Abstract
IgG and albumin are the most abundant proteins in the circulation and have the longest half-lives. These properties are due to a unique receptor, the neonatal Fc receptor (FcRn). Although FcRn is named for its function of transferring IgG across the placenta from maternal to fetal circulation, FcRn functions throughout life to maintain IgG and albumin concentrations. FcRn protects IgG and albumin from intracellular degradation and recycles them back into the circulation. Clinical trials have confirmed that pathogenic antibodies can be depleted by blocking this homeostatic function of FcRn. Moreover, understanding the molecular interactions between IgG and FcRn has resulted in the design of therapeutic monoclonal antibodies with more efficacious pharmacokinetics. As a result of genetic engineering these monoclonals can be delivered at lower doses and at longer intervals. More recent findings have demonstrated that FcRn enhances phagocytosis by neutrophils, immune complex clearance by podocytes and antigen presentation by dendritic cells, macrophages and B cells. This minireview highlights the relevance of FcRn to transplantation.
Summary
The receptor Brambell correctly theorized to control catabolism of IgG as well as transport IgG across placental and mucosal barriers is now known to perform multiple other functions. More refined models of FcRn function have allowed the rational design of therapeutic biologics with longer or shorter half-lives. Various strategies to block the salvage of IgG by FcRn are in clinical trials for treatment of autoimmune diseases and could be applied to deplete alloantibodies in transplant recipients. Further research is needed to define the function of FcRn in phagocytosis of IgG opsonized pathogens, clearance of immune complexes by podocytes and presentation of antigen complexed to IgG.
Introduction
IgG has a long half-life compared to other immunoglobulin classes. Normally, IgG1, 2 and 4 subclasses have half-lives over 3 weeks in the circulation and interstitial fluids of humans. By comparison, IgA and IgM have half-lives of 5–7 days. The long half-life of IgG is dependent on a unique Fc receptor now known as Fc receptor neonate (FcRn). Among many functions, FcRn protects IgG from intracellular degradation and recycles it to the circulation. In the absence of FcRn catabolism of IgG is increased and the serum concentration of IgG decreases to levels similar to IgM or IgA (1). Understanding the functions of FcRn has manifold implications to transplantation. Translational applications include strategies of depleting pathological antibodies and the rational design of Fc-containing biologics with longer or shorter half-lives. More basic mechanistic considerations include the impact of FcRn on phagocytosis by neutrophils, immune complex clearance by podocytes and antigen presentation by dendritic cells, macrophages and B cells.
Discovery and Characterization of FcRn
The discovery of FcRn is noteworthy because 20 years before the receptor was isolated Rogers Brambell and his colleagues hypothesized the existence of an Fc receptor (FcR) dependent salvage pathway for IgG (Fig. 1). Brambell had already established that an FcR was responsible for transporting IgG across the placenta of rabbits and intestinal epithelium in rodents (2). First, he demonstrated that the transport of IgG across these tissues was saturable by excess quantities of IgG. Then, with the use of papain that had been newly reported in 1959 by Rodney Porter as a method to dissect the immunoglobulin structure (3), Brambell discovered the receptor bound the Fc domain of IgG. With these insights, he analyzed data published by John Fahey that related the concentration of IgG in the circulation to its half-life. Based on this analysis, Brambell, Hemmings and Morris proposed a model in which IgG molecules became “attached to specific receptors on, or in, the cells and are ultimately returned to the circulation, while the remainder are degraded” (4). For 20 years, this hypothetical receptor was known as the Brambell receptor or FcRB. Then in 1984, the transport receptor for IgG was isolated from rat intestinal epithelium (5), and in 1989, the transport receptor was cloned and the structure categorized in the family of MHC class I molecules (6). Unlike other FcR structures, FcRn is a heterodimer of β2-microglobulin and a heavy chain homologous to MHC class I. In 1996, two groups of investigators demonstrated that IgG catabolism was increased in β2-microglobulin deficient mice and concluded that the MHC class I related Fc receptor was responsible for protecting IgG (7, 8). Since then, mutations in β2-microglobulin have been found in humans that result in decreased IgG levels (1).
Figure 1.
