Abstract
Background and Purpose
Effective stroke prevention depends on accurate stroke risk prediction. We determined the discriminative ability of neurofilament light chain (NfL) levels for distinguishing between adults with diabetes who develop incident stroke and those who remain stroke-free during a seven-year follow-up period.
Method
We performed a case-control study of participants selected from the previously completed Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Cases were all ACCORD subjects who were stroke-free at enrollment and developed incident stroke during follow-up (n=113). Control subjects (n=250) were randomly selected ACCORD subjects who had no stroke events either prior to or after randomization. NfL was measured in baseline samples using Single Molecule Array technology (Quanterix).
Results
Baseline NfL levels were higher in stroke subjects, compared to controls, after adjusting for age, race, blood pressure, weight and the Framingham Stroke Risk Score (FSRS)). Relative to the subjects in the lowest quintile of NfL levels, the hazard ratio of incident stroke for subjects in the 2nd to 5th quintiles were: 3.91 (1.45, 10.53); 4.05 (1.52, 10.79); 5.63 (2.16, 14.66) and 9.75 (3.84, 27.71) respectively, after adjusting for race and FSRS. Incorporating NfL levels into a predictive score that already included race and FSRS increased the score’s c-statistic from 0.71 [95% CI: 0.66, 0.77] to 0.78 [95% CI: 0.73, 0.83], p <0.001. Older age, non-Caucasian race, higher systolic blood pressure, glomerular filtration rate<60, and higher hemoglobin A1c were independent predictors of serum NfL in this cohort but diastolic blood pressure, durations of hypertension or diabetes, and lipid levels were not. In total, cardiovascular disease risk factors explained 19.2% of the variability in baseline NfL levels.
Conclusions
Serum NfL levels predict incident stroke and add considerably to the discriminatory power of the FSRS in a cohort of middle-aged and older adults with diabetes.
Keywords: Cerebrovascular Disease/Stroke, diagnostic testing, risk factors, diabetes, primary prevention
Introduction
Inaccurate stroke risk prediction constitutes a critical barrier to effective stroke prevention. Prevention of stroke is better than treatment of stroke and it depends on accurate identification of at-risk individuals. Blood biomarkers of brain injury offer a complementary approach to improving the accuracy of stroke risk prediction.1 Neurofilament light chain (NfL) is a potential biomarker of axonal injury. It is an intermediate filament protein abundantly expressed in neurons and specifically in myelinated axons. Elevated blood NfL levels are found in a number of neurodegenerative conditions including: amyotrophic lateral sclerosis,2 multiple sclerosis,3 frontotemporal dementia4 and traumatic brain injury.5 Given that axonal injury often occurs in subclinical cerebrovascular disease, as evidenced by the detection of white matter lesions on magnetic resonance imaging,6 and that subclinical cerebrovascular disease predicts clinical stroke,7 NfL, a biomarker of axonal injury, is likely to be an important predictor of stroke risk. Serum NfL levels are markedly elevated in acute cerebral small vessel disease stroke and remain elevated for at least 3 months post-stroke.8,9
We hypothesized that among subjects without a history of stroke, those with high serum levels of NfL will have a higher risk of incident stroke than those with lower levels. We also examined the associations of serum NfL levels with known cerebrovascular disease risk factors within the study population.
Materials and Methods
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
We conducted a case-control study nested within The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial.10 The ACCORD cohort was selected because it has a relatively large number of stroke cases, detailed information on cerebrovascular risk factors and available biospecimen. The ACCORD trial is a previously completed randomized, multicenter double 2 × 2 factorial design trial of participants with type 2 diabetes who were initially randomized to either intensive glycemic control or standard therapy and then subsequently randomized to either intensive or standard blood pressure control; OR intensive or standard lipid control.
ACCORD participants were 40–79 years old with a history of type 2 diabetes for more than 3 months and a history of cardiovascular disease or 55–79 years old with a history of type 2 diabetes for more than 3 months and anatomical evidence of significant atherosclerosis, albuminuria, left ventricular hypertrophy, or at least two risk factors for cardiovascular disease. Subjects were excluded from the ACCORD trial if they had: hypoglycemia within the preceding 3 months; BMI>45 kg/m2; serum creatinine >1.5 mg/dl; elevated transaminase; cardiovascular disease event/hospitalization within preceding 3 months; symptomatic heart failure; medical condition likely to limit survival; any factors that limit adherence to interventions; any organ transplant; weight loss>10% in the last 6 months; or were pregnant/trying to become pregnant. A total of 10,251 subjects were enrolled in ACCORD and 162 of them had incident stroke during the follow-up period. Written informed consent was obtained and included consent to use blood samples for future studies.
