from 924 to 3888 bp in the six comparable chloroplast genomes. The ndhF gene was 32, 37, 42, 136,136 and 251 bp from the IRb and SSC border in Z. spectabile, A. kravanh, A. oxyphylla, C. roscoeana, C. flaviflora and A. zerumbet, respectively. The rps19 gene is usually one of the most abundant transcripts among the plant chloroplast genome, including five comparable chloroplasts. However, the rps19 gene was completely located in the LSC region in the Z. spectabile chloroplast genome. Our results suggest that the IR/LSC boundary might be conversed among the chloroplast genomes of closely related family species, but greater diversity also occurs between relatively distantly related family species, such as Z. spectabile [33,34].
Fig 2. Comparison of the borders of the LSC, SSC and IR regions among the six chloroplast genomes.
Ψ, pseudogenes.
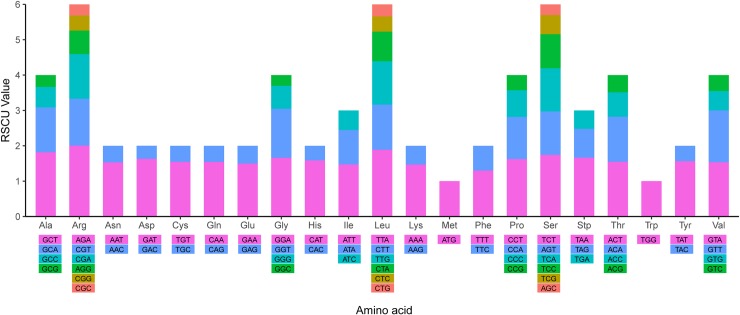
Codon usage
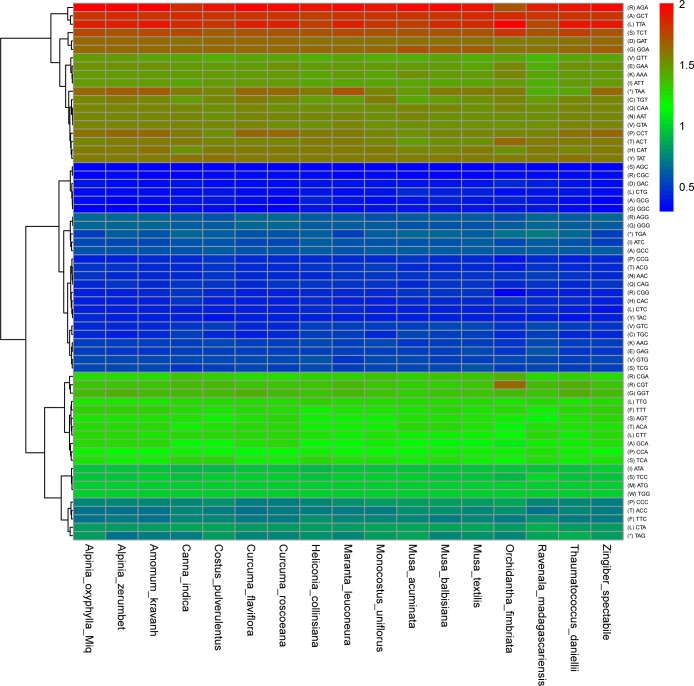
The standard ATG codon is typically the start codon for most protein-coding genes. However, ATA and ATC are also used as alternatives to ATG as the initiation codon under certain circumstances [35]. The initiation codon ATG of three genes was replaced among the A. oxyphylla chloroplast protein-coding genes, which were ATC for rps12 and orf79 and ATA for rp12(S1 Table). The codon usage frequency and relative synonymous codon usage (RSCU) were analysed based on sequences of 87 distinct protein-coding genes in the A. oxyphylla chloroplast genome (Fig 3). The high RSCU value was probably attributed to the function of the amino acid or the structure of the peptide to avoid error in transcription [35]. As shown in Fig 4, the result of the distributions and the visualization of codon usage in the form of heatmaps of 17 species of Zingiberales suggested that approximately one-third of the codons were not frequently used. These codons are shown in blue, which indicates an RSCU value of <1 and weak codon bias. The results showed evident codon use preferences for A. oxyphylla, among which AGA, TTA, GCT, TCT, and AGA were used most frequently (Fig 4). Approximately two-thirds of all codons of A. oxyphylla that had high RSCU values showed a high A/T preference in the third codon. This phenomenon is common in the chloroplast genomes of higher plants [36].