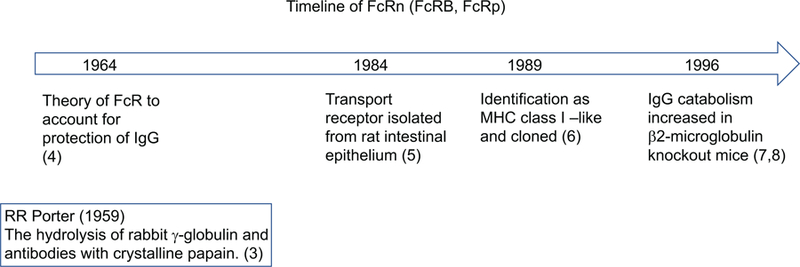
Historical time line from the hypothesis of an FcRn to the isolation and characterization of FcRn.
Although studies with early β2-microglobulin deficient mice and humans with mutations in β2-microglobulin confirmed that FcRn was essential for the long half-life of IgG, this deficiency altered many additional immunological parameters related to MHC class I. The production of fcgrt−/− mice with a specific deficiency in FcRn heavy chain provided more precise insights. Experiments on bone marrow chimeras between fcgrt−/− and normal mice established that both hematopietic and parenchymal cells contributed to the long half-life of IgG (9). More detailed studies on mice with the FcRn deficiency limited to endothelial cells demonstrated that vascular endothelium was responsible for about half of the IgG salvage (10). Conversely, overexpression of FcRn was found to prolong the half-life of IgG in mice. This latter construct has been exploited to produce higher titers of therapeutic antibodies in animals such as rabbit anti-thymocyte globulin (11).
Function of FcRn
FcRn differs from other Fc receptors not only in structure, but also in function. FcRn is expressed on most hematopoietic cells, endothelial cells and epithelial cells. Although FcRn is present on the plasma membrane of these cells, it does not bind IgG at pH7.4, but at acidic pH of 6 and lower. Therefore, FcRn is engaged after IgG is internalized along with other proteins by pinocytosis (Fig. 2). When the pH decreases to 6 in the endosomal compartments, two FcRn molecules bind and retain an IgG molecule while other proteins including excess IgG are shunted to lysosomes for degradation. The FcRn-bound IgG is shuttled back to the plasma membrane where at pH7.4 it is released. In addition to IgG, FcRn simultaneously salvages albumin. Importantly, the binding sites for IgG and albumin on FcRn are different and can be independently blocked (12, 13). This salvage mechanism accounts for the data Brambell analyzed and fulfills his hypothesis. Specifically, the finite number of receptors accounts for the saturation curves he calculated and the shorter half-life of IgG at higher concentrations.
Figure 2.
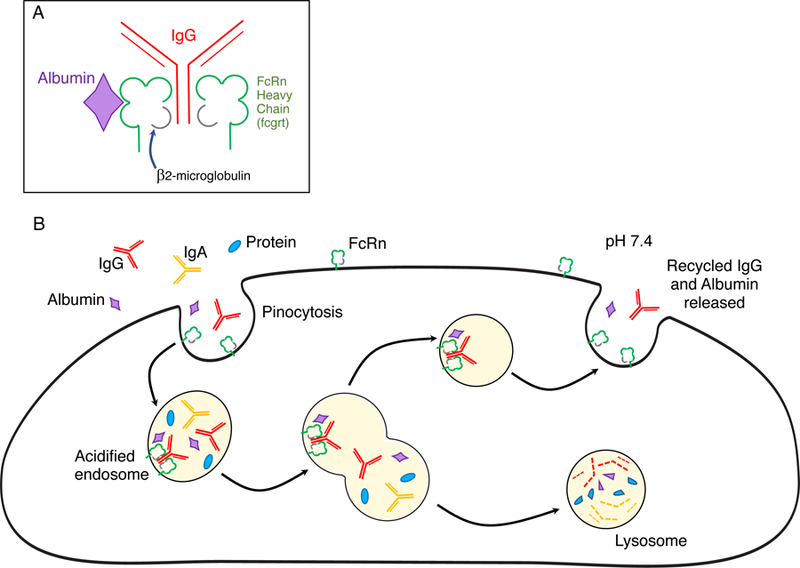
(A) FcRn structure. FcRn consist of β2 microglobulin associated with an MHC class I-like heavy chain encoded by the fcrgt gene. FcRn binds IgG in a 2:1 ratio. Albumin binds FcRn at a separate site. (B) FcRn salvage pathway for IgG. IgG, albumin and other serum proteins are ingested by pinocytosis. Pinocytotic vesicles fuse with acidic endosomes in which FcRn can bind IgG. Albumin binds a different site on FcRn. Excess unbound IgG and albumin as well as other proteins enter lysosomes and are degraded. Bound IgG and albumin are recycled and released by exocytosis. The MHC class I-related FcRn requires both β2-microglobulin (gray) and fcgrt encoded heavy chain (green) to function. Blocking the FcRn binding sites with small peptides, genetically engineered Fc regions or high affinity antibodies to FcRn causes more IgG to be shunted into lysosomal degradation.
