Abstract
Existing studies evaluating the survival benefit of kidney transplantation were unable to incorporate time-updated information on decisions related to each organ offer. We used national registry data, including organ turn-down data, to evaluate the survival benefit of accepting vs. turning down kidney offers in candidates waitlisted from 2007–2013. Among candidates who declined their first offer, only 43% ultimately received organ transplantations. Recipients who were later received organ transplantations after declining their first offer had markedly longer wait-times than recipients who accepted their first offer, and 56% received kidney transplants that were similar or lower quality compared to their initial offer. In marginal structural modeling analyses accounting for time-updated offer characteristics (including Kidney Donor Profile Index, Public Health System risk status, and pumping), after three months post-transplantation, there was a significant survival benefit of accepting an offer (adjusted hazard ratio 0.76, 95% confidence interval 0.66–0.89) that was similar among diabetics, candidates aged >65 years, and candidates living in donor service areas with the longest waitlist times. After carefully accounting for the effect of donor quality, we confirm that the survival benefit of accepting an organ offer is clinically meaningful and persistent beyond three months post-kidney transplantation, including among high-risk subgroups of organ transplantation candidates.
1. INTRODUCTION
Widespread use of lower quality kidneys (i.e. kidneys with elevated risks of allograft failure or disease transmission) has increased in the United States over the past two decades as a necessary response to the growing demand for kidney transplantation (1). Despite growing acceptance of these lower quality donor kidneys, many viable deceased donor kidneys are declined, and ultimately discarded (2). Contributing factors to high ongoing rates of decline include insufficient data supporting the use of lower quality kidneys and uncertainty about how best to identify those candidates who are most likely to benefit from accepting them.
In 1999, Wolfe et al. published a landmark paper that was the first to clearly demonstrate a survival benefit of deceased-donor kidney transplantation over remaining on the waitlist using national registry data (3). Several subsequent studies have used registry data to investigate the survival benefit of transplantation among select subsets of lower quality donor kidneys, including expanded criteria donor kidneys (4), high kidney donor profile index (KDPI) kidneys (5), and diabetic donor kidneys (6) compared to remaining on the waitlist.
Despite having access to rich national registry data and use of rigorous study design techniques, these previous studies had methodologic constraints which may have limited their applicability to real-world practice. These studies were unable to identify which candidates actually had access to lower quality donor kidneys (i.e. because the candidate received an organ offer, as opposed to being on the waitlist without having necessarily received an offer), and how declining those kidneys (either directly by their transplant physician, or by refusing them after discussion with the physician) impacted their long-term outcomes. Specifically, these investigators lacked access to time-updated information about individual organ offers to wait-listed candidates. These data are critical as they account for differences in the quality of each donor; this type of information is essential to being able to evaluate causal relationships between accepting an organ offer and survival (7). Additionally, there are numerous important reasons for accepting or declining a particular organ. This type of confounding (i.e. confounding by indication) cannot be readily addressed using classic model adjustment; to eliminate this confounding requires either randomized assignment to a given treatment arm (which is neither ethically nor logistically feasible in the case of organ allocation), or methods that attempt to mimic this process, such as careful matching or weighting based on the decision to accept or decline a kidney (7–10).
The goal of this study was to leverage match-run data to more accurately evaluate the survival benefit of accepting an organ offer compared to turning it down and remaining on the waitlist for a “better” offer, particularly among high-risk subgroups of candidates including the elderly, diabetics, and individuals in donor service areas (DSAs) with the longest wait-times. Unlike previous studies, we are able to address differences in the quality of each donor offer, as well as the role of accepting versus declining each offer (rather than only comparing organ transplant recipients to everyone who remained on the waitlist but may not have necessarily even received, or been eligible for, an offer). Understanding limitations of previously available data and analytic techniques, we used a novel approach accounting for time-updated confounding to better evaluate how accepting or turning down an organ offer contributes to long-term kidney transplant candidate survival, especially in high-risk populations.
2. METHODS
2.1. Data Source
We used the Organ Procurement and Transplantation Network (OPTN) database, including data on all US donors, waitlisted candidates, and donor offers in the match-run that resulted in successful organ transplantation, submitted by members of the OPTN. The Health Resources and Services Administration (HSRA), US Department of Health and Human Services provides oversight to the activities of the OPTN contractor. We did not have access to match-run data for kidneys that were ultimately discarded. The study was approved by the Institutional Review Board at the University of Pennsylvania and the HRSA.
2.2. Subjects
Our cohort included all individuals aged ≥18 years who were waitlisted for a kidney transplant between May 1, 2007 and July 3, 2013. Organ offers were only included that were made between May 1, 2007 and July 3, 2013. Patients were followed from the date of waitlisting through the date of death (as reported by transplant centers) or June 1, 2015 (the last day of follow-up in the dataset), whichever was first. We excluded match-runs in which candidates were bypassed during the match-run process (11, 12) and donor kidneys that were DSA paybacks (12, 13). Individual organ offers that were accepted but that did not result in a successful transplantation were omitted from the analyses.
