Abstract
Purpose of Review
This review synthesizes recent findings in humans pertaining to the relationships between marinobufagenin (MBG), a steroidal Na+/K+-ATPase inhibitor and salt-sensitivity biomarker, and early cardiovascular risk markers.
Recent Findings
Twenty-four-hour urinary MBG strongly associates with habitual salt intake in young healthy adults (aged 20–30 years). Furthermore, in young healthy adults free of detected cardiovascular disease, MBG associates with increased large artery stiffness and left ventricular mass independent of blood pressure. These findings in human studies corroborate mechanistic data from rat studies whereby stimulation of MBG by a high salt intake or MBG infusion increased vascular fibrosis and cardiac hypertrophy.
Summary
Twenty-four-hour urinary MBG may be a potential biomarker of early cardiovascular risk. Adverse associations between MBG—which increases with salt consumption—and early cardiovascular risk markers support the global efforts to reduce population-wide salt intake in an effort to prevent and control the burden of non-communicable diseases.
Keywords: Early cardiovascular risk, Humans, Marinobufagenin, Women, Salt-sensitivity
Background and Introduction
There is currently little doubt that high salt intake significantly increases the risk for hypertension and cardiovascular disease [1]. At present, the global mean salt intake is twice the amount recommended by the World Health Organization (namely 10 g salt/day [2] vs < 5 g salt/day) [3]. Thus, with 1.65 million cardiovascular-related deaths in 2010 [4] and 2.3 million all-cause related deaths in 2016 [5] being attributed to a high salt diet, reducing excessive dietary salt intake remains a key priority. Global organizations including the World Health Organization [6], the United Nations [7, 8], and the Resolve to Save Lives initiative [9] have taken steps to commit to the initiation and implementation of sodium reduction strategies, in an effort to reduce the growing burden of non-communicable diseases.
In light of the aforementioned, the importance of understanding underlying mechanisms whereby salt intake increases cardiovascular risk is vital. This review specifically focuses on the cardiotonic steroid and Na/K-ATPase inhibitor, marinobufagenin (MBG), as a possible novel biomarker directly relating to salt intake and implicated in increased cardiovascular risk [10••].
The purpose ofthis review is to highlight recent evidence from human studies that supports previous animal studies demonstrating a link between elevated MBG and cardiovascular risk. As portrayed in Fig. 1, the subsequent sections will review research on the physiological functions of MBG and how it relates to measures of cardiovascular risk in humans.
Fig. 1.
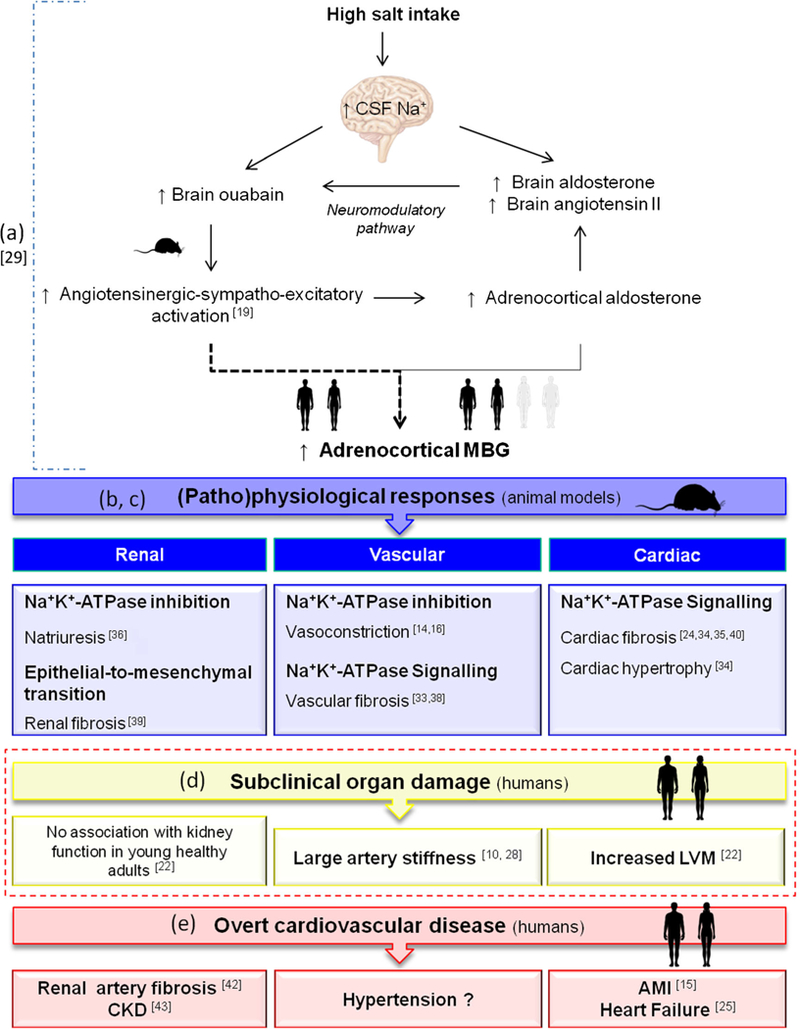
Summary of the evidence linking MBG to an increased cardiovascular risk. a Salt intake stimulates MBG synthesis and secretion via the angiotensinergic-sympatho-excitatory pathway. b Under normal physiological conditions, MBG acts as a natriuretic hormone to stimulate natriuresis as a compensation for increased salt intake. c Excessive MBG production promotes pathophysiological responses including vasoconstriction, vascular, renal, and cardiac fibrosis. d Evidence from human studies demonstrate that elevated MBG associates with measures of subclinical target organ damage that may promote the development of cardiovascular disease. e Elevated MBG has already been observed in several overt cardiovascular diseases. AMI, acute myocardial infarction; CKD, chronic kidney disease; CSF, cerebral spinal fluid; MBG, marinobufagenin
Endogenous Marinobufagenin
Bufadienolides were firstly recognized as playing a regulatory role in the salt acclimation of amphibians via its inhibitory function on skin Na+/K+-ATPase [11, 12]. Later, it was found that one of the compounds of the venom of the Bufo marinus toad, MBG, is structurally and functionally similar to digitalis-like steroid from mammalian plasma and urine that inhibited the Na+/K+-ATPase and exhibited vasoconstrictive properties [13–16]. This digitalis-like steroid was identified as MBG [15, 16] and shown to be stimulated by sodium- induced volume loading [17]. Fedorova et al. have previously demonstrated MBG synthesis in adrenal cortical and placental cells under control of the bile acid CYP27A1 enzyme, but recognize that MBG synthesis may not be limited to these areas [18•]. It was also demonstrated that mammalian MBG production is stimulated by angiotensin II (ANGII) in the animal model of salt-sensitive hypertension and in the adrenocortical cell culture [19].
Mammalian MBG has since been extracted from the plasma and urine of humans [10••, 20•, 21•, 22••, 23••, 24•], and we have recently demonstrated the reliability of the non-invasive measurement of 24-h urinary MBG in the presence of other steroidal hormones, using a solid-phase dissociation-enhanced lanthanide fluorescent immunoassay, based on a 4G4 anti-MBG mouse monoclonal antibody [10••]. In the clinical study with the salt-loaded subjects [21•] and in the animal model of salt-sensitive hypertension with an enhanced MBG production, urine MBG exceeded the concomitant changes in plasma MBG [25, 26•, 27], though the MBG changes in both biological fluids exhibited a similar profile. Notably, one of the studies reported plasma MBG increases in the absence of MBG excretion changes on a high salt diet [20•], while the other study demonstrated exclusively urine MBG changes in the presence of high salt intake [28•]. This discrepancy may be due to the differences in the experimental designs (sequence of the dietary interventions, amount of sodium chloride in the diet, habitual style life prior to the study, etc.), and is discussed in detail in the “Marinobufagenin and blood pressure” section.
