Abstract
It is unclear whether structural findings in the kidneys of living kidney donors predict post-donation kidney function. We studied living kidney donors who had a kidney biopsy during donation. Nephron size was measured by glomerular volume, cortex volume per glomerulus, and mean cross-sectional tubular area. Age-specific thresholds were defined for low nephron number (calculated from CT and biopsy measures) and nephrosclerosis (global glomerulosclerosis, interstitial fibrosis/tubular atrophy (IF/TA), and arteriosclerosis). These structural measures were assessed as predictors of post-donation measured GFR, 24-hour urine albumin, and hypertension. Analyses were adjusted for baseline age, gender, body mass index, systolic and diastolic blood pressure, hypertension, measured GFR, urine albumin, living related donor status, and time since donation. Of 2,673 donors, 1,334 returned for a follow-up visit at a median 4.4 months after donation, with measured GFR <60 ml/min/1.73m2 in 34%, urine albumin >5 mg/24h in 13%, and hypertension in 5.3%. Larger glomerular volume and IF/TA predicted follow-up measured GFR <60 ml/min/1.73 m2. Larger cortex volume per glomerulus and low nephron number predicted follow-up urine albumin >5 mg/24h. Arteriosclerosis predicted hypertension. Microstructural findings predict GFR <60 ml/min/1.73m2, modest increases in urine albumin, and hypertension shortly after kidney donation.
1. Introduction
The evaluation of living kidney donor candidates seeks to identify any clinical findings that would portend an increased risk of harm with living kidney donation. While most kidney donors do well post-donation, rarely they may be at risk for end-stage renal disease or premature mortality.(1, 2) To minimize this risk, much of the evaluation of potential kidney donors focuses on detection of kidney disease from blood and urine biomarkers (in particular, glomerular filtration rate [GFR] and urine albumin excretion) and screening for chronic kidney disease (CKD) risk factors. Detection of kidney pathology on imaging (e.g., polycystic kidney disease or kidney stones) is also employed.(3) However, a pre-donation kidney biopsy is not typically obtained, both due to its invasive nature and because kidney biopsies in this carefully screened population may contribute little additional information on kidney health.
Studies of kidney biopsies from living kidney donors obtained at the time of transplantation do identify underlying pathology and structural variation,(4) but the clinical significance of these findings to the donor are unclear. Structural findings of living kidney donor biopsies can be categorized into two major categories: larger nephrons (size of the glomeruli and/or tubules) and nephrosclerosis.(4) Larger nephrons (nephron hypertrophy) occur when metabolic demand exceeds nephron supply. Nephrosclerosis is characterized by arteriosclerosis leading to ischemia with global glomerulosclerosis, tubular atrophy, and surrounding interstitial fibrosis (sometimes referred to as “chronic changes” or “histological abnormalities”). Nephrosclerosis in a donor kidney contributes to macrostructural findings on imaging, including renal cysts and a decrease in cortex to medulla ratio.(4) Nephrosclerosis leads to loss of functioning nephrons resulting in a lower number of nephrons.(5) Since there is a marked increase in nephrosclerosis and decrease in nephron number with healthy aging,(4–6) age-specific thresholds for nephrosclerosis and nephron number may be particularly informative. Age-specific thresholds for global glomerulosclerosis have previously been defined with living kidney donors (Table S1).(7) Only when global glomerulosclerosis exceeds these age-specific thresholds does it predict chronic kidney disease progression in patients with native kidney disease.(8, 9)
Prior studies predicting outcomes from kidney biopsy findings in living kidney donors have been single center, limited in sample size, had limited characterization of the biopsy findings, lacked sufficient adjustment for clinical characteristics, and only assessed GFR as an outcome.(10–14) Thus, we studied a large cohort of living kidney donors at three transplant centers in the Aging Kidney Anatomy study with thoroughly characterized biopsy findings.(4, 5) The objectives were to determine whether increased nephron size, reduced nephron number (less than expected for age), or nephrosclerosis (more than expected for age) were predictive of lower GFR, higher urine albumin, or hypertension after kidney donation.
2. Materials and Methods
2.1. Study Population
We studied living kidney donors who were at least 18 years of age at the Mayo Clinic (Rochester, MN and Scottsdale, AZ) and the Cleveland Clinic (Cleveland, OH), who had a pre-donation CT scan and a renal biopsy at the time of kidney donation from 1996 to 2016. These transplant programs had a post-donation follow-up visit recommended to all donors; however, the targeted timing of this visit varied by site and time period (usually targeting 3, 6, or 12 months after donation). For this study, we included any donor that returned for a post-donation follow-up visit between 1 and 18 months after kidney donation.