2.3. Outcomes and Covariates
The primary outcome was mortality. Candidates were censored at the time of organ transplantation if they received a living donor kidney transplant and at the time of removal from the waitlist. We selected factors for inclusion in the multivariable models a priori that have been previously demonstrated to be associated with mortality on the waitlist and following transplantation (6, 12–16). Please see the Supplemental Methods and Table S1 for detailed description of the selected covariates.
Understanding that kidney transplantation has historically been associated with an initial increased perioperative mortality risk that diminishes over time (3–6), we divided the hazard of mortality in all analyses into post-transplantation time intervals at 90-days, 183-days, 1-year, 2-years, 3-years, and beyond 3-years (6).
2.4. Statistical Analyses
We performed all statistical analyses using STATA version 15.0 (Statacorp LP, College Station, TX) with 2-sided hypothesis testing and a p-value of <0.05 to determine statistical significance. Median follow-up times were calculated using the Kaplan-Meier method to account for censoring.
2.4.1. “Classic Approach”: Time-Varying Cox Models to Address Immortal Time Bias
Given that patients enter the study at the time of waitlisting and not all patients receive an organ transplantation, analyses need to address differences in the timing of the exposure relative to being able to achieve the outcome (17, 18). We used Cox regression to estimate the adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for mortality after time-varying exposure to kidney transplantation (the “classic approach” previously performed by others (3–5) in non-contemporary cohorts). We also performed sensitivity analyses using Cox regression with time-varying exposure to any kidney allograft offer that was either accepted or declined, adjusting for offer characteristics (the “classic approach accounting for time-updated turn-down,” which we anticipated would produce biased results due to limitations of this analytic approach in its ability to address time-varying confounding (19); the results are reported in Tables S2–S3 and Figures S1–S2).
Although previous studies used the classic approach to address immortal time bias, they were unable to identify if patients actually received an organ offer (i.e. were eligible to receive organ transplant); they could only identify actual transplantation events. To better address this limitation using the added information in the match-run dataset, sensitivity analyses addressed mortality risk by restricting the cohort to candidates that 1) received at least one offer, with follow-up starting at the time of the first offer (Table S2, “The classic approach accounting for time-updated organ turn-down decisions, starting at the first offer”), 2) were always active (i.e. eligible to receive offers, Figure S2) on the waitlist, and 3) were preemptively waitlisted (i.e. waitlisted prior to initiating dialysis, Figure S3). We did not adjust for time-varying active status because of some general quality concerns with the OPTN status history file; active status was often changed several times in a short period, making it difficult to interpret the correct active status, and offers did not consistently corroborate with active status. Table S4 demonstrates the differences in baseline characteristics between candidates that were always active and those that were not. Additional sensitivity analyses closely evaluated donor and candidate risk factors by 1) stratifying the analyses by high-KDPI (>85%) vs. low-KDPI (≤85%) kidney offers among the overall cohort, and restricting to 2) diabetic candidates, 3) older candidates (>65 years), and 4) candidates in DSAs in the longest tertile of waitlist time (≥1058 days). All models incorporated robust sandwich estimation of the standard error to address clustering by donor and candidate (20).
2.4.2. Marginal Structural Modeling with Cox Regression to Address Time-Updated Confounding and Confounding by Indication
Marginal structural modeling was also applied to Cox regression analyses; marginal structural modeling is an analytical technique that incorporates time-updated confounders into a model, facilitating causal estimation of the relationship between a time-updated exposure and the outcome (7, 21–23). Weights (23) were calculated using each candidate’s probability of accepting or declining each successive organ offer, taking into account factors that change with each offer (including the KDPI of the donor offer, PHS risk status, pumping, and number of previous donor offers the candidate has received; see Table S1). Applying these weights creates a pseudo-population in which covariates are balanced across the population to attempt to mimic randomization at the time of each organ offer. Please see the Supplemental Methods for a detailed description of this methodologic approach.
Similar to the time-varying Cox models, sensitivity analyses restricted the cohort to candidates that 1) received at least one offer, with follow-up starting at the time of the first offer, and 2) were always active on the waitlist, 3) were preemptively waitlisted, 4) diabetic candidates, 5) older candidates (>65 years), and 6) candidates in DSAs in the longest tertile of waitlist time. We were not able to perform analyses stratifying by the KDPI of the kidney offer because marginal structural modeling can only be stratified using baseline characteristics, and because we expect KDPI to be on the causal pathway between decisions about organ offers and candidate survival (7).