In the current nested case-control study, we identified all ACCORD subjects who were stroke-free at enrollment, had sufficient baseline serum samples (obtained at enrollment) available and had an incident stroke during follow-up (n=113). Control subjects (n=250) were randomly selected ACCORD subjects who had no stroke events either prior to or after randomization, and had serum samples available. This study was approved by the Institutional Review Board at the University of Michigan.
Measurements
Incident Stroke
Our primary outcome was time until incident fatal or non-fatal stroke (ischemic or hemorrhagic) occurring within 7 years of randomization. Stroke outcomes in ACCORD were adjudicated by a central committee of physicians who used a standard protocol and were blinded to study arm assignment. Definite stroke was defined as: CT or MRI scan within 14 days of onset of a focal neurological deficit lasting more than 24 hours with evidence of brain infarction or confirmation of intraparenchymal hemorrhage in a compatible location with CT/MRI scan within 14 days of the deficit onset, or at autopsy, or by lumbar puncture. Ischemic and hemorrhagic strokes were combined within the ACCORD database. Subarachnoid hemorrhage was included in the definition of stroke in the ACCORD cohort. Surveillance MRI to detect silent brain infarcts were not performed in the ACCORD study.
Serum NfL
Frozen serum was thawed on ice and then spun prior to NfL measurement using Simoa HD-1 Analyzer (Quanterix) and Single Molecule Array (Simoa) technology according to manufactors instructions. The assay for these samples has a lower limit of detection of 0.054 pg/mL, a lower limit of quantitation (LLOQ) of 0.686 pg/ml, a dynamic range of 0–2,000 pg/ml and a co-efficient of variation of 7% at the LLOQ. Measurements were performed in duplicates and batches were completed by a scientist who was blinded to all clinical data including outcome measures. Samples with a co-efficient of variation of >20% between measurements were repeated as per standard practice.
Other demographic and clinical characteristics
Subject demographics and baseline clinical characteristics were ascertained from ACCORD case report forms. For each subject, we calculated the Framingham Stroke Risk Score (FSRS). Demographics included subject race, sex, and age. Clinical characteristics included weight, height, systolic BP, diastolic BP, current smoking status, history of cardiovascular disease (e.g. myocardial infarction, angina, coronary artery bypass, percutaneous coronary intervention), stroke, years of hypertension, years of diabetes, glomerular filtration rate (GFR), glycemic control (measured by hemoglobin A1C), years of hyperlipidemia, high density lipoprotein (HDL), low density lipoprotein (LDL) level, triglyceride, and total cholesterol levels. The FSRS score combined stroke risk factors. We calculated the FSRS from these variables using the standard scoring rules.11
Statistical analysis
We began with a descriptive analysis of the sample on each demographic and clinical characteristic. We then conducted two adjusted survival analyses using Cox regression. First, we analyzed the effect of baseline NfL levels on post-baseline time-to-stroke. Based on exploratory analyses, we found three basic levels of stroke risk as a function of NfL level—low (bottom quantile), medium (middle three quintiles), high (top quintile)—which we used in descriptive analyses of the survival curves. Models included the FSRS score, race and NfL.
Next, we derived a stroke risk score that incorporated NfL levels. To determine score contributions we: 1) fit a logistic regression model with race, FSRS, and baseline NfL level, and 2) scaled by the minimum regression coefficient and rounded to the nearest integer, in analogous fashion to the FSRS.12 Since there were few subjects that did not identify as either Caucasian or African American, we dichotomized race as Caucasian or Non-Caucasian. We estimated the out-of-sample discriminatory power using leave-one-out cross validation, which operates by sequential exclusion of each data point, deriving the score using the non-excluded data, and predicting the left out case. Leave-one-out cross validation shows the accuracy of a study while preventing the bias that comes from testing a model on it’s own data. We used the complete FSRS, rather than each individual variable, in part because our sample size was likely too small for 5 more predictor variables. We quantified discriminatory power using the area under the ROC curve (AUC), or c-statistic. Some control subjects were lost to follow-up before the end of the 7-year follow-up period. To examine the impact of potential misclassification of controls as a result of censoring, we performed a sensitivity analysis limiting the follow-up period to the minimum follow-up period among controls (3.5 years); this restriction resulted in the exclusion of 39 stroke cases that occurred between years 3.5 and 7.