Biologics that are Designed to Interact with FcRn
These general concepts of FcRn function provide the foundation for designing Fc fusion biologics such as CTLA4-Ig with long serum half-lives as well as very short acting Fab or F(ab’)2 products that lack Fc such as abciximab that is used to inhibit platelet aggregation (14). With greater definition of the interaction of FcRn and IgG, more refined adjustments to Fc-containing biologics have been integrated at the molecular level (15). Increased half-lives of therapeutic monoclonal antibodies have been achieved by increasing their binding to FcRn at pH6 while also maintaining or increasing release at pH7.4 (16). As an example, eculizumab (a recombinant humanized monoclonal antibody to human C5) has a relatively short half-life in the circulation of about 11 days. The introduction of 2 selected amino acid substitutions in the Fc region to increase the affinity to FcRn doubled the longevity of the antibody and permitted treatment intervals as long as every 12 weeks. The pharmacodynamics of these modified biologics can be developed with in vitro testing on cells that express FcRn and then validated in transgenic mice that express human FcRn (17–21). The half-lives of monoclonal antibodies currently in clinical use generally correlate with measurements of the affinity of their Fc for FcRn (15). As a result, FcRn binding characteristics are now routinely assessed to confirm biosimilarity of therapeutic monoclonal antibodies.
Nonetheless variations in half-lives of therapeutic monoclonal antibodies such as infliximab (anti-TNFα) and rituximab (anti-CD20)have been observed among different patient populations. In some cases, this has been attributed to variations in distribution of IgG allotypes among different ethnic populations (22). For example, infliximab has a shorter half-life in patients homozygous for G1m17,1 allotype of IgG. This difference is due to a higher affinity of G1m17,1 allotypes for FcRn. As a result, the endogenous IgG of these patients competes more effectively for recycling by FcRn and shortens the half-life of infliximab.
Polymorphisms in the promoter of the fcgrt gene have been associated with minor variations in the pharmacokinetics and compartmentalization of therapeutic antibodies (23–25). These polymorphisms have been demonstrated to alter binding capacities of FcRn on monocytes.
Additional pharmacodynamic considerations related to the partitioning of molecules during FcRn recycling include the release of antigens bound to the IgG. Antibodies that retain antigen bound to their Fab and are recycled into the circulation complexed with the antigen do not effectively clear the antigen. In contrast, antibodies that are engineered to release antigen in the acidic endosome result in antigen-free antibodies being recycled into the circulation. This allows an antibody to bind to its target antigen multiple times, and decreases the dose of therapeutic antibody required for treatment (20).
Other strategic modifications have been incorporated into therapeutic monoclonal antibodies to maintain long half-lives and avert activation of complement or interaction with cells that express Fcγ receptors (FcγR). These modifications are possible because FcRn binds to a site within the CH2-CH3 region of IgG that is distinct from the binding site for C1 or FcγR. This separation of binding sites has been exploited to engineer an antibody that will block CD154 but not induce thromboembolism by interacting with the FcγR on platelets (26).
Improved pharmacokinetics of biologics have also been achieved by exploiting the recycling of albumin by FcRn. This has been accomplished by linking the therapeutic agent either directly or indirectly to albumin (27, 28). An early example of this approach was the fusion of human soluble complement receptor type 1 to the albumin-binding domains from Streptococcal protein G (29).
Depletion of IgG by Blocking FcRn
More precise knowledge of FcRn structure also has been exploited to design biologics that can block FcRn function and increase the catabolism of endogenous pathological antibodies. A key feature of these biologics is high affinity for FcRn at pH 7.4 to preempt endogenous IgG binding. This has been accomplished by engineering small peptides, or the Fc or Fab regions of antibodies (Table 1). All of these strategies are effective in decreasing IgG levels and ameliorating pathology in models of antibody-mediated autoimmune diseases in rats and mice (17, 21). More recently, these strategies have been advanced to non-human primates and clinical trials in humans with promising results (18, 30–33).