3. RESULTS
3.1. Cohort Characteristics
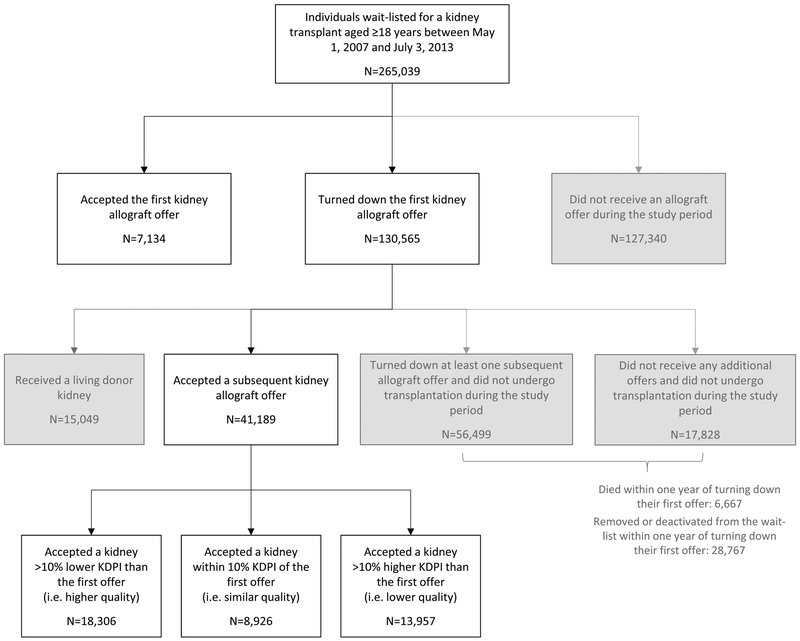
Median duration of follow-up of the cohort was 4.8 years. Median time from waitlisting until the first allograft offer was 2.5 years. Median time from waitlisting to accepting an allograft offer was 5.8 years. A total of 265,039 kidney transplant candidates met inclusion criteria (Figure 1), of which 137,699 (52%) ever received a kidney offer during follow-up. Although the marginal structural modeling analyses included all offers that candidates received, comparisons of decisions around the first offer also provide important insights supplementary to the modeling results. Among candidates who received offers, 7,134 (5%) accepted their first offer and 130,565 (95%) declined their first offer. A total of 74,327 candidates who declined their first offer never successfully received organ transplantations during follow-up; 6,667 candidates died and 28,767 candidates were delisted within one year of declining their first offer.
Figure 1.
Transplantation outcomes of waitlisted candidates based on decisions related to organ offers
Candidates who accepted their first kidney allograft offer, as opposed to declining their first offer, were slightly younger (Table 1; median age 51, interquartile range [IQR] 37–61 vs. 53, IQR 42–61 years), with longer dialysis vintage (median 370, IQR 66–993 vs. 272, IQR 0–692 days), located in DSAs with shorter wait-time (median 854, IQR 727–1194 days vs. 1129, IQR 823–1437 days), had greater sensitization (panel reactive antibody ≥80% in 25% vs. 10%), and were less likely to be male (53% vs. 63%), African American (27% vs. 29%), diabetic (33% vs 42%), and to be listed in competitive DSAs (28% vs. 35%). Candidates who accepted their first allograft offer were also more likely to be always active on the waitlist since initial waitlisting (64% vs. 43%) compared to candidates who declined their first offer. There were no clinically meaningful differences in body mass index or functional status across individuals who accepted vs. declined their first organ offer.
Table 1.
Transplant candidate characteristics at waitlisting among candidates that accepted vs. turned down the first organ offer
| Candidate’s first offer accepted N=7,134 | Candidate’s first offer turned down N=130,565 | P-value | |
|---|---|---|---|
| Candidate characteristics | |||
| Median age, years (IQR) | 51 (37, 61) | 53 (42, 61) | <0.001 |
| Male sex, n (%) | 3,800 (53%) | 81,670 (63%) | <0.001 |
| African American race, n (%) | 1,902 (27%) | 38,456 (29%) | <0.001 |
| Median body mass index, kg/m2 (IQR) | 27 (23, 31) | 28 (24, 32) | <0.001 |
| Cause of kidney disease, n (%) | <0.001 | ||
| Diabetes | 41,225 (32%) | 1,685 (24%) | |
| Hypertension | 29,825 (23%) | 1,330 (19%) | |
| Glomerular disease | 16,417 (13%) | 952 (13%) | |
| Cystic disease | 9,762 (7%) | 601 (8%) | |
| Other | 19,969 (15%) | 1,651 (23%) | |
| Unknown | 13,367 (10%) | 915 (13%) | |
| Diabetes mellitus, n (%) | 2,352 (33%) | 54,007 (42%) | <0.001 |
| Insurance type, n (%) | <0.001 | ||
| Private | 2,701 (38%) | 57,526 (44%) | |
| Public | 4,417 (62%) | 72,564 (56%) | |
| Self or donation | 10 (<1%) | 267 (<1%) | |
| Unknown | 6 (<1%) | 208 (<1%) | |
| Recipient functional status, n (%) | <0.001 | ||
| No Assistance | 4,842 (68%) | 90,062 (69%) | |
| Some Assistance | 1,896 (27%) | 30,417 (23%) | |
| Total Assistance | 124 (2%) | 1,240 (1%) | |
| Unknown | 272 (4%) | 8,846 (7%) | |
| Always active on the waitlist, n (%) | 4,599 (64%) | 55,938 (43%) | <0.001 |
| Median days from candidate waitlisting to offer (IQR) | 131 (41, 361) | 114 (43, 257) | <0.002 |
| Median dialysis vintage at waitlisting, days (IQR) | 370 (66, 993) | 272 (0, 692) | <0.001 |
| Maximum panel reactive antibody, n (%) | <0.001 | ||
| ≤20% | 4,264 (60%) | 97,793 (75%) | |
| 21–79% | 1,028 (14%) | 19,219 (15%) | |
| ≥80% | 1,812 (25%) | 13,510 (10%) | |
