Abstract
The immune system plays a critical role in white adipose tissue (WAT) energy homeostasis and, by extension, whole-body metabolism. Substantial evidence from mouse and human studies firmly establishes that insulin sensitivity deteriorates as a result of subclinical inflammation in the adipose tissue of individuals with diabetes. However, the relationship between adipose tissue expandability and immune cell infiltration remains a complex problem important for understanding the pathogenesis of obesity. Notably, a large body of work challenges the idea that all immune responses are deleterious to WAT function. This review highlights recent advances that describe how immune cells and adipocytes coordinately enable WAT expansion and regulation of energy homeostasis.
The prevalence of obesity doubled in the last 25 years (1), increasing the burden of care on medical centers and government (2). The epidemiologic associations of obesity with type 2 diabetes mellitus (T2DM) and cardiovascular disease are unequivocal, but detailed mechanisms accounting for these links are not well understood. Nonetheless, it is clear that obesity promotes chronic alterations in energy storage and utilization resulting in lipid deposition in nonadipose tissues, insulin resistance, and T2DM.
White adipose tissue (WAT) is an endocrine organ that dynamically expands and contracts to meet the metabolic demands of the organism. WAT also secretes peptides, hormones, and metabolites that contribute to insulin sensitivity in other peripheral tissues. WAT mass characterizes obesity and correlates with a strong predisposition for insulin resistance, T2DM, and cardiovascular disease. Adipocytes remain the singular cell type capable of sequestering lipids and protecting the periphery from lipotoxicity.
Subcutaneous (peripheral) and visceral (central) WAT depots broadly constitute the bulk of adipose tissues in adults. In humans, the visceral (omental) fat resembles mouse epididymal fat based on gene expression profiling, inflammation, and expandability, despite anatomical differences; subcutaneous fat depots are anatomically and functionally similar in mice and humans (3–7). Excess calorie intake evokes WAT expansion through both increased adipocyte size (hypertrophy) and number (hyperplasia). Hyperplasia has been linked to increased gene expression of transcriptional regulators essential for adipose tissue formation, such as peroxisome proliferator–activated receptor γ (PPARγ) (8). In chronic states of positive energy balance, such as consuming a Western diet, subcutaneous WAT differentiation becomes impaired (9) and visceral WAT expands (10–13), driving metabolic maladaptation.
Increased hypertrophy is a hallmark of WAT enlargement in obesity and is typically associated with metabolic alterations, proinflammatory response, and increased risk of developing T2DM independent of total fat mass (14–16). Experimental observations indicate that larger, hypertrophic fat cells behave differently than do smaller, hyperplastic adipocytes, namely in responses to lipolytic stimuli, secretory functions, and the anabolic effects of insulin. Consequently, the failure of integral lipid metabolism and insulin sensitivity effectors reflects low subcutaneous adipocyte differentiation and thus limits WAT expandability. Subcutaneous WAT safely sequesters excess energy and promotes insulin sensitivity in the face of diet-induced obesity, though the molecular mechanisms underpinning these observations remain incompletely understood.
Distinct Precursor Cells and Microenvironments Contribute to WAT Mass
WAT depots develop in a spatiotemporal manner, which likely engenders functional identity. Adipocyte progenitor cells express overlapping progenitor and mesenchymal cell surface markers, including platelet-derived growth factor receptor-α (PDGFR-α), CD29, CD34, stem cell antigen-1 (SCA1), and CD24 (17–20). Mural and smooth muscle–related cells that express Acta2, Myh11, or Pdgfrb also contribute to adipocyte formation under certain conditions (21–25). Lineage tracing studies performed in mice argue that subcutaneous and intra-abdominal depots emanate from distinct lineages (26). Visceral adipocytes descend from cells expressing the mesothelial cell marker Wilms tumor 1 (26, 27), whereas subcutaneous adipocytes can be marked with paired related homeobox 1 (PRRX1)–Cre (28, 29) and myxovirus 1 (Mx1)–Cre transgenes (6). Adding to the complexity, anatomically distinct depots may contain a network of adipocytes and other stromal precursor cells that express molecular and secretory programs to restrict or enable responses to dietary challenges (7, 30–35). Such functional cell–cell interactions likely exert stop-and-go signals for WAT development and metabolic plasticity.
Of note, murine and human adipose tissue depots perform physiological and endocrine behaviors based on distinct anatomical locations. Modern molecular and single-cell approaches have partly revealed the specific origins of subcutaneous and visceral adipocytes in mice and humans (30, 34, 36, 37). A few studies indicate that specific anatomical niches provide adipose precursors in various human tissues (38–40). Recent work discovered that DPP4+ interstitial progenitors give rise to committed populations of preadipocytes poised to undergo adipocyte differentiation (36). These cells reside in the fluid-filled collagen network of collagen and elastin fibers that surround adipose tissues and many other organs. Other efforts identified distinct populations of adipocyte progenitor cells in human WAT that vary in endocrine function and anatomical distribution by differential CD34 expression (37). These types of studies ultimately elaborate the specification of anatomical fat depots and how fat cells interpret microenvironment cues.
Obesity as an Inflammatory Disease
Obesity-induced chronic inflammation in adipose tissues contributes to the manifestation of insulin resistance and T2DM. However, the precise triggers of obesity-associated inflammation remain poorly characterized. Numerous mechanisms have been investigated in rodent models of dietary and genetic obesity. It is likely that the trigger of inflammation in adipose tissue originates from the anabolic pressure of positive energy balance. The catabolic inflammatory response alleviates anabolic pressure and supports the expansion of adipose tissues to meet the need for increased lipid storage. However, over time, the persistent stress of obesity permanently skews the reparative immune response and new thresholds for adipose tissue expansion cannot be met. This concept suggests that the insults that ultimately constrain fat cell expandability must be buffered appropriately to counter the energetic demands of dietary stress.
Many early observations in humans corroborate links between inflammation and T2DM. The initial observations noted that patients with meningitis also exhibited transient hyperglycemia (41). A large volume of studies in humans continue to underscore the importance of immune cells in T2DM [reviewed in (42)]. Although most of the direct evidence linking chronic inflammation to the comorbidities of obesity stems from rodent studies, primary human cells ex vivo support the notion that inflammation disrupts the metabolic flexibility in adipocytes (43–50).
The causal role for inflammation in the etiology of obesity and T2DM remains unclear in humans. Genome-wide association studies mostly identify loci that predict T2DM risk near genes critical for insulin secretion and processing (51, 52). The lack of T2DM risk loci that enrich in immune pathways may weaken the genetic links between obesity and inflammation. However, human genetic variability likely only partly contributes to the heritability of complex traits. Many questions remain unanswered, including how to restore the endocrine and anti-lipotoxic functions of WAT during excess nutrient intake.
WAT expandability and nutrient storage are closely linked to hypoxia. As reviewed elsewhere, abundant evidence suggests that adipocytes experience hypoxic stress that triggers local inflammatory signatures (53). Hypoxia develops as adipose tissue grows in size due to lack of tissue perfusion, mechanical stress, or increased oxygen consumption (54). The ensuing hypoxia activates hypoxia-inducible factor 1 (HIF1), which stimulates transcription of numerous inflammatory genes and chemokine release. HIF1 knockout (KO) prevents obesity-induced inflammation and insulin resistance (55), which firmly demonstrates that HIF1 mediates maladaptive responses to hypoxia in adipose tissues. Likewise, immunostaining of WAT from obese rodents and humans with obesity reveals that regions of hypoxia correlate with macrophage infiltration and fat cell necrosis (56, 57). Despite the evidence that links hypoxia and inflammation in WAT, it remains uncertain whether hypoxia is a consequence of adipose tissue expansion or a direct causative contributor to obesity-associated metabolic disease.
Hypoxia and inflammation likely foster fibrosis to restrict WAT expandability. During early WAT expansion, hypoxia induces HIF1-regulated profibrotic genes associated with increased inflammation, insulin resistance, and adipocyte cell hypertrophy (58). Notably, subcutaneous WAT fibrosis is negatively correlated with the effectiveness of weight loss following gastric bypass surgery (53). Clinical studies show a strong correlation between increased subcutaneous WAT fibrosis with insulin resistance and obesity (59–61). Conversely, inhibition of fibrosis through collagen VI or HIF1 KO reduces inflammation, glucose intolerance, and adipocyte cell size (62, 63). Hasegawa et al. (64) similarly demonstrated that repression of WAT fibrosis improves glucose homeostasis and insulin sensitivity. Fibrosis is often viewed as a mere consequence of obesity, but these studies suggest that fibrosis may be a pathogenic factor that restricts WAT expansion in obesity and degrades insulin sensitivity.