2.2. Clinical characteristics at donation
All kidney donors underwent a thorough medical evaluation prior to donation that included a prescheduled series of tests. The pre-donation evaluation included serum creatinine to estimate GFR using the CKD-EPI equation, urinary iothalamate clearance to measure GFR, 24-hour urine albumin excretion (except at Cleveland Clinic), body mass index (BMI), and office blood pressure. Acceptance criteria for donation varied by site and era, but in general included 24-hour urine albumin excretion < 30 mg and GFR normal for age.(15) Mild hypertension in older donors and moderate obesity (BMI 30 to 35 kg/m2; occasionally up to 40 kg/m2 in older donors) were allowed. Patients with diabetes mellitus or cardiovascular disease were not accepted as donors. Hypertension was defined as a pre-existing known diagnosis of hypertension, an office blood pressure >140/90 mm Hg, or use of antihypertensive medication to lower blood pressure. Hypertension in donors pre-donation was considered “mild” as it was either 140–159/90–99 mm Hg or controlled with one anti-hypertensive medication (with or without a thiazide diuretic). Potential donors with more severe hypertension were excluded from donation. Living related donors were defined by being a blood relative of the kidney recipient.
2.3. Microstructural findings at donation
Intraoperative needle core biopsy of the renal cortex was performed at the time of donation (pre-implantation at the Cleveland Clinic site and post-perfusion at the two Mayo Clinic sites). The tissue specimen was fixed in formalin and embedded in paraffin. Unstained sections from the tissue block were sent to the Mayo Clinic in Minnesota from the other sites for staining. Two sections (2- to 3-mm thickness) from the biopsy core were stained, one with periodic acid–Schiff and one with Masson trichrome, and then scanned into high-resolution digital images (Aperio XT digital scanner; Leica Biosystems). The Supplementary Methods describes the stereologic measurements and equations used to characterize the microstructure from these images. Nephron size on biopsy was characterized by mean non-sclerotic glomerular volume, cortex volume per glomerulus (inverse of nonsclerotic glomerular volumetric density), and mean cross-sectional tubular area as previously described.(4) Nephrosclerosis on biopsy was characterized by the number of globally sclerotic glomeruli, the percentage interstitial fibrosis/tubular atrophy (IF/TA), the number of distinct IF/TA foci, and severity of arteriosclerosis. The severity of arteriosclerosis was determined by the percentage of luminal stenosis by intimal thickening in the small-medium artery (if any) most orthogonal to its axis.(4)
2.4. Macrostructural findings at donation
The CT images from the angiogram/cortical phase were downloaded onto a workstation for processing. The kidney cortical and medullary volumes were segmented in a random order and blinded to other donor characteristics using a semiautomated algorithm (ITK-SNAP software, version 2.2; University of Pennsylvania, Philadelphia, PA).(4) The cortex volume (average between both kidneys) divided by medulla volume (average between both kidneys) and the diameter of the largest parenchymal cyst (if present) were evaluated as measures of CT-based nephrosclerosis as both are correlated with biopsy-based nephrosclerosis.(4) Nephron number per kidney was calculated from the product of cortical volume and non-sclerotic glomerular volumetric density (See Supplementary Methods).(5) Thus, the two determinants of cortical volume: nephron number and nephron size (specifically cortex volume per glomerulus) were analyzed as structural predictors.
2.5. Kidney function outcomes
Kidney function at the one follow-up visit was assessed by estimated GFR (eGFR), measured GFR (mGFR), 24h urine albumin, and new onset hypertension. Residual eGFR (follow-up eGFR/pre-donation eGFR × 100%), residual mGFR (follow-up mGFR/pre-donation mGFR × 100%), eGFR <60 ml/min/1.73 m2, and mGFR <60 ml/min/1.73 m2 were all assessed. The GFR threshold of 60 was chosen because it is often used to label former living kidney donors with CKD, even if inappropriate.(16) Urine albumin was dichotomized at ≥5 mg/24h as the distribution was very right-skewed and even very low levels of albuminuria are predictive of mortality and other adverse outcomes in healthy adults.(17–21) Urine albumin ≥ 5 mg/24h at follow-up was only assessed in donors with urine albumin <5 mg/24h prior to donation. Hypertension at follow-up was only assessed in donors without hypertension prior to donation.