To identify independent determinants of serum NfL levels, we constructed linear regression models with locally-weighted smoothing (LOESS) and examined the association between serum NfL and known predictors of cerebrovascular disease risk. Baseline systolic BP, diastolic BP, and the use of antihypertensive medications were analyzed as measures of BP control. Baseline hemoglobin A1C was analyzed as a measure of glycemic control. Total cholesterol, HDL, LDL, and triglyceride levels were analyzed as measures of lipid control. To determine the influence of renal clearance on NfL levels, we utilized glomerular filtration rate (GFR) dichotomized as <60 or ≥60 ml/min/1.73m2, since a graphical plot of the association between GFR and NfL levels revealed a strong association between GFR values <60 ml/min/1.73m2 and NfL levels. Other predictors that were analyzed include age, race, cigarette smoking, prior cardiovascular disease, and left ventricular hypertrophy. Variables that had significant univariate associations (p<0.05) with NfL were included in a multivariable model. Statistical analyses were conducted in R, version 3.3.2 (R Core Team, 2013).
Results
Descriptive Analyses
Characteristics of the study sample are presented in Table 1. There were demographic and clinical differences between incident stroke cases and stroke-free controls. Subjects with incident stroke were more likely to be older, and less likely to be Caucasian than stroke-free controls. Subjects with incident stroke had higher baseline systolic BP levels, lower weight, and higher FSRS than those who remained stroke-free. Median follow-up was 4.67 years (interquartile range, 3.69 – 5.68).
Table 1: Description of the Sample.
The group analyzed had n=363 people (113 that had stroke within seven years, and 250 that did not). In Table 1, the full sample, and the two groups are compared on demographic characteristics, baseline clinical characteristics, and Neurofilament light chain (NfL) concentration.
| Total Sample (n=363) | Stroke (n=113) | No Stroke (n=250) | |
|---|---|---|---|
| Demographics | |||
| Non-Caucasian (n, %)*** | 92 (25.3%) | 42 (37.2%) | 50 (20.0%) |
| Female (n, %) | 149 (41.0%) | 51 (45.1%) | 98 (39.2%) |
| Age (mean, SD)*** | 62.8 (6.4) | 64.6 (6.8) | 62.0 (6.0) |
| Clinical Characteristics (Baseline) | |||
| Weight in Kg (mean, SD)*** | 91.7 (17.4) | 88.0 (18.4) | 93.3 (16.7) |
| BMI (mean, SD) | 31.9 (5.1) | 31.3 (5.5) | 31.2 (4.9) |
| Systolic BP (mean, SD)*** | 137.3 (18.0) | 144.5 (17.8) | 134.0 (17.1) |
| Years of hypertension (mean, SD) | 10.2 (9.5) | 10.7 (9.5) | 9.9 (9.5) |
| Years of diabetes (mean, SD) | 10.7 (7.4) | 11.7 (7.7) | 10.2 (7.2) |
| Hemoglobin A1C (mean, SD)* | 8.5 (0.9) | 8.7 (1.0) | 8.4 (0.8) |
| Smoke (n, %) | 40 (11.0%) | 11 (9.7%) | 29 (11.6%) |
| Cardiovascular disease history (n, %) | 124 (34.2%) | 39 (34.5%) | 85 (34.0%) |
| Left Ventricular Hypertrophy (n, %) | 5 (1.4%) | 4 (3.5%) | 1 (0.4%) |
| On Hypertensive Medication (n, %) | 300 (82.6%) | 98 (86.7%) | 202 (80.8%) |
| Atrial Fibrillation (n, %) | 3 (0.8%) | 1 (0.9%) | 2 (0.8%) |
| Framingham Score (mean, SD)*** | 9.3 (2.9) | 10.4 (2.7) | 8.8 (2.9) |
| GFR<60ml/min/1.73m2 (n, %) | 36 (9.9%) | 15 (13.4%) | 21 (8.4%) |
| Total cholesterol (mean, SD)* | 184.5 (43.2) | 192.2 (46.0) | 181.1 (41.6) |
| High density lipoprotein (mean, SD) | 41.9 (12.4) | 43.4 (15.1) | 41.2 (10.9) |
| Low density lipoprotein (mean, SD) | 105.2 (33.7) | 110.4 (32.6) | 102.9 (34.0) |
| Triglycerides (mean, SD) | 192.5 (130.2) | 198.8 (166.2) | 189.7 (110.5) |
| Years of hyperlipidemia (mean, SD) | 6.6 (6.0) | 7.2 (6.7) | 6.3 (5.7) |
| Neurofilament light chain (median, IQR) | 15.2 (11.0, 22.8) | 19.8 (14.7, 30.9) | 13.8 (9.9, 19.6) |