Table 1:
Overview of monoclonal biologics that block FcRn in preclinical and clinical trials.
| Biologic | Trial | Dose | Route | Decrease in IgG relative to baseline | Return to baseline IgG | Reference |
|---|---|---|---|---|---|---|
| Fully human monoclonal antibody to FcRn * | cynomolgus monkeys | 5–20 mg/kg q7 days x 2 doses | i.v. or s.c. | 60% at 4–14 days | >15 days | (32) |
| Humanized monoclonal antibody to FcRn ** | cynomolgus monkeys | 150 mg/kg q3 days x 4 weeks | i.v. or s.c. | 80–90% at 7–35 days | 70–77 days | (30) |
| Humanized monoclonal antibody to FcRn** | Humans | Single 7mg/kg | i.v. or s.c. | 50% at 5–15 days | 45–56 days | (30) |
| Fully human monoclonal antibody to FcRn *** | Humans | Single 30–60mg/kg | i.v. | 75–80% at 7–21 days | >56 days | (31) |
| Fully human monoclonal antibody to FcRn *** | Humans | 15 or 30 mg/kg q7days x 4 doses | i.v. | 80% at 14–35 days | 84– >98 days | (31) |
| Modified human Fc fragment**** | cynomolgus | Single 20mg/kg | i.v. | 50% at 3–10 days | >15 days | (33) |
| Modified human Fc fragment**** | cynomolgus | 20 mg/kg q4 days x 4 doses | i.v. | 50% at 4–16 days | >20 days | (33) |
| Modified human Fc fragment**** | humans | Single 10mg/kg | i.v. | 50% at 5–15 days | >28 days | (33) |
| Modified human Fc fragment**** | humans | 10mg/kg q4 days x 6 doses | i.v. | 50% at 5–40 days | 63–77 days | (33) |
DX-2504 a fully human monoclonal antibody that binds with high affinity to human-FcRn at both neutral (pH 7.0) and acidic (pH 6.4) environments.
Rozanolixizumab a humanized high-affinity IgG4P monoclonal antibody to FcRn.
M281 a fully human, effectorless monoclonal IgG1 antibody to FcRn antibody that binds with high affinity at both endosomal pH 6.0 and extracellular pH 7.6
Efgartigimod a human IgG1–derived Fc fragment modified to increase affinity for FcRn at both neutral and acidic pH, but with higher affinity at pH 6.0
Biologics that effectively block FcRn cause a prolonged (weeks to months) dose-dependent decrease in circulating IgG (18, 30–33). One human monoclonal antibody to FcRn (DX-2504) decreased serum IgG levels by 40–60% for 2 weeks when 2 doses of 5mg/kg were administered intravenously or subcutaneously to cynomolgus monkeys (32). Another humanized high-affinity monoclonal antibody to human FcRn, Rozanolixizumab, was even more effective. In a dose-escalation study of rozanolixizumab in humans, a single dose of 4–7mg/kg intravenously or subcutaneously decreased serum IgG levels by 50% for over 2 weeks (30). Blocking FcRn with these antibodies has no effect on IgM or IgA because these classes of immunoglobulin are not protected from degradation by FcRn. In addition, the recycling of albumin is not disrupted because the antibodies specifically block the IgG binding site of FcRn. Finally, these antibodies are designed to avoid complement activation (Table 2).
Table 2:
Comparison of depletion of pathogenic antibodies by FcRn blockade, IVIg and plasmapheresis.
| FcRn Blockade | IVIg | Plasmapheresis | |
|---|---|---|---|
| Administration | sc or iv injection | iv infusion | Catheterization of large vein |
| IgG effects | Global decrease* | Global decrease | Global decrease |
| IgM, IgA effects | No decrease | Global decrease** | Global decrease |
| Complement effects | No decrease | Variable effects | Consumption*** |
| Coagulation effects | No decrease | No decrease | Consumption*** |
| Treatment Parameters | Low dose (4–20 mg/kg) long acting (weeks) | High dose (1–2 g/kg) | Repeated treatments required to diminish re-compartmentalization |
Can be circumvented by Seldeg
Dependent on IVIg composition (34)
Components of the complement and coagulation pathway can be activated and depleted by contact with bioincompatible surfaces.