Several studies have shown that elevated MBG associates with increased salt intake in animals [25, 26•] and humans [10••, 21•, 28•]. The suggested stimulatory pathway was described in detail by Fedorova et al. [19] Briefly, an increase in salt intake promotes increased angiotensin II, aldosterone, and sympathetic activity, which in turn stimulates adrenocortical MBG synthesis and secretion [19]. In support, we have recently shown in a human cohort that increased autonomic activity and aldosterone are associated with increased MBG excretion [29••].
Physiological Function
In a review by Bagrov et al., the (patho) physiological interaction of MBG with the Na+/K+-ATPase pump was thoroughly described [30•], clearly distinguishing between the two pathways through which MBG acts on the Na+/K+-ATPase pump [30•] (Fig. 2).
Fig. 2.
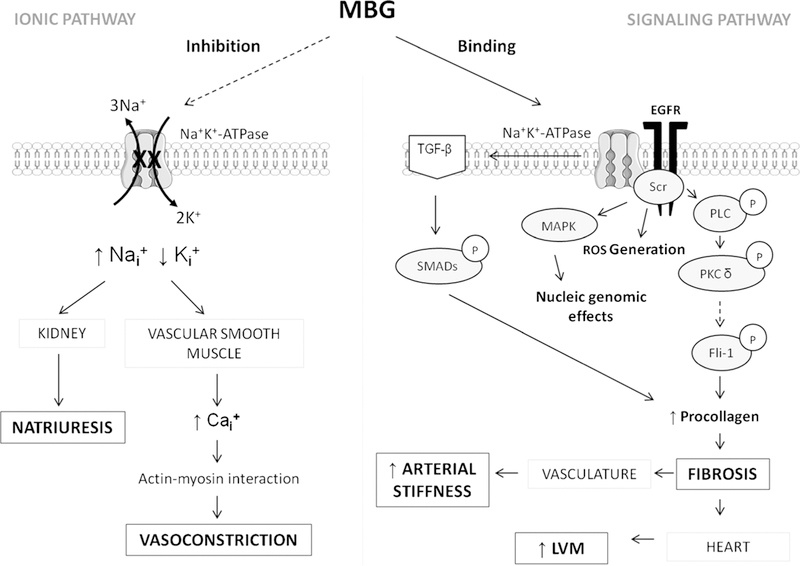
Mechanisms whereby MBG has been implicated increasing cardiovascular risk. Ca+, calcium; Fli-1, friend leukemia integration factor-1; K+, potassium; LVM, left ventricular mass; MBG, marinobufagenin; MAPK, mitogen-activated protein kinase; Na+, sodium; PKC, protein kinase C; PLC, phospholipase C; ROS, reactive oxygen species; TGF-β, transforming growth factor beta; P, phosphorylated form of the protein
The first, identified as the classic “ionic pathway,” involves the inhibition of membrane-bound Na+/K+-ATPase and concurrently altered transmembrane ion transport. It is via the inhibition of the renal Na+/K+-ATPase pump that MBG was described to promote natriuresis as part of its normal physiological function in response to sodium-induced volume loading [25,26•]. This acts as a compensatory mechanism to lower blood pressure. However, MBG has also been shown to promote vasoconstriction via the inhibition of Na+/K+-ATPase in the vascular smooth muscle cells [14, 16]—the proposed result of excessive MBG production. Vascular Na+/K+-ATPase inhibition increases intracellular sodium concentrations and concurrently reverses the function of the vascular Na+/Ca2+-exchanger. This reversed functionality results in an influx of calcium ions into the vascular smooth muscle cells that further stimulates calcium-induced calcium release from the sarcoplasmic reticulum. The elevated intracellular calcium concentration increases the vascular actin-myocin interactions thereby promoting vasoconstriction (Fig. 2) [30•, 31].
In contrast, the second pathway namely the “signaling pathway,” involves the binding of MBG to the Na+/K+-ATPase, which activates several downstream signaling cascades [30•]. These include the activation of mitogen-activated protein kinases and reactive oxygen species and the promotion of fibrosis [30•, 32]. It is via these signaling pathways through which MBG has been shown to promote vascular [32, 33] and cardiac fibrosis [34, 35], which will be described in more detail further on. The aforementioned, also demonstrated in Fig. 2, indicates the mechanistic pathways through which excessive MBG production could promote cardiovascular disease, overriding the normal physiological function thereof.
Marinobufagenin and Cardiovascular Risk
Investigations into the mechanistic pathways whereby MBG contributes to cardiovascular disease have been predominantly performed in rats. Increased plasma MBG, either brought about by salt loading or osmotic pump infusion, was shown to promote pressor responses [19, 25, 26•, 36•], microvascular alterations [37], vascular [33, 38], renal [39], and cardiac fibrosis [24•, 34, 35, 40•].
Indeed, human studies focusing on diseased populations have also observed elevated plasma levels of MBG in patients with primary aldosteronism [41], heart failure [24•], renal artery stenosis [42•] and chronic kidney disease [43•, 44•], and elevated urinary MBG in patients with acute myocardial infarction [15]. However, it remains unclear whether elevated urinary levels of MBG, specifically 24-h MBG excretion—due to excessive dietary salt intake—in young healthy populations would already confer increased cardiovascular risk prior to the onset of disease. If so, MBG may be considered a biomarker of early cardiovascular risk.
We recently investigated in young healthy adults whether urinary MBG is associated with blood pressure and measures of early cardiovascular risk, including large artery stiffness [10••] and increased left ventricular mass (LVM) [22••]— thereby evaluating MBG’s potential as a biomarker of early cardiovascular risk.
Marinobufagenin and Blood Pressure
When taking into consideration the well-known relationship between salt, and especially salt-sensitivity, and blood pressure [45], it is not surprising that elevated MBG is associated with pressor responses [21•, 23••, 28•] (Table 1). Indeed, MBG was shown to exhibit both natriuretic as well as vasoconstrictive properties via the inhibition of renal and vascular Na+/K+-ATPase [16, 26•]. Although the relationship between MBG and blood pressure was originally largely confirmed in rats [19, 25, 26•, 36•, 46], recent investigations have been performed in humans [20•, 21•, 23••, 28•], with one study including young adults with clinic blood pressures < 140/90 mmHg [23••].
Table 1.