Nutrient excess and activation of the immune response coincides with the pathogenesis of obesity and its comorbidities, including fatty liver, insulin resistance, T2DM, and cardiovascular disease. Moreover, obesity-induced inflammation involves multiple metabolic and endocrine organs, including WAT, pancreas, skeletal muscle, heart, and brain. Although obesity-induced inflammation occurs in many tissues, WAT remains a primary site for complex skewing of the immune system. The low level of persistent WAT inflammation in obesity should not be confused with acute infection responses. Successful immune responses require short-lived reactions necessary for survival of the organism. In contrast, the nature of obesity-induced inflammation involves sustained low-level activation of the immune response. Macronutrient overload ultimately leads to compromised WAT function accompanied by infiltration of proinflammatory cells, including fibrogenic cells (30), neutrophils (65), M1 macrophages (66), CD8+ lymphocytes (67, 68), helper T cells (69), and adipogenesis regulatory cells (34). Supporting these observations, proinflammatory mediators secreted by immune cells and other fibroinflammatory progenitors, such as interferon γ (IFNγ) and TNFα, correlate with insulin resistance and central WAT accumulation (45, 46, 70, 71).
Macrophages comprise up to 40% of all stromal vascular cells (72) and supply inflammatory cytokines that disrupt homeostatic WAT function. Recruitment of polarized M1 macrophages defines the adipose tissue inflammation that accompanies obesity. Although the spectrum of polarization varies across adipose tissue volume (73–75), the term M1 depicts the proinflammatory state of recruited adipose tissue macrophages that express CD11c. In obesity, free fatty acids (FFAs) promote polarization of adipose tissue M1 macrophages, which secrete factors and perform functions that can block insulin action. Indeed, numerous studies demonstrate that genetic alterations that deplete the function of M1 macrophages protect against obesity-induced insulin resistance and glucose intolerance (76). Many other immune cell types, including dendritic cells, mast cells, eosinophils, and lymphocytes, also contribute to the impact of inflammation in WAT. Despite the numerous discoveries linking these cells to the metabolic profile of obesity, the tissue-dependent and pleiotropic functions of the immune system foster inherent challenges that slow development of therapies to minimize the dysfunction caused by diet-induced inflammation.
Immune Signals Mediate Adipose Tissue Expansion
WAT expansion in response to excess nutrients depends greatly on a microenvironment composed of immune cells, blood vessels, and stromal cells. In particular, crosstalk between adipocytes and immune cells involves an intricate signaling network to modulate inflammation and consequently whole-body energy homeostasis. Broadly, insulin sensitivity is preserved when WAT expansion maintains an anti-inflammatory state, primarily through the actions of M2 macrophages (77), innate lymphoid type 2 cells, and regulatory T cells (Tregs) (78). Tregs play a prominent role in WAT to promote an anti-inflammatory environment (79). In contrast, several mouse models of obesity [ob/ob, high-fat diet (HFD), Ay/a, New Zealand obese mice] exhibit dramatically reduced numbers of Tregs in epididymal WAT (eWAT) (79–81). Treg depletion in obesity correlates with infiltration of WAT by Th1 CD4+ T cells, cytotoxic CD8+ T cells, and classically activated macrophages (M1), leading to a proinflammatory milieu that is highly associated with obesity and insulin resistance (79, 81, 82). These observations highlight the complex relationship between Treg suppressive functions, cytokines, and insulin sensitivity in adipocytes.
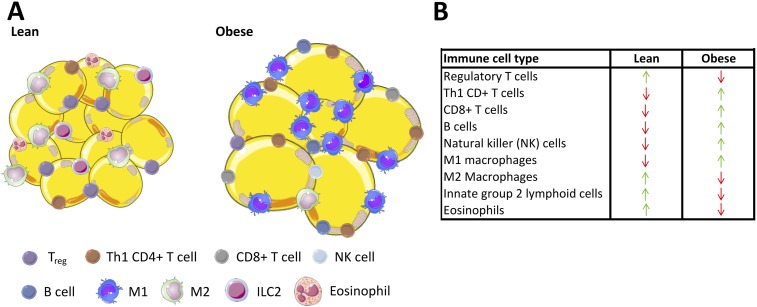
The type 1 immune phenotype in obesity derives from M1 macrophages, cytotoxic CD8 T cells, CD4 type 1 helper T cells, natural killer cells, and B cells (Fig. 1). Type 2 immunity in WAT reflects the behavior of M2-like macrophages, eosinophils, group 2 innate lymphoid cells (ILC2s), CD4 type 1 helper T cells, and Tregs. Type 1 inflammation in WAT can be counteracted by the immunosuppressive cytokine IL-10, secreted from Tregs and other type 2 immune cells. eWAT Tregs produce higher levels of IL-10 compared with Tregs from lymphoid tissues and exhibit enhanced IL-10 signaling (79). IL-10 suppresses M1 macrophage polarization and prevents macrophage recruitment by decreasing the adipocyte-derived chemokine MCP-1 (83, 84). Notably, systemic overexpression of IL-10 in mice reduces weight gain and improves insulin sensitivity with fewer eWAT macrophages (85). IL-10 also protects against TNFα-induced expression of inflammatory genes (MCP-1, IL-6, MMP-3, SAA-3, RANTES) in cultured mouse and human adipocytes (72, 79).
Figure 1.
White adipose immune cell composition in obesity. (A) WAT from lean individuals is primarily composed of Tregs, alternatively activated M2 macrophages, ILC2s, and eosinophils that suppress proinflammatory responses and support insulin-sensitive adipocytes. In contrast, adipocytes from individuals with obesity are hypertrophic and insulin resistant due, in part, to infiltrating B cells and various T cells (CD4+ Th1, CD8+, and natural killer), as well as differentiation and polarization of M1 macrophages with reductions in Tregs and ILC2s. (B) The inset table summarizes skewing of immune cells in obesity. This proinflammatory environment impinges on adipocyte insulin signaling, adipocyte hyperplasia, and function.
IL-10 and forkhead box protein p3 (Foxp3) show reduced expression in both rodent and human eWAT during obesity (79, 80, 86, 87). Decreased Foxp3 expression and Treg function can be attributed, at least in part, to proinflammatory cytokine secretion by adipocytes and infiltrating CD4+ Th1 T cells, causing an altered Treg phenotype marked by upregulation of IFNγ (79, 80, 86, 87). This shift in Treg phenotype might promote Treg fragility (88) and may underlie some loss of eWAT Tregs in obesity. IL-2 treatment of HFD-fed mice increased eWAT Tregs and IL-10 expression, although adoptive transfers of eWAT Tregs into obese mice have been limited by isolation of sufficient Tregs, among other technical challenges (79). Induction of Tregs by anti-CD3 and β-glucosylceramide administration reduced WAT inflammation and restored insulin sensitivity in ob/ob mice (89). Although these studies suggest that expansion of IL-10–producing Foxp3+ Tregs in eWAT might be an effective therapeutic strategy, further studies are needed to understand how Tregs and adipocytes communicate and mediate metabolic effects.
Eosinophils and ILC2s support Treg suppression of type 1 immunity. ILC2 secretion of IL-5 stimulates the maturation and infiltration of eosinophils, and both cell types promote M2 macrophage polarization through type 2 cytokines IL-4 and IL-13 (90–92). However, HFD-induced obesity reduces ILC2s and eosinophils in mice, whereas ILC2s are decreased in WAT of humans with obesity (90, 92–94). Activation of the ILC2–eosinophil axis induces metabolic effects related with subcutaneous WAT expandability and “browning,” which may involve immune cell secretion of peptides and other hormones (93–96).