| Median time to transplantation by listing DSA (IQR) | 854 (727, 1194) | 1,129 (823, 1437) | <0.001 |
| Competitive DSA, n (%) | 1,981 (28%) | 45,541 (35%) | <0.001 |
| Waitlisted at an aggressive transplant center (top 25th percentile of centers accepting high-KDPI kidneys), n (%) | 1,501 (21%) | 31,747 (24%) | <0.001 |
Abbreviations: IQR = interquartile range; DSA = Donor Service Area; KDPI = Kidney Donor Profile Index; PHS = Public Health Service
Among candidates who accepted their first kidney offer as opposed to declining it, the donor tended to have a lower KDPI (median KDPI 40%, IQR 18–64 vs. 52%, IQR 27–76), and was less likely to be KDPI >85% (Table 2; 8% vs. 13% with KDPI >85%), PHS increased risk status (9% vs. 14%), donation after circulatory death (13% vs 18%), diabetic (6% vs. 10%), hypertensive (22% vs. 29%), or pumped (41% vs. 53%).
Table 2.
Donor characteristics at the time of the first organ offer and ultimately accepted offer among candidates that accepted vs. turned down the first organ offer
| Candidate’s first offer accepted | Candidate’s first offer turned down | P-value | ||
|---|---|---|---|---|
| Accepted donor characteristics N=7,134 | Characteristics of the donor kidney that was declined N=130,565 | Subsequent accepted donor kidney characteristics N=41,189 | ||
| Median days from candidate waitlisting to offer (IQR) | 131 (41, 361) | 114 (43, 257) | 748 (371, 1,155) | <0.001 |
| Donor Characteristics | ||||
| Median KDPI, % (IQR) | 40 (18,64) | 52 (27, 76) | 46 (24, 69) | <0.001 |
| High KDPI (>85%), n (%) | 570 (8%) | 17,139 (13%) | 4,084 (10%) | <0.001 |
| Median age, years (IQR) | 35 (21, 49) | 40 (24, 51) | 40 (25, 51) | <0.001 |
| Median body mass index, kg/m2 (IQR) | 26 (22, 30) | 27 (22, 32) | 26 (23, 31) | <0.001 |
| PHS increased risk donor, n (%) | 617 (9%) | 17,754 (14%) | 4,959 (12%) | <0.001 |
| Donation after circulatory death, n (%) | 945 (13%) | 23,766 (18%) | 6,343 (15%) | <0.001 |
| African American race, n (%) | 951 (13%) | 18,120 (14%) | 5,591 (14%) | <0.001 |
| Terminal creatinine ≥1.5 mg/dL, n (%) | 1,116 (16%) | 31,296 (24%) | 7,392 (18%) | <0.001 |
| Terminal creatinine ≥3.0 mg/dL, n (%) | 141 (2%) | 7,689 (6%) | 1,330 (3%) | <0.001 |
| Diabetes mellitus, n (%) | 428 (6%) | 12,522 (10%) | 3,113 (8%) | <0.001 |
| Hepatitis C virus antibody positive, n (%) | 219 (3%) | 943 (1%) | 794 (2%) | <0.001 |
| Hypertension, n (%) | 1,583 (22%) | 37,631 (29%) | 11,525 (28%) | <0.001 |
| Donor kidney pumped, n (%) | 2,827 (41%) | 67,979 (53%) | 18,349 (45%) | <0.001 |
| Median cold ischemia time, hours (IQR) | 15 (11, 21) | 20 (15, 29) | 16 (11, 22) | <0.001 |
| Weekend procurement, n (%) | 2,948 (41%) | 56,589 (43%) | 17,227 (42%) | <0.001 |
Abbreviations: IQR = interquartile range; KDPI = Kidney Donor Profile Index; PHS = Public Health Service
3.2. Organ Transplantation Outcomes for Patients who Declined their First Kidney Allograft Offer
Among those candidates who declined their first offer (N=130,565), 56,238 (43%) ultimately received organ transplantations before the end of the observation period (Figure 1, Table 2, Table S5), of which 41,189 were from deceased donors and 15,049 were from living donors. The median time from waitlisting to accepting an offer among those candidates who declined their first offer was 6.1 years. Compared to the initial donor kidneys that were declined, the donor kidneys that were eventually accepted had slightly lower frequencies of high KDPI (13% vs. 10%). However, 34% of the kidneys that were eventually accepted had at least 10% higher KDPI (i.e. were lower quality) compared to the initial donor kidneys that were declined, and 22% had similar KDPIs compared to the declined kidneys. The kidney donors that were ultimately accepted were similarly likely to be PHS increased risk status (14% vs. 12%), donation after circulatory death (18% vs. 15%), hypertensive (29% vs. 28%), diabetic (10% vs. 8%), pumped (53% vs. 45%), and procured on a weekend (43% vs. 42%).