Twenty-four-hour urinary marinobufagenin in human cohorts without reported kidney or heart disease
| Population description Mean urinary MBG excretion |
Population salt intake | Study results (observed associations with MBG) |
|
|---|---|---|---|
| Anderson et al. [21•] | Women (n = 28): 1.86 nmol/day 2.45 nmol/day |
Low salt diet (2.86 g/day) High salt diet (16.32 g/day) |
MBG associated inversely with SBP-natriuretic response |
| Strauss et al. [10••, 22••, 23••] | The African-PREDICT study White women (n = 112): 2.52 nmol/day [23••] |
Habitual salt intake (mean 6.68 g/day) |
Trend of a negative association with SBP [23••] |
| Black women (n = 74): 2.82 nmol/day [23••] |
Habitual salt intake (mean 6.65 g/day) |
MBG associated positively with central SBP [23••] |
|
| White men (n = 77) 4.69 nmol/day [23••] |
Habitual salt intake (mean 8.91 g/day) |
No association with blood pressure [23••] |
|
| Black men (n = 68) 3.99 nmol/day [23••] |
Habitual salt intake (mean 8.52 g/day) |
No association with blood pressure [23••] |
|
| Black and white women (n = 415): 2.69 nmol/day [10••] |
Habitual salt intake (mean 7.27 g/day) |
MBG positively associated with arterial stiffness [10••] |
|
| Black and white men (n = 296): 4.13 nmol/day [10••] |
Habitual salt intake (mean 8.32 g/day) |
No association with large artery stiffness |
|
| Black and white men and women within the highest quartile of MBG excretion (n = 179) from the total study population of n = 711 | |||
| 6.92 nmol/day [22••] | Habitual salt intake (mean 11.8 g/day) [22••] | MBG associates positively with increased LVMi [22••] | |
| Jablonski et al. [28•] | Men and women (n = 11): 2.04 nmol/day 2.45 nmol/day |
Low salt diet (3.75 g/day) Habitual salt diet (9 g/day) |
MBG positively associated with SBP MBG positively associated with arterial stiffness |
| Fedorova et al. [20•] | Men (n = 20): 1.30 nmol/day 1.19 nmol/day 1.13 nmol/day |
Habitual high salt intake (mean > 9 g/day) High salt diet (9 g/day) Low salt diet (3 g/day) |
No relationships of urinary MBG and systolic blood pressure were evident during either condition. |
| Women (n =19): 1.06 nmol/day 0.97 nmol/day 0.98 nmol/day |
Habitual high salt intake (mean ≥ 9 g/day) High salt diet (9 g/day)Low salt diet (3 g/day) |
No relationships of urinary MBG and systolic blood pressure were evident during either condition. |
|
LVMi, left ventricular mass index; MBG, marinobufagenin; SBP, systolic blood pressure
The first human study investigating the relationship between MBG and blood pressure included 28 normotensive white women (aged 53 ± 1.6 years) who underwent 12 days of dietary sodium intervention (a 6-day low sodium diet of approximately 2.86 g/day, followed by a 6-day high sodium diet of 16.32 g salt per day) [21•]. They demonstrated a 35% increase in 24-h urinary MBG, from approximately 1.83 to 2.45 nmol/day, when comparing sodium interventions (Table 1). Only during the high salt diet, did systolic blood pressure (SBP) inversely correlate with MBG excretion, possibly reflecting the natriuretic function of MBG in this cohort of the normotensive subjects [21•]. This study was performed over a short-time period that might reflect the short-term homeostatic mechanism whereby increased natriuresis may lower blood pressure as a protective mechanism to excessive salt intake in the healthy subjects. Nonetheless, we also demonstrated non-significant inverse relationship between the MBG/ Na+ ratio (but not MBG) and SBP measures in young normotensive white women (aged 25.6 ±2.78 years) from the African Prospective study on the Early Detection and Identification of Cardiovascular disease and Hypertension (African-PREDICT) [47•], consuming approximately 6.68 g of salt per day (n =112) [23••]. The MBG/Na+ ratio was used as an indication of Na+ excretion resistance to elevated levels of urinary MBG [23••] and may be reflective of the natriuretic functionality of MBG.
In an intervention study performed by Jablonski et al. in 11 men and women, with SBP ranging from 130 to 159 mmHg and DBP < 99 mmHg, they also found that MBG was significantly attenuated (2.04 ±0.16 nmol/day) during 5 weeks of low salt intake (mean 3.75 g/day), compared to MBG levels (2.45 ±0.17 nmol/day) during a high salt intake (mean 9 g/day). However, they found that MBG related positively with SBP only during the high salt diet intervention [28•]. In contrast to the short-term dietary intervention performed by Anderson et al. [21•], the study performed by Jablonski and colleagues may be reflective of a long-term homeostatic mechanisms where the natriuretic function of MBG to high salt intake may be overridden by the vasoconstrictive properties of MBG [28•]. Indeed, the authors alluded to the vasoconstrictive characteristic of MBG as a possible explanation for the observed positive association between MBG and blood pressure in their cohort [27]. Additionally, possible kidney dysfunction in these prehypertensive and hypertensive participants may overbalance and diminish the natriuretic function of MBG, which would cause an additional stimulation of MBG production and will initiate and feed a vicious circle of salt-sensitivity.
We also demonstrated a significant positive association between the MBG/Na+ ratio and central SBP in young black women (n = 74) (aged 24.3 ±3.64 years), that was in contrast to our finding of a borderline negative association between MBG/Na+ and SBP in white women from the African-PREDICT study [23••]. There was no relationship between the MBG/Na+ ratio and SBP in either black or white men [23••].
In contrast to the abovementioned studies, Fedorova et al. found that while both men (n = 20) and women (n =19) (aged 53 ± 11 years) displayed increases in SBP with salt loading, neither plasma nor urinary MBG levels changed significantly [20•]. In the total group, however, only plasma MBG was significantly lower during low compared to high salt intake. While men and women were included into the study based on no-reported history of hypertension, it was evident that some of the participants did indeed have hypertension with the mean SBP being 139 ± 13.3 mmHg and DBP 86.3 ±7.4 mmHg [20•]. Participants from this study were firstly examined at baseline so to take into account their habitual salt diet, after which they participated in a double-blind cross-over study. Participants were placed on a strict diet containing only 3 g of salt per day, for the entire study period of 8 weeks. Additionally, participants received 6 g of salt or placebo capsules that were randomly taken for two periods of 4 weeks each. Thus, each participant consumed a 4-week high salt diet (9 g salt per day) and a 4-week low salt diet (3 g salt per day). Mean baseline 24-h urinary MBG in men and women were 1.30nmol/day and 1.06 nmol/day (on a habitual diet), and after 4 weeks of a high salt diet 1.19 nmol/day and 0.97 nmol/day, respectively [20•]—noticeably lower compared to normotensive adults from another study [10••]. Twenty-four-hour urinary MBG was 1.13 nmol/day and 0.98 nmol/day for men and women after 4 weeks of low sodium intervention. Notably, baseline 24-h urinary sodium as well as MBG was higher in comparison to urinary sodium and MBG after the high salt intervention. This may suggest that participants consumed a habitual high salt diet in access of the high sodium intervention of 9 g of salt per day. Fedorova et al. found that the changes in the plasma MBG levels were related to the changes in SBP from a high to a low salt diet, although no relationship was evident between urinary MBG and SBP [20•].
From the abovementioned studies, it seems that the reported relationships between urinary MBG and blood pressure in humans are inconsistent. While animal studies have provided compelling evidence on the functionality of MBG and the effect to increase blood pressure, more evidence in human studies are needed. Importantly, the differences in the study designs, population characteristics, and sample sizes of these studies cannot be overlooked when bearing in mind the discrepancies (Table 1). Still, some intriguing observations on the contrasting relationships between MBG and blood pressure in specific groups, including white and black women, support the potential divergent properties of MBG on blood pressure in humans.
Marinobufagenin and Arterial Stiffness
Several studies have established large artery stiffness as a predictor of increased cardiovascular risk and mortality [48] in young [49•], middle-aged, and older populations [50–53] beyond blood pressure. Salt intake was shown to be associated with arterial stiffness—not only in hypertensive [54, 55] but also young healthy adults [56•]. Arterial stiffness measured as the pulse wave velocity (PWV) within the carotid to femoral (cf) section of the arterial tree is currently considered as the gold standard measurement of large artery stiffness [57]. The first human study investigating the relationship between arterial stiffness and MBG was performed by Jablonski et al. and included 11 participants, namely men (n = 8) and women (n = 3), aged 62 ± 2 years, with high or hypertensive blood pressures [28•]. They demonstrated a positive correlation between cfPWV and MBG excretion [28•]. In support, in young healthy women (aged 24.8 ±3.08 years; N =415) consuming a habitual high salt diet (mean 7.27 g/day), we have recently found that cfPWV associated positively with MBG excretion, independent of salt intake [10••]. When performing sensitivity analyses for salt intake on the relationship between MBG and arterial stiffness, salt intake remained non-significant [10••]—possibly implying that relationships observed between salt intake and arterial stiffness [54, 55, 56•] may be via MBG as opposed to salt in itself. This relationship was also independent of mean arterial pressure [10••]. Since arterial stiffness may precede the development of hypertension [58], this relationship in young normotensive healthy adults, and the possible blood pressure independent effect of MBG on large artery stiffness, is highlighted [10••].