Fig 3. Codon content of 20 amino acids and stop codons in all protein-coding genes of the A. oxyphylla chloroplast genome.
The colour of the histogram corresponds to the colour of codons.
Fig 4. Heatmap analysis for codon distribution of all protein-coding genes of all considered species.
Colour key: higher red values indicate higher RSCU values, and lower blue values indicate lower RSCU values.
Analysis of SSRs and repeats
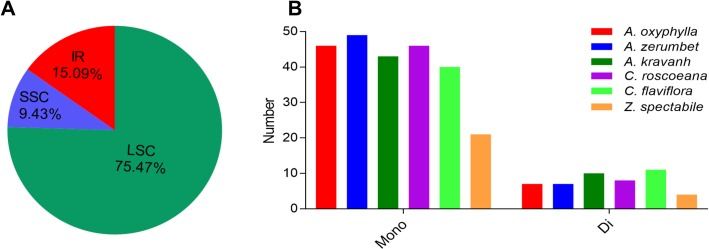
Simple sequence repeats (SSRs), also known as microsatellites, are a group of tandem repeated sequences, generally ranging in length from 1–6 or more base pairs and are widely distributed in chloroplast genomes [37]. A total of 53 SSRs were detected from the A. oxyphylla chloroplast genome, including 46 mono- and 7 di-nucleotide SSRs, which were located in the LSC region (75.47%), IR region (15.09%) and SSC region (9.43%), respectively (Fig 5 and S2 Table). Furthermore, the distribution pattern and number of SSRs among the six Zingiberaceae chloroplast genomes (A. oxyphylla, A. kravanh, A. zerumbet, C. flaviflora, C. roscoeana, and Z. spectabile) were compared, and the results suggested that there was little difference in the distribution pattern and number of SSRs among the six chloroplast genomes (Fig 5). In addition, a total of 80 repeats were detected in the A. oxyphylla chloroplast genome, including 4 complement, 35 forward (direct) repeats, 36 palindrome (inverted) repeats and 14 reverse repeats (S3 Table). Fifty-seven repeats were located in the intergenic spacers (IGS) regions, 19 repeats were located in coding sequence (CDS) regions, and 4 repeats were located in intron regions. These SSRs and repeats can be made into lineage-specific markers, which can provide genetic diversity analysis for A. oxyphylla and its related species [38,39].
Fig 5. SSR analysis of the six Zingiberaceae chloroplast genomes.
(A) Presence of SSRs in the LSC, SSC and IR regions (A. oxyphylla); (B) Presence of polymers in the chloroplast genome of A. oxyphylla, A. zerumbet, A. kravanh, C. roscoeana, C. flaviflora, and Z. spectabile.
CCT map
CCT is a package for visually comparing circular bacterial, plasmid, chloroplast, or mitochondrial genome sequences [40]. The A. oxyphylla chloroplast genome was compared with 16 previously reported chloroplast genomes of Zingiberales by using CCT (Fig 6). The results showed that the highest sequence similarity (>90%) was between the chloroplast genomes of A. oxyphylla and A. zerumbet, which was consistent with the result of the phylogenetic analysis (Figs 6 and 7). The most similar region appears in the IR region, and diversity exists in the LSC and SSC regions among 17 chloroplast genomes. This evolutionary feature of the chloroplast genome has also been reported in other plants [41].
Fig 6. CCT map comparison of 17 chloroplast genomes of A. oxyphylla to Orchidantha fimbriata.
The four outer rings are the protein-coding gene positions based on the A. oxyphylla chloroplast genome. The innermost ring displays AT skew in A. oxyphylla. The remaining rings display regions of similarity among the 17 compared chloroplast genomes.