In unadjusted analyses, candidates who declined their first offer but accepted a subsequent deceased donor kidney offer experienced 88% three-year all-cause allograft survival and 94% three-year death-censored allograft survival. In comparison, the ultimate recipients of kidneys that were declined as a first offer had 84% three-year all-cause allograft survival and 93% three-year death-censored allograft survival.
3.3. Survival Benefit of Accepting an Organ Offer
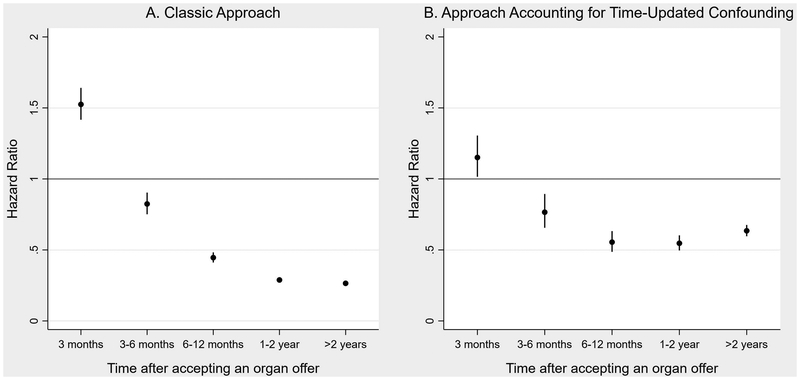
Using the classic approach applied to our more recent cohort, after adjusting for baseline candidate and donor covariates, there was a significantly elevated risk of mortality in the first three months post-transplantation compared to remaining on the waitlist (Figure 2A, Table S2; aHR 1.54, 95% CI 1.43–1.65), which dissipated after the first three months (3–6 month aHR 0.83, 95% CI 0.76–0.91; 2–3 year aHR 0.38, 95% CI 0.35–0.40).
Figure 2.
Survival benefit of organ transplantation vs. remaining on the waitlist based on A) the classic approach and B) marginal structural modeling taking into account organ turn-downs and time-updated confounding
Using marginal structural modeling accounting for time-updated confounders, accepting an organ offer was associated with an attenuated but significantly increased risk of mortality in the first three months compared to declining it and remaining on the waitlist (Figure 2B, Table S6; aHR 1.15, 95% CI 1.01–1.30); the mortality risk again declined after the first three months (3–6 month aHR 0.76, 95% CI 0.65–0.89; 2–3 year aHR 0.53, 95% CI 0.48–0.59).
3.4. Survival Benefit of Accepting an Organ Offer Among High-Risk Candidates
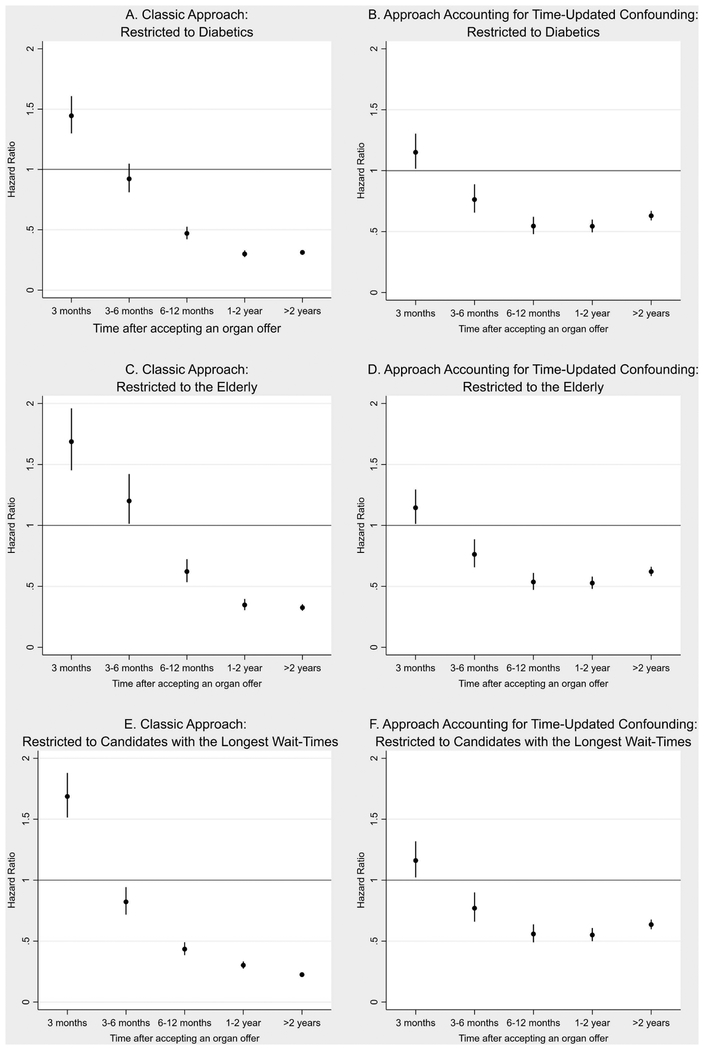
Using the classic approach, diabetic candidates had a similarly increased risk of mortality in the first three months post-transplantation as the overall population (Figure 3A, Table S7; aHR 1.46, 95% CI 1.31–1.62); however, they did not achieve a survival benefit from organ transplantation until after six months post-transplantation (3–6 month aHR 0.93, 95% CI 0.82–1.06; 6–12 month aHR 0.47, 95% CI 0.43–0.53). Using marginal structural modeling accounting for donor quality of each offer, diabetic candidates had a similarly increased risk of mortality in the first 3 months after accepting an offer as the overall population (Figure 3B, Table S8; aHR 1.14, 95% CI 1.00–1.28), with survival benefit appreciated beginning at 3 months after accepting an organ offer (3–6 month aHR 0.74, 95% CI 0.63–0.88).