Although there are no human studies demonstrating mechanistic links between MBG and arterial stiffness, MBG was shown to promote vascular fibrosis in rat aortic explants [33] and increase in collagen production in the cultured rat vascular smooth muscle cells [32]—which indicate a pressure-independent effect of MBG on vascular fibrosis. Both Fedorova [33] and Elkareh et al. [35] have described the MBG-dependent signaling pathway in the promotion of collagen deposition—initiated by MBG binding to Na+K+-ATPase [33, 35]. Findings from these studies indicated a significant downregulation of transcription factor Friend leukemia integration-1 (Fli-1), in response to MBG, and concurrently increased collagen-1 synthesis [33, 35]. The scaffolding protein collagen reduces the arterial wall elasticity, thereby adversely influences large artery function [59]. In support, recently published findings from Grigorova et al. demonstrated that increased dietary sodium resulted in concurrent increased MBG excretion, aortic collagen expression, and arterial stiffness via TGF-β in normotensive rats [32]. Contrarily, sodium reduction and concurrent attenuation of MBG excretion resulted in decreased aortic collagen abundance and restored large artery elasticity [32]. If this applies to humans, it may further strengthen current strategies to reduce salt intake.
All together, the positive findings from the two studies investigating the relationship between MBG and arterial stiffness in humans support the role of MBG in the development of arterial stiffness. However, whether the exact mechanisms shown in rats, whereby MBG may promote arterial stiffness as a result of vascular fibrosis, holds true in humans, remains to be investigated.
Marinobufagenin and Structural Cardiac Alterations
Left ventricular mass (LVM) determined by echocardiography is a predictor of increased cardiovascular risk and mortality [60]. Findings from the Coronary Artery Risk Development in Young Adults study (CARDIA) indicated that 24-h urinary sodium associated positively with LVM in young adults (30.1 ±3.6 years), although this relationship was confounded by obesity [61]. We therefore speculated that higher levels of MBG as a result of increased salt intake would also be associated with increased LVM [22••]. Indeed, our research group found a significant positive association between MBG and LVM index in young adults with excessively high MBG levels [22••]. In accordance with international guidelines, the LVM index takes into account intra-individual body composition and was normalized for body surface area [62]. The association between LVM index and MBG excretion was therefore independent of obesity. In addition, the latter relationship was also independent blood pressure, suggesting alternate mechanisms whereby MBG promotes cardiac hypertrophy [22••]. Although there are no human studies investigating histological cardiac changes in response to MBG, others have demonstrated increased cardiac myocyte hypertrophy and fibrosis in response to MBG infusion in Sprague-Dawley rats. These observations were parallel with an increased cardiac mass in these animals [34, 35].
It is, therefore, likely that excessively high levels of MBG may cause corresponding histological changes in the cardiac tissue of humans—thereby increasing the cardiac mass. Although our findings suggest that the structural cardiac changes associated with elevated MBG may precede cardiac dysfunction at an early age, it is possible that cardiac functionality may be adversely altered at a later stage.
Marinobufagenin and Ethnicity
It is well known that black ethnicity is associated with increased salt-sensitivity and abnormal sodium handling [63–65]. It would therefore be important to investigate whether MBG—a marker of salt sensitivity—is elevated in black populations. Contradictory to expectations, Anderson et al. indicated that white adults (n = 40) had higher concentrations of 24-h urinary MBG (mean 2.7 ± 0.2pmol) compared to black adults (n = 40) (mean 2.1 ± 0.2 pmol) who participated in the Baltimore Longitudinal Study on Aging [66]. A limitation of this study, however, was that the researchers did not report the salt intake of participants [66], which highly correlates with 24-h urinary MBG [10••]. It is therefore not possible to accurately interpret findings on the observed ethnic differences in 24-h urinary MBG concentrations.
We have also investigated whether there are ethnic differences in 24-h urinary MBG excretion between young healthy black and white adults from the African-PREDICT study while also reporting their estimated salt intake based on 24-h urinary sodium [23••]. We found no significant difference in estimated salt intake or the 24-h urinary MBG excretion when comparing black and white men and black and white women [23••]. Also, unexpectedly, no interaction of ethnicity was evident on the relationship of MBG with arterial stiffness [10••] or left ventricular mass [22••]. The absences of these interactions were unforeseen, especially with salt intake shown to be associated with large artery stiffness in black but not white adults [55, 56•]. As previously described in detail, we did however observed a difference in the relationship of the MBG/Na+ ratio and SBP between black and white women. While the MBG/Na+ ratio associated positively with central SBP in black women, a tendency for a negative association was evident in white women [23••]. Future studies may look at relationships between the MBG/Na+ ratio and cardiovascular risk markers between ethnic groups, especially if the ratio is used as an indication of Na+ excretion resistance to elevated urinary MBG [23••]—taking into account the differential sodium handling between black and white populations [63].
These findings bring rise to the question with regard to salt-sensitivity, MBG-sensitivity, and black ethnicity. Does salt-sensitivity associated with black ethnicity [64] automatically imply increased sensitivity to the cardiovascular effects of MBG? Our results suggest that while increased salt intake may increase cardiovascular risk in blacks, they may not at this young age be as susceptible to the adverse effects of elevated MBG.
Nonetheless, a phenomenon of increased autonomic activity during stress [67–69] and cardiovascular sensitivity to sympathetic outflow [70] as observed in black adults may at a later stage exaggerate MBG production resulting in excessive MBG levels to increase their cardiovascular risk at an older age. This suggestion is supported by our recent findings of increased autonomic activity being positively associated with MBG excretion only in black men and women, but not their white counterparts [29••].
Still, there is limited research on MBG in ethnic groups, and at this stage, the young age and healthy status of the African-PREDICT participants may mask the influence of ethnicity on MBG levels. More in-depth research is needed to further investigate ethnic differences and the cardiovascular effects of MBG.
Marinobufagenin and Sex
While reports on the relationship between MBG and blood pressure have been inconsistent in different sex groups [20•, 21•, 23••, 28•], the relationship between MBG and cardiovascular risk factors including increased arterial stiffness and left ventricular mass seems more prominent in young women [10••, 22••]. We have previously suggested that women may likely be more sensitive to the cardiovascular effects of MBG, despite having lower salt intake and lower MBG levels than men [22••]. In support of this suggestion, women have been shown to be more salt-sensitive compared to men when consuming similar amounts of salt [71, 72•, 73•], and exhibit greater increases in aldosterone levels, in response to ANGII infusion [73•].
Importantly, the possible role of sex hormones cannot be disregarded. While there is no human study to our knowledge investigating the direct relationship between MBG and sex hormones, we have demonstrated the possible confounding effect thereof. While exploring the association between MBG and arterial stiffness in women, we performed a sensitivity analyses for hormonal contraceptive use and repeated subgroup analyses in women who made use of hormonal contraceptives (N = 140) and those who did not (N =217) [10••]. Our finding of a positive association between MBG and arterial stiffness remained significant only in women who did not make use of hormonal contraceptives [10••]. These findings suggest an interaction between the steroidal hormone, MBG, and other sex hormones, which exhibit regular cyclic changes, that require further research.