IL-33 secretion from adipocytes and stromal cells activates ILC2s (90, 94, 95, 97, 98). Interestingly, IL-33 also acts directly on eWAT Tregs through the ST2 receptor (Il1rl1) (81). IL-33–deficient mice show reduced numbers of Tregs specifically in eWAT, indicating the significance of IL-33 to maintain eWAT Tregs. IL-33 administration in HFD and New Zealand obese mice restored eWAT Tregs with increased Foxp3 and PPARγ expression, resulting in improved glucose tolerance. One study suggested that ILC2s are not required for IL-33–induced Treg effects (99); in contrast, Brestoff et al. (93) demonstrate IL-33–elicited ILC2 induction of uncoupling protein 1 (UCP1)+ beige adipocytes in subcutaneous WAT from mice lacking adaptive immune cells. Although Tregs have not been shown to promote recruitment of UCP1+ adipocytes in subcutaneous WAT, Tregs and ILC2s interact through ICOS and ICOSL costimulatory molecules, which are inhibited by IFNγ (100). Perhaps the ILC2–eosinophil axis and Tregs act as integrated or partly redundant systems to suppress type 1 inflammation and enable WAT expandability and insulin sensitivity.
Treg-Derived Signals Promote Adipocyte Insulin Sensitivity and Metabolism
In addition to Treg-mediated suppression of proinflammatory immune cells, Tregs might also directly affect adipocyte function and metabolism. Depletion of Tregs in Foxp3–diphtheria toxin receptor mice worsens the inflammatory profile and degrades insulin action in eWAT (79). Most studies suggest that Tregs likely promote insulin signaling in adipocytes via IL-10–directed repression of inflammatory cytokine synthesis (72, 79). Seminal work by Hotamisligil and many others demonstrated that inflammatory cytokines (TNFα, IL1β, and IFNγ) interfere with insulin receptor signaling in adipocytes (43, 44, 72, 101, 102). Accordingly, TNFα and IFNγ lower Glut4 expression in adipocytes, contributing to reduced insulin-stimulated glucose uptake (72, 79). However, treatment of adipocytes with IL-10 enhances insulin-stimulated glucose uptake and antagonizes TNFα blockade of insulin receptor signaling and glucose uptake (72). These studies suggest an important role of Tregs to modulate adipocyte function through IL-10 signaling.
Several studies, however, established divergent effects of IL-10 on metabolism and insulin resistance in IL-10 KO mice (103–106). A recent study demonstrated that injection of IL-10 receptor (IL-10Ra)–targeted antisense oligonucleotides decreased body weight and fat mass in chow-fed mice and increased UCP1 expression in subcutaneous WAT (107). Accordingly, IL-10 treatment of subcutaneous WAT ex vivo suppressed UCP1 and other thermogenic genes and oxygen consumption. As a direct target of PPARγ, IL-10Ra expression likely coincides with adipogenesis and other critical insulin sensitivity genes in adipocytes (107). It is also unclear whether other type 2 cytokines (such as IL-4, IL-13) and associated signaling pathways might be altered in the absence of IL-10. Treg depletion studies in vivo and IL-10–stimulated adipocytes in vitro present (72, 79, 108) a strong argument for their role in adipocyte metabolism and insulin sensitivity; however, the adipose microenvironment is a diverse network of cells and signals that requires further study given recent findings.
Inter-Cell Crosstalk Performs Metabolic Functions in WAT
While cytokines comprise a substantial portion of intercellular communication within the WAT microenvironment, adipokines and extracellular vesicles also play a role. In particular, Tregs express receptors for leptin, adiponectin, and FFAs (109–113). The suppressive actions of leptin on Treg function and proliferation have been well described (110, 111, 114). De Rosa et al. (110) demonstrated that in vitro stimulation of activated human Tregs reduced proliferation and the ability to suppress CD4+ T effector cells, which was partially restored with addition of a leptin monoclonal antibody. In vivo, Treg proliferation and Foxp3 expression increased with leptin monoclonal antibody administration in wild-type mice, although transfer of wild-type Tregs into ob/ob leptin-deficient mice also exhibited a higher degree of proliferation. The nutrient-sensing mTOR signaling cascade mediates leptin effects in Tregs while leptin receptor (db/db)–deficient Tregs exhibited reduced mTOR signaling and higher proliferative capacity (111). In contrast, leptin stimulates proliferation of CD4+ Th1 T cells coupled with increased IFNγ expression (110, 114, 115). Thus, elevated leptin production during obesity might contribute to Treg depletion and expansion of CD4+ Th1 T cells, thereby altering the inflammatory environment in WAT.
Adiponectin production by adipocytes exerts anti-inflammatory, antidiabetic, and cardioprotective effects (116–118). Adiponectin KO mice have fewer Tregs in eWAT (119) and, although not directly tested to date, might induce IL-10 production by Tregs, as observed in macrophages (79, 120). Ramos-Ramírez et al. (112) showed that eWAT-resident Tregs expressed higher levels of adiponectin receptor 1 than did Tregs in the spleen, and its expression on adipose tissue Helios+ Tregs correlated negatively with eWAT mass. Thus, it is possible that the inverse relationship between adiponectin and leptin reflects the dynamic regulation of Tregs by adipocytes during changes in the nutritional status of lean individuals and those with obesity.
FFAs derived from WAT may also contribute to peripheral insulin sensitivity (121). For example, palmitoleate treatment of eWAT-derived adipocytes reduces proinflammatory cytokine expression (MCP-1, TNFα). Interestingly, anti-inflammatory IL-4 may also induce lipolysis, in addition to dampening of WAT inflammation and mitigating effects of diet-induced obesity (122, 123). FFAs are the preferred substrate for functional Tregs (114, 124, 125) and therefore might secrete lipolytic cytokines to increase the local concentration of FFAs for use as fuel (122). Kahn and colleagues (126) identified a new class of lipids called fatty acid esters of hydroxy fatty acids, which include palmitic acid–hydroxystearic acids (PAHSAs) abundantly found in mouse serum, WAT, brown adipose tissue, and, to a lesser extent, liver. PAHSAs correlate with insulin sensitivity in serum and subcutaneous WAT in humans. PAHSAs also regulate insulin-stimulated glucose uptake through GLUT4 translocation in adipocytes in vitro and decrease inflammatory cytokine production by macrophages in HFD-fed mice. Future studies should help illustrate how specific classes of lipids act on Treg metabolism and function, and how they might impact Tregs during obesity.
Aside from adipokines, local insulin levels are abundant in WAT. Whereas insulin acts on adipocytes to suppress lipolysis and promote energy storage, insulin stimulation of Tregs decreases IL-10 production and reduces the ability to suppress CD4+ Th1 T cells in vitro (86). In obese, hyperinsulinemic mice, elevated serum insulin was associated with decreased Foxp3+ Tregs in the eWAT, expressing lower levels of IL-10 with a switch toward increased expression of the type 1 cytokine, IFNγ (86). A recent report showed a moderate reduction in insulin production (Ins1−/−;Ins2fl/+;Pdx1-CreER mice) induced weight loss on an HFD, mainly affecting eWAT mass, without altering glucose tolerance (127). It will be interesting to consider how eWAT Tregs and other immune cell populations might vary in the setting of reduced insulin. However, anecdotal evidence suggests that Treg-specific insulin receptor deletion might not impact glucose tolerance (128). Thus, although reducing insulin appears important for adipocyte metabolism, it might not be a key signal during the obesity-related shift to a proinflammatory environment and the suppression of Treg function.
Another potential signaling mechanism between immune cells and adipocytes may occur through extracellular vesicles, such as exosomes. Local and circulating exosomes carry cargo-containing proteins, RNA, and particularly miRNAs that can affect function of acceptor cells [reviewed in detail in (129)]. Multiple studies have shown that adipocyte-derived circulating exosomes regulate gene expression and insulin sensitivity of peripheral tissues (130–133). Mainly, miRNAs within circulating exosomes from obese mice confer glucose intolerance and insulin resistance when transferred to lean mice (130, 134). Obese adipose tissue secretes twice as many exosomes as do lean mice, which induce differentiation of bone marrow–derived cells into macrophages (135). Similar findings were observed with human adipocyte exosomes, which induced differentiation of monocytes into macrophages that, in turn, could reduce adipocyte insulin signaling (136). Adipose-derived exosomes from ob/ob mice also activate circulating macrophages marked by increased serum IL-6 and TNFα levels (131). In contrast, adipose-derived stem cell exosomes from lean mice induced M2 macrophage polarization in obese mice associated with improved insulin resistance and hepatic steatosis (137). Additionally, macrophages within adipose tissue also secrete exosomes that influence glucose tolerance and insulin sensitivity. Exosomes derived from macrophages in lean mice improve insulin sensitivity, whereas macrophages collected from obese mice secrete exosomes that promote insulin resistance (134). Of note, miR-155 within macrophages targets PPARγ, which might lead to restricted WAT expansion (134).
