Abstract
Objective
To assess the performance of reusable pulse oximeter probe and microprocessor box combinations, of varying price‐points, in the context of a low‐income pediatric setting.
Methods
A prospective, randomized cross‐over study comparing time to biologically plausible oxygen saturation (SpO2) between: (1) Lifebox LB‐01 probe with Masimo Rad‐87 box (L + M) and (2) a weight‐appropriate reusable Masimo probe with Masimo Rad‐87 box (M + M). A post hoc secondary analysis comparison with historical usability testing data with the Lifebox LB‐01 probe and Lifebox V1.5 box (L + L) was also conducted. Participants, children aged 0 to 35 months, were recruited from pediatric wards and outpatient clinics in the central region of Malawi. The primary outcome was time taken to achieve a biologically plausible SpO 2 measurement, compared using t tests for equivalence.
Results
We recruited 572 children. Plausible SpO2 measurements were obtained in less than 1 minute, 71%, 70%, and 63% for the M + M, L + M, and L + L combinations, respectively. A similar pattern was seen for less than 2 minutes, however, this effect disappeared at less than 5 minutes with 96%, 96%, and 95% plausible measurements. Using a ±10 second threshold for equivalence, we found L + M and M + M to be equivalent, but were under‐powered to assess equivalence for L + L.
Conclusions
The novel reusable pediatric Lifebox probe can achieve a quality SpO2 measurement within a pragmatic time range of weight‐appropriate Masimo equivalent probes. Further research, which considers the cost of the devices, is needed to assess the added value of sophisticated motion tolerance software.
Keywords: hypoxemia, LMIC, pediatric, pulse oximeter
1. INTRODUCTION
Hypoxemia, an oxygen saturation (SpO2) less than 90%, is a considerable risk for child pneumonia mortality in low‐middle income countries (LMIC).1 Pulse oximetry allows for accurate and noninvasive diagnosis of hypoxemia, but in the absence of oximetry, health providers rely on clinical observations to diagnose severe pneumonia and determine the need for oxygen therapy.2 Clinical signs lack accuracy in predicting hypoxemia with pulse oximetry identifying 20% to 30% more hypoxemic cases than clinical signs alone.3, 4, 5 Additionally, identifying clinical signs of severe pneumonia, often by nonphysician clinicians or community health workers, remains inconsistent and unreliable.6, 7, 8, 9
Given oxygen availability, universal implementation of pulse oximetry in the 15 highest pneumonia burden countries could avert 148 000 deaths annually.10 Despite this, evidence on the uptake of pulse oximetry in LMIC is limited. Available estimates suggests it remains low, ranging from less than 30% to more than 70% across different LMIC settings.11, 12 There are examples of pulse oximeter implementation being feasible in LMIC settings, including Malawi and Nigeria, and resulting in improved referral decision‐making.13, 14 Barriers to wider implementation include cost, lack of training and supervision, and lack of robust pulse oximeters and probes. In pediatric populations additional barriers include the lack of a high‐quality, reusable, low‐cost probe that fits all ages of children and is tolerant to movement.15
To facilitate routine pulse oximeter implementation and scale‐up, evidence of low‐cost but high‐quality devices being usable in busy clinical settings, typical of many LMIC settings is needed. In response to this call, the Lifebox Foundation led a project to develop a universal pediatric probe in 2016.16 Using a human centered design approach to probe development with end‐user usability testing in the United Kingdom, Bangladesh, and Malawi, Lifebox developed a novel probe.17 Usability testing found that among 1307 SpO2 results, 81% biologically plausible measurements were achieved in less than 2 minutes.17
This study builds on this work, assessing how the redesigned Lifebox probe functions when paired with a market leading oximeter microprocessor from Masimo that includes motion and low‐perfusion tolerance software. We aimed to compare this performance with the same Masimo microprocessor and its weight‐appropriate Masimo probe on the same child, to give a direct Lifebox vs Masimo probe comparison. As a secondary objective, we also sought to compare these measurements to historical results that used the redesigned probe with the standard Lifebox V1.5 oximeter microprocessor, which is not enhanced with motion tolerance or low‐perfusion software.