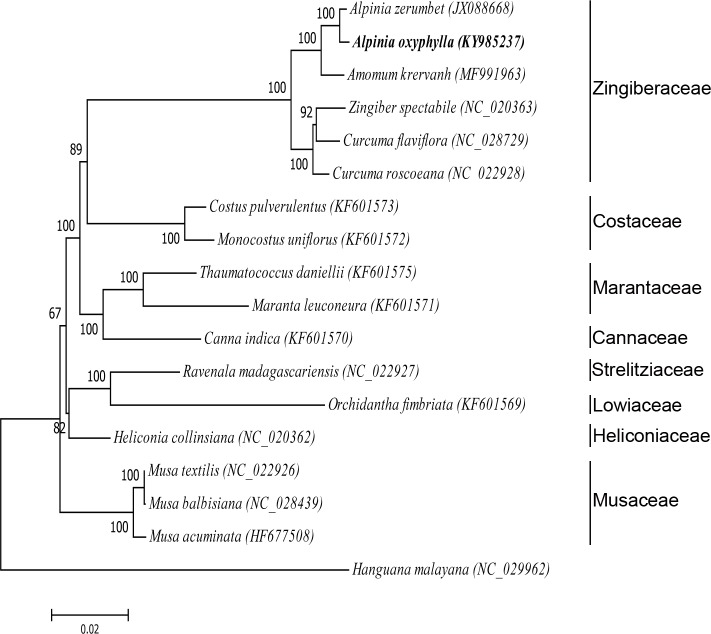
Fig 7. The maximum parsimony (MP) phylogenetic tree based on chloroplast genome sequences.
The numbers in each node were tested by bootstrap analysis with 1000 replicates.
Phylogenetic analysis
Complete chloroplast genomes contain a substantial amount of phylogenetic information, which has been used for phylogenetic analysis of deep relationships among the primary clades of Zingiberales [14,16,34,42]. To identify the evolutionary position of A. oxyphylla within Zingiberales, an improved resolution of phylogenetic relationships was achieved by using these complete chloroplast genome sequences of 17 Zingiberales species. The maximum likelihood (ML) bootstrap values had values of 100% bootstrap support for the Zingiberaceae family except the node of Z. spectabile and C. flaviflora (92%) (Fig 7). The A. oxyphylla chloroplast genome was closely related to A. zerumbet and A. kravanh, which then formed a cluster with Z. spectabile, C. roscoeana, and C. flaviflora with 100% bootstrap supports. In addition, the four ginger families form a well-supported clade within which the families Zingiberaceae and Costaceae, Marantaceae and Cannaceae are sisters. Therefore, the results are expected to be useful in resolving the deeper branches of the phylogenetic tree and will help expand the understanding of the evolutionary history of Zingiberaceae, particularly regarding the role of A. oxyphylla in plant systematics and evolution.
Adaptive evolution analysis
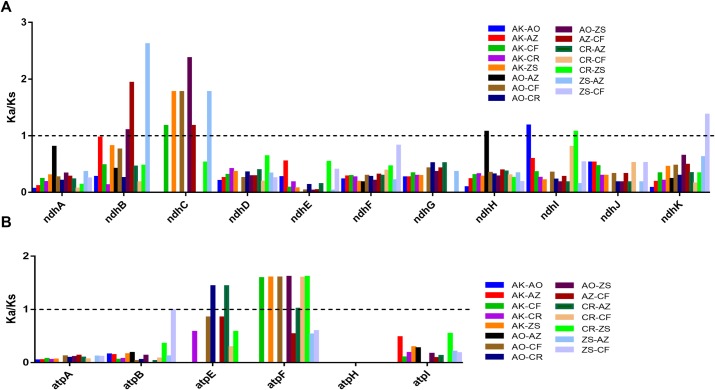
The Zingiberaceae family is divided into two main categories, A. oxyphylla, A. kravanh and A. zerumbet clustered into one branch, C. roscoeana, C. flaviflora and Z. spectabile clustered into another (Fig 7). Interesting, the former are typical shade-loving plants whose natural habitats are shade forests of South China, and the latter is sun-loving plants. To investigate the evolutionary process of light adaptation of Zingiberaceae plants, we calculated Ka/Ks ratios of NADH-dehydrogenase, photosystem I, photosystem II, cytochrome b/f complex and ATP synthase coding genes associated with the photosystem. The Ka/Ks is a powerful approach for measuring selective pressure at the protein-coding level. The genes with positive selection played key roles in the adaptation to diverse environment [43]. As a result, NADH-dehydrogenase (ndhB, ndhC, ndhH, ndhI, ndhK), photosystem II (psbZ) and ATP synthase (atpE, atpF) coding genes with Ka/Ks > 1 were detected, indicating that these genes are undergoing positive selection (Fig 8 and S4 Table). Moreover, the Ka/Ks ratios of the gene ndhC and atpF in four pairwise comparisons of A. kravanh—C. flaviflora, A. kravanh—Z. spectabile, A. oxyphylla—C. flaviflora and A. oxyphylla—Z. spectabile were both > 1, indicating that these two genes are critical in adapting to light (Fig 8). Our results reveal the phototrophic component of NADH-dehydrogenase (ndhB and ndhC), photosystem II (psbZ) and ATP synthase (atpE, atpF) exhibit adaptive evolution under different environments, and the strength of light is an important trigger for the adaptations at the chloroplast level.