Figure 3.
Survival benefit of organ transplantation compared to remaining on the waitlist A) restricted to diabetic candidates using the classic approach, B) restricted to diabetic candidates using marginal structural modeling taking into account organ turn-down and time-updated confounding, C) restricted to patients >65 years of age using the classic approach, D) restricted to patients >65 years of age using marginal structural modeling taking into account organ turn-down and time-updated confounding, E) restricting to candidates in the longest tertile of waiting time using the classic approach, and F) restricting to candidates in the longest tertile of waiting time using marginal structural modeling taking into account organ turn-down and time-updated confounding
Among candidates older than age 65 at the time of waitlisting, using the classic approach, there was a significantly increased mortality risk for the first six months following organ transplantation (Figure 3C, Table S7; <3 month aHR 1.70, 95% CI 1.46–1.97; 3–6 month aHR 1.21, 95% CI 1.02–1.44), with a survival benefit beginning after six months (6–12 month aHR 0.63, 95% CI 0.54–0.73). Using marginal structural modeling, older candidates had a slightly increased mortality risk in the first 3 months after accepting an organ offer (Figure 3D, Table S8; aHR 1.13, 95% CI 1.00–1.28) that resolved at 3 months (3–6 month aHR 0.76, 95% CI 0.64–0.89).
Restricting the analyses to candidates in DSAs with the longest waitlist times (≥1058 days) and using the classic approach, there was again a significantly increased risk of mortality in the first three months after transplantation (Figure 3E, Table S7; aHR 1.69, 95% CI 1.52–1.89) that improved after three months (3–6 month aHR 0.83, 95% CI 0.72–0.95). Using marginal structural modeling, there was also a significantly increased mortality risk in the first three months after accepting an organ offer (Figure 3F, Table S8; aHR 1.14, 95% CI 1.01–1.30), with a survival benefit beginning after that time (3–6 month aHR 0.75, 95% CI 0.63–0.88).
3.5. Survival Benefit of Accepting an Organ Offer with Survival Time Measured from the First Offer and Among Always-Active Candidates
Applying marginal structural modeling, there was a significantly increased mortality risk in the first three months after accepting an organ offer (Figure S1, Table S2, Table S6; <3 month aHR 1.39, 95% CI 1.23–1.60), with a survival benefit beginning at six months (3–6 month aHR 0.98, 95% CI 0.85–1.16; 6–12 month aHR 0.61, 95% CI 0.56–0.73). Among always-active candidates, there was an attenuated mortality risk in the first three months after accepting an organ offer (Figure S2, Table S5, Table S6; aHR 1.11, 95% CI 0.96–1.27), with a survival benefit beginning at three months (3–6 month aHR 0.73, 95% CI 0.62–0.86).
4. DISCUSSION
In this study, we found a significant survival benefit to accepting a kidney allograft offer (beginning at three months following acceptance) compared to declining an offer and remaining on the waitlist in hopes of receiving a “better” offer; this finding persisted across multiple high-risk candidate populations. Turning down an organ offer was associated with a markedly longer wait until transplantation without clear benefit; the majority of kidneys that were ultimately accepted by those candidates who declined their first offer were similar or lower quality compared to the initial offer. Correspondingly, the three-year allograft survival of recipients of donor kidneys that were declined as a first offer was similar to that achieved by recipients who declined their first offer to theoretically wait for a “better” offer (all-cause allograft survival 84% vs. 88%; death-censored allograft survival 93% vs. 94%).