We know less about how exosomes affect Treg function in obesity and metabolic diseases. In a mouse model of type 1 diabetes, treatment with exosomes derived from adipose tissue mesenchymal stem cells increased the percentage of splenic Tregs, along with increased type 2 cytokines IL-4, IL-10, and TGF-β (138). In support of these observations, human mesenchymal stem cell exosomes induced differentiation of Tregs with enhanced suppressive capacity (139, 140). Studies have also shown in various contexts that Tregs generate exosomes (141–144). Okoye et al. (142) demonstrated that Tregs produce miRNA-containing exosomes dependent on Dicer (required for biogenesis of most miRNAs) expression and canonical exosomal extrusion pathways. The authors subsequently showed that Treg exosomes suppress Th1 T cell proliferation and IFNγ production through let-7d. Treg-derived exosomes also skew dendritic cells toward a type 2 phenotype, marked by increased IL-10 and decreased IL-6 production, which involved transfer of miR-150-5p and miR-142-3p (144). Whether resident adipose Tregs exhibit similar exosomal suppressive activity upon type 1 immune cells during obesity will be an important question in the emerging area of the adipose tissue exosomes. Additionally, Treg exosomes might also influence adipocyte-specific inflammatory pathways, such as IFNγ and TNFα, to modulate insulin sensitivity. Meanwhile, adipocyte-derived exosomes carry lipids (135) that could influence Treg metabolism and function.
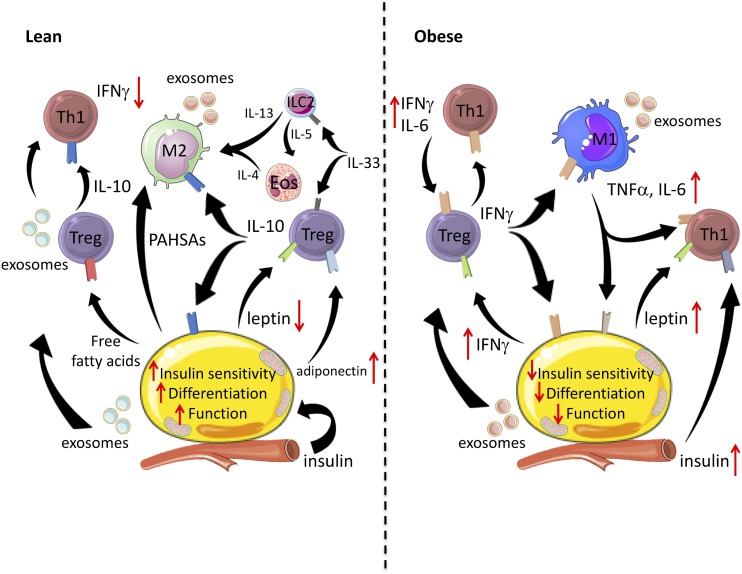
In summary, the adipose microenvironment involves complex and delicate signaling between adipocytes and immune cells. In insulin-sensitive WAT, Tregs produce IL-10 to suppress CD4+ Th1 T cell function, polarize M2 macrophages, and repress adipocyte-derived inflammatory cytokines (Fig. 2). IL-33 derived from adipocytes and stromal cells activates Tregs and ILC2s. ILC2s recruit and activate esosinophils, and in combination, they produce type 2 cytokines that polarize M2 macrophages. Adipocytes secrete adiponectin and FFAs, which also suppress macrophage cytokine production and might influence Treg function, whereas the relative absence of leptin allows Tregs to proliferate and maintain suppressive activity. Both adipocytes and Tregs produce exosomes, with adipocyte-derived exosomes influencing macrophage differentiation and function, although Treg exosomes suppress T cells in some contexts. Lastly, adipocytes secrete fatty acid esters of hydroxy fatty acids, such as PAHSAs, that regulate macrophages and peripheral insulin sensitivity. In contrast, obese insulin-resistant adipocytes secrete inflammatory cytokines (IFNγ, IL-6, RANTES, SAA) and recruit M1 macrophages (via MCP-1). Tregs, ILC2s, and eosinophils are reduced, with Tregs shifting toward IFNγ production. High levels of leptin and insulin also reduce Treg proliferation, function, and fatty acid metabolism. Accumulation of CD4+ Th1 T cells and M1 macrophages contributes to the increasing inflammatory milieu that restricts adipocyte insulin signaling, function, and expansion. Obesity-derived adipocyte or macrophage exosomes also reduce insulin sensitivity. Notably, these observations largely focused on immune cell–adipocyte signaling in eWAT given its propensity for greater immune cell infiltration in obese mice; however, some studies are beginning to uncover roles of immune cells in subcutaneous WAT and the signals that might preserve insulin sensitivity and WAT expansion (43, 145). Finally, the complexity of the adipose tissue microenvironment suggests that therapeutic intervention will likely require targeting of multiple signals and cell types to restore adipocyte expansion and insulin sensitivity in individuals with obesity.
Figure 2.
Inter-cell crosstalk regulates metabolic and inflammatory functions in WAT. In lean WAT, insulin-sensitive adipocytes expand appropriately to anabolic pressure and secrete adipokines (adiponectin), FFAs and PAHSAs, and exosomes. Tregs produce IL-10 to suppress CD4+ Th1 T cell function, polarize M2 macrophages, and repress adipocyte-derived inflammatory cytokines. IL-33 derived from adipocytes and stromal cells activates Tregs and ILC2s. ILC2s recruit and activate esosinophils and, in combination, they produce type 2 cytokines that polarize M2 macrophages. Adipocytes secrete adiponectin and FFAs, which also suppress macrophage cytokine production and might influence Treg function, whereas the relative absence of leptin allows Tregs to proliferate and maintain suppressive activity. Both adipocytes and Tregs produce exosomes, with adipocyte-derived exosomes influencing macrophage differentiation and function, whereas Treg exosomes suppress T cells in some contexts. Lastly, lean adipocytes secrete fatty acid esters of hydroxy fatty acids, such as PAHSAs, that regulate macrophages and peripheral insulin sensitivity. In summary, type 2 immune cells promote adipocyte insulin sensitivity, differentiation, and function through cytokines IL-4, IL-10, and M2 macrophage-derived exosomes. In obese adipose tissue, insulin resistance develops, and adipocyte differentiation is restricted, which contributes to ectopic lipid deposition. Adipocytes secrete inflammatory cytokines (IFNγ, TNFα, IL-6, RANTES, SAA) and recruit M1 macrophages (via MCP-1). Tregs, ILC2s, and eosinophils are reduced, with Tregs shifting toward IFNγ production. High levels of leptin and insulin also reduce Treg proliferation, function, and fatty acid metabolism. Obese adipocyte- or macrophage-derived exosomes also reduce insulin sensitivity. Collectively, accumulation of CD4+ Th1 T cells and M1 macrophages contributes to the increasing inflammatory milieu (IFNγ, TNFα, IL-6, and exosomes) that restricts adipocyte insulin sensitivity, expansion, and function.
Future Perspectives
More than 20 years of detailed mechanistic and physiological studies firmly establish that chronic inflammation in WAT depots leads to systemic glucose and lipid dysregulation. Although WAT inflammation is one conserved pathway that links obesity to insulin resistance and T2DM, numerous questions remain unanswered, including how to sustain healthy noninflamed subcutaneous WAT expansion during excess nutrient intake. For people with T2DM, we are hopeful that the immune system will be leveraged as a tool to maintain insulin sensitivity. However, targeting the immune component of obesity has proved elusive. For example, antibodies that neutralize TNF improve insulin sensitivity in obese mice, but humans showed no metabolic improvements (146). In clinical trials, anti-inflammatory salicylates improved glycemia and adipose inflammation profiles in individuals with obesity and T2D, but insulin sensitivity was unaffected (147–149). New strategies that target higher baseline inflammation in adipose tissues appear promising (150) in small clinical trials. Note that several well-established antidiabetic drugs influence anti-inflammatory endpoints. Metformin inhibits reactive oxygen species production and the production of numerous inflammatory cytokines (151, 152). Thiazolidinediones also exert anti-inflammatory effects in macrophages and adipocytes (153). In both cases, these drugs block nuclear factor κB (151, 154) and restore the M2 phenotype of macrophages (134, 155). Further exploration into how biologics targeting inflammation in rheumatoid arthritis and Crohn disease (156) may reveal therapeutic vulnerabilities for obesity. A thoughtful survey of how manipulating the immune response impacts physiology is warranted. Importantly, note that general disruption of inflammatory pathways may compromise immune responses, but also result in tissue damage, dysbiosis, and amplification of other autoimmune conditions (157).