2. MATERIALS AND METHODS
We conducted a prospective, randomized cross‐over study comparing (1) the novel Lifebox LB‐01 probe paired with a Masimo Rad‐87 oximeter box (L + M) and (2) a weight‐appropriate reusable Masimo probe paired with the Masimo Rad‐87 oximeter box (M + M; Box 1). The LB‐01 probe used with the Masimo Rad‐87 box was specifically adapted to be compatible for the purposes of this study and is not standard for devices available in the market. Data collection was conducted in May 2018.
Box 1. Testing protocol for different age and weight categories.

|
We conducted a post hoc secondary comparison with existing data on the LB‐01 probe paired with the Lifebox V1.5 box (L + L), to explore the added value of motion tolerance capacity. The methods for this study have been reported previously, and data collection was done in February to July 2017.17
2.1. Settings
Testing was conducted in the central region of Malawi, across three hospitals: Kamuzu Central Hospital (KCH) and Bwaila Hospital in Lilongwe district, and Mchinji District Hospital, in Mchinji district. KCH is a large, tertiary, referral hospital, Bwaila Hospital provides outpatient care only; L + M and M + M data were collected from these sites. Mchinji district hospital provides inpatient and outpatient care, and L + L data were collected from this site.
2.2. Recruitment
Children were purposefully recruited using convenience sampling from inpatient and outpatient settings. All children recruited during the cross‐over equivalence study contributed data to the analyses; Figure S1 shows participant inclusion from the historical data. Patients were eligible if they were 0 to 35 months of age excluding those: receiving oxygen therapy; with a nasogastric tube; with a congenital limb malformation; and simultaneously receiving care from a healthcare worker.
2.3. Sample size
For the cross‐over study, we were powered to determine equivalence. This required 340 patients to be tested with both L + M and M + M for 80% power to determine equivalence in time to successful measurement within a ±10 second range, with a standard deviation of 40 and a mean time to measurement of 51 seconds. The sample for the L + L testing was based on those meeting eligibility criteria within the existing data set; we did not conduct an a priori power calculation for this analysis.
2.4. Data collection
All measurements were conducted by physicians with expertize in pediatric pulse oximetry (TM, EDM, KS, BZ, and NB), following training in the study protocol. For the cross‐over study, two pulse oximetry readings were conducted per child, separated by a 5‐minute washout period, allowing the child to settle and reduce potential measurement bias by the tester. The order in which the probes were used was randomly assigned using a random number generator at the point of testing, within the ODK software used for data collection.18
The measurement procedure was the same for L + L and the cross‐over study. The tester placed the probe on the foot, toe, or finger of the child, depending on age and weight (Box 1 and Figure 1). An independent observer, a researcher who had received training in the study protocol and was experienced in pulse oximetry but not necessarily clinically qualified, recorded the time from when probe placement was completed to a biologically plausible reading announced by the tester, by them stating “stop.” Biologically plausible was defined as having an age appropriate pulse rate above the approximate 10th centile for age,19 and a consistent waveform or quality signal, depending on the oximeter box. The observer noted the condition of the child, location of probe placement, number of adjustments, and any issues during the measurement. Neither the tester, observer, or participants were blinded due to the nature of the measurement; however, randomization was done at the point of testing after a participant was recruited, and the tester could not see the timer during measurements.
Figure 1.

Photograph of the LB‐01 probe in use [Color figure can be viewed at wileyonlinelibrary.com]
2.5. Analysis
The primary outcome was the difference in time to a plausible SpO2 reading between L + M and M + M, based on a cross‐over design. The primary analysis approach was testing equivalence, defined as ±10 seconds in the mean time to successful measurement (ie, a measurement between 50 and 70 seconds is equivalent to 60 seconds). We chose equivalence, rather than noninferiority, as we did not hypothesize that M + M would necessarily outperform the L + M combination. We deemed ±10 seconds to be a pragmatic range that would not significantly impact routine care in a busy LMIC pediatric setting. We evaluated this through two one‐sided t tests, using the ‐tostt‐ command in Stata 14.20 The comparison between L + M and M + M took into account the paired nature of the data.
A post hoc secondary analysis was conducted to compare the M + M measurements to historical L + L measurements, using the same definition of equivalence. Additionally, we described the median time to SpO2, and proportion of SpO2 readings within less than 1, 2, and 5 minutes, and conducted a multivariable analysis to examine factors associated with a successful SpO2 in less than 1 and 2 minutes, with robust standard errors to account for clustering at the participant level. These models included, probe and box combination, order of the measurement, child's condition, age, and weight. Other potential confounders were investigated for an association between testing rounds and were included if there was a difference. All analyses were conducted using Stata 14.