2.6. Statistical Analysis
Age-based thresholds were calculated for all measures of nephrosclerosis. We used thresholds we previously published that identify the 95th percentile for number of globally sclerotic glomeruli per section based on age and the number of glomeruli present per section (Table S1).(7) For other nephrosclerosis measures, we identified the 5 percent that would be abnormal within the age groups of 18–29, 30–39, 40–49, 50–59, and 60+ years. For largest cyst diameter, % IF/TA, number of IF/TA foci, and % luminal stenosis, >95th percentile for each age group identified abnormal. For cortex to medulla ratio and nephron number, <5th percentile for each age group identified abnormal.
Each nephron size, nephron number, and nephrosclerosis measure was evaluated for its association with post-donation kidney function. Reported estimates, odds ratios, confidence intervals, and p-values for analyses were determined using linear regression for residual mGFR or residual eGFR and logistic regression for mGFR<60 ml/min/1.73 m2, eGFR<60 ml/min/1.73 m2, urine albumin ≥5 mg/24h, and hypertension. Outcomes were evaluated using unadjusted models, models adjusted for age and follow-up time since kidney donation, and models further adjusted for baseline clinical characteristics: gender, BMI, blood pressure, eGFR (or mGFR), urine albumin, living related donor (versus living unrelated donor), and hypertension. All calculated p-values were 2 sided, and p <0.05 was considered statistically significant. Statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc, Cary, NC) and R software, version 3.4.1.
3. Results
3.1. Donor Characteristics
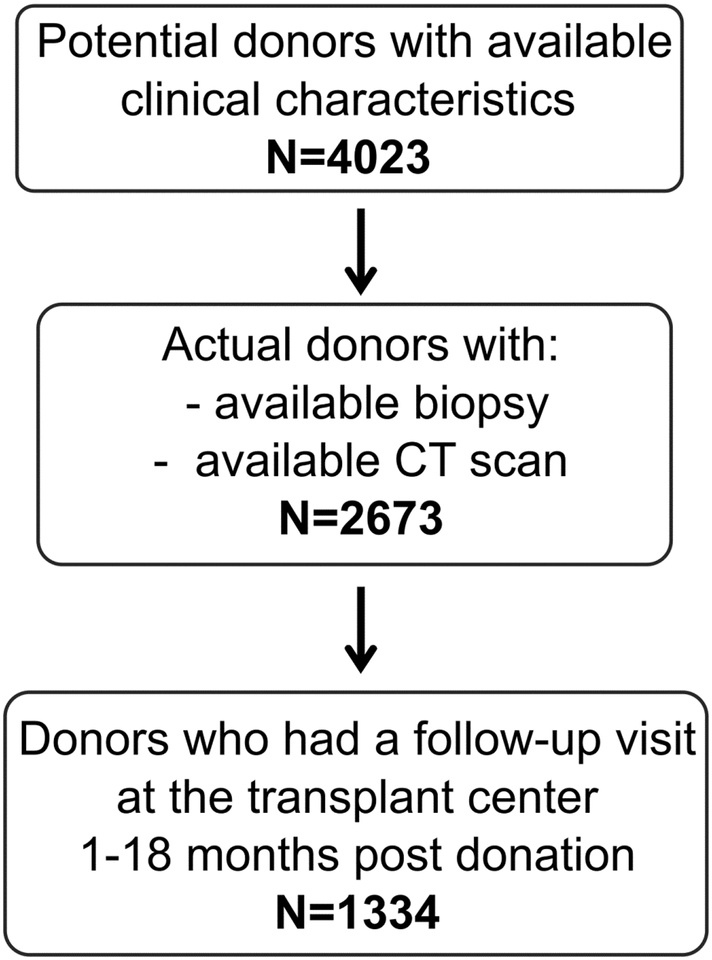
There were 2,673 adult living kidney donors that had both a renal biopsy at the time of donation and a pre-donation contrast CT scan of their kidneys (Figure 1). A subset of 1,334 donors (50%) returned to their transplant center for follow-up visit at a median of 4.41 (IQR: 2.93, 7.50) months after donation. Table 1 depicts the pre-donation clinical and CT characteristics and kidney biopsy findings at the time of donation for the 2,673 donors studied. As expected, kidney donors had healthy kidney function, with only 15.9% having hypertension. Donors who returned for follow-up tended to be older with baseline higher BMI, lower GFR, lower urine albumin, living unrelated donation, hypertensive, larger and fewer nephrons, larger cysts, and more nephrosclerosis on biopsy (Table S2). The age-specific biopsy and CT-based nephrosclerosis thresholds (calculated to be 5% abnormal) are shown in Table 2.