SD = standard deviation
p < 0.001
p < 0.05
Baseline NfL level and stroke risk
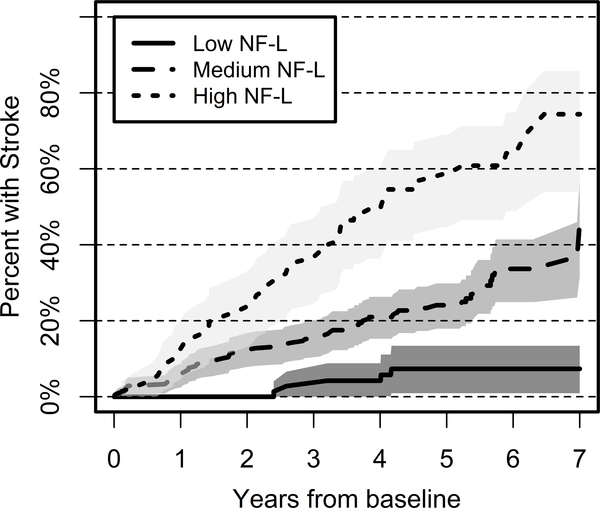
Baseline NfL levels were higher among subjects with incident stroke, compared to controls. The levels remained higher in stroke subjects after adjusting for age, race, systolic BP, weight and FSRS. Among those in the first, middle three, and fifth quintiles of baseline NfL levels, stroke within 7 years following baseline was observed in 5 (6.8%), 66 (30.4%) and 42 (57.5%) individuals respectively. Figure 1 shows the Kaplan-Meier survival curve estimates with NfL levels categorized into top quintile, middle three quintiles, and bottom quintile. There is a clear gradient of stroke risk across those three groups, with subjects with higher NfL levels experiencing higher strike hazard during the follow-up period. Prior to covariate adjustment, the highest quintile of NfL had 11.4-fold higher hazard of stroke than the lowest quintile; the 2nd-4th quintiles had unadjusted stroke hazards of 4.16, 4.91, and 6.49, respectively, relative to the lowest quintile. Baseline NfL levels remained significantly associated with incident stroke after adjustment for race and FSRS score. Table 2 shows the adjusted stroke risk. Relative to the lowest quintile, the highest quintile NfL group had over 9.8-fold hazard of stroke, after controlling for FSRS and race. The three middle quintiles showed similar effect sizes to one another, with excess hazards of between 3.91 and 5.63, relative to the low NfL group. The proportional hazards assumption was not violated.
Figure 1: Survival Analysis of Stroke Risk as a function of baseline NfL.
This figure displays the probability of incident stroke according to serum NfL level. Low NfL represents subjects with NfL levels in the lowest quintile (n=73), medium NfL represents subjects with NfL levels in quintiles 2–4 (n=217) and high NfL represents subjects with NfL levels in the highest quintile (n=73). Subjects with high NfL levels were more likely to develop incident stroke than those with medium or low NfL values.
Table 2:
Hazard Ratios of the Framingham Stroke Risk Score and Baseline Serum NfL Levels for Predicting Incident Stroke in the ACCORD cohort.
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| African American | 3.13 (1.98, 4.96) | 3.18 (2.00, 5.06) | 2.88 (1.81, 4.59) |
| Framingham Risk Score | 1.16 (1.09, 1.23) | 1.14 (1.07, 1.21) | |
| Baseline NfL (2nd Quintile) | 4.09 (1.52, 11.03) | 3.91 (1.45, 10.53) | |
| Baseline NfL (3rd Quintile) | 4.47 (1.68, 11.87) | 4.05 (1.52, 10.79) | |
| Baseline NfL (4th Quintile) | 6.54 (2.52, 16.99) | 5.63 (2.16, 14.66) | |
| Baseline NfL (5th Quintile) | 10.58 (4.18, 26.78) | 9.75 (3.84, 27.71) | |
| Concordance | 0.67 | 0.71 | 0.74 |
Model 1: Race and Framingham Stroke Risk Score only
Model 2: Race and serum NfL levels only
Model 3: Race, Framingham Stroke Risk Score and serum NfL levels
Stroke Prediction Score
We derived a stroke prediction score that incorporates race and NfL levels using the ACCORD data. The logistic model including race, FSRS, and baseline NfL levels produced coefficients of 1.99 for race, 0.19 for each 1-point increase of the FSRS, and 0.06 for each 1 pg/mL increase in NfL level. Scaling and rounding resulted in point contributions of 32 for black race, 3 for each point on the FSRS, and 1 for each pg/ml increase in the NfL level. The addition of serum NfL levels significantly improved the discrimination of the FSRS for stroke risk prediction compared with a score analogously derived and including only race and FSRS (area under the curve, 0.78 [(95% CI: 0.73, 0.83] vs. 0.71 [95% CI: 0.66, 0.77]; p<0.001).