2.6. Ethics
This study was approved by the National Health Science Research Committee of Malawi (ref: 16/4/1570), University College London (ref: 8075/003), and Johns Hopkins (IRB00047406). Verbal consent was obtained from all caregivers.
3. RESULTS
3.1. Patient characteristics
Overall 572 children were recruited, 232 in L + L testing and 340 in L + M and M + M testing (Table S1). There were significant differences in the presenting diagnoses of children recruited for different testing rounds, with 37% of L + L participants classified as healthy compared with 22% of L + M/M + M (ie, attending for routine postnatal care or vaccination clinics), and 27% of L + L children with acute respiratory infections vs 43% of L + M/M + M children (P < 0.001). There was a higher proportion of agitated and crying children in the L + L testing (28%), compared with L + M (12%) and M + M measurements (13%; P < 0.001).
3.2. Testing procedures
Overall 174 of 340 (51%) of the cross‐over study measurements were randomized to L + M first, and we did not observe any differences in patient characteristics based on randomization order (Table 1). Seventy‐five percent of SpO2 measurements were on the child's toe, followed by 23% on the child's foot. As recorded by the independent observer, there was no difference in the number of probe repositions between the different probe and oximeter combinations (0 repositions: 85% M + M, 84% L + M, 80% L + L; P = 0.704). There were 13 cases where issues during the measurement were attributed to the Lifebox oximeter box, three to the Masimo oximeter box, seven to the Lifebox probe, and 10 for the Masimo probes (9 = wrap and 1 = pediatric clip). Reported issues were similar between devices and included: poor quality signals, slow or no presentation of SpO2 results, and implausibly low pulse rates.
Table 1.
Description of patient's recruited to the cross‐over study and characteristics according to the order of randomization
| Participant characteristics | Overall (N = 340) | M+M first (N = 166) | L+M first (N = 174) | P value a | |
|---|---|---|---|---|---|
| Age, median (IQR), mo | 6 (0‐16) | 6 (1‐14) | 6 (1‐13) | 0.985 | |
| Weight, mean (SD), kg | 7.3 (3.1) | 7.5 (3.1) | 7.2 (3.0) | 0.777 | |
| Skin color | |||||
| Black | 336 (99%) | 164 (99%) | 173 (99%) | 0.535 | |
| White | 3 (1%) | 2 (1%) | 1 (1%) | ||
| Primary diagnosis | |||||
| ARI | 145 (43%) | 70 (42%) | 75 (43%) | 0.855 | |
| Fever | 77 (23%) | 40 (24%) | 37 (21%) | ||
| Healthy | 75 (22%) | 34 (20%) | 41 (24%) | ||
| Other | 43 (13%) | 22 (13%) | 21 (12%) | ||
| Recruitment location | |||||
| Lilongwe (outpatient) | 167 (49%) | 78 (47%) | 89 (51%) | 0.443 | |
| Lilongwe (inpatient) | 173 (51%) | 88 (53%) | 85 (49%) | ||
| SpO2, mean (SD) | 96.2 (3.48) | 96.1 (3.38) | 96.3 (3.59) | 0.559 | |
| Time to measurement, median (IQR) | 33 (23‐66) | 33 (23‐69) | 33 (23‐58) | 0.311 | |
Abbreviations: ARI, acute respiratory infections; IQR, interquartile range; L + M, Lifebox LB‐01 probe with Masimo Rad‐87 box; M + M, a weight‐appropriate reusable Masimo probe with Masimo Rad‐87 box.
χ2 test for binary and categorical variables, and t test for continuous variables.
3.3. Equivalence
The mean time to L + M measurement was 51.9 seconds (95% confidence interval [CI]: 47.1, 56.7) and 52.5 seconds (95% CI: 47.6, 57.5) for the M + M combination (Figure 2). Using the ±10 second threshold for equivalence, we found L + M and M + M to be equivalent (P < 0.001 and 0.002). They were equivalent down to a threshold of ±7 seconds (P = 0.003 and 0.033).
Figure 2.