The anatomical and functional differences of WAT depots portend distinct immune cell niches that contribute to adipocyte function and expansion during obesity. The subcutaneous WAT has a tremendous capacity to expand and store excess nutrients, as demonstrated by MitoNEET transgenic mice (158, 159). In addition to varied WAT depots, a recent study suggested that beige fat may also exist in multiple distinct forms, conventional and glycolytic beige fat (160). The immune cell composition also greatly varies, with the eWAT containing a greater abundance of immune cells, even during metabolic homeostasis, in contrast to the less infiltrated brown adipose tissue. The tissue- and depot-specific roles for immune cells is unknown, but there is clearly an extensive communication network between adipocytes and immunity through multiple signaling mechanisms (e.g., hormones, cytokines, exosomes, polyunsaturated fatty acids) within anatomical niches. The characterization of immune cells within WAT marches forward, as highlighted by the identification of multiple macrophage subtypes in WAT beyond the traditional M1/M2 subtypes (161). Ultimately, treatment of obesity and T2DM will require a multipronged approach to address adipocyte insulin sensitivity and nutrient storage while maintaining an anti-inflammatory environment. Advances in immunotherapy and gene editing tools might provide unique opportunities to skew macrophage and T cell populations toward an anti-inflammatory state. Collaborative efforts to couple cell biology with immunology and genetics will be pivotal to address the current knowledge gaps and identify new therapeutic strategies to improve insulin sensitivity and nutrient storage for patients with obesity and T2DM.
Acknowledgments
We apologize to our colleagues in the field for not being able to discuss all of the outstanding studies that detail how immune cells regulate WAT function. We thank Robb Moses for critical reading of the manuscript and useful discussions.
Financial Support: This work was supported by American Diabetes Association Grant 1-18-IBS-105 and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK114356.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- eWAT
epididymal WAT
- FFA
free fatty acid
- Foxp3
forkhead box protein p3
- HFD
high-fat diet
- HIF1
hypoxia-inducible factor 1
- ILC2
group 2 innate lymphoid cell
- IFNγ
interferon γ
- KO
knockout
- PAHSA
palmitic acid–hydroxystearic acid
- PPARγ
peroxisome proliferator–activated receptor γ
- T2DM
type 2 diabetes mellitus
- Treg
regulatory T cell
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
References and Notes
- 1. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GB, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL; GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gesta S, Blüher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA. 2006;103(17):6676–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harman-Boehm I, Blüher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Klöting N, Stumvoll M, Bashan N, Rudich A. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab. 2007;92(6):2240–2247. [DOI] [PubMed] [Google Scholar]
- 5. Kralova Lesna I, Kralova A, Cejkova S, Fronek J, Petras M, Sekerkova A, Thieme F, Janousek L, Poledne R. Characterisation and comparison of adipose tissue macrophages from human subcutaneous, visceral and perivascular adipose tissue. J Transl Med. 2016;14(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee KY, Luong Q, Sharma R, Dreyfuss JM, Ussar S, Kahn CR. Developmental and functional heterogeneity of white adipocytes within a single fat depot. EMBO J. 2019;38(3):e99291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61(7):1691–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tchoukalova Y, Koutsari C, Jensen M. Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia. 2007;50(1):151–157. [DOI] [PubMed] [Google Scholar]
- 9. van Tienen FH, van der Kallen CJ, Lindsey PJ, Wanders RJ, van Greevenbroek MM, Smeets HJ. Preadipocytes of type 2 diabetes subjects display an intrinsic gene expression profile of decreased differentiation capacity. Int J Obes. 2011;35(9):1154–1164. [DOI] [PubMed] [Google Scholar]
- 10. Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol. 2015;17(4):376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeffery E, Wing A, Holtrup B, Sebo Z, Kaplan JL, Saavedra-Peña R, Church CD, Colman L, Berry R, Rodeheffer MS. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metab. 2016;24(1):142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim SM, Lun M, Wang M, Senyo SE, Guillermier C, Patwari P, Steinhauser ML. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 2014;20(6):1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19(10):1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Acosta JR, Douagi I, Andersson DP, Bäckdahl J, Rydén M, Arner P, Laurencikiene J. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia. 2016;59(3):560–570. [DOI] [PubMed] [Google Scholar]
- 15. Veilleux A, Caron-Jobin M, Noël S, Laberge PY, Tchernof A. Visceral adipocyte hypertrophy is associated with dyslipidemia independent of body composition and fat distribution in women. Diabetes. 2011;60(5):1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43(12):1498–1506. [DOI] [PubMed] [Google Scholar]
- 17. Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15(3):302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15(4):480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135(2):240–249. [DOI] [PubMed] [Google Scholar]
- 20. Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322(5901):583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berry DC, Jiang Y, Graff JM. Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat Commun. 2016;7(1):10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang Y, Berry DC, Tang W, Graff JM. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Reports. 2014;9(3):1007–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, Castellot JJ Jr, Rosen ED, Spiegelman BM. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19(5):810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shao M, Vishvanath L, Busbuso NC, Hepler C, Shan B, Sharma AX, Chen S, Yu X, An YA, Zhu Y, Holland WL, Gupta RK. De novo adipocyte differentiation from Pdgfrß+ preadipocytes protects against pathologic visceral adipose expansion in obesity. Nat Commun. 2018;9(1):890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vishvanath L, MacPherson KA, Hepler C, Wang QA, Shao M, Spurgin SB, Wang MY, Kusminski CM, Morley TS, Gupta RK. Pdgfrβ+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab. 2016;23(2):350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chau YY, Bandiera R, Serrels A, Martínez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, Stimson RH, Walker BR, Chapuli RM, Schedl A, Hastie N. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16(4):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, Wu J, Gunawardana SC, Banks AS, Camporez JP, Jurczak MJ, Kajimura S, Piston DW, Mathis D, Cinti S, Shulman GI, Seale P, Spiegelman BM. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156(1–2):304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krueger KC, Costa MJ, Du H, Feldman BJ. Characterization of Cre recombinase activity for in vivo targeting of adipocyte precursor cells. Stem Cell Reports. 2014;3(6):1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanchez-Gurmaches J, Hsiao WY, Guertin DA. Highly selective in vivo labeling of subcutaneous white adipocyte precursors with Prx1-Cre. Stem Cell Reports. 2015;4(4):541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hepler C, Shan B, Zhang Q, Henry GH, Shao M, Vishvanath L, Ghaben AL, Mobley AB, Strand D, Hon GC, Gupta RK. Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. eLife. 2018;7:e39636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee KY, Russell SJ, Ussar S, Boucher J, Vernochet C, Mori MA, Smyth G, Rourk M, Cederquist C, Rosen ED, Kahn BB, Kahn CR. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes. 2013;62(3):864–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morgan-Bathke M, Harteneck D, Jaeger P, Sondergaard E, Karwoski R, Espinosa De Ycaza A, Carranza-Leon BG, Faubion WA Jr, Oliveira AM, Jensen MD. Comparison of methods for analyzing human adipose tissue macrophage content. Obesity (Silver Spring). 2017;25(12):2100–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5(1):4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwalie PC, Dong H, Zachara M, Russeil J, Alpern D, Akchiche N, Caprara C, Sun W, Schlaudraff KU, Soldati G, Wolfrum C, Deplancke B. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature. 2018;559(7712):103–108. [DOI] [PubMed] [Google Scholar]
- 35. Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Merrick D, Sakers A, Irgebay Z, Okada C, Calvert C, Morley MP, Percec I, Seale P. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science. 2019;364(6438):eaav2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Raajendiran A, Ooi G, Bayliss J, O’Brien PE, Schittenhelm RB, Clark AK, Taylor RA, Rodeheffer MS, Burton PR, Watt MJ. Identification of metabolically distinct adipocyte progenitor cells in human adipose tissues. Cell Rep. 2019;27(5):1528–1540.e7. [DOI] [PubMed] [Google Scholar]