We compared two analytic techniques, time-varying Cox modeling (“the classic approach”) and marginal structural modeling, to address methodologic challenges related to understanding the outcomes of decisions surrounding kidney allograft offers. While the classic approach addresses important biases related to follow-up time (17, 18), it cannot appropriately address factors that differ with each organ offer that can greatly impact the decision to accept or decline a particular organ (24–26) (see Table S2 for an example of how the results of the “classic approach” become biased after attempting to account for time-updated confounders associated with successive organ offers). When evaluating the survival benefit of accepting vs. turning down organ offers, marginal structural modeling is the more appropriate approach, because it aptly addresses important factors that change with each donor offer, such as KDPI and HLA matching. Using marginal structural modeling, the initial three month post-transplant period was associated with an increased mortality risk (versus staying on the waiting list) that was attenuated compared to the classic approach. There continued to be a survival benefit beginning at three months after accepting a kidney allograft offer, but this benefit was also diminished compared to the classic approach. This attenuation in the results compared to the classic approach is due to the distinct ability of marginal structural modeling to properly account for candidate and donor characteristics that vary with each organ offer. Marginal structural modeling demonstrated a similar survival benefit among several populations of high-risk candidates, including diabetics, the elderly, and candidates with longer DSA waiting times.
Using standard time-varying Cox modeling, Massie et al. previously demonstrated that there was a survival benefit to accepting a high-KDPI kidney, specifically among older individuals who had long anticipated wait-times (5). In contrast to this and other existing studies (3–6), we took advantage of match-run data, which allowed us to take into account not only candidates who accepted organ offers (i.e. those patients who received organ transplantations), but also to analyze follow-up data related to those candidates who declined organ offers. A recent study by Wey et al. used these data to develop a prediction model for allograft survival based on individual organ acceptances and turn-downs (27). In the study, Wey et al. provide important insights with regard to decision-making around organ offers, and demonstrated attenuated survival benefit from high-KDPI kidneys among candidates at the top of the match-run or with restricted donor pools. Instead of using a time-varying approach, Wey et al. modeled accepted and declined organs separately to focus on specific predictors of individual offer outcomes. Distinct from our study, the models were not intended to adjust for time-varying confounders related to the broader, population-level survival benefit of accepting vs. turning down organ offers. Nonetheless, unlike Massie (5) and Wey’s (27) approaches, we were not able to use marginal structural modeling to assess for differences in outcomes across low-KDPI vs. high-KDPI kidneys, only to assess survival benefit of accepting an offer after accounting for each offer’s KDPI status.
When we altered the start of follow-up to the date of the first offer and restricted the analyses to only individuals who received at least one offer, marginal structural modeling demonstrated a delayed survival benefit of accepting an organ offer until six months after acceptance. These analyses highlight the importance of selecting the appropriate start date for the question at hand, and the possibility of (intentionally or unintentionally) introducing bias based on that decision. Starting at the time of waitlisting addresses the question of survival benefit of accepting a kidney offer among all potential transplant candidates, including those patients on the waitlist who may not truly be eligible for organ transplantation and who never survive to receive an organ offer. Starting at the time of the first offer restricts the study to only those individuals who survive long enough, and remain active on the waitlist long enough, to receive organ offers. Accordingly, the comparison population is likely to be have more robust overall health, consistent with our findings of the delayed survival benefit of accepting a kidney allograft offer in this population.
Our study has several limitations, primarily related to the quality and breadth of the data currently available. While marginal structural modeling is a valuable tool for addressing time-updated confounding, it is prone to bias from unmeasured confounding (28). We did not have data available on time-updated waitlisted candidate factors that may increase mortality risk, such as development of new comorbidities (e.g. new diabetes, or worsening functional status). We also lacked information on some reasons for turndown that may contribute to organ outcomes, such as anatomical abnormalities, cross-clamping prior to the offer, or anticipated cold ischemia time at the time of the offer. Future studies with more details time-updated data could make valuable additional contributions to our understanding of survival benefit from transplantation if used with marginal structural modeling techniques. We also did not have accurate time-updated information on active status on the waitlist, nor information on donor offers of organs that were ultimately discarded. Additionally, we did not have the ability to discriminate if organ turn-downs were patient- or center-level decisions. Correspondingly, while we adjusted for DSA-level wait-time and competition, we could not account for heterogeneous, center-specific behaviors related to offers. For example, when receiving high-risk donor offers, some centers may select sicker candidates further down on the waitlist, intentionally passing over candidates with greater anticipated post-transplant survival (i.e. who may have more to gain from a higher quality organ). Consequently, our results are best interpreted at the population-level, rather than the distinct center-level.
In conclusion, the survival benefit of accepting an organ offer may be somewhat overestimated by traditional methods, and declining an offer to wait for a “better” offer often results in negative consequences. This study provides clear evidence that accepting a kidney allograft offer provides considerable long-term survival benefit beginning three months after the organ offer. While the specific clinical circumstances and preferences of individual patients must be taken into account (particularly in light of waitlist priority changes due to the 2014 modified allocation policy), our study contributes one more piece of evidence that transplant clinicians should cautiously expand acceptance patterns at the margin. Our study leveraged high-dimensional data and advanced epidemiologic methods to account for time-updated donor-offer quality. Improved collection of time-updated candidate data by transplant centers and organ procurement organizations would promote greater precision in the investigation of the risks and benefits of transplantation among high-risk donors and candidates.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported in part by the National Institutes of Health grant number K23-HL133843 (NHLBI, PI: Cohen). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the National Institutes of Health.