Figure 1.
Flowchart showing the selection of the study sample.
Table 1.
Baseline characteristics of 2,673 donors
| Clinical Characteristics | Mean ± SD or n (%) |
|---|---|
| Age at Biopsy, years | 43.7 ± 11.8 |
| Male | 1,095 (41.0%) |
| BMI, kg/m2 | 27.5 ± 4.7 |
| Systolic blood pressure, mmHg | 118.7 ± 14.4 |
| Diastolic blood pressure, mmHg | 73.4 ± 9.5 |
| eGFR, ml/min/1.73 m2 | 92.8 ± 16.2 |
| mGFR, ml/min/1.73 m2 | 104.1 ± 19.9 |
| Urine Albumin, mg† | 4.59 ± 11.44 |
| Urine Albumin ≥5mg† | 651 (33.5%) |
| Living related donor | 1350 (51.3%) |
| Hypertension | 426 (15.9%) |
| Nephron size and number measures* | |
| Glomerular Volume, mm3 | 0.0025 ± 0.0010 |
| Cortex volume per glomerulus, mm3 | 0.071 ± 0.036 |
| Tubular cross-sectional area, μm2 | 4,241 ± 1,438 |
| Nephron number per kidney | 942,000 ± 430,000 |
| CT nephrosclerosis measures | |
| Cortex volume / Medulla volume | 2.59 ± 0.66 |
| Diameter of largest cyst, cm‡ | 0.17 ± 0.60 |
| Biopsy nephrosclerosis measures* | |
| Number of IF/TA Foci | |
| 0 | 1953 (73%) |
| 1 | 439 (16%) |
| 2 | 120 (4.5%) |
| 3 | 84 (3.1%) |
| 4 | 45 (1.7%) |
| ≥5 | 32 (1.1%) |
| Percentage IF/TA Groups | |
| 0% | 2263 (85%) |
| 1–5% | 330 (12%) |
| 6–10% | 63 (2.4%) |
| >10% | 17 (0.6%) |
| Luminal Stenosis, % | 33.6 ± 21.2 |
| Globally sclerotic glomeruli, % | 3.2 ± 6.0 |
Biopsies used had a mean ± SD cortical area of 6.7 ± 3.0 mm2 and number of glomeruli of 19 ± 11 per section.
Not obtained in 731 donors.
If no cysts were present, these patients were given a value of “0” cm for size.
Table 2.
Age-based thresholds used to define nephrosclerosis in 2673 donors (targeting as close to 5% abnormal as possible).
| Nephrosclerosis measures | n | Age | Threshold | n(%) Abnormal |
|---|---|---|---|---|
| CT-based | ||||
| Cortex / Medulla volume | ||||
| 344 | 18–29 | <1.97 | 18 (5.2%) | |
| 616 | 30–39 | <1.81 | 30 (4.9%) | |
| 706 | 40–49 | <1.73 | 34 (4.8%) | |
| 545 | 50–59 | <1.60 | 27 (5.0%) | |
| 222 | 60+ | <1.53 | 11 (5.0%) | |
| Largest kidney cyst diameter, cm | ||||
| 372 | 18–29 | ≥0.1 | 17 (4.6%) | |
| 665 | 30–39 | ≥0.5 | 41 (6.2%) | |
| 770 | 40–49 | ≥1.0 | 45 (5.8%) | |
| 584 | 50–59 | ≥1.3 | 34 (5.8%) | |
| 242 | 60+ | ≥2.6 | 14 (5.8%) | |
| Biopsy-based | ||||
| Globally sclerotic glomeruli | ||||
| 376 | 18–29 | * | 16 (4.3%) | |
| 676 | 30–39 | * | 55 (8.1%) | |
| 784 | 40–49 | * | 52 (6.6%) | |
| 592 | 50–59 | * | 26 (4.4%) | |
| 245 | 60+ | * | 18 (7.4%) | |
| % IF/TA | ||||
| 376 | 18–29 | ≥1% | 25 (6.7%) | |
| 676 | 30–39 | ≥1% | 61 (9.0%) | |
| 784 | 40–49 | ≥1% | 112 (14.3%) | |
| 592 | 50–59 | ≥5% | 20 (3.4%) | |
| 245 | 60+ | ≥10% | 13 (5.3%) | |
| Number of IF/TA foci | ||||
| 376 | 18–29 | ≥2 | 9 (2.4%) | |
| 676 | 30–39 | ≥2 | 36 (5.3%) | |
| 784 | 40–49 | ≥3 | 39 (5.0%) | |
| 592 | 50–59 | ≥3 | 47 (7.9%) | |
| 245 | 60+ | ≥5 | 19 (7.7%) | |
| % Luminal stenosis | ||||
| 332 | 18–29 | ≥59.5% | 17 (5.1%) | |
| 604 | 30–39 | ≥62.8% | 31 (5.1%) | |
| 690 | 40–49 | ≥68.9% | 34 (4.9%) | |
| 533 | 50–59 | ≥74.0% | 27 (5.1%) | |
| 225 | 60+ | ≥76.9% | 11 (4.9%) | |
| CT- and Biopsy-based | ||||
| Nephron number† | ||||
| 344 | 18–29 | <393,000 | 17 (4.9%) | |
| 616 | 30–39 | <443,000 | 31 (5.0%) | |