Understanding the underlying mechanisms of MBG and salt-sensitive hypertension, and particularly the role of sex, is challenging since studies investigating the relationships and relevant mechanisms of MBG with salt sensitive hypertension [26•], arterial stiffness [74], cardiac hypertrophy [34, 40•, 75], and cardiac [34, 40•], vascular [33], and renal fibrosis [39] have all been performed in male rats except the studies on the model of preeclampsia [76, 77]. Therefore, none of these studies investigated nor compared the mechanisms whereby MBG promotes cardiovascular dysfunction in female rats. In the one study including both male and female rats, the SBP and plasma MBG (and its regulatory enzyme CYP27A1), were significantly increased after 4 weeks of sodium loading in both sexes [18•]. However, consistent with reports of lower 24-h urinary MBG in women [10••], female Dahl salt- sensitive rats had lower levels of plasma MBG and CYP27A1 mRNA expression at the baseline and after 4 weeks of a high salt diet compared to male rats, despite consuming similar amounts of salt [18•].
Taking into consideration the abovementioned, it is unclear why the adverse relationship between MBG and early markers of cardiovascular risk is predominantly seen in women, despite their lower MBG. One possible mechanism includes the sensitization of the α1-Na+/K+-ATPase to MBG. Indeed, elevated levels of protein kinase C β2 expression have been found in female rats [78], previously shown to sensitize α1-Na+/K+-ATPase to MBG [27].
In view of the recent findings in women, it would seem that women may be more sensitive to the cardiovascular effects of steroidal MBG compared to men. The female’s childbearing function demands disparate requirements to salt handling compared to men, which may be one of the explanations of the above difference. Normal pregnancy is accompanied by plasma volume expansion involving retention of sodium ions and fluid [79–81], which concurrently increases the levels of MBG as a natriuretic factor to control the water/salt balance. It was found that in women with normal pregnancies, plasma MBG increased up to twofold compared to non-pregnant age-matched controls [82] with a further dramatic elevation (up to eightfold) in preeclampsia [82, 83]. In the rat model of preeclampsia, BP increase was achieved by an addition of 1.8% NaCl to the drinking water [46, 77] or by a combination of high NaCl (0.9% in the water) and deoxycorticosterone acetate treatments for the duration of their pregnancy [76]. In rats, 24-h urinary MBG and BP were higher in pregnant and non-pregnant animals on a high salt intake in comparison to normal pregnancies and non-pregnant controls [46, 76, 77]. Similarly to the humans, even normal pregnancies exhibited significantly higher MBG levels than non-pregnant controls [46, 76, 77]. The exaggerated production of MBG in preeclampsia contributes to BP increase via direct vasoconstriction [46, 76, 77, 82–84], and to the pathologies associated with the Fli-1-dependent fibrotic changes in the umbilical arteries [83] and in the placenta [85] (Fig. 2). The latter would affect fetal blood supply and placentation. MBG impairs the proliferation, migration, and invasion of the cultured first-trimester human cytotrophoblast cells. This is done through the activation of Jnk, P38, and Src leading to augmented apoptosis [86, 87], which provides a mechanistic insight on the impaired placentation. Still, normal pregnancy is accompanied by an increase in MBG due to the association of normal pregnancy with salt and water retention [79–81]. It is possible that the sensitivity of Na+/K+-ATPase to MBG inhibition in normal pregnancies predominantly promotes the normal physiological natriuretic function of MBG [77]. The rat model of preeclampsia is accompanied by the increased salt intake, which indicates that the water/salt balance is vulnerable in pregnancy. This outlines the necessity of dietary salt control during pregnancy in order to ensure balanced functioning of the renal and cardiovascular systems. Still, there are no clear answers when it comes to the role of sex, especially female sex, on the functionality of MBG. Thus, the multifaceted role of MBG in non-pregnancy, pregnancy, and preeclampsia merits future investigations.
Future Directions and Conclusion
Twenty-four-hour urinary MBG may serve as a potential biomarker of early cardiovascular risk in young adults who consume a habitual high salt diet. This review highlights recent findings on the associations between MBG—which markedly increases with increased salt intake—and established cardiovascular risk factors in young healthy adults, including large artery stiffness and increased left ventricular mass. These important new findings on the potential harmful role of MBG in adults with no-detected cardiovascular disease add to a body of literature indicating elevated levels of MBG in older populations with reported pathology. These results also support mechanistic studies in rats demonstrating the pathophysiological mechanisms promoted by increased MBG, including vasoconstriction, vascular, and cardiac fibrosis as demonstrated in Fig. 2. Evidently, sodium reduction may be pivotal in reducing the cardiovascular risk associated with elevated MBG.
The most recent body of work investigating MBG and early cardiovascular risk in young healthy adults forms part of the African-PREDICT study [47•]. The study enrolled young black and white men and women (20–30 years of age) with no prior history of cardiovascular disease, and who were screened to be healthy and clinic normotensive upon inclusion into the study. The African-PREDICT study is the first longitudinal study that will measure and track the MBG levels of healthy adults over a time period, providing a unique insight into the possible prognostic value of MBG [47•].
Establishing MBG as an early biomarker of increased cardiovascular risk, furthermore, will support the efforts of several international legislations to lower salt intake of populations.
Acknowledgments
Funding The sources of funding of this research are the South African Medical Research Council (SAMRC), the South African Research Chairs Initiative (SARChI) of the Department of Science and Technology, and National Research Foundation (NRF) of South Africa (UID 86895 and 111862). This research was supported in part by the Intramural research Program of the NIH, National Institute on Aging, Baltimore, Maryland, USA.
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Ethical Considerations This manuscript does not contain patient data.
Disclaimer Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors, and therefore, the NRF does not accept any liability in regard.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.He FJ, MacGregor GA. Role of salt intake in prevention of cardiovascular disease: controversies and challenges. Nat Rev Cardiol. 2018;15:371–7. 10.1038/s41569-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 2.Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013;3:e003733. 10.1136/bmjopen-2013-003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Guideline: sodium intake for adults and children. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 4.Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–34. 10.1056/NEJMoa1304127. [DOI] [PubMed] [Google Scholar]
- 5.Gakidou E, Afshin A, Abajobir AA, Abate KH, Abbafati C, Abbas KM, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1345–422. 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Global action plan for the prevention and control of NCDs 2013–2020. Geneva: World Health Organization; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United Nations General Assembly Resolution 66/2. Political declaration of the high-level meeting of the general assembly on the prevention and control of non-communicable diseases, A/RES/66/2. New York: United Nations; 19 September 2011. Available from undocs.org/A/RES/66/2. [Google Scholar]
- 8.United Nations General Assembly Resolution 73/2. Political declaration of the third high-level meeting of the General Assembly on the prevention and control of non-communicable diseases, a/res/73/2. 10 October 2018. Available from
- 9.Resolve to Save Lives Initiative. Sodium reduction [press release]. Available from: https://www.resolvetosavelives.org/resources. Accessed 28 Dec 2018.