Sensitivity Analysis
Results were similar in a sensitivity analysis restricting the follow-up period to the shortest time interval that ensures complete follow-up in controls (3.5 years). The logistic model including race, FSRS, and NfL level, applied to only those with known stroke status during the 3.5 years following baseline produced coefficients of 1.99, 0.21, and 0.06 for race, FSRS, and NfL, respectively. The newly derived stroke prediction score with NfL levels had significantly greater stroke risk prediction different (p =0.002) than a score analogously derived and including only race and FSRS score (area under the curve, 0.80 [95% CI: 0.75, 0.86] vs. 0.73 [95% CI: 0.66, 0.79].
Correlates of serum NfL levels
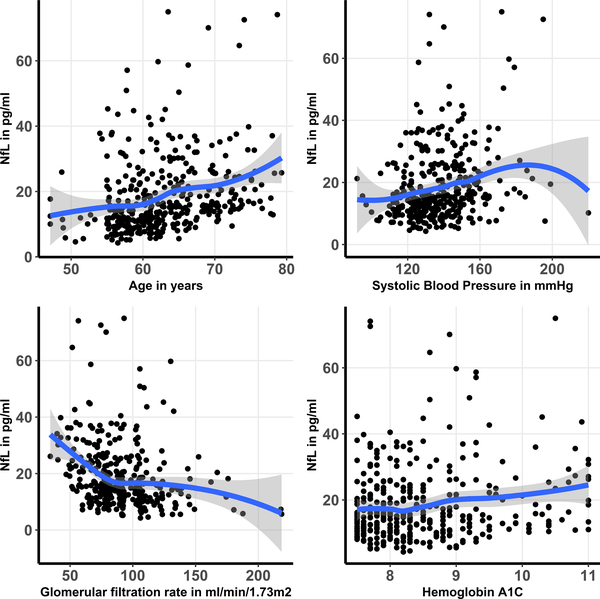
Older age, non-Caucasian race, higher systolic BP, GFR<60, higher HDL and LDL cholesterol levels, duration of diabetes, and higher hemoglobin A1C each had significant univariable associations with NfL levels (Table 3). After multivariable adjustment, older age, non-Caucasian race, higher systolic BP, GFR<60, and higher hemoglobin A1C remained significantly associated with serum NfL levels (Table 3). In combination, the independent predictors identified explained 19% of the variability in NfL levels. The associations between older age, higher systolic BP and higher hemoglobin A1C levels and NfL levels were approximately linear throughout the majority of the range of these variables (Figure 2). Sex was not associated with NfL levels.
Table 3: Determinants of serum NfL levels within the study population.
This table examines univariable and multivariable associations between risk factors for cardiovascular disease and serum NfL values. The unadjusted values are univariable associations with NfL. The adjusted values are multivariable associations after adjusting for variables with significant univariable associations (age, race, systolic blood pressure, glomerular filtration rate, high density lipoprotein, low density lipoprotein, years of diabetes and hemoglobin A1C).
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| β(SE) | p-Value | β(SE) | p-Value | |
| Age in years | 0.48 (0.09) | <0.01 | 0.36 (0.09) | <0.01 |
| Female | −0.95 (1.24) | 0.44 | ||
| Non-Caucasian race | 4.71 (1.38) | <0.01 | 3.57 (1.33) | <0.01 |
| Cardiovascular disease history | 1.66 (1.29) | 0.20 | ||
| Cigarette smoking history | 0.31 (1.85) | 0.87 | ||
| Left ventricular hypertrophy | 0.73 (1.16) | 0.53 | ||
| Antihypertensive medication use | −0.003 (1.61) | 0.99 | ||
| Systolic blood pressure | 0.16 (0.03) | <0.01 | 0.11 (0.03) | <0.01 |
| Diastolic blood pressure | −0.01 (0.06) | 0.81 | ||
| Glomerular filtration rate <60 ml/min/1.73m2 | 9.87 (1.97) | <0.01 | 8.01 (1.92) | <0.01 |
| Total cholesterol | 0.03 (0.01) | 0.07 | ||
| High density lipoprotein | 0.12 (0.05) | 0.01 | 0.03 (0.05) | 0.36 |
| Low density lipoprotein | 0.04 (0.02) | 0.04 | 0.02 (0.02) | 0.13 |
| Triglycerides | −0.004(0.004) | 0.37 | ||
| Years of diabetes | 0.25 (0.08) | <0.01 | 0.09 (0.08) | 0.27 |
| Hemoglobin A1C | 1.95 (0.67) | <0.01 | 1.85 (0.64) | <0.01 |
Figure 2: A graphical display of the association between independent predictors and serum NfL levels.