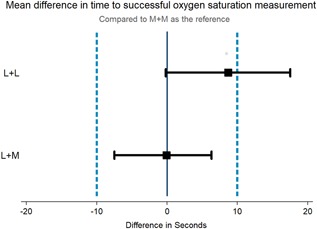
Mean difference in time to biologically plausible oxygen saturation measurement. L + L, Lifebox LB‐01 probe and Lifebox V1.5 box; L + M, Lifebox LB‐01 probe with Masimo Rad‐87 box; [Color figure can be viewed at wileyonlinelibrary.com]
The mean time to measurement for the L + L combination was 61.2 seconds (95% CI: 53.3, 69.1)—an average of 8.7 seconds longer than the M + M measurements. We did not have sufficient power to test for equivalence between L + L and M + M measurements.
3.4. Time to successful SpO2 measurement
Table 2 shows the proportion of biologically plausible SpO2 measurements for device combinations across age and weight groups. Plausible SpO2 measurements were obtained in less than 1 minute, 71%, 70%, and 63% for the M + M, L + M, and L + L combinations, respectively. A similar pattern was seen for less than 2 minutes, however, this effect disappeared at less than 5 minutes. Performance across age and weight groups showed a clear trend favoring M + M for the less than 2‐month age group, with 78%, 62%, and 48% measurements in less than 1 minutes for M + M, L + M, and L + L, respectively. However, by less than 2 minutes this distinction was no longer observed for M + M and L + M (89% and 88%). All device combinations performed better in children more than 10 kg, while performance was mixed when comparing less than 2 months with 2 to 11 months.
Table 2.
Description of time to reading, comparing the three different probe and device combinations, stratified by age and weight groups
| Test round | Total SpO2 tests | Biologically plausible SpO2 < 1 min, n (%) | 95% CI | Biologically plausible SpO2 < 2 min n, (%) | 95% CI | Biologically plausible SpO2 < 5 min, n (%) | 95% CI | Time, median (IQR), s |
|---|---|---|---|---|---|---|---|---|
| M + M | ||||||||
| Overall | 340 | 240 (71) | 65‐75 | 297 (87) | 83‐91 | 328 (96) | 94‐98 | 33 (23‐66) |
| 0‐2, mo | 94 | 73 (78) | 68‐86 | 84 (89) | 81‐95 | 93 (99) | 94‐100 | 28 (23‐49) |
| 2‐11, mo | 137 | 83 (61) | 52‐69 | 112 (82) | 74‐88 | 127 (93) | 87‐96 | 44 (26‐82) |
| 12‐35, mo | 109 | 84 (77) | 68‐85 | 101 (93) | 86‐97 | 108 (99) | 95‐100 | 29 (22‐57) |
| <10, kg | 271 | 179 (66) | 60‐72 | 230 (85) | 80‐89 | 260 (96) | 93‐98 | 37 (24‐74) |
| ≥10, kg | 68 | 60 (88) | 78‐95 | 66 (97) | 90‐100 | 67 (99) | 92‐100 | 26 (21‐40) |
| L + M | ||||||||
| Overall | 340 | 237 (70) | 65‐75 | 306 (90) | 86‐93 | 328 (96) | 94‐98 | 34 (24‐68) |
| 0‐2, mo | 95 | 59 (62) | 52‐72 | 84 (88) | 80‐94 | 92 (97) | 91‐99 | 39 (26‐86) |
| 2‐11, mo | 137 | 91 (66) | 57‐73 | 118 (86) | 78‐91 | 130 (95) | 89‐97 | 38 (27‐69) |
| 12‐35, mo | 109 | 88 (81) | 73‐89 | 105 (96) | 92‐99 | 107 (98) | 95‐100 | 28 (20‐41) |
| <10, kg | 271 | 177 (65) | 59‐71 | 238 (88) | 83‐91 | 259 (96) | 92‐98 | 38 (26‐73) |
| ≥10, kg | 68 | 59 (87) | 76‐94 | 67 (99) | 92‐100 | 68 (100) | 95‐100 | 25 (19‐35) |
| L + L | ||||||||
| Overall | 232 | 147 (63) | 57‐70 | 186 (80) | 74‐85 | 221 (95) | 92‐98 | 35 (20‐84) |
| 0‐2, mo | 71 | 34 (48) | 36‐60 | 53 (75) | 63‐84 | 69 (97) | 90‐100 | 65 (31‐113) |
| 2‐11, mo | 65 | 38 (58) | 46‐71 | 49 (75) | 63‐85 | 59 (91) | 81‐97 | 39 (20‐95) |
| 12‐35, mo | 96 | 75 (78) | 69‐86 | 84 (88) | 79‐93 | 93 (97) | 91‐99 | 22 (16‐46) |
| 10, kg | 164 | 93 (57) | 49‐64 | 127 (77) | 70‐84 | 155 (95) | 90‐97 | 44 (20‐100) |
| ≥10, kg | 59 | 48 (81) | 69‐90 | 52 (88) | 77‐95 | 57 (97) | 88‐100 | 23 (16‐46) |
Abbreviations: CI, confidence interval; IQR, interquartile range; L + L, Lifebox LB‐01 probe and Lifebox V1.5 box; L + M, Lifebox LB‐01 probe with Masimo Rad‐87 box; M + M, a weight‐appropriate reusable Masimo probe with Masimo Rad‐87 box.