- 38. Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. [DOI] [PubMed] [Google Scholar]
- 39. Yuan SM, Guo Y, Zhou XJ, Shen WM, Chen HN. PDGFR-β (+) perivascular cells from infantile hemangioma display the features of mesenchymal stem cells and show stronger adipogenic potential in vitro and in vivo. Int J Clin Exp Pathol. 2014;7(6):2861–2870. [PMC free article] [PubMed] [Google Scholar]
- 40. Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Péault B, Rubin JP, Donnenberg AD. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77(1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fox MJ, Kuzma JF, Washam WT. Transitory diabetic syndrome associated with meningococcic meningitis. Arch Intern Med (Chic). 1947;79(6):614–621. [DOI] [PubMed] [Google Scholar]
- 42. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–185. [DOI] [PubMed] [Google Scholar]
- 43. Koh EH, Chernis N, Saha PK, Xiao L, Bader DA, Zhu B, Rajapakshe K, Hamilton MP, Liu X, Perera D, Chen X, York B, Trauner M, Coarfa C, Bajaj M, Moore DD, Deng T, McGuire SE, Hartig SM. miR-30a remodels subcutaneous adipose tissue inflammation to improve insulin sensitivity in obesity. Diabetes. 2018;67(12):2541–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McGillicuddy FC, Chiquoine EH, Hinkle CC, Kim RJ, Shah R, Roche HM, Smyth EM, Reilly MP. Interferon γ attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. J Biol Chem. 2009;284(46):31936–31944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95(5):2409–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Blüher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28(7):1304–1310. [DOI] [PubMed] [Google Scholar]
- 47. Laurencikiene J, van Harmelen V, Arvidsson Nordström E, Dicker A, Blomqvist L, Näslund E, Langin D, Arner P, Rydén M. NF-κB is important for TNF-α-induced lipolysis in human adipocytes. J Lipid Res. 2007;48(5):1069–1077. [DOI] [PubMed] [Google Scholar]
- 48. Creely SJ, McTernan PG, Kusminski CM, Fisher M, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292(3):E740–E747. [DOI] [PubMed] [Google Scholar]
- 49. Xu H, Uysal KT, Becherer JD, Arner P, Hotamisligil GS. Altered tumor necrosis factor-alpha (TNF-α) processing in adipocytes and increased expression of transmembrane TNF-α in obesity. Diabetes. 2002;51(6):1876–1883. [DOI] [PubMed] [Google Scholar]
- 50. Liu LS, Spelleken M, Röhrig K, Hauner H, Eckel J. Tumor necrosis factor-alpha acutely inhibits insulin signaling in human adipocytes: implication of the p80 tumor necrosis factor receptor. Diabetes. 1998;47(4):515–522. [DOI] [PubMed] [Google Scholar]
- 51. Dimas AS, Lagou V, Barker A, Knowles JW, Mägi R, Hivert MF, Benazzo A, Rybin D, Jackson AU, Stringham HM, Song C, Fischer-Rosinsky A, Boesgaard TW, Grarup N, Abbasi FA, Assimes TL, Hao K, Yang X, Lecoeur C, Barroso I, Bonnycastle LL, Böttcher Y, Bumpstead S, Chines PS, Erdos MR, Graessler J, Kovacs P, Morken MA, Narisu N, Payne F, Stancakova A, Swift AJ, Tönjes A, Bornstein SR, Cauchi S, Froguel P, Meyre D, Schwarz PE, Häring HU, Smith U, Boehnke M, Bergman RN, Collins FS, Mohlke KL, Tuomilehto J, Quertemous T, Lind L, Hansen T, Pedersen O, Walker M, Pfeiffer AF, Spranger J, Stumvoll M, Meigs JB, Wareham NJ, Kuusisto J, Laakso M, Langenberg C, Dupuis J, Watanabe RM, Florez JC, Ingelsson E, McCarthy MI, Prokopenko I; MAGIC Investigators. Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes. 2014;63(6):2158–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Strawbridge RJ, Dupuis J, Prokopenko I, Barker A, Ahlqvist E, Rybin D, Petrie JR, Travers ME, Bouatia-Naji N, Dimas AS, Nica A, Wheeler E, Chen H, Voight BF, Taneera J, Kanoni S, Peden JF, Turrini F, Gustafsson S, Zabena C, Almgren P, Barker DJ, Barnes D, Dennison EM, Eriksson JG, Eriksson P, Eury E, Folkersen L, Fox CS, Frayling TM, Goel A, Gu HF, Horikoshi M, Isomaa B, Jackson AU, Jameson KA, Kajantie E, Kerr-Conte J, Kuulasmaa T, Kuusisto J, Loos RJ, Luan J, Makrilakis K, Manning AK, Martínez-Larrad MT, Narisu N, Nastase Mannila M, Ohrvik J, Osmond C, Pascoe L, Payne F, Sayer AA, Sennblad B, Silveira A, Stancáková A, Stirrups K, Swift AJ, Syvänen AC, Tuomi T, van ’t Hooft FM, Walker M, Weedon MN, Xie W, Zethelius B, Ongen H, Mälarstig A, Hopewell JC, Saleheen D, Chambers J, Parish S, Danesh J, Kooner J, Ostenson CG, Lind L, Cooper CC, Serrano-Ríos M, Ferrannini E, Forsen TJ, Clarke R, Franzosi MG, Seedorf U, Watkins H, Froguel P, Johnson P, Deloukas P, Collins FS, Laakso M, Dermitzakis ET, Boehnke M, McCarthy MI, Wareham NJ, Groop L, Pattou F, Gloyn AL, Dedoussis GV, Lyssenko V, Meigs JB, Barroso I, Watanabe RM, Ingelsson E, Langenberg C, Hamsten A, Florez JC; DIAGRAM Consortium; GIANT Consortium; MuTHER Consortium; CARDIoGRAM Consortium; C4D Consortium. Genome-wide association identifies nine common variants associated with fasting proinsulin levels and provides new insights into the pathophysiology of type 2 diabetes. Diabetes. 2011;60(10):2624–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18(4):470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee YS, Kim JW, Osborne O, Oh DY, Sasik R, Schenk S, Chen A, Chung H, Murphy A, Watkins SM, Quehenberger O, Johnson RS, Olefsky JM. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell. 2014;157(6):1339–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM, Gonzalez FJ. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes. 2011;60(10):2484–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumié A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clément K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–2286. [DOI] [PubMed] [Google Scholar]
- 57. Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes. 2008;32(3):451–463. [DOI] [PubMed] [Google Scholar]
- 58. Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29(16):4467–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Henegar C, Tordjman J, Achard V, Lacasa D, Cremer I, Guerre-Millo M, Poitou C, Basdevant A, Stich V, Viguerie N, Langin D, Bedossa P, Zucker JD, Clement K. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 2008;9(1):R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lackey DE, Burk DH, Ali MR, Mostaedi R, Smith WH, Park J, Scherer PE, Seay SA, McCoin CS, Bonaldo P, Adams SH. Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity. Am J Physiol Endocrinol Metab. 2014;306(3):E233–E246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reggio S, Rouault C, Poitou C, Bichet J-C, Prifti E, Bouillot J-L, Rizkalla S, Lacasa D, Tordjman J, Clément K. Increased basement membrane components in adipose tissue during obesity: links with TGFβ and metabolic phenotypes. J Clin Endocrinol Metab. 2016;101(6):2578–2587. [DOI] [PubMed] [Google Scholar]
- 62. Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29(6):1575–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sun K, Halberg N, Khan M, Magalang UJ, Scherer PE. Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol Cell Biol. 2013;33(5):904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hasegawa Y, Ikeda K, Chen Y, Alba DL, Stifler D, Shinoda K, Hosono T, Maretich P, Yang Y, Ishigaki Y, Chi J, Cohen P, Koliwad SK, Kajimura S. Repression of adipose tissue fibrosis through a PRDM16-GTF2IRD1 complex improves systemic glucose homeostasis. Cell Metab. 2018;27(1):180–194.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ, Murphy AJ. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19(5):821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Anderson EK, Gutierrez DA, Kennedy A, Hasty AH. Weight cycling increases T-cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes. 2013;62(9):3180–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–920. [DOI] [PubMed] [Google Scholar]
- 69. Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S, Blin-Wakkach C, Anty R, Iannelli A, Gugenheim J, Tran A, Bouloumié A, Gual P, Wakkach A. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61(9):2238–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dahlman I, Forsgren M, Sjögren A, Nordström EA, Kaaman M, Näslund E, Attersand A, Arner P. Downregulation of electron transport chain genes in visceral adipose tissue in type 2 diabetes independent of obesity and possibly involving tumor necrosis factor-α. Diabetes. 2006;55(6):1792–1799. [DOI] [PubMed] [Google Scholar]
- 71. Schmidt FM, Weschenfelder J, Sander C, Minkwitz J, Thormann J, Chittka T, Mergl R, Kirkby KC, Faßhauer M, Stumvoll M, Holdt LM, Teupser D, Hegerl U, Himmerich H. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS One. 2015;10(3):e0121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, Landerholm RW, Crouthamel M, Gozal D, Hwang S, Singh PK, Becker L. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20(4):614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57(12):3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282(48):35279–35292. [DOI] [PubMed] [Google Scholar]
- 76. Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12(1):15–28. [DOI] [PubMed] [Google Scholar]
- 77. McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41(1):36–48. [DOI] [PubMed] [Google Scholar]