Antibodies that bind FcRn with high affinity by their antigen binding sites are more efficient than unmodified intravenous immunoglobulin (IVIg) preparations that compete with endogenous IgG to bind FcRn by their Fc regions. For this reason, the doses of genetically engineered antibody required to block FcRn (4–20mg/kg) are much lower than the doses of unmodified IVIg (1–2 g/kg) that are needed to modulate pathogenic antibodies (34). However, biologics with Fc regions that are genetically engineered to bind FcRn with high affinity can decrease pathogenic antibodies effectively at lower doses (33, 35). Although IVIg has the potential to modulate immune responses by multiple mechanisms, in some diseases such as autoimmune blistering skin diseases IVIg functions primarily through FcRn (36).
The pharmacodynamics of the FcRn blocking agents in clinical trials are also superior to depleting Ig by plasmapheresis, a procedure that requires repeated interventions to counteract extravascular to intravascular re-equilibrations of IgG (37). The need for repeated invasive interventions to deplete Ig by plasmapheresis contrasts with the finding that at least some of the FcRn blocking biologics achieve prolonged depletion of IgG when administered safely by subcutaneous injections (Table 1). This property might permit FcRn blocking biologics to be combined effectively with plasmapheresis. For example, plasmapheresis could be used to achieve an immediate depletion of DSA in a sensitized patient and then an FcRn blocking antibody could be used to maintain the depletion over a longer time. The development of different monoclonal antibodies to block FcRn has the added advantage that these could be applied in succession in patients who develop neutralizing antibodies to a given biologic. Additional benefits for transplant recipients could be incorporated into monoclonal antibodies that are engineered to block FcRn via their Fc region. For example, the Fc region of monoclonal antibodies to CMV or other pathogens could be engineered to bind to FcRn in order to diminish the risk for infections in immunosuppressed patients during the period of hypogammaglobulinemia.
Although less invasive and more efficient than plasmapheresis, blocking FcRn function causes global depletion of IgG. In cynomolgus monkeys, DX-2504 did not interfere with the IgM antibody response to a primary challenge with keyhole limpet hemocyanin (KLH) or a secondary IgM immune response to tetanus toxoid, but did decrease the IgG responses to both antigens. These data support the premise that blocking FcRn would decrease preformed or de novo pathogenic IgG antibody responses. However, as the interactions of FcRn with more antibodies are investigated, new factors are discovered that influence the recycling mechanisms. For example, IgG3 binds to FcRn less avidly than other sublcasses of IgG because IgG3 has an arginine instead of a histidine at position 435 that is unique among IgG subclasses (38). Other subtler factors include differences in the variable region of Fab that alter the charge of the antibody (39–41). These factors may cause blockade of the FcRn to be more effective in decreasing some pathogenic antibodies than others.
The risks of global IgG depletion have been recognized and strategies to circumvent this disadvantage are under development. One strategy to deplete antigen specific antibodies through the FcRn pathway has been tested and has been named Seldeg (for selective degradation of antigen-specific antibodies). Seldeg employs chimeric molecules composed of a monomeric antigenic epitope linked to a dimeric IgG Fc region. The antigen acts as a lure to engage the specific antibody. In addition, the Fc is engineered to bind to FcRn with high affinity at neutral pH on the cell surface and be retained at acidic pH in endosomes. The FcRn delivers the bound antibody into lysosomes and then recycles to the cell surface. The monomeric antigen avoids crosslinking that could result in inflammatory immune responses. Test constructs of modified Fc fused to the encephalitic myelin oligodendrocyte glycoprotein or the tumor target HER2 have been demonstrated to deplete specific radiolabeled antibodies from the circulation of mice (42). Depletion of a polyclonal IgG response will require a cocktail of different antigenic epitopes fused to Fc molecules. This could be particularly problematic for designing reagents to treat sensitized patients, who have a wide range of antibodies to very polymorphic antigens such as HLA. Alternatively, depletion of a narrow range of antibodies may be sufficient to permit the transplantation of a partially matched organ.
Role of FcRn in Phagocytosis and Antigen Presentation
Although preclinical and clinical data have established that inhibition of FcRn depletes circulating IgG effectively, the potential consequences of modifying other functions of FcRn need to be considered including the impact of FcRn on phagocytosis by neutrophils, immune complex clearance by podocytes and antigen presentation by dendritic cells, macrophages and B cells.