Compared with M + M, the adjusted odds of plausible SpO2 measurement in less than 1 minute was 9% (95% CI: 0.65, 1.27) lower for L + M and 16% (95% CI: 0.52, 1.35) lower for L + L (Table 3). Notably, neither of these differences was statistically significant when adjusted for age, weight, child's condition, order of SpO2 measurement, presenting diagnosis, and accounting for the clustered nature of the data. Plausible SpO2 measurement in less than 1 minute was associated with an age of 12 to 35 months (adjusted odds ratio [aOR]: 3.36; 95% CI: 1.76, 6.42); more than 10 kg (aOR: 3.34; 95% CI: 1.90, 6.26); and being asleep (aOR: 2.36; 95% CI: 1.50, 3.68). Using less than 2 minutes as the outcome showed similar magnitude and direction of associations, except L + M showed a higher but nonsignificant odds of plausible measurement (aOR: 1.28; 95% CI: 0.80, 2.05; Table S2).
Table 3.
Associations between biologically plausible measurement in less than 1 minute and probe and device combinations, adjusted for confounders
| Characteristics | SpO2 <1 min | SpO2 >1 min | OR (95% CI) | P value | aOR (95% CI) a | P value |
|---|---|---|---|---|---|---|
| Oximeter and probe | ||||||
| Masimo + Masimo | 240 | 100 | 1.00 | 1.00 | ||
| Masimo + Lifebox | 237 | 103 | 0.96 (0.72‐1.28) | 0.774 | 0.91 (0.65‐1.27) | 0.573 |
| Lifebox + Lifebox | 147 | 85 | 0.72 (0.50‐1.05) | 0.087 | 0.84 (0.52‐1.35) | 0.477 |
| Testing order | ||||||
| First measure | 393 | 179 | 1.00 | 1.00 | ||
| Second measure | 231 | 109 | 0.97 (0.74‐1.26) | 0.796 | 0.81 (0.58‐1.13) | 0.215 |
| Age, mo | ||||||
| 0‐2 | 165 | 94 | 1.00 | 1.00 | ||
| 2‐11 | 212 | 127 | 0.95 (0.66‐1.38) | 0.791 | 1.34 (0.81‐2.21) | 0.248 |
| 12‐35 | 247 | 67 | 2.10 (1.41‐ 3.14) | <0.001 | 3.36 (1.76‐6.42) | <0.001 |
| Weight, kg | ||||||
| <10 | 449 | 257 | 1.00 | 1.00 | ||
| ≥10 | 167 | 28 | 3.41 (2.15‐5.41) | <0.001 | 3.34 (1.90‐6.26) | <0.001 |
| Child's condition | ||||||
| Calm | 349 | 143 | 1.00 | 1.00 | ||
| Agitated | 30 | 47 | 0.26 (0.15‐0.44) | <0.001 | 0.14 (0.08‐0.27) | <0.001 |
| Crying | 30 | 44 | 0.28 (0.16‐0.48) | <0.001 | 0.12 (0.07‐0.23) | <0.001 |
| Sleeping | 215 | 54 | 1.63 (1.12‐2.38) | 0.011 | 2.36 (1.50‐3.68) | <0.001 |
| Child's diagnosis | ||||||
| ARI | 231 | 122 | 1.00 | 1.00 | ||
| Fever | 147 | 47 | 1.65 (1.08, 2.52) | 0.020 | 1.13 (0.71‐1.80) | 0.614 |
| Healthy | 153 | 83 | 0.97 (0.66, 1.43) | 0.892 | 1.05 (0.63‐1.76) | 0.838 |
| Other | 93 | 36 | 1.36 (0.83, 2.24) | 0.219 | 1.22 (0.69‐2.15) | 0.503 |
Abbreviations: aOR, adjusted odds ratio; ARI, acute respiratory infections; CI, confidence interval; SpO2, peripheral oxyhemoglobin saturation.