| 705 | 40–49 | <382,000 | 35 (5.0%) | |
| 543 | 50–59 | <348,000 | 28 (5.2%) | |
| 222 | 60+ | <314,000 | 12 (5.4%) |
3.2. Predictors of follow-up GFR
At follow-up, the mean residual eGFR was 66.7%, with 55% (739/1,334) of donors having an eGFR <60 ml/min/1.73 m2; the mean residual mGFR was 65.2% with 34.4% (368/1071) having a mGFR <60 ml/min/1.73 m2. After adjusting for age and follow-up time, higher cortex volume per glomerulus and lower nephron number were structural predictors for follow-up eGFR <60 ml/min/1.73 m2 (Table S3). However, these associations were no longer statistically significant after adjusting for other clinical characteristics (including baseline eGFR). After adjusting for age and follow-up time, larger glomerular volume and a high number of IF/TA foci were structural predictors for a follow-up mGFR <60 ml/min/1.73 m2 (Table 3). After further adjustment for other clinical characteristics, mGFR <60 ml/min/1.73 m2 was still predicted by glomerular volume per SD (OR=1.19, p=0.04) and IF/TA foci >95% threshold (OR=1.99, p=0.04). There were no significant structural predictors found for residual eGFR or residual mGFR (Table 3 and Table S3).
Table 3.
Predictors of mGFR measures at follow-up among 1,057 donors
| Residual mGFR (mGFRfollow-up/mGFRpre-donation ×100%) | Follow-up mGFR <60 (y/n) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline clinical characteristics | Unadjusted | Adjusted for age and follow-up time | Further adjusted for all clinical characteristics | Unadjusted | Adjusted for age and follow-up time | Further adjusted for all clinical characteristics | ||||||
| Est | P | Est | P | Est | P | OR | P | OR | P | OR | P | |
| Age, per year | −0.2% | <0.001 | - | - | - | - | 1.11 | <0.001 | - | - | - | - |
| Male | 1.4% | 0.06 | 0.9% | 0.21 | - | - | 0.93 | 0.56 | 1.18 | 0.28 | - | - |
| BMI, per kg/m2 | −0.1% | 0.10 | −0.1% | 0.12 | - | - | 1.04 | 0.009 | 1.04 | 0.01 | - | - |
| Systolic blood pressure, per 10 mmHg | −0.3% | 0.26 | −0.3% | 0.34 | - | - | 1.15 | 0.002 | 1.01 | 0.81 | - | - |
| Diastolic blood pressure, per 10 mmHg | −0.5% | 0.17 | −0.2% | 0.58 | - | - | 1.26 | 0.001 | 1.07 | 0.42 | - | - |
| mGFR, per 10 ml/min/1.73 m2* | −2.2% | <0.001 | −3.2% | <0.001 | - | - | 0.48 | <0.001 | 0.56 | <0.001 | - | - |
| Urine Albumin ≥5mg | 1.2% | 0.13 | −0.1% | 0.92 | - | - | 0.94 | 0.67 | 1.31 | 0.11 | - | - |
| Living related donor | 1.4% | 0.06 | 0.0% | 0.95 | - | - | 0.60 | <0.001 | 1.02 | 0.92 | - | - |
| Hypertension | −3.0% | 0.001 | −1.4% | 0.14 | - | - | 2.56 | <0.001 | 1.20 | 0.33 | - | - |
| Nephron Size | ||||||||||||
| Glomerular volume, per SD | 0.3% | 0.47 | 0.0% | 0.91 | 0.1% | 0.82 | 1.05 | 0.46 | 1.18 | 0.02 | 1.19 | 0.04 |
| Cortex volume per glomerulus, per SD | 0.2% | 0.57 | 0.2% | 0.67 | 0.1% | 0.83 | 1.13 | 0.04 | 1.09 | 0.20 | 1.02 | 0.80 |
| Tubular cross-sectional area, per SD | −0.1% | 0.71 | −0.3% | 0.36 | −0.2% | 0.63 | 1.05 | 0.44 | 1.05 | 0.47 | 1.09 | 0.32 |
| Nephron number | ||||||||||||
| Nephron number, <5% threshold | −0.3% | 0.87 | −0.7% | 0.67 | −1.9% | 0.19 | 1.53 | 0.11 | 1.72 | 0.08 | 1.32 | 0.42 |
| CT-based nephrosclerosis | ||||||||||||