Fig 8.
Ka/Ks ratios of NADH-dehydrogenase (A) and ATP synthase (B) coding genes. Ka, nonsynonymous; Ks, synonymous; AO, A. Oxyphylla; AK, A. kravanh; AZ, A. Zerumbet; CR, C. Roscoeana; CF, C. Flaviflora; ZS, Z. Spectabile.
Conclusions
The complete chloroplast sequence of A. oxyphylla contains LSC, SSC, and IR regions. A total of 53 SSRs and 80 repeats were identified in the A. oxyphylla chloroplast genome. The CCT analytical results indicated that the A. oxyphylla chloroplast genome shared the highest sequence similarity with A. zerumbet. Phylogenetic trees strongly supported classification of the characteristics of sun-like and shade-like in Zingiberaceae plants. Moreover, the results will help expand the understanding of the evolutionary history of Zingiberaceae, particularly regarding the phototrophic component of NADH-dehydrogenase (ndhB and ndhC), photosystem II (psbZ) and ATP synthase (atpE, atpF) exhibit adaptive evolution under different environments.
Supporting information
(XLS)
(XLS)
(XLS)
(XLSX)
Acknowledgments
This work is supported by the National Natural Science Foundation of China (No. 81560611), Hainan Provincial Keypoint Research and Invention Program (No. ZDYF2018138), China Scholarship Council (No. 201708460079), and the Program of Hainan Association for Science and Technology Plans to Youth R & D Innovation (No. 201513).
Data Availability
The complete chloroplast genome of A. oxyphylla are available from GenBank accession number: KY985237.
Funding Statement
Funded by National Natural Science Foundation of China (No. 81560611), Hainan Provincial Keypoint Research and Invention Program (No. ZDYF2018138), China Scholarship Council (No. 201708460079), and the Program of Hainan Association for Science and Technology Plans to Youth R & D Innovation (No. 201513).
References
- 1.Liu H, Han CR, Liu HX, Liu YF, He MX. Study on IR fingerprint spectra of Alpinia oxyphylla Miq. Guang Pu Xue Yu Guang Pu Fen Xi. 2008; 28: 2557–2560. [PubMed] [Google Scholar]
- 2.Chang YM, Tamilselvi S, Lin HJ, Tsai CC, Lin YM, Day CH, et al. Alpinia oxyphylla Miq extract ameliorates cardiac fibrosis associated with D-galactose induced aging in rats. Environ Toxicol. 2018; 1–7. [DOI] [PubMed] [Google Scholar]
- 3.Sharifi-Rad M, Varoni EM, Salehi B, Sharifi-Rad J, Matthews KR, Ayatollahi SA, et al. Plants of the Genus Zingiber as a Source of Bioactive Phytochemicals: From Tradition to Pharmacy. Molecules.2017; 22: E2145 10.3390/molecules22122145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang YM, Tamilselvi S, Lin HJ, Tsai CC, Lin YM, Day CH, et al. Alpinia oxyphylla Miq. fruit extract activates IGFR-PI3K/Akt signaling to induce Schwann cell proliferation and sciatic nerve regeneration. BMC Complement Altern Med. 2017; 17: 184 10.1186/s12906-017-1695-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Q, Zheng Y, Hu X, Hu X, Lv W, Lv D, et al. Ethnopharmacological uses, phytochemistry, biological activities, and therapeutic applications of Alpinia oxyphylla Miquel: A review. J Ethnopharmacol. 2018; 224: 149–168. 10.1016/j.jep.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 6.Xie Y, Xiao M, Ni Y, Jiang S, Feng G, Sang S, Du G. Alpinia oxyphylla Miq. Extract Prevents Diabetes in Mice by Modulating Gut Microbiota. J Diabetes Res. 2018; 4230590 10.1155/2018/4230590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu X, An L, Wang Y, Zhao H, Gao C. Neuroprotective effect of Alpinia oxyphylla Miq. fruits against glutamate-induced apoptosis in cortical neurons. Toxicology letters. 2003; 144: 205–212. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Hu X, Hui F, Song Q, Cui C, Wang C, Zhao Q. Ethanol extract and its dichloromethane fraction of Alpinia oxyphylla Miquel exhibited hepatoprotective effects against CCl4-induced oxidative damage in vitro and in vivo with the involvement of Nrf2. Biomed Pharmacother. 2017; 91: 812–822. 10.1016/j.biopha.2017.04.131 [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Zhou T, Bai G, Zhao Y. Complete chloroplast genome sequence of Fagopyrum dibotrys: genome features, comparative analysis and phylogenetic relationships. Scientific reports. 