FcRn is expressed at low levels on B and T lymphocytes, strongly on monocytes and at even higher levels on neutrophils (43, 44). Besides functioning as an IgG scavenger receptor in phagocytic cells, FcRn enhances phagocytic function. For this process, FcγR on the plasma membrane bind opsonized bacteria by the CH2 domain of IgG at neutral pH and initiate internalization. As the pH decreases in phagosomes, the opsonized bacteria are transferred from FcγR to FcRn that bind distinct residues within the CH2-CH3 region of IgG. The contribution of FcRn to these steps was documented using neutrophils from fcgrt−/− mice. These fcgrt−/− neutrophils bind opsonized bacteria by FcγR normally, but have impaired phagocytosis (44). This finding was confirmed by experiments using antibodies modified to prevent binding to FcRn, but retain binding to FcγR normally. Bacteria opsonized with these modified antibodies failed to engage FcRn and resulted in decreased phagocytosis. The possible risk of increased infection due to blocking this aspect of FcRn function has not been fully examined.
The transfer of immune complexes from FcγR to FcRn has also been found to be a critical component of the presentation of antigens complexed to IgG. This process is initiated by FcγR internalizing immune complexes and delivering them to acidified compartments within antigen presenting cells where the antibody and antigen are transferred to FcRn (45, 46). FcRn shields immune complexes from catabolism and directs antigen to processing for loading on MHC class II molecules and presentation to CD4 T cells (47). FcRn-directed intracellular sorting of IgG immune complexes has also been shown to be critical to cross-presentation of antigen on MHC class I in monocyte-derived dendritic cells (45). The outcome of IgG immune complex internalization may depend on context. A recent report demonstrated that maternal IgG immune complexes induce regulatory T cells in neonates. This process requires FcRn on dendritic cells of the offspring. It is possible that blocking antigen presenting functions of FcRn might decrease antibody-directed recognition of antigen in presensitized patients.
Acknowledgements
This work was supported by grant 1P01 AI087586 from NIAID (to R.F., W.M.B., and A.V.).
Abbreviations
- FcRn
neonatal Fc receptor
- FcγR
Fcγ receptors
- IVIg
intravenous immunoglobulin
- Seldeg
Selective degradation of antigen-specific antibodies
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Ardeniz O, Unger S, Onay H, Ammann S, Keck C, Cianga C, et al. beta2-Microglobulin deficiency causes a complex immunodeficiency of the innate and adaptive immune system. J Allergy Clin Immunol 2015;136(2):392–401. [DOI] [PubMed] [Google Scholar]
- 2.Brambell FW. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet 1966;2(7473):1087–93. [DOI] [PubMed] [Google Scholar]
- 3.Porter RR. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J 1959;73:119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brambell FW, Hemmings WA, Morris IG. A Theoretical Model of Gamma-Globulin Catabolism. Nature 1964;203:1352–4. [DOI] [PubMed] [Google Scholar]
- 5.Rodewald R, Kraehenbuhl JP. Receptor-mediated transport of IgG. J Cell Biol 1984;99(1 Pt 2):159s–64s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature 1989;337(6203):184–7. [DOI] [PubMed] [Google Scholar]
- 7.Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol 1996;26(3):690–6. [DOI] [PubMed] [Google Scholar]
- 8.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A 1996;93(11):5512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akilesh S, Christianson GJ, Roopenian DC, Shaw AS. Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol 2007;179(7):4580–8. [DOI] [PubMed] [Google Scholar]