- 10.••.Strauss M, Smith W, Wei W, Bagrov AY, Fedorova OV, Schutte AE. Large artery stiffness is associated with marinobufagenin in young adults: the African-PREDICT study. J Hypertens. 2018;36: 2333–9. 10.1097/HJH.0000000000001866 This study indicates a blood pressure independent relationship between MBG and arterial stiffness in young healthy adults. Results from this study support findings from animal studies demonstrating increased vascular fibrosis, and concurrently arterial stiffness, in response to increased MBG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lichtstein D, Gati I, Babila T, Haver E, Katz U. Effect of salt acclimation on digitalis-like compounds in the toad. BBA. 1991;1073:65–8. [DOI] [PubMed] [Google Scholar]
- 12.Flier J, Edwards MW, Daly JW, Myers CW. Widespread occurrence in frogs and toads of skin compounds interacting with the ouabain site of Na+, K+-ATPase. Science. 1980;208:503–5. [DOI] [PubMed] [Google Scholar]
- 13.Bagrov AY, Roukoyatkina NI, Fedorova OV, Pinaev AG, Ukhanova MV. Digitalis-like and vasoconstrictor effects of endogenous digoxin-like factor(s) from the venom of Bufo marinus toad. Eur J Pharmacol. 1993;234:165–72. [DOI] [PubMed] [Google Scholar]
- 14.Bagrov AY, Dmitrieva RI, Fedorova OV, Kazakov GP, Roukoyatkina NI, Shpen VM. Endogenous marinobufagenin-like immunoreactive substance. A possible endogenous Na, K-ATPase inhibitor with vasoconstrictor activity. Am J Hypertens. 1996;9: 982–90. 10.1016/0895-7061(96)00148-3. [DOI] [PubMed] [Google Scholar]
- 15.Bagrov AY, Fedorova OV, Dmitrieva RI, Howald WN, Hunter AP, Kuznetsova EA, et al. Characterization of a urinary bufodienolide Na+,K+-ATPase inhibitor in patients after acute myocardial infarction. Hypertension. 1998;31:1097–103. 10.1161/01.hyp.31.5.1097. [DOI] [PubMed] [Google Scholar]
- 16.Bagrov AY, Fedorova OV. Effects of two putative endogenous digitalis-like factors, marinobufagenin and ouabain, on the Na+, K+-pump in human mesenteric arteries. J Hypertens. 1998;16: 1953–8. [DOI] [PubMed] [Google Scholar]
- 17.Fedorova OV, Doris PA, Bagrov AY. Endogenous marinobufagenin-like factor in acute plasma volume expansion. Clin Exp Hypertens. 1998;20:581–91. [DOI] [PubMed] [Google Scholar]
- 18.•.Fedorova OV, Zernetkina VI, Shilova VY, Grigorova YN, Juhasz O, Wei W, et al. Synthesis of an endogenous steroidal Na pump inhibitor marinobufagenin, implicated in human cardiovascular diseases, is initiated by CYP27A1 via bile acid pathway. Circ Cardiovasc Genet. 2015;8:736–45. 10.1161/circgenetics.115.001217 This study demonstrates the biosynthesis of mammalian MBG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fedorova OV, Agalakova NI, Talan MI, Lakatta EG, Bagrov AY. Brain ouabain stimulates peripheral marinobufagenin via angiotensin II signalling in NaCl-loaded Dahl-S rats. J Hypertens. 2005;23: 1515–23. [DOI] [PubMed] [Google Scholar]
- 20•.Fedorova OV, Lakatta EG, Bagrov AY, Melander O. Plasma level of the endogenous sodium pump ligand marinobufagenin is related to the salt-sensitivity in men. J Hypertens. 2015;33:534–41. 10.1097/hjh.0000000000000437 This study is one of 4 studies investigating the relationship between MBG and blood pressure in adults with no kidney or heart disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Anderson DE, Fedorova OV, Morrell CH, Longo DL, Kashkin VA, Metzler JD, et al. Endogenous sodium pump inhibitors and age-associated increases in salt sensitivity of blood pressure in normotensives. Am J Physiol Regul Integr Comp Physiol. 2008;294: R1248–54. 10.1152/ajpregu.00782.2007 This is the first human study investigating the relationship between MBG and blood pressure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22••.Strauss M, Smith W, Kruger R, Wei W, Fedorova OV, Schutte AE. Marinobufagenin and left ventricular mass in young adults: The African-PREDICT study. Eur J Prev Cardiol. 2018;25:1587–95. 10.1177/2047487318788140 This cross-sectional study for the first time indicates that excessive levels of MBG is associated with increased left ventricular mass, independent of blood pressure, in young healthy adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Strauss M, Smith W, Wei W, Fedorova OV, Schutte AE. Marinobufagenin is related to elevated central and 24-h systolic blood pressures in young black women: the African-PREDICT Study. Hypertens Res. 2018;41:183–92. 10.1038/s41440-017-0009-x This is the first study to investigate the relationship between MBG and SBP in a young healthy biethnic cohort - including young black and white, men and women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Kennedy DJ, Shrestha K, Sheehey B, Li XS, Guggilam A, Wu Y, et al. Elevated plasma marinobufagenin, an endogenous cardiotonic steroid, is associated with right ventricular dysfunction and nitrative stress in heart failure. Circ Heart Fail. 2015;8:1068–76. 10.1161/circheartfailure.114.001976 This study demonstrates the effect of increased MBG on the cardiac structure of rats, that supported the need to investigate the relationship between MBG and cardiac structure in humans with excessively high levels of salt intake and 24h MBG excretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fedorova OV, Lakatta EG, Bagrov AY. Endogenous Na,K pump ligands are differentially regulated during acute NaCl loading of dahl rats. Circulation. 2000;102:3009–14. 10.1161/01.cir.102.24.3009. [DOI] [PubMed] [Google Scholar]
- 26.•.Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. Endogenous ligand of alpha(1) sodium pump, marinobufagenin, is a novel mediator of sodium chloride–dependent hypertension. Circulation. 2002;105:1122–7. 10.1161/hc0902.104710 Here Fedorova et al. describe that sustained high levels of MBG may promote a vasoconstrictive response, thereby increasing blood pressure, due to the blunted natriuretic functionality of MBG. [DOI] [PubMed] [Google Scholar]
- 27.Fedorova OV, Talan MI, Agalakova NI, Droy-Lefaix M-T, Lakatta EG, Bagrov AY. Myocardial PKC β2 and the sensitivity of Na/K-ATPase to marinobufagenin are reduced by cicletanine in Dahl hypertension. Hypertension. 2003;41:505–11. 10.1161/01.hyp.0000053446.43894.9f. [DOI] [PubMed] [Google Scholar]
- 28•.Jablonski KL, Fedorova OV, Racine ML, Geolfos CJ, Gates PE, Chonchol M, et al. Dietary sodium restriction and association with urinary marinobufagenin, blood pressure, and aortic stiffness. Clin J Am Soc Nephrol. 2013;8:1952–9. 10.2215/cjn.00900113 This is the first human study demonstrating a relationship between increased salt intake, MBG and arterial stiffness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Strauss M, Smith W, Wei W, Fedorova OV, Schutte AE. Autonomic activity and its relationship with the endogenous cardiotonic steroid marinobufagenin: the African-PREDICT study. Nutr Neurosci. 2019;7:1–11. 10.1080/1028415X.2018.1564985 This observational study is the first study in a human cohort demonstrating associations of salt intake, autonomic activity and aldosterone with MBG. This study supports the proposed angiotensinergic-sympatho-excitatory pathway implicated in MBG sythesis and secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61:9–38. 10.1124/pr.108.000711 This useful review thoroughly describes the pathways whereby MBG may effect the cardiovasculature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adrogué HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med. 2007;356:1966–78. [DOI] [PubMed] [Google Scholar]