2018; 8: 12379 10.1038/s41598-018-30398-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian N, Han L, Chen C, Wang Z. The complete chloroplast genome sequence of Epipremnum aureum and its comparative analysis among eight Araceae species. PloS ONE. 2018; 13: e0192956 10.1371/journal.pone.0192956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang R, Li Q, Gao J, Qu M, Ding P. The complete chloroplast genome sequence of the medicinal plant Morinda officinalis (Rubiaceae), an endemic to China. Mitochondrial DNA A DNA Mapp Seq Anal. 2016; 27: 4324–4325. 10.3109/19401736.2015.1089484 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Deng J, Li Y, Gao G, Ding C, Zhang L, et al. The complete chloroplast genome sequence of Curcuma flaviflora (Curcuma). Mitochondrial DNA A DNA Mapp Seq Anal. 2016; 27:3644–3645. 10.3109/19401736.2015.1079836 [DOI] [PubMed] [Google Scholar]
- 13.Wu M, Li Q, Hu Z, Li X, Chen S. The Complete Amomum kravanh Chloroplast Genome Sequence and Phylogenetic Analysis of the Commelinids. Molecules. 2017; 22: E1875 10.3390/molecules22111875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett CF, Baker WJ, Comer JR, Conran JG, Lahmeyer SC, Leebens-Mack JH, et al. Resolving ancient radiations: can complete plastid gene sets elucidate deep relationships among the tropical gingers (Zingiberales)? Ann Bot-London. 2014; 113: 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett CF, Davis JI, Leebens-Mack J, Conran JG, Stevenson DW. Plastid genomes and deep relationships among the commelinid monocot angiosperms. Cladistics. 2013, 29: 65–87. [DOI] [PubMed] [Google Scholar]
- 16.Gevu KV, Lima HRP, Kress J, Da Cunha M. Morphological analysis of vessel elements for systematic study of three Zingiberaceae tribes. Journal of Plant Research.2017;130: 527–538. 10.1007/s10265-017-0911-y [DOI] [PubMed] [Google Scholar]
- 17.Wyman SK, Jansen RK, Boore JL. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004; 20: 3252–3255. 10.1093/bioinformatics/bth352 [DOI] [PubMed] [Google Scholar]
- 18.Schattner P., Brooks A. N. & Lowe T. M. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Research. 2005; 33: W686–W689. 10.1093/nar/gki366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiths-Jones S, Bateman A, Marshall M, Khanna A, Eddy SR. Rfam: an RNA family database. Nucleic Acids Research, 2003; 31: 439–441. 10.1093/nar/gkg006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe T, Ikemura T, Sugahara J, et al. tRNADB-CE 2011: tRNA gene database curated manually by experts. Nucleic Acids Research. 2011; 39: D210–D213. 10.1093/nar/gkq1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohse M, Drechsel O, Bock R. OrganellarGenomeDRAW (OGDRAW): a tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Current Genetics. 2007; 52: 267–274. 10.1007/s00294-007-0161-y [DOI] [PubMed] [Google Scholar]
- 22.Sharp PM, Li WH. The Codon Adaptation Index—a Measure of Directional Synonymous Codon Usage Bias, and Its Potential Applications. Nucleic Acids Research. 1987; 15: 1281–1295. 10.1093/nar/15.3.1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurtz S. Choudhuri JV, Ohlebusch E, Schleiermacher C, Stoye J, Giegerich R. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001; 29: 4633–4642. 10.1093/nar/29.22.4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999; 27: 573–580. 10.1093/nar/27.2.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang XM, Sun JT, Xue XF, Zhu WC, Hong XY. Development and characterization of 18 novel EST-SSRs from the western flower Thrips, Frankliniella occidentalis (Pergande). Int J Mol Sci. 2012; 13: 2863–2876. 10.3390/ijms13032863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Wan JM. SSRHunter: development of a local searching software for SSR sites. Yi Chuan. 2005; 27: 808–810. [PubMed] [Google Scholar]