- 10.Montoyo HP, Vaccaro C, Hafner M, Ober RJ, Mueller W, Ward ES. Conditional deletion of the MHC class I-related receptor FcRn reveals the sites of IgG homeostasis in mice. Proc Natl Acad Sci U S A 2009;106(8):2788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baranyi M, Cervenak J, Bender B, Kacskovics I. Transgenic rabbits that overexpress the neonatal Fc receptor (FcRn) generate higher quantities and improved qualities of anti-thymocyte globulin (ATG). PLoS One 2013;8(10):e76839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenniston JA, Taylor BM, Conley GP, Cosic J, Kopacz KJ, Lindberg AP, et al. Structural basis for pH-insensitive inhibition of immunoglobulin G recycling by an anti-neonatal Fc receptor antibody. J Biol Chem 2017;292(42):17449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sand KM, Bern M, Nilsen J, Noordzij HT, Sandlie I, Andersen JT. Unraveling the Interaction between FcRn and Albumin: Opportunities for Design of Albumin-Based Therapeutics. Front Immunol 2014;5:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudson PJ, Souriau C. Engineered antibodies. Nat Med 2003;9(1):129–34. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki T, Ishii-Watabe A, Tada M, Kobayashi T, Kanayasu-Toyoda T, Kawanishi T, et al. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: a comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J Immunol 2010;184(4):1968–76. [DOI] [PubMed] [Google Scholar]
- 16.Yeung YA, Leabman MK, Marvin JS, Qiu J, Adams CW, Lien S, et al. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J Immunol 2009;182(12):7663–71. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Garcia AM, Santoro H, Zhang Y, McDonnell K, Dumont J, et al. Amelioration of experimental autoimmune myasthenia gravis in rats by neonatal FcR blockade. J Immunol 2007;178(8):5390–8. [DOI] [PubMed] [Google Scholar]
- 18.Mezo AR, McDonnell KA, Hehir CA, Low SC, Palombella VJ, Stattel JM, et al. Reduction of IgG in nonhuman primates by a peptide antagonist of the neonatal Fc receptor FcRn. Proc Natl Acad Sci U S A 2008;105(7):2337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roth A, Rottinghaus ST, Hill A, Bachman ES, Kim JS, Schrezenmeier H, et al. Ravulizumab (ALXN1210) in patients with paroxysmal nocturnal hemoglobinuria: results of 2 phase 1b/2 studies. Blood Adv 2018;2(17):2176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheridan D, Yu ZX, Zhang Y, Patel R, Sun F, Lasaro MA, et al. Design and preclinical characterization of ALXN1210: A novel anti-C5 antibody with extended duration of action. PLoS One 2018;13(4):e0195909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaccaro C, Zhou J, Ober RJ, Ward ES. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol 2005;23(10):1283–8. [DOI] [PubMed] [Google Scholar]
- 22.Ternant D, Arnoult C, Pugniere M, Dhommee C, Drocourt D, Perouzel E, et al. IgG1 Allotypes Influence the Pharmacokinetics of Therapeutic Monoclonal Antibodies through FcRn Binding. J Immunol 2016;196(2):607–13. [DOI] [PubMed] [Google Scholar]
- 23.Passot C, Azzopardi N, Renault S, Baroukh N, Arnoult C, Ohresser M, et al. Influence of FCGRT gene polymorphisms on pharmacokinetics of therapeutic antibodies. MAbs 2013;5(4):614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachs UJ, Socher I, Braeunlich CG, Kroll H, Bein G, Santoso S. A variable number of tandem repeats polymorphism influences the transcriptional activity of the neonatal Fc receptor alpha-chain promoter. Immunology 2006;119(1):83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billiet T, Dreesen E, Cleynen I, Wollants WJ, Ferrante M, Van Assche G, et al. A Genetic Variation in the Neonatal Fc-Receptor Affects Anti-TNF Drug Concentrations in Inflammatory Bowel Disease. Am J Gastroenterol 2016;111(10):1438–45. [DOI] [PubMed] [Google Scholar]
- 26.Kim SC, Wakwe W, Higginbotham LB, Mathews DV, Breeden CP, Stephenson AC, et al. Fc-Silent Anti-CD154 Domain Antibody Effectively Prevents Nonhuman Primate Renal Allograft Rejection. Am J Transplant 2017;17(5):1182–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen MT, Kuhlmann M, Hvam ML, Howard KA. Albumin-based drug delivery: harnessing nature to cure disease. Mol Cell Ther 2016;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyzik M, Rath T, Lencer WI, Baker K, Blumberg RS. FcRn: The Architect Behind the Immune and Nonimmune Functions of IgG and Albumin. J Immunol 2015;194(10):4595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makrides SC, Nygren PA, Andrews B, Ford PJ, Evans KS, Hayman EG, et al. Extended in vivo half-life of human soluble complement receptor type 1 fused to a serum albumin-binding receptor. J Pharmacol Exp Ther 1996;277(1):534–42. [PubMed] [Google Scholar]