| Cortex / Medulla volume, <5% threshold | 2.1% | 0.22 | 1.6% | 0.33 | 1.3% | 0.39 | 1.10 | 0.75 | 1.10 | 0.79 | 0.96 | 0.90 |
| Largest cyst diameter, >95% threshold | −2.4% | 0.12 | −2.1% | 0.17 | −0.5% | 0.72 | 1.28 | 0.36 | 1.16 | 0.63 | 1.38 | 0.36 |
| Biopsy-based nephrosclerosis | ||||||||||||
| Globally sclerotic glomeruli >95% threshold | −1.8% | 0.22 | −1.7% | 0.24 | −1.6% | 0.22 | 0.91 | 0.72 | 0.84 | 0.56 | 0.75 | 0.40 |
| % IF/TA, >95% threshold | −0.1% | 0.93 | −0.4% | 0.78 | −0.9% | 0.49 | 1.42 | 0.14 | 1.61 | 0.07 | 1.67 | 0.08 |
| Number of IF/TA foci, >95% threshold | −1.4 | 0.35 | −0.2% | 0.89 | −0.7% | 0.59 | 2.80 | <0.001 | 1.97 | 0.02 | 1.99 | 0.04 |
| Artery luminal stenosis, >95% threshold | 1.6% | 0.43 | 0.9% | 0.63 | 1.4% | 0.42 | 0.40 | 0.03 | 0.45 | 0.08 | 0.38 | 0.05 |
Association reflects regression to the mean.
3.3. Predictors of follow-up urine albumin
Among donors without a pre-donation urine albumin >5 mg/24h, 13% (92/712) developed a post-donation urine albumin >5 mg/24h. After adjusting for age and follow-up time, larger glomerular volume, more cortex volume per glomerulus, and lower nephron number were structural predictors for a follow-up urine albumin >5 mg/24h (Table 4). After further adjustment for other clinical characteristics, urine albumin >5 mg/24h was still predicted by higher cortex volume per glomerulus per SD (OR 1.39, p=0.003) and low nephron number <5% threshold (OR 4.67, p=0.0004).
Table 4.
Urine albumin ≥5 mg at follow-up, among donors with baseline urine albumin <5 mg (n=712)
| Follow-up urine albumin ≥5mg | ||||||
|---|---|---|---|---|---|---|
| Baseline clinical characteristics | Unadjusted | Adjusted for age and follow-up time | Further adjusted for all clinical characteristics | |||
| OR | P | OR | P | OR | P | |
| Age, per year | 0.99 | 0.53 | - | - | - | - |
| Male | 1.02 | 0.95 | 1.01 | 0.95 | - | - |
| BMI, per kg/m2 | 1.05 | 0.05 | 1.05 | 0.04 | - | - |
| Systolic blood pressure, per 10 mmHg | 1.31 | <0.001 | 1.28 | 0.005 | - | - |
| Diastolic blood pressure, per 10 mmHg | 0.96 | 0.75 | 0.94 | 0.61 | - | - |
| eGFR, per 10 ml/min/1.73 m2 | 1.16 | 0.03 | 1.25 | 0.01 | - | - |
| Living related donor | 1.08 | 0.74 | 1.02 | 0.94 | - | - |
| Hypertension | 0.92 | 0.79 | 0.96 | 0.90 | - | - |
| Nephron Size | ||||||
| Glomerular volume, per SD | 1.29 | 0.02 | 1.29 | 0.03 | 1.25 | 0.07 |
| Cortex volume per glomerulus, per SD | 1.38 | 0.002 | 1.36 | 0.003 | 1.39 | 0.003 |
| Tubular cross-sectional area, per SD | 1.26 | 0.04 | 1.21 | 0.11 | 1.18 | 0.17 |
| Nephron number | ||||||
| Nephron number, <5% threshold | 3.41 | 0.002 | 3.22 | 0.004 | 4.67 | <0.001 |
| CT-based nephrosclerosis | ||||||
| Cortex / Medulla volume, <5% threshold | 0.97 | 0.96 | 0.88 | 0.81 | 1.02 | 0.97 |
| Largest cyst diameter, >95% threshold | 0.93 | 0.89 | 0.94 | 0.90 | 1.02 | 0.97 |
| Biopsy-based nephrosclerosis | ||||||
| Globally sclerotic glomeruli >95% threshold | 0.89 | 0.80 | 0.81 | 0.65 | 0.74 | 0.53 |
| % IF/TA, >95% threshold | 1.98 | 0.07 | 1.60 | 0.23 | 1.40 | 0.41 |
| Number of IF/TA foci, >95% threshold | 0.48 | 0.22 | 0.50 | 0.26 | 0.48 | 0.24 |
| Artery luminal stenosis, >95% threshold | 1.43 | 0.58 | 1.46 | 0.57 | 1.20 | 0.79 |