- 78. Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. 2013;14(10):1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cipolletta D, Cohen P, Spiegelman BM, Benoist C, Mathis D. Appearance and disappearance of the mRNA signature characteristic of Treg cells in visceral adipose tissue: age, diet, and PPARγ effects. Proc Natl Acad Sci USA. 2015;112(2):482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, Fagarasan S, Mielke LA, Afshar-Sterle S, Masters SL, Nakae S, Saito H, Wentworth JM, Li P, Liao W, Leonard WJ, Smyth GK, Shi W, Nutt SL, Koyasu S, Kallies A. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells [published correction appears in Nat Immunol. 2015;16(5):544]. Nat Immunol. 2015;16(3):276–285. [DOI] [PubMed] [Google Scholar]
- 82. Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15(8):921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Park-Min KH, Antoniv TT, Ivashkiv LB. Regulation of macrophage phenotype by long-term exposure to IL-10. Immunobiology. 2005;210(2–4):77–86. [DOI] [PubMed] [Google Scholar]
- 85. Gao M, Zhang C, Ma Y, Bu L, Yan L, Liu D. Hydrodynamic delivery of mIL10 gene protects mice from high-fat diet-induced obesity and glucose intolerance. Mol Ther. 2013;21(10):1852–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Han JM, Patterson SJ, Speck M, Ehses JA, Levings MK. Insulin inhibits IL-10–mediated regulatory T cell function: implications for obesity. J Immunol. 2014;192(2):623–629. [DOI] [PubMed] [Google Scholar]
- 87. Pettersson US, Waldén TB, Carlsson P-O, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One. 2012;7(9):e46057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, Horne W, Moskovitz JM, Kolls JK, Sander C, Shuai Y, Normolle DP, Kirkwood JM, Ferris RL, Delgoffe GM, Bruno TC, Workman CJ, Vignali DAA. Interferon-γ drives Treg fragility to promote anti-tumor immunity. Cell. 2017;169(6):1130–1141.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, Wu HY, Weiner HL. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci USA. 2010;107(21):9765–9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210(3):535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang H-E, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502(7470):245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wu D, Molofsky AB, Liang H-E, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332(6026):243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, Artis D. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519(7542):242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ding X, Luo Y, Zhang X, Zheng H, Yang X, Yang X, Liu M. IL-33-driven ILC2/eosinophil axis in fat is induced by sympathetic tone and suppressed by obesity. J Endocrinol. 2016;231(1):35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160(1–2):74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157(6):1292–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wood IS, Wang B, Trayhurn P. IL-33, a recently identified interleukin-1 gene family member, is expressed in human adipocytes. Biochem Biophys Res Commun. 2009;384(1):105–109. [DOI] [PubMed] [Google Scholar]
- 98. Zeyda M, Wernly B, Demyanets S, Kaun C, Hämmerle M, Hantusch B, Schranz M, Neuhofer A, Itariu BK, Keck M, Prager G, Wojta J, Stulnig TM. Severe obesity increases adipose tissue expression of interleukin-33 and its receptor ST2, both predominantly detectable in endothelial cells of human adipose tissue. Int J Obes. 2013;37(5):658–665. [DOI] [PubMed] [Google Scholar]
- 99. Zeng Q, Sun X, Xiao L, Xie Z, Bettini M, Deng T. A unique population: Adipose-resident regulatory T cells. Front Immunol. 2018;9:2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Molofsky AB, Van Gool F, Liang H-E, Van Dyken SJ, Nussbaum JC, Lee J, Bluestone JA, Locksley RM. Interleukin-33 and interferon-γ counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity. 2015;43(1):161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature. 1997;389(6651):610–614. [DOI] [PubMed] [Google Scholar]
- 102. Xu H, Hirosumi J, Uysal KT, Guler AD, Hotamisligil GS. Exclusive action of transmembrane TNFα in adipose tissue leads to reduced adipose mass and local but not systemic insulin resistance. Endocrinology. 2002;143(4):1502–1511. [DOI] [PubMed] [Google Scholar]
- 103. Clementi AH, Gaudy AM, van Rooijen N, Pierce RH, Mooney RA. Loss of Kupffer cells in diet-induced obesity is associated with increased hepatic steatosis, STAT3 signaling, and further decreases in insulin signaling. Biochim Biophys Acta. 2009;1792(11):1062–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. den Boer MA, Voshol PJ, Schröder-van der Elst JP, Korsheninnikova E, Ouwens DM, Kuipers F, Havekes LM, Romijn JA. Endogenous interleukin-10 protects against hepatic steatosis but does not improve insulin sensitivity during high-fat feeding in mice. Endocrinology. 2006;147(10):4553–4558. [DOI] [PubMed] [Google Scholar]
- 105. Faulkner JL, Gomolak JR, Didion SP. Interleukin-10 deficiency limits the development of obesity and insulin resistance produced by a high fat diet. FASEB J. 2013;27(Suppl 1):1183.6. [Google Scholar]
- 106. Miller AM, Wang H, Bertola A, Park O, Horiguchi N, Ki SH, Yin S, Lafdil F, Gao B. Inflammation-associated interleukin-6/signal transducer and activator of transcription 3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in interleukin-10–deficient mice. Hepatology. 2011;54(3):846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rajbhandari P, Thomas BJ, Feng AC, Hong C, Wang J, Vergnes L, Sallam T, Wang B, Sandhu J, Seldin MM, Lusis AJ, Fong LG, Katz M, Lee R, Young SG, Reue K, Smale ST, Tontonoz P. IL-10 signaling remodels adipose chromatin architecture to limit thermogenesis and energy expenditure. Cell. 2018;172(1–2):218–233.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kälin S, Becker M, Ott VB, Serr I, Hosp F, Mollah MM, Keipert S, Lamp D, Rohner-Jeanrenaud F, Flynn VK, Scherm MG, Nascimento LF, Gerlach K, Popp V, Dietzen S, Bopp T, Krishnamurthy P, Kaplan MH, Serrano M, Woods SC, Tripal P, Palmisano R, Jastroch M, Blüher M, Wolfrum C, Weigmann B, Ziegler AG, Mann M, Tschöp MH, Daniel C. A Stat6/Pten axis links regulatory T cells with adipose tissue function. Cell Metab. 2017;26(3):475–492.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486(7404):549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. De Rosa V, Procaccini C, Calì G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26(2):241–255. [DOI] [PubMed] [Google Scholar]
- 111. Procaccini C, De Rosa V, Galgani M, Abanni L, Calì G, Porcellini A, Carbone F, Fontana S, Horvath TL, La Cava A, Matarese G. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33(6):929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ramos-Ramírez P, Malmhäll C, Johansson K, Lötvall J, Bossios A. Weight gain alters adiponectin receptor 1 expression on adipose tissue-resident Helios+ regulatory T cells. Scand J Immunol. 2016;83(4):244–254. [DOI] [PubMed] [Google Scholar]
- 113. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gerriets VA, Danzaki K, Kishton RJ, Eisner W, Nichols AG, Saucillo DC, Shinohara ML, MacIver NJ. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur J Immunol. 2016;46(8):1970–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394(6696):897–901. [DOI] [PubMed] [Google Scholar]
- 116. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83. [DOI] [PubMed] [Google Scholar]
- 117. Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8(7):731–737. [DOI] [PubMed] [Google Scholar]
- 118. Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, Summer R, Walsh K. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285(9):6153–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Onodera T, Fukuhara A, Jang MH, Shin J, Aoi K, Kikuta J, Otsuki M, Ishii M, Shimomura I. Adipose tissue macrophages induce PPARγ-high FOXP3+ regulatory T cells. Sci Rep. 2015;5(1):16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323(2):630–635. [DOI] [PubMed] [Google Scholar]
- 121. Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134(6):933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. DiSpirito JR, Mathis D. Immunological contributions to adipose tissue homeostasis. Semin Immunol. 2015;27(5):315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tsao CH, Shiau MY, Chuang PH, Chang YH, Hwang J. Interleukin-4 regulates lipid metabolism by inhibiting adipogenesis and promoting lipolysis. J Lipid Res. 2014;55(3):385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, Winter PS, Liu X, Priyadharshini B, Slawinska ME, Haeberli L, Huck C, Turka LA, Wood KC, Hale LP, Smith PA, Schneider MA, MacIver NJ, Locasale JW, Newgard CB, Shinohara ML, Rathmell JC. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest. 2015;125(1):194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186(6):3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yore MM, Syed I, Moraes-Vieira PM, Zhang T, Herman MA, Homan EA, Patel RT, Lee J, Chen S, Peroni OD, Dhaneshwar AS, Hammarstedt A, Smith U, McGraw TE, Saghatelian A, Kahn BB. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. 2014;159(2):318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Page MM, Skovsø S, Cen H, Chiu AP, Dionne DA, Hutchinson DF, Lim GE, Szabat M, Flibotte S, Sinha S, Nislow C, Rodrigues B, Johnson JD. Reducing insulin via conditional partial gene ablation in adults reverses diet-induced weight gain. FASEB J. 2018;32(3):1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bettini M, personal communication.