All variables were included in the multivariable model. Multiple testing within individual children has been accounted for using robust standard errors.
4. DISCUSSION
Hypoxemia, a key risk for child mortality, can easily be measured using pulse oximetry, but barriers to widespread implementation in LMIC settings are commonly cited as cost and the lack of devices specifically designed for children in these settings. We found that, independent of the oximeter box, the Lifebox and Masimo probes were equivalent in achieving biologically plausible SpO2 measurements within as little as ±7 seconds of one another. However, the Lifebox probe in combination with the Lifebox oximeter box was marginally but inconclusively slower.
Equivalence of the probes is an important finding. The technical specification of the novel universal Lifebox probe used across age ranges has been openly published, allowing any manufacturers to make this design.21 It is projected to retail for $25, in comparison to the two probes recommended by Masimo for this age range commercially retailing at approximately $100 to $125 each at the time of publication, plus specialty cables to connect the Masimo probes to the oximeter boxes (≈$140/cable). Similarly, there is a price difference between the Rad‐87 and the Lifebox oximeter box, with Masimo oximeter boxes retailing at ≈$450 to $700 vs $250 for Lifebox. Establishing that a more affordable device, of comparable quality to a market leading oximeter, would challenge the assumption that cost is a barrier to scale‐up.
The overall achievement of biologically plausible SpO2 achieved in less than 2 minutes for 90% of L + M measurements shows improvement on usability testing conducted in Malawi, Bangladesh, and the United Kingdom with a range of healthcare providers to develop the LB‐01 probe (81% <2 minutes).17 This suggests the LB‐01 probe was being limited in its performance by the software in the Lifebox oximeter box. A key finding was the trend toward the Masimo microprocessor box being quicker, independent of probe, indicating more sophisticated motion tolerance software improves performance. Despite being unable to test equivalence for M + M and L + L measurements, the L + L group were on average the longest measurements. As this patient group was slightly older, healthier and more agitated, it suggests these children may have been more mobile during testing, possibly accounting for the poorer performance. This emphasizes the importance of software that can account for motion, a key challenge that has been highlighted by healthcare providers in small children.15
Crucial to innovation in this field is ensuring lower‐cost oximeter boxes designed for LMIC settings are not poor quality. Masimo announced the development of the Rad‐G device, designed specifically for spot‐checking for the LMIC market.22 It will be crucial to subject this new device to pragmatic testing in the field, to ensure it maintains the current low‐perfusion and motion tolerant software, which we believe to be important. Other initiatives for low‐cost devices for LMIC settings include smartphone based oximetry.23, 24 With multiple initiatives, generating clear evidence for policy makers and procurement agencies will be crucial to support implementation and scale‐up. Our testing approach could be expanded to benchmark usability, as continuing to evaluate the added value of novel devices in real‐world settings is as important as laboratory‐based accuracy testing.
An important finding was the difference in device performances according to age. The M + M combination was more successful than L + M combination in children less than 2 months within less than 1 minute, although this effect disappeared within less than 2 minutes. A Y‐sensor was used for this age group in the M + M measurements according to Masimo probe specifications (Box 1), suggesting a difference between Y and clip probe designs in smaller infants. It is important to note that we did not record time to probe placement and all testing was done by experts, therefore, taking this into consideration among healthcare workers with less training may increase the measurement time of the Y‐sensor. We would consider our comparison of M + M to L + M in the children less than 12 months to be conservative, with L + M potentially outperforming if we had included time to probe placement. As spot‐checking needs to be quick and easy, straightforward placement is important and future research should consider healthcare provider preference and technique for site selection and placement with different probes. Additionally, as the majority of pneumonia burden is seen in less than 24‐months old,25 a universal design may favor a clip.