The figure displays a scatter plot of the association between variables that were found to be independently associated with serum NfL and NfL levels. Locally weighted smoothing (LOESS) was used to create a smooth line through the scatter plot to visually depict the association between predictors and NfL.
Discussion
We found that serum NfL levels strongly predict incident stroke in people with diabetes who were stroke-free at baseline. This finding is dose-dependent. Compared to people with NfL levels in the lowest quintile, those with NfL levels in the fifth quintile had a hazard ratio nearly 10 times greater, after adjusting for FSRS and race. The addition of NfL values to the FSRS substantially improved the prognostic accuracy of the FSRS. The predictive capacity of NfL was substantially greater than known risk factors. NfL levels outperformed both the FSRS and a modified FSRS score that accounts for race in terms of discriminating cases from controls. The addition of NfL values to the FSRS significantly improved the discriminatory power of the FSRS.
Our results suggest that serum NfL is a biomarker that predicts incident stroke and adds incremental discriminatory power to a conventional stroke risk score even in high-risk individuals, such as those with diabetes. White matter disease is an established predictor of life-long risk of strike, cognitive impairment ad functional disability.13 In fact, subjects with either genetically defined or sporadic cerebral small vessel disease (SVD) both have higher NfL levels than healthy controls.8,14 Serum NfL levels are also associated with brain imaging markers of SVD.14 Serum NfL levels are further established as a marker of cerebral infarction because they are markedly elevated in acute cerebral SVD stroke (by 6-fold),8,9 and remain elevated for 3 months (by 5-fold) post-stroke.8 Serum NfL levels obtained 7 days after the onset of ischemic stroke are strongly correlated with infarct size and functional outcome.15 Our findings are consistent with the existing literature and extend prior work by establishing the prognostic importance of serum NfL levels for future stroke in stroke-free individuals. Our results suggest a scientific need to determine whether serum NfL also predicts stroke risk in a population-based cohort, where it will also be possible to calibrate to determine absolute risk.
We hypothesize that most of the elevated NfL in our sample is caused by subclinical ischemic vascular disease, which in turn predicts clinical ischemic vascular disease.7 Serum NfL has been strongly associated with subclinical and clinical ischemic cerebrovascular events. Subclinical ischemic cerebrovascular events increase the risk of, and often precede, clinically overt stroke.16–18 These subclinical cerebrovascular ischemic events result in brain cellular death accompanied by a release of brain structural proteins (including axonal proteins such as NfL) into the circulatory system through a compromised blood brain barrier.19 The resulting molecular biosignature of brain injury may provide an early warning sign and marker of the future risk of stroke.
We also found evidence that serum NfL levels contribute new prognostic information regarding stroke, above and beyond information obtained from traditional cardiovascular risk factors. Our analysis demonstrates that only 19% of the variability in serum NfL levels is explained by age, race, systolic blood pressure, decreased renal function, and glycemic control measured by hemoglobin A1C. The combination of two weakly correlated biomarkers both independently associated with the outcome of interest, is more likely to significantly increase the c-statistic than the combination of two strongly correlated biomarkers.20 Therefore the lack of a strong correlation between serum NfL levels and traditional cardiovascular risk factors may explain why the addition of NfL to the FSRS significantly improved its c-statistic for stroke risk prediction.