3.4. Association of age-specific nephrosclerosis and nephron size with follow-up hypertension
Among donors without pre-donation hypertension, 5.3% (58/1092) developed post-donation hypertension. After adjusting for age and follow-up time, larger glomerular volume, more cortex volume per glomerulus, larger tubular area, high number of IF/TA foci, and increased luminal stenosis were structural predictors for hypertension at follow-up (Table 5). After further adjustment for other clinical characteristics, only increased artery luminal stenosis >95% threshold (OR=4.66, p=0.002) predicted post-donation hypertension.
Table 5.
Hypertension at follow-up, among donors without hypertension at baseline (n=1092)
| Follow-up hypertension | ||||||
|---|---|---|---|---|---|---|
| Baseline clinical characteristics | Unadjusted | Adjusted for age and follow-up time | Further adjusted for all clinical characteristics | |||
| OR | P | OR | P | OR | P | |
| Age, per year | 1.04 | 0.003 | - | - | - | - |
| Male | 2.52 | 0.001 | 2.85 | <0.001 | - | - |
| BMI, per kg/m2 | 1.11 | <0.001 | 1.11 | <0.001 | - | - |
| Systolic blood pressure, per 10 mmHg | 2.29 | <0.001 | 2.08 | <0.001 | - | - |
| Diastolic blood pressure, per 10 mmHg | 1.78 | 0.001 | 1.64 | 0.007 | - | - |
| eGFR, per 10 ml/min/1.73 m2 | 0.81 | 0.02 | 0.91 | 0.39 | - | - |
| Urine Albumin ≥5mg | 3.49 | <0.001 | 3.83 | <0.001 | - | - |
| Living related donor | 1.37 | 0.25 | 1.64 | 0.080 | - | - |
| Nephron Size | ||||||
| Glomerular volume, per SD | 1.59 | <0.001 | 1.61 | <0.001 | 1.30 | 0.07 |
| Cortex volume per glomerulus, per SD | 1.46 | <0.001 | 1.35 | 0.004 | 1.13 | 0.35 |
| Tubular cross-sectional area, per SD | 1.58 | <0.001 | 1.53 | <0.001 | 1.06 | 0.73 |
| Nephron number | ||||||
| Nephron number, <5% threshold | 1.34 | 0.59 | 0.97 | 0.96 | 0.84 | 0.77 |
| CT-based nephrosclerosis | ||||||
| Cortex / Medulla volume, <5% threshold | 3.13 | 0.26 | 2.63 | 0.35 | 2.40 | 0.40 |
| Largest cyst diameter, >95% threshold | 0.91 | 0.88 | 0.91 | 0.88 | 0.70 | 0.61 |
| Biopsy-based nephrosclerosis | ||||||
| Globally sclerotic glomeruli >95% threshold | 0.54 | 0.40 | 0.57 | 0.44 | 0.80 | 0.77 |
| % IF/TA, >95% threshold | 2.01 | 0.10 | 2.03 | 0.10 | 1.40 | 0.50 |
| Number of IF/TA foci, >95% threshold | 2.70 | 0.00 | 2.64 | 0.025 | 1.86 | 0.21 |
| Artery luminal stenosis, >95% threshold | 4.74 | <0.001 | 4.49 | <0.001 | 4.66 | 0.002 |
Discussion
Microstructural findings on the biopsies of living kidney donors not detected on pre-donation clinical, laboratory, or imaging evaluations predicted adverse changes in kidney function early after donation. Several measures of larger nephron size predicted a low mGFR or a modest increase in albuminuria. Low nephron number predicted a modest increase in albuminuria. Several measures of nephrosclerosis on biopsy (but not by CT scan) predicted a low mGFR or new onset hypertension. These associations persisted after adjusting for clinical characteristics and risk factors evident at the time of kidney donation. Thus, some living kidney donors have underlying microstructural pathology that is only detectable by a kidney biopsy and is informative regarding the anticipated kidney function after donation.