We had three key limitations: firstly, the tester in the cross‐over study could not be blinded. The tester may have had an inherent preference for one probe or box over another, either through prior personal experience or experience during the testing. This could have influenced their decision on when to accept a measurement as biologically plausible. We were aware of this potential bias during study design, and decided to randomize at the point of measurement rather than in‐advance to reduce the potential for selective recruitment of participants. In addition, the independent observer served both a pragmatic and quality control role, to reduce non‐standardized testing. The second limitation was the potential for both the child's and the tester's behavior to be modified between the first and second measurement. For example, children may have become calmer on second measurement as they were familiar with the process and tester, or the tester may have modified where and how they chose to place the probe based on their recent experience. Again, we were aware of this in the design stage and included the 5‐minute washout period between measurements, and included standardized locations where the first placement of the probe should be according to age and weight in the protocol. We included the order of measurement in the adjusted analyses, and found that it was not significantly associated with successful measurements, suggesting these biases were not present. Finally, we lacked sufficient power from the historical L + L testing to conduct an equivalence analysis, limiting our ability to make a conclusion for this comparison. A prospective three‐way cross‐over could have overcome this limitation, however, it was beyond the scope of testing at the time.
We found the novel universal reusable pediatric Lifebox clip probe can achieve a quality SpO2 measurement within a pragmatically equivalent time as the Masimo reusable Y‐wrap sensor on children less than 10 kg and the reusable Masimo pediatric clip probe on children more than 10 kg. As cost and sustainability is frequently cited as a key barrier to pulse oximeter implementation and scale‐up in LMICs, this is an exciting finding as Lifebox probes are typically available at a fraction of the cost of market leading reusable probes, and requires a single probe for all children rather than multiple specialty designs. Further work is needed to improve motion tolerance in low‐cost oximeter boxes to fully realize the potential of pulse oximetry as a routine point‐of‐care diagnostic for pediatric hypoxemia in low‐resource settings. Additionally, as new devices are released, including multi‐model devices with multiple integrated functions, it will be crucial to continue benchmarking these devices not only on cost and laboratory accuracy, but on real‐world applicability.
AUTHOR CONTRIBUTIONS
The study was designed by CK, EDM, IWa, IWi, and MB, with input from TM and KS. The data collection tools were developed by CK. Data collection was conducted by KS with supervision from TM and EDM for the cross‐over equivalence, and by BZ, EDM, and NB, with supervision from TP and CM for the historical usability testing. Data cleaning and analysis was conducted by CK. The manuscript was written by CK, TM, and KS with significant input from EDM. All authors read, commented and approved the final manuscript.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Supporting information
Supplementary information
Supplementary information
Supplementary information
ACKNOWLEDGMENTS
We would like to thank all the caregivers and children who took part in the testing for their time and co‐operation, along with the staff at Kamuzu Central Hospital, Bwaila Hospital, and Mchinji District Hospital for their support in this study. We would like to acknowledge Laura Ruegsegger and Innocent Ndindi (UNC Project Malawi) for data collection in the cross‐over study, and the remaining members of The Lifebox Study group: Dr Tim Colbourn and Dr Bejoy Nambiar (University College London); Ms Katie Fernandez (the Lifebox Foundation). The historical usability testing was funded by the Bill and Melinda Gates Foundation (OPP1133291). The cross‐over equivalence testing was not supported by a specific project grant. Kristen Session received salary support from the Doris Duke Charitable Foundation. Eric D McCollum received support from the National Institutes of Health through the Fogarty International Center (K01TW009988).
King C, Mvalo T, Sessions K, et al. Performance of a novel reusable pediatric pulse oximeter probe. Pediatric Pulmonology. 2019;54:1052‐1059. 10.1002/ppul.24295
Contributor Information
Carina King, Email: c.king@ucl.ac.uk.
Eric D. McCollum, Email: emccoll3@jhmi.edu.