- 32.Grigorova Y, Wei W, Petrashevskaya N, Zernetkina V, Juhasz O, Fenner R, et al. Dietary sodium restriction reduces arterial stiffness, vascular TGF-β-dependent fibrosis and marinobufagenin in young normotensive rats. Int J Mol Sci. 2018;19:3168 10.3390/ijms19103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fedorova OV, Emelianov IV, Bagrov KA, Grigorova YN, Wei W, Juhasz O, et al. Marinobufagenin-induced vascular fibrosis is a likely target for mineralocorticoid antagonists. J Hypertens. 2015; 33 : 1602–10. 10.197/hjh0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elkareh J, Kennedy DJ, Yashaswi B, Vetteth S, Shidyak A, Kim EG, et al. Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension. 2007;49:215–24. 10.1161/01.hyp.0000252409.36927.05. [DOI] [PubMed] [Google Scholar]
- 35.Elkareh J, Periyasamy SM, Shidyak A, Vetteth S, Schroeder J, Raju V, et al. Marinobufagenin induces increases in procollagen expression in a process involving protein kinase C and Fli-1: implications for uremic cardiomyopathy. Am J Physiol Renal Physiol. 2009;296: F1219–26. 10.1152/ajprenal.90710.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Fedorova OV, Kolodkin NI, Agalakova NI, Lakatta EG, Bagrov AY. Marinobufagenin, an endogenous α−1 sodium pump ligand, in hypertensive Dahl salt-sensitive rats. Hypertension. 2001;37: 462–6. 10.1161/01.hyp.37.2.462 This study firstly investigated the role of MBG in Dahl salt-sensitive hypertension. [DOI] [PubMed] [Google Scholar]
- 37.Uddin MN, Horvat D, Childs EW, Puschett JB. Marinobufagenin causes endothelial cell monolayer hyperpermeability by altering apoptotic signaling. Am J Physiol-Regul Integ Comp Physiol. 2009;296:R1726–R34. 10.1152/ajpregu.90963.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fedorova OV, Shilova V, Zernetkina V, Zhang Y, Lehrmann E, Becker KG, et al. A monoclonal antibody to an endogenous Na/K-ATPase ligand, marinobufagenin, reverses expression of pro-fibrotic genes and reduces cardiovascular fibrosis in aged rats. Artery Res. 2013;7:169 10.1016/j.artres.2013.10.027. [DOI] [Google Scholar]
- 39.Fedorova LV, Raju V, El-Okdi N, Shidyak A, Kennedy DJ, Vetteth S, et al. The cardiotonic steroid hormone marinobufagenin induces renal fibrosis: implication of epithelial-to-mesenchymal transition. Am J Physiol Renal Physiol. 2009;296:F922–F34. 10.1152/ajprenal.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, Khouri S, et al. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47:488–95. 10.1161/01.hyp.0000202594.82271.92 This study demonstrates the effect of increased MBG on the cardiac structure of rats, that supported the need to investigate the relationship between MBG and cardiac structure in humans with excessively high levels of salt intake and 24h MBG excretion. [DOI] [PubMed] [Google Scholar]
- 41.Tomaschitz A, Piecha G, Ritz E, Meinitzer A, Haas J, Pieske B, et al. Marinobufagenin in essential hypertension and primary aldosteronism: a cardiotonic steroid with clinical and diagnostic implications. Clin Exp Hypertens. 2015;37:108–15. 10.3109/10641963.2014.913604. [DOI] [PubMed] [Google Scholar]
- 42.•.Tian J, Haller S, Periyasamy S, Brewster P, Zhang H, Adlakha S, et al. Renal ischemia regulates marinobufagenin release in humans. Hypertension. 2010;56:914–9. 10.1161/hypertensionaha.110.155564 This study indicates elevated levels of MBG in patients with renal artery stenosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.•.Kolmakova EV, Haller ST, Kennedy DJ, Isachkina AN, Budny GV, Frolova EV, et al. Endogenous cardiotonic steroids in chronic renal failure. Nephrol Dial Transplant. 2011;26:2912–9. 10.1093/ndt/gfq772 This study indicates elevated levels of MBG in patients with chronic kidney disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.•.Piecha G, Kujawa-Szewieczek A, Kuczera P, Skiba K, Sikora-Grabka E, Wiecek A. Plasma marinobufagenin immunoreactivity in patients with chronic kidney disease: a case control study. Am J Physiol Renal Physiol. 2018;315:F637–F43. 10.1152/ajprenal.00046.2018 This study indicates elevated levels of MBG in patients with chronic kidney disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, et al. Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension. 2016;68:e7–e46. 10.1161/hyp.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 46.Fedorova OV, Simbirtsev AS, Kolodkin NI, Kotov AY, Agalakova NI, Kashkin VA, et al. Monoclonal antibody to an endogenous bufadienolide, marinobufagenin, reverses preeclampsia-induced Na/K-ATPase inhibition and lowers blood pressure in NaCl-sensitive hypertension. J Hypertens. 2008;26:2414–25. 10.1097/HJH.0b013e328312c86a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.•.Schutte AE, Gona PN, Delles C, Uys AS, Burger A, Mels CM, et al. The African prospective study on the early detection and identification of cardiovascular disease and hypertension (African-PREDICT): design, recruitment and initial examination. Eur J Prev Cardiol. 2019;6:2047487318822354. 10.1177/2047487318822354 The African-PREDICT study is a unique longitudinal study tracking the early development of hypertension in black and white men and women from South-Africa. Follow up data from this study will provide valuable prognostic information on the role of MBG in the development and progression of cardiovascular disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J Am Coll Cardiol. 2010;55: 1318–27. 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 49.•.Koivistoinen T, Lyytikainen L-P, Aatola H, Luukkaala T, Juonala M, Viikari J, et al. Pulse wave velocity predicts the progression of blood pressure and development of hypertension in young adults novelty and significance. Hypertension. 2018;71:451–6. 10.1161/HYPERTENSI0NAHA.117.10368 This study highlights the predictive value of arterial stiffness for the development of hypertension, supporting the significance of a relationship observed between MBG and arterial stiffness at an early age. [DOI] [PubMed] [Google Scholar]
- 50.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–70. 10.1161/circulationaha.105.579342. [DOI] [PubMed] [Google Scholar]
- 51.Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, et al. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension. 2010;55:799–805. 10.1161/hypertensionaha.109.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–83. https://doi.org/10.1016zj.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events. Circulation. 2010;121:505–11. 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Todd AS, Macginley RJ, Schollum JB, Johnson RJ, Williams SM, Sutherland WH, et al. Dietary salt loading impairs arterial vascular reactivity. Am J Clin Nutr. 2010;91:557–64. 10.3945/ajcn.2009.28645. [DOI] [PubMed] [Google Scholar]
- 55.He FJ, Marciniak M, Visagie E, Markandu ND, Anand V, Dalton RN, et al. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and asian mild hypertensives. Hypertension. 2009;54:482–8. 10.1161/hypertensionaha.109.133223. [DOI] [PubMed] [Google Scholar]
- 56.•.Strauss M, Smith W, Kruger R, van der Westhuizen B, Schutte AE. Large artery stiffness is associated with salt intake in young healthy black but not white adults: the African-PREDICT study. Eur J Nutr. 2018;57:2649–56. 10.1007/s00394-018-1791-1 This study cross-sectional study demonstrates the relationship between salt intake and arterial stiffness in young adults who consume large amounts of salt. [DOI] [PubMed] [Google Scholar]