This does not necessarily imply that a kidney biopsy is warranted as part of pre-donation evaluation. Although we found biopsy findings to be predictive of adverse changes in kidney function after donation, the potential harm of a pre-operative kidney biopsy (pain and bleeding) may not be justified for this very modest contribution to risk prediction. Furthermore, it is unknown if these biopsy findings predict ESRD or mortality in donors. Efforts are underway to identify urine and blood biomarkers that might detect this occult kidney pathology.(22, 23) Such biomarkers may be useful in kidney donor evaluations if they reflect this microstructural pathology that goes undetected with current pre-donation evaluations. The intra-operative kidney biopsy used in this study has very low risk for bleeding complications, provides baseline histology for the recipient, and may identify donors at risk for adverse changes in kidney function.
This study used age-based thresholds for measures of nephrosclerosis. A single threshold to identify nephrosclerosis (irrespective of age) would have been “prejudiced” against older living kidney donors, since nephrosclerosis occurs with even healthy aging. (4, 6) Instead, this age-based approach avoids medicalizing age-related structural pathology. This approach is supported by older living kidney donors having a good prognosis,(24) while some of the concerns with living kidney donation are focused on younger donors.(25) Further, hyperfiltration (an increase in single nephron GFR) only occurs in donors when nephrosclerosis exceeds that expected for the age of the donor.(4)
Structural predictors had generally modest associations with GFR that were somewhat inconsistent between eGFR and mGFR in our study. Some prior studies have found nephrosclerosis to predict a lower eGFR recovery post-donation,(10, 12, 14) whereas other studies have not found this.(11, 13) We found only one measure of nephrosclerosis predicted a lower mGFR and none predicted a lower eGFR after adjusting for baseline characteristics. We found low nephron number to predict a post-donation eGFR <60 mL/min/1.73 m2, but not after adjusting for baseline characteristics including baseline eGFR. A compensatory increase in GFR occurs early on after donation and continues during decades of follow-up without explaining any increased risk of ESRD.(26) Thus, GFR may not be the most predictive kidney function parameter early after donation to estimate long term outcomes.
Unlike post-donation GFR, there has been less study of biopsy findings predicting post-donation albuminuria. We found that more cortex volume per glomerulus predicted higher albuminuria post-donation, even after adjusting for clinical predictors. Low nephron number was also a strong predictor of higher albuminuria post-donation, and this also persisted after adjusting for clinical predictors. Compensatory enlargement of glomeruli after donation increases single nephron GFR to meet metabolic demand.(27) Glomerular enlargement in persons who already have enlarged glomeruli from low nephron number may leak albumin across the glomerular filtration barrier via a more disorganized glomerular structure.(28) While the amount of albuminuria predicted by microstructural findings was modest (≥5mg/24h), even modest levels of albuminuria are associated with a higher risk of mortality in the general population.(17)
Nephrosclerosis in patients with a history of hypertension is a well described finding in autopsy studies(29) and living kidney donors.(4, 6) The extent to which hypertension is a cause versus a result of nephrosclerosis has long been debated.(30) This study could uniquely assess the temporal relationship between nephrosclerosis and hypertension. We found that nephrosclerosis, and specifically, arteriosclerosis (increased luminal stenosis) predicted the onset of post-donation hypertension, independent of other structural and clinical predictors (including pre-donation blood pressure). Hypertension is reported to be common after kidney donation, (31, 32) and arteriosclerosis in the remaining kidney after a nephrectomy may be part of the mechanism. While the mechanism is unclear, ischemia from the stenosis of small to medium sized arteries in the cortical parenchyma may stimulate renin, contributing to the risk of hypertension via the renin-angiotensin-aldosterone-system activation. A meta-analysis of 48 studies estimated a 5 mmHg increase in blood pressure over that expected with aging alone .(33) However, a small prospective study of 203 kidney donors with paired controls followed for up to 3 years post-donation found no difference in blood pressure at