- 30.Kiessling P, Lledo-Garcia R, Watanabe S, Langdon G, Tran D, Bari M, et al. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: A randomized phase 1 study. Sci Transl Med 2017;9(414). [DOI] [PubMed] [Google Scholar]
- 31.Ling LE, Hillson JL, Tiessen RG, Bosje T, van Iersel MP, Nix DJ, et al. M281, an Anti-FcRn Antibody: Pharmacodynamics, Pharmacokinetics, and Safety Across the Full Range of IgG Reduction in a First-in-Human Study. Clin Pharmacol Ther 2018. [DOI] [PMC free article] [PubMed]
- 32.Nixon AE, Chen J, Sexton DJ, Muruganandam A, Bitonti AJ, Dumont J, et al. Fully human monoclonal antibody inhibitors of the neonatal fc receptor reduce circulating IgG in non-human primates. Front Immunol 2015;6:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulrichts P, Guglietta A, Dreier T, van Bragt T, Hanssens V, Hofman E, et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Invest 2018;128(10):4372–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spath PJ, Schneider C, von Gunten S. Clinical Use and Therapeutic Potential of IVIG/SCIG, Plasma-Derived IgA or IgM, and Other Alternative Immunoglobulin Preparations. Arch Immunol Ther Exp (Warsz) 2017;65(3):215–31. [DOI] [PubMed] [Google Scholar]
- 35.Maeda A, Iwayanagi Y, Haraya K, Tachibana T, Nakamura G, Nambu T, et al. Identification of human IgG1 variant with enhanced FcRn binding and without increased binding to rheumatoid factor autoantibody. MAbs 2017;9(5):844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li N, Zhao M, Hilario-Vargas J, Prisayanh P, Warren S, Diaz LA, et al. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J Clin Invest 2005;115(12):3440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaplan AA. Therapeutic plasma exchange: a technical and operational review. J Clin Apher 2013;28(1):3–10. [DOI] [PubMed] [Google Scholar]
- 38.Irani V, Guy AJ, Andrew D, Beeson JG, Ramsland PA, Richards JS. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol Immunol 2015;67(2 Pt A):171–82. [DOI] [PubMed] [Google Scholar]
- 39.Piche-Nicholas NM, Avery LB, King AC, Kavosi M, Wang M, O’Hara DM, et al. Changes in complementarity-determining regions significantly alter IgG binding to the neonatal Fc receptor (FcRn) and pharmacokinetics. MAbs 2018;10(1):81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoch A, Kettenberger H, Mundigl O, Winter G, Engert J, Heinrich J, et al. Charge-mediated influence of the antibody variable domain on FcRn-dependent pharmacokinetics. Proc Natl Acad Sci U S A 2015;112(19):5997–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Lu P, Fang Y, Hamuro L, Pittman T, Carr B, et al. Monoclonal antibodies with identical Fc sequences can bind to FcRn differentially with pharmacokinetic consequences. Drug Metab Dispos 2011;39(9):1469–77. [DOI] [PubMed] [Google Scholar]
- 42.Devanaboyina SC, Khare P, Challa DK, Ober RJ, Ward ES. Engineered clearing agents for the selective depletion of antigen-specific antibodies. Nat Commun 2017;8:15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Bilsen K, van Hagen PM, Bastiaans J, van Meurs JC, Missotten T, Kuijpers RW, et al. The neonatal Fc receptor is expressed by human retinal pigment epithelial cells and is downregulated by tumour necrosis factor-alpha. Br J Ophthalmol 2011;95(6):864–8. [DOI] [PubMed] [Google Scholar]
- 44.Vidarsson G, Stemerding AM, Stapleton NM, Spliethoff SE, Janssen H, Rebers FE, et al. FcRn: an IgG receptor on phagocytes with a novel role in phagocytosis. Blood 2006;108(10):3573–9. [DOI] [PubMed] [Google Scholar]
- 45.Baker K, Qiao SW, Kuo TT, Aveson VG, Platzer B, Andersen JT, et al. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. Proc Natl Acad Sci U S A 2011;108(24):9927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiao SW, Kobayashi K, Johansen FE, Sollid LM, Andersen JT, Milford E, et al. Dependence of antibody-mediated presentation of antigen on FcRn. Proc Natl Acad Sci U S A 2008;105(27):9337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X, Lu L, Yang Z, Palaniyandi S, Zeng R, Gao LY, et al. The neonatal FcR-mediated presentation of immune-complexed antigen is associated with endosomal and phagosomal pH and antigen stability in macrophages and dendritic cells. J Immunol 2011;186(8):4674–86. [DOI] [PubMed] [Google Scholar]