Our findings regarding the determinants of serum NfL levels provide mechanistic insights regarding factors that contribute to subclinical cerebrovascular ischemic events. Interestingly, systolic but not diastolic BP was associated with increasing NfL levels. These findings are consistent with a prior study that higher mean systolic but not diastolic BP is associated with a higher number of brain infarcts including gross and microinfarcts.21 Further studies are needed to elucidate mechanisms underlining the association between systolic blood BP and serum NfL. Our study also found an association between decreased GFR and serum NfL levels, with subjects whose GFR was <60 ml/min/1.73m2 having 8 times higher odds of higher serum NfL levels than those with a GFR within a normal range. This suggests that serum NfL may be cleared by the kidneys and therefore renal function ought to be considered when interpreting serum NfL levels. Poor glycemic control and not the duration of diabetes was also found to be independently associated with serum NfL. This finding is in contrast to a prior study that reported the duration of diabetes to be a more important predictor of clinically overt ischemic stroke than glycemic control.22 However, in that study, glycemic control and duration of diabetes were dichotomized and not examined as a continuous variable. Additionally, the association between diabetes and subclinical stroke may be different from its association with clinically overt stroke. Until now, the primary modality for studying subclinical brain injury is MRI. Blood-based biomarkers offer a promising complementary approach to quantifying subclinical brain injury.
Our study has several methodologic strengths. These include using a well-phenotyped cohort and a highly sensitive digital immunoassay for measuring NfL levels. NfL levels were also measured in technical replicates. Furthermore, we compared the predictive accuracy of Nfl to an established and highly cited stroke risk prediction score.
Our study also has limitations. First, the study cohort consists of trial subjects with diabetes and at least 2 risk factors for cardiovascular disease and therefore findings are not necessarily generalizable to all populations. Relatedly, the case/control nature of our study population prevented calibration of the risk score to estimate absolute risk of future stroke. Future studies will examine the prognostic accuracy of serum NfL for stroke risk prediction in population-based samples and individuals at lower risk for stroke. Second, some participants in the control group were censored due to loss to follow-up. However, our sensitivity analysis restricted to those with complete follow-up at 3.5 years generated similar results. Third, the ACCORD dataset does not distinguish between ischemic and hemorrhage stroke, therefore, we are unable to examine the association between NfL and stroke subtype.
Summary
Serum NfL level is associated with incident stroke in a cohort of adults with diabetes and cardiovascular disease risk factors. The addition of serum NfL levels to the FSRS significantly improved the FSRS’s discriminative ability for predicting incident stroke. While these findings are preliminary, if validated in other studies we believe serum NfL levels could become a central feature of stroke prevention.
Acknowledgments
Sources of Funding:
Drs. Korley, Goldstick, Mastali, Levine and Van Eyk are supported by 5R21HL140274 from the National Heart Lung and Blood Institute (NHLBI)
Dr. Jennifer Van Eyk is supported by the Erika J. Glazer chair in Women’s Heart Health and the Advanced Clinical Biosystems Institute at Cedars Sinai Medical Center.
Dr. Jeremy Sussman is supported by the Veterans Affairs Career Development Award 13–021 and the Veterans Affairs Investigator-Initiated Research Award 15–432.
Dr. Levine reports research grants R01 NS102715 (National Institute of Neurological Disorders and Stroke), R01 AG051827 (National Institute on Aging); and consulting for NIH grants (modest) and University of California San Francisco on the Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) Trial (modest).
Footnotes
Social Media Handles
@UMichiganEM
@fkorley
All authors report no conflicts of interest.
Contributor Information
Frederick K. Korley, Department of Emergency Medicine, University of Michigan, 24 Frank Lloyd Wright Drive, Suite H3100, Ann Arbor, MI 48105
Jason Goldstick, Injury Prevention Center, Department of Emergency Medicine, University of Michigan, 2800 Plymouth Road, Suite B10-G080, Ann Arbor, MI 48109-2800.
Mitra Mastali, Advanced Clinical Biosystems Research Institute, The Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA.
Jennifer E. Van Eyk, Advanced Clinical Biosystems Research Institute, The Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA.
William Barsan, Department of Emergency Medicine, University of Michigan, 24 Frank Lloyd Wright Drive, Suite H3100, Ann Arbor, MI 48105.
William J. Meurer, Department of Emergency Medicine, University of Michigan, 24 Frank Lloyd Wright Drive, Suite H3100, Ann Arbor, MI 48105.
Jeremy Sussman, Department of General Medicine, University of Michigan, 2215 Fuller Road, Primary Care, Floor 1, Station 5, VA Ann Arbor Healthcare System, Ann Arbor, MI 48105
Hayley Falk, Department of Emergency Medicine, University of Michigan, 24 Frank Lloyd Wright Drive, Suite H3100, Ann Arbor, MI 48105.
Deborah Levine, Departments of Internal Medicine and Neurology, University of Michigan, 2800 Plymouth Road, NCRC 16-430W, Ann Arbor, MI 48109.