References
REFERENCES
- 1. Lazzerini M, Sonego M, Pellegrin MC. Hypoxaemia as a mortality risk factor in acute lower respiratory infections in children in low and middle‐income countries: systematic review and meta‐analysis. PLOS One. 2015;10(9):e0136166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Health A. Handbook IMCI: Integrated management of childhood illness. Geneva: WHO; 2005. [Google Scholar]
- 3. Alwadhi V, Dewan P, Malhotra RK, Shah D, Gupta P. Tachypnea and other danger signs vs pulse oximetry for prediction of hypoxia in severe pneumonia/very severe disease. Indian Pediatr. 2017;54(9):729‐734. [DOI] [PubMed] [Google Scholar]
- 4. Basnet S, Adhikari RK, Gurung CK. Hypoxemia in children with pneumonia and its clinical predictors. Indian J Pediatr. 2006;73(9):777‐781. [DOI] [PubMed] [Google Scholar]
- 5. Duke T, Subhi R, Peel D, Frey B. Pulse oximetry: technology to reduce child mortality in developing countries. Ann Trop Paediatr. 2009;29(3):165‐175. [DOI] [PubMed] [Google Scholar]
- 6. McCollum ED, Bjornstad E, Preidis GA, Hosseinipour MC, Lufesi N. Multicenter study of hypoxemia prevalence and quality of oxygen treatment for hospitalized Malawian children. Trans R Soc Trop Med Hyg. 2013;107(5):285‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sazawal S, Black RE, PCMT Group . Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta‐analysis of community‐based trials. Lancet Infect Dis. 2003;3(9):547‐556. [DOI] [PubMed] [Google Scholar]
- 8. Uwemedimo OT, Lewis TP, Essien EA, et al. Distribution and determinants of pneumonia diagnosis using Integrated Management of Childhood Illness guidelines: a nationally representative study in Malawi. BMJ Glob Health. 2018;3(2):e000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCollum ED, Ginsburg AS. Outpatient management of children with World Health Organization chest indrawing pneumonia: implementation risks and proposed solutions. Clin Infect Dis. 2017;65(9):1560‐1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Floyd J, Wu L, Hay burgess D, Izadnegahdar R, Mukanga D, Ghani AC. Evaluating the impact of pulse oximetry on childhood pneumonia mortality in resource‐poor settings. Nature. 2015;528(7580):S53‐S59. [DOI] [PubMed] [Google Scholar]
- 11. Ginsburg AS, Gerth‐Guyette E, Mollis B, Gardner M, Chham S. Oxygen and pulse oximetry in childhood pneumonia: surveys of clinicians and student clinicians in Cambodia. Trop Med Int Health. 2014;19(5):537‐544. [DOI] [PubMed] [Google Scholar]
- 12. Ginsburg AS, Van Cleve WC, Thompson MIW, English M. Oxygen and pulse oximetry in childhood pneumonia: a survey of healthcare providers in resource‐limited settings. J Trop Pediatr. 2012;58(5):389‐393. [DOI] [PubMed] [Google Scholar]
- 13. McCollum ED, King C, Deula R, et al. Pulse oximetry for children with pneumonia treated as outpatients in rural Malawi. Bull World Health Organ. 2016;94(12):893‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Graham HR, Bakare AA, Gray A, et al. Adoption of paediatric and neonatal pulse oximetry by 12 hospitals in Nigeria: a mixed‐methods realist evaluation. BMJ Glob Health. 2018;3(3):e000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. King C, Boyd N, Walker I, et al. Opportunities and barriers in paediatric pulse oximetry for pneumonia in low‐resource clinical settings: a qualitative evaluation from Malawi and Bangladesh. BMJ Open. 2018;8(1):e019177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lifebox . Pulse Oximetry; 2018. http://www.lifebox.org/. Accessed July, 2, 2018.
- 17. Boyd N, King C, Walker I, et al. Usability testing of a reusable pulse oximeter probe developed for healthcare workers caring for children. Am J Trop Med Hygiene. 2018;99(4):1096‐1104. 10.4269/ajtmh.18-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Open Data Kit. https://opendatakit.org/. Accessed July 02, 2018.
- 19. Fleming S, Thompson M, Stevens R, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377(9770):1011‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schuirmann DJ. A comparison of the two one‐sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987;15(6):657‐680. [DOI] [PubMed] [Google Scholar]
- 21.Lifebox Foundation. Lifebox LB‐01 sensor IP disclosure. Research Disclosure 2017; November 2017.
- 22.Masimo Introduces Rad‐G™ Pulse Oximeter In: Practice IMa, editor; 2017.
- 23. Dunsmuir D, Petersen C, Karlen W, Lim J, Dumont G, Ansermino MJ 2012 28/03/2018. The phone oximeter for mobile spot‐check. http://www.phoneoximeter.org/. Accessed March 28, 2018.
- 24. Sarah T, Sydney B, James C, Marisa L, Andrew H. Accuracy of smartphone‐based pulse oximetry compared with hospital‐grade pulse oximetry in healthy children. Telemedicine and e‐Health. 2018;24:527‐535. [DOI] [PubMed] [Google Scholar]
- 25. Walker CLF, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary information
Supplementary information