- 27.Grant JR, Stothard P. The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res. 2008, 36: W181–184. 10.1093/nar/gkn179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006; 22: 2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Zhang Y, Zhang Z, Zhu J, Yu J. KaKs_Calculator 2.0: A toolkit incorporating Gamma-Series methods and sliding window strategies. Genomics, Proteomics & Bioinformatics. 2010; 8: 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas GE, Geetha KA, Augustine L, Mamiyil S, Thomas G. Analyses between Reproductive Behavior, Genetic Diversity and Pythium Responsiveness in Zingiber spp. Reveal an Adaptive Significance for Hemiclonality. Frontiers in Plant Science. 2016; 7: 1913 10.3389/fpls.2016.01913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu CH, Tembrock LR, Zheng SY, Wu ZQ. The Complete Chloroplast Genome of Catha edulis: A Comparative Analysis of Genome Features with Related Species. International Journal of Molecular Sciences. 2018;19: 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu ML, Li Q, Xu J, Li XW. Complete chloroplast genome of the medicinal plant Amomum compactum: gene organization, comparative analysis, and phylogenetic relationships within Zingiberales. Chin Med-Uk. 2018; 13: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park E, Caplan JL, Dinesh-Kumar SP. Dynamic coordination of plastid morphological change by cytoskeleton for chloroplast-nucleus communication during plant immune responses. Plant Signaling & Behavior. 2018; 13: e1500064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin G, Baurens FC, Cardi C, Aury JM, D'Hont A. The Complete Chloroplast Genome of Banana (Musa acuminata, Zingiberales): Insight into Plastid Monocotyledon Evolution. PloS ONE. 2013; 8: e67350 10.1371/journal.pone.0067350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Kuang XJ, Zhu XX, Zhu YJ, Sun C. Codon usage bias of Catharanthus roseus. Zhongguo Zhong Yao Za Zhi. 2016; 41: 4165–4168. 10.4268/cjcmm20162213 [DOI] [PubMed] [Google Scholar]
- 36.Gichira AW, Li ZZ, Saina JK, Long Z, Hu G, Gituru RW, et al. The complete chloroplast genome sequence of an endemic monotypic genus Hagenia (Rosaceae): structural comparative analysis, gene content and micro satellite detection. Peerj. 2017; 5: e2846 10.7717/peerj.2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng J, Li X, Li H, Yang J, Wang H, He J. Comparative Analysis of the Complete Chloroplast Genomes of Four Aconitum Medicinal Species. Molecules. 2018; 23: E1015 10.3390/molecules23051015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu W, Kong H, Zhou J, Fritsch PW, Hao G, Gong W. Complete Chloroplast Genome of Cercis chuniana (Fabaceae) with Structural and Genetic Comparison to Six Species in Caesalpinioideae. Int J Mol Sci. 2018;19: E1286 10.3390/ijms19051286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W, Liu Y, Yang Y, Xie X, Lu Y, Yang Z, et al. Interspecific chloroplast genome sequence diversity and genomic resources in Diospyros. BMC Plant Biol. 2018;18: 210 10.1186/s12870-018-1421-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant JR, Arantes AS, Stothard P. Comparing thousands of circular genomes using the CGView Comparison Tool. Bmc Genomics. 2012; 13: 202 10.1186/1471-2164-13-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Zhan DF, Jia X, Mei WL, Dai HF, Chen XT, Peng SQ. Complete Chloroplast Genome Sequence of Aquilaria sinensis (Lour.) Gilg and Evolution Analysis within the Malvales Order. Frontiers in Plant Science. 2016; 7: 280 10.3389/fpls.2016.00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kress WJ, Liu AZ, Newman M, Li QJ. The molecular phylogeny of Alpinia (Zingiberaceae): A complex and polyphyletic genus of gingers. American Journal of Botany. 2005; 92: 167–178. 10.3732/ajb.92.1.167 [DOI] [PubMed] [Google Scholar]
- 43.Gao C, Deng Y, Wang J. The complete chloroplast genomes of Echinacanthus species (Acanthaceae): phylogenetic relationships, adaptive evolution, and screening of molecular markers. Front. Plant Sci. 2019; 9:1989 10.3389/fpls.2018.01989 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(XLS)
(XLS)
(XLSX)
Data Availability Statement
The complete chloroplast genome of A. oxyphylla are available from GenBank accession number: KY985237.