References
- (1).Williams SR, Lorenzano S. Seeking the “holy grail” of biomarkers to improve stroke risk prediction of clinical scores. Neurology. 2016;87:1194–1195. [DOI] [PubMed] [Google Scholar]
- (2).Lu CH, Macdonald-Wallis C, Gray E, Pearce N, Petzold A, Norgren N, et al. Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology. 2015;84:2247–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Kuhle J, Barro C, Andreasson U, Derfuss T, Lindberg R, Sandelius A, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. 2016;54:1655–1661. [DOI] [PubMed] [Google Scholar]
- (4).Rohrer JD, Woollacott IO, Dick KM, Brotherhood E, Gordon E, Fellows A, et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology. 2016;87:1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Oliver JM, Jones MT, Kirk KM, Gable DA, Repshas JT, Johnson TA, et al. Serum Neurofilament Light in American Football Athletes over the Course of a Season. J Neurotrauma. 2016;33:1784–1789. [DOI] [PubMed] [Google Scholar]
- (6).Kovacs KR, Czuriga D, Bereczki D, Bornstein NM, Csiba L. Silent brain infarction--a review of recent observations. Int J Stroke. 2013;8:334–347. [DOI] [PubMed] [Google Scholar]
- (7).Gupta A, Giambrone AE, Gialdini G, Finn C, Delgado D, Gutierrez J, et al. Silent Brain Infarction and Risk of Future Stroke: A Systematic Review and Meta-Analysis. Stroke. 2016;47:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Gattringer T, Pinter D, Enzinger C, Seifert-Held T, Kneihsl M, Fandler S, et al. Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology. 2017;89:2108–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).De Marchis GM, Katan M, Barro C, Fladt J, Traenka C, Seiffge DJ, et al. Serum neurofilament light chain in patients with acute cerebrovascular events. Eur J Neurol. 2018;25:562–568. [DOI] [PubMed] [Google Scholar]
- (10).Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Framingham Heart Study. Stroke (based on D’Agostino, Wolf, Belanger, Kannel ‘Stroke Risk Profile: Adjustment for Antihypertensive Medication’, Stroke 1994). 2018; Available at: https://www.framinghamheartstudy.org/fhs-risk-functions/stroke/. Accessed 6/27, 2018. [DOI] [PubMed]
- (12).Sullivan LM, Massaro JM, D’Agostino RBS. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23:1631–1660. [DOI] [PubMed] [Google Scholar]
- (13).Chutinet A, Rost NS. White matter disease as a biomarker for long-term cerebrovascular disease and dementia. Curr Treat Options Cardiovasc Med. 2014;16:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Duering M, Konieczny MJ, Tiedt S, Baykara E, Tuladhar AM, Leijsen EV, et al. Serum Neurofilament Light Chain Levels Are Related to Small Vessel Disease Burden. J Stroke. 2018;20:228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Tiedt S, Duering M, Barro C, Kaya AG, Boeck J, Bode FJ, et al. Serum neurofilament light: A biomarker of neuroaxonal injury after ischemic stroke. Neurology. 2018; 91:e1338–e1347 [DOI] [PubMed] [Google Scholar]
- (16).Bernick C, Kuller L, Dulberg C, Longstreth WT Jr, Manolio T, Beauchamp N, et al. Silent MRI infarcts and the risk of future stroke: the cardiovascular health study. Neurology. 2001;57:1222–1229. [DOI] [PubMed] [Google Scholar]
- (17).Bokura H, Kobayashi S, Yamaguchi S, Iijima K, Nagai A, Toyoda G, et al. Silent brain infarction and subcortical white matter lesions increase the risk of stroke and mortality: a prospective cohort study. J Stroke Cerebrovasc Dis. 2006;15:57–63. [DOI] [PubMed] [Google Scholar]
- (18).Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol. 2013;9:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Pepe MS, Thompson ML. Combining diagnostic test results to increase accuracy. Biostatistics. 2000;1:123–140. [DOI] [PubMed] [Google Scholar]
- (21).Arvanitakis Z, Capuano AW, Lamar M, Shah RC, Barnes LL, Bennett DA, et al. Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology. 2018;91:e517–e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Ashburner JM, Go AS, Chang Y, Fang MC, Fredman L, Applebaum KM, et al. Effect of Diabetes and Glycemic Control on Ischemic Stroke Risk in AF Patients: ATRIA Study. J Am Coll Cardiol. 2016;67:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.