The data reported here have been supplied by the United Network of Organ Sharing as the contractor for the OPTN. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
Abbreviations:
- aHR
adjusted hazard ratio
- CI
confidence interval
- DSA
donor service area
- HRSA
Health Resources and Services Administration
- IQR
interquartile range
- KDPI
kidney donor profile index
- OPTN
Organ Procurement and Transplantation Network
- PHS
Public Health Service
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Data for this study were supplied by the United Network of Organ Sharing. The statistical code used to generate the cohort and perform the analyses is available upon request from the corresponding author.
SUPPORTING INFORMATON
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Mohan S, Tanriover B, Ali N, et al. Availability, utilization and outcomes of deceased diabetic donor kidneys; analysis based on the UNOS registry. Am J Transplant. 2012;12(8):2098–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrero WJ, Naik AS, Friedewald JJ, et al. Predictors of Deceased Donor Kidney Discard in the United States. Transplantation. 2016. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–30. [DOI] [PubMed] [Google Scholar]
- 4.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294(21):2726–33. [DOI] [PubMed] [Google Scholar]
- 5.Massie AB, Luo X, Chow EK, et al. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant. 2014;14(10):2310–6. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JB, Eddinger KC, Locke JE, et al. Survival Benefit of Transplantation with a Deceased Diabetic Donor Kidney Compared with Remaining on the Waitlist. Clin J Am Soc Nephrol. 2017;12(6):974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–60. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbaum PR. Attributing effects to treatment in matched observational studies. J Am Statist Assoc. 2002;97(457):183–92. [Google Scholar]
- 9.Li YP, Propert KJ, Rosenbaum PR. Balanced risk set matching. J Am Statist Assoc. 2001;96(455):870–82. [Google Scholar]
- 10.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.United Network for Organ Sharing [password-protected]. DonorNet guidance document. https://portal.unos.org/Donor_Net/help/donornet/Complete_PTR_Close_Match.htm. Accessed 10 Jul 2018. [Google Scholar]
- 12.Cohen JB, Shults J, Goldberg DS, et al. Kidney allograft offers: Predictors of turndown and the impact of late organ acceptance on allograft survival. Am J Transplant. 2018;18(2):391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith JM, Biggins SW, Haselby DG, et al. Kidney, pancreas and liver allocation and distribution in the United States. Am J Transplant. 2012;12(12):3191–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erickson KF, Zheng Y, Winkelmayer WC, et al. Consolidation in the Dialysis Industry, Patient Choice, and Local Market Competition. Clin J Am Soc Nephrol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Israni AK, Salkowski N, Gustafson S, et al. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol. 2014;25(8):1842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seem DL, Lee I, Umscheid CA, et al. PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation. Public Health Rep. 2013;128(4):247–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sylvestre MP, Huszti E, Hanley JA. Do Oscar winners live longer than less successful peers? A reanalysis of the evidence. Ann Intern Med. 2006;145(5):361–3; discussion 92. [DOI] [PubMed] [Google Scholar]
- 18.Beyersmann J, Gastmeier P, Wolkewitz M, et al. An easy mathematical proof showed that time-dependent bias inevitably leads to biased effect estimation. J Clin Epidemiol. 2008;61(12):1216–21. [DOI] [PubMed] [Google Scholar]
- 19.Mansournia MA, Etminan M, Danaei G, et al. Handling time varying confounding in observational research. BMJ. 2017;359:j4587. [DOI] [PubMed] [Google Scholar]
- 20.Lin DY, Wei LJ. The Robust Inference for the Cox Proportional Hazards Model. J Am Statist Assoc. 1989;84(408):1074–8. [Google Scholar]
- 21.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–25. [DOI] [PubMed] [Google Scholar]
- 22.Joffe MM, Ten Have TR, Feldman HI, et al. Model selection, confounder control, and marginal structural models: Review and new applications. American Statistician. 2004;58(4):272–9. [Google Scholar]
- 23.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austin PC, Platt RW. Survivor treatment bias, treatment selection bias, and propensity scores in observational research. J Clin Epidemiol. 2010;63(2):136–8. [DOI] [PubMed] [Google Scholar]
- 25.Daniel RM, Cousens SN, De Stavola BL, et al. Methods for dealing with time-dependent confounding. Stat Med. 2013;32(9):1584–618. [DOI] [PubMed] [Google Scholar]
- 26.Greenland S Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology. 2003;14(3):300–6. [PubMed] [Google Scholar]
- 27.Wey A, Salkowski N, Kremers WK, et al. A kidney offer acceptance decision tool to inform the decision to accept an offer or wait for a better kidney. Am J Transplant. 2018;18(4):897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brumback BA, Hernan MA, Haneuse SJ, et al. Sensitivity analyses for unmeasured confounding assuming a marginal structural model for repeated measures. Stat Med. 2004;23(5):749–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.