- 57.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–8. 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 58.Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension. 2014;64:210–4. 10.1161/hypertensionaha.114.03449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005;18(S1):3S–10S. 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 60.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990; 322 : 1561–6. 10.1056/nejm199005313222203.. [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez CJ, Bibbins-Domingo K, Jin Z, Daviglus ML, Goff DC Jr, Jacobs DR Jr. Association of sodium and potassium intake with left ventricular mass: coronary artery risk development in young adults. Hypertension. 2011;58:410–6. 10.1161/hypertensionaha.110.168054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J - Cardiovasc Imag. 2015;16: 233–71. 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 63.Bochud M, Staessen JA, Maillard M, Mazeko MJ, Kuznetsova T, Woodiwiss A, et al. Ethnic differences in proximal and distal tubular sodium reabsorption are heritable in black and white populations. J Hypertens. 2009;27:606–12. 10.1097/HJH.0b013e32832104b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Falkner B, Kushner H. Effect of chronic sodium loading on cardiovascular response in young blacks and whites. Hypertension. 1990;15:36–43. [DOI] [PubMed] [Google Scholar]
- 65.Palacios C, Wigertz K, Martin BR, Jackman L, Pratt JH, Peacock M, et al. Sodium retention in black and white female adolescents in response to salt intake. J Clin Endocrinol Metab. 2004;89:1858–63. 10.1210/jc.2003-031446. [DOI] [PubMed] [Google Scholar]
- 66.Anderson DE, Scuteri A, Agalakova N, Parsons DJ, Bagrov AY. Racial differences in resting end-tidal CO2 and circulating sodium pump inhibitor. Am J Hypertens. 2001;14:761–7. [DOI] [PubMed] [Google Scholar]
- 67.Reimann M, Hamer M, Schlaich M, Malan NT, Rudiger H, Ziemssen T, et al. Autonomic responses to stress in Black versus Caucasian Africans: the SABPA study. Psychophysiology. 2012;49:454–61. 10.1111/j.1469-8986.2011.01328.x. [DOI] [PubMed] [Google Scholar]
- 68.Abate NI, Mansour YH, Tuncel M, Arbique D, Chavoshan B, Kizilbash A, et al. Overweight and sympathetic overactivity in black Americans. Hypertension. 2001;38:379–83. [DOI] [PubMed] [Google Scholar]
- 69.Calhoun DA, Mutinga ML, Collins AS, Wyss JM, Oparil S. Normotensive blacks have heightened sympathetic response to cold pressor test. Hypertension. 1993;22:801–5. [DOI] [PubMed] [Google Scholar]
- 70.Vranish JR, Holwerda SW, Young BE, Credeur DP, Patik JC, Barbosa TC, et al. Exaggerated vasoconstriction to spontaneous bursts of muscle sympathetic nerve activity in healthy young black men. Hypertension. 2018;71:192–8. 10.1161/HYPERTENSIONAHA.117.10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He J, Gu D, Chen J, Jaquish CE, Rao DC, Hixson JE, et al. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt Study. J Hypertens. 2009;27:48–54. This study indicates increased salt-sensitivity in women, that may be important considering the sex specific relationships observed between MBG and markers of early cardiovascular risk.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.•.Murao S, Takata Y, Yasuda M, Osawa H, Kohi F. The influence of sodium and potassium intake and insulin resistance on blood pressure in normotensive individuals is more evident in women. Am J Hypertens. 2018;31:876–85. 10.1093/ajh/hpy041 This study indicates increased salt-sensitivity in women, that may be important considering the sex specific relationships observed between MBG and markers of early cardiovascular risk. [DOI] [PubMed] [Google Scholar]
- 73.•.Shukri MZ, Tan JW, Manosroi W, Pojoga LH, Rivera A, Williams JS, et al. Biological sex modulates the adrenal and blood pressure responses to angiotensin II. Hypertension. 2018;71:1083–90. 10.1161/hypertensionaha.117.11087 This study indicates increased salt-sensitivity in women, that may be important considering the sex specific relationships observed between MBG and markers of early cardiovascular risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fedorova O, Grigorova Y, Hagood M, Mcdevitt R, Long J, Mcpherson R, et al. Age-dependent hypertension and vascular remodeling in dahl-s rats are associated with elevated levels of marinobufagenin and cognitive decline. J Hypertens. 2018;36: e47 10.1097/01.hjh.0000539088.99851.d2. [DOI] [Google Scholar]
- 75.Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. Coordinated shifts in Na/K-ATPase isoforms and their endogenous ligands during cardiac hypertrophy and failure in NaCl-sensitive hypertension. J Hypertens. 2004;22:389–97. 10.1097/00004872-200402000-00025. [DOI] [PubMed] [Google Scholar]
- 76.Vu HV, Ianosi-Irimie MR, Pridjian CA, Whitbred JM, Durst JM, Bagrov AY, et al. Involvement of marinobufagenin in a rat model of human preeclampsia. Am J Nephrol. 2005;25:520–8. 10.1159/000088461. [DOI] [PubMed] [Google Scholar]
- 77.Fedorova OV, Kolodkin NI, Agalakova NI, Namikas AR, Bzhelyansky A, St-Louis J, et al. Antibody to marinobufagenin lowers blood pressure in pregnant rats on a high NaCl intake. J Hypertens. 2005;23:835–42. 10.1097/01.hjh.0000163153.27954.33. [DOI] [PubMed] [Google Scholar]
- 78.Goel A, Zhang Y, Anderson L, Rahimian R. Gender difference in rat aorta vasodilation after acute exposure to high glucose: involvement of protein kinase C β and superoxide but not of Rho Kinase. Cardiovasc Res. 2007;76:351–60. 10.1016/j.cardiores.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barron WM. Volume homeostasis during pregnancy in the rat. Am J Kidn Dis. 1987;9:296–302. 10.1016/S0272-6386(87)80125-7. [DOI] [PubMed] [Google Scholar]
- 80.Lindheimer MD, Katz AI, Nolten WE, Oparil S, Ehrlich EN. Sodium and mineralocorticoids in normal and abnormal pregnancy. Adv Nephrol Necker Hosp. 1977;7:33–59. [PubMed] [Google Scholar]
- 81.Luft FC, Gallery EDM, Lindheimer MD. Chapter 15 - Normal and abnormal volume homeostasis In: Lindheimer MD, Roberts JM, Gary Cunningham F, editors. Chesley’s hypertensive disorders in pregnancy. 3rd ed. San Diego: Academic Press; 2009. p. 269–85. [Google Scholar]
- 82.Lopatin DA, Ailamazian EK, Dmitrieva RI, Shpen VM, Fedorova OV, Doris PA, et al. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J Hypertens. 1999;17: 1179–87. [DOI] [PubMed] [Google Scholar]
- 83.Nikitina ER, Mikhailov AV, Nikandrova ES, Frolova EV, Fadeev AV, Shman VV, et al. In preeclampsia endogenous cardiotonic steroids induce vascular fibrosis and impair relaxation of umbilical arteries. J Hypertens. 2011;29:769–76. 10.1097/HJH.0b013e32834436a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fedorova OV, Tapilskaya NI, Bzhelyansky AM, Frolova EV, Nikitina ER, Reznik VA, et al. Interaction of Digibind with endogenous cardiotonic steroids from preeclamptic placentae. J Hypertens. 2010;28:361–6. 10.1097/HJH.0b013e328333226c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fedorova O, Ishkaraeva V, Grigorova Y, Reznik V, Kolodkin N, Zazerskaya I, et al. Antibody to marinobufagenin reverses placenta-induced fibrosis of umbilical arteries in preeclampsia. Int J Mol Sci. 2018;19:2377 10.3390/ijms19082377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uddin MN, Horvat D, Glaser SS, Mitchell BM, Puschett JB. Examination of the cellular mechanisms by which marinobufagenin inhibits cytotrophoblast function. J Biol Chem. 2008;283:17946–53. 10.1074/jbc.M800958200. [DOI] [PubMed] [Google Scholar]
- 87.Uddin MN, Horvat D, Glaser SS, Danchuk S, Mitchell BM, Sullivan DE, et al. Marinobufagenin inhibits proliferation and migration of cytotrophoblast and CHO cells. Placenta. 2008;29:266–73. https://doi.org/10.1016zj.placenta.2007.12.009. [DOI] [PubMed] [Google Scholar]