- 129. Huang-Doran I, Zhang CY, Vidal-Puig A. Extracellular vesicles: novel mediators of cell communication in metabolic disease. Trends Endocrinol Metab. 2017;28(1):3–18. [DOI] [PubMed] [Google Scholar]
- 130. Castaño C, Kalko S, Novials A, Párrizas M. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci USA. 2018;115(48):12158–12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Deng ZB, Poliakov A, Hardy RW, Clements R, Liu C, Liu Y, Wang J, Xiang X, Zhang S, Zhuang X, Shah SV, Sun D, Michalek S, Grizzle WE, Garvey T, Mobley J, Zhang HG. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58(11):2498–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hubal MJ, Nadler EP, Ferrante SC, Barberio MD, Suh J-H, Wang J, Dohm GL, Pories WJ, Mietus-Snyder M, Freishtat RJ. Circulating adipocyte-derived exosomal microRNAs associated with decreased insulin resistance after gastric bypass. Obesity (Silver Spring). 2017;25(1):102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, Gorden P, Kahn CR. Adipose-derived circulating miRNAs regulate gene expression in other tissues [published correction appears in Nature. 2017;545(7653):252]. Nature. 2017;542(7642):450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, Li P, Olefsky JM. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171(2):372–384.e12. [DOI] [PubMed] [Google Scholar]
- 135. Flaherty SE III, Grijalva A, Xu X, Ables E, Nomani A, Ferrante AW Jr. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science. 2019;363(6430):989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kranendonk ME, Visseren FL, van Balkom BW, Nolte-’t Hoen EN, van Herwaarden JA, de Jager W, Schipper HS, Brenkman AB, Verhaar MC, Wauben MH, Kalkhoven E. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring). 2014;22(5):1296–1308. [DOI] [PubMed] [Google Scholar]
- 137. Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, Zhang Q, Guo C, Zhang L, Wang Q. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018;67(2):235–247. [DOI] [PubMed] [Google Scholar]
- 138. Nojehdehi S, Soudi S, Hesampour A, Rasouli S, Soleimani M, Hashemi SM. Immunomodulatory effects of mesenchymal stem cell–derived exosomes on experimental type-1 autoimmune diabetes. J Cell Biochem. 2018;119(11):9433–9443. [DOI] [PubMed] [Google Scholar]
- 139. Du YM, Zhuansun YX, Chen R, Lin L, Lin Y, Li JG. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp Cell Res. 2018;363(1):114–120. [DOI] [PubMed] [Google Scholar]
- 140. Zhang Q, Fu L, Liang Y, Guo Z, Wang L, Ma C, Wang H. Exosomes originating from MSCs stimulated with TGF-β and IFN-γ promote Treg differentiation. J Cell Physiol. 2018;233(9):6832–6840. [DOI] [PubMed] [Google Scholar]
- 141. Azimi M, Ghabaee M, Moghadasi AN, Noorbakhsh F, Izad M. Immunomodulatory function of Treg-derived exosomes is impaired in patients with relapsing-remitting multiple sclerosis. Immunol Res. 2018;66(4):513–520. [DOI] [PubMed] [Google Scholar]
- 142. Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, Seabra MC, Wilson MS. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41(1):89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Smyth LA, Ratnasothy K, Tsang JYS, Boardman D, Warley A, Lechler R, Lombardi G. CD73 expression on extracellular vesicles derived from CD4+CD25+Foxp3+ T cells contributes to their regulatory function. Eur J Immunol. 2013;43(9):2430–2440. [DOI] [PubMed] [Google Scholar]
- 144. Tung SL, Boardman DA, Sen M, Letizia M, Peng Q, Cianci N, Dioni L, Carlin LM, Lechler R, Bollati V, Lombardi G, Smyth LA. Regulatory T cell-derived extracellular vesicles modify dendritic cell function. Sci Rep. 2018;8(1):6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Chung KJ, Chatzigeorgiou A, Economopoulou M, Garcia-Martin R, Alexaki VI, Mitroulis I, Nati M, Gebler J, Ziemssen T, Goelz SE, Phieler J, Lim JH, Karalis KP, Papayannopoulou T, Blüher M, Hajishengallis G, Chavakis T. A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity. Nat Immunol. 2017;18(6):654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Moller DE. Potential role of TNF-α in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11(6):212–217. [DOI] [PubMed] [Google Scholar]
- 147. Goldfine AB, Conlin PR, Halperin F, Koska J, Permana P, Schwenke D, Shoelson SE, Reaven PD. A randomised trial of salsalate for insulin resistance and cardiovascular risk factors in persons with abnormal glucose tolerance. Diabetologia. 2013;56(4):714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE; TINSAL-T2D (Targeting Inflammation Using Salsalate in Type 2 Diabetes) Study Team. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152(6):346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Salastekar N, Desai T, Hauser T, Schaefer EJ, Fowler K, Joseph S, Shoelson SE, Goldfine AB; TINSAL-CVD study team. Salsalate improves glycaemia in overweight persons with diabetes risk factors of stable statin-treated cardiovascular disease: a 30-month randomized placebo-controlled trial. Diabetes Obes Metab. 2017;19(10):1458–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Oral EA, Reilly SM, Gomez AV, Meral R, Butz L, Ajluni N, Chenevert TL, Korytnaya E, Neidert AH, Hench R, Rus D, Horowitz JF, Poirier B, Zhao P, Lehmann K, Jain M, Yu R, Liddle C, Ahmadian M, Downes M, Evans RM, Saltiel AR. Inhibition of IKKε and TBK1 improves glucose control in a subset of patients with type 2 diabetes. Cell Metab. 2017;26(1):157–170.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, Schönbeck U, Libby P. Metformin inhibits proinflammatory responses and nuclear factor-κB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26(3):611–617. [DOI] [PubMed] [Google Scholar]
- 152. Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, Glasauer A, Dufour E, Mutlu GM, Budigner GS, Chandel NS. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife. 2014;3:e02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20(4):573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Peraldi P, Xu M, Spiegelman BM. Thiazolidinediones block tumor necrosis factor-alpha-induced inhibition of insulin signaling. J Clin Invest. 1997;100(7):1863–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Prieur X, Mok CY, Velagapudi VR, Núñez V, Fuentes L, Montaner D, Ishikawa K, Camacho A, Barbarroja N, O’Rahilly S, Sethi JK, Dopazo J, Orešič M, Ricote M, Vidal-Puig A. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60(3):797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13(6):465–476. [DOI] [PubMed] [Google Scholar]
- 157. Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, Gettinger S, Sznol M, Young A, Rushakoff R, Lee J, Bluestone JA, Anderson M, Herold KC. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. 2018;67(8):1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, Askew GR, Simcox JA, McClain DA, Li C, Scherer PE. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012;18(10):1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kusminski CM, Park J, Scherer PE. MitoNEET-mediated effects on browning of white adipose tissue. Nat Commun. 2014;5(1):3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Chen Y, Ikeda K, Yoneshiro T, Scaramozza A, Tajima K, Wang Q, Kim K, Shinoda K, Sponton CH, Brown Z, Brack A, Kajimura S. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature. 2019;565(7738):180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155(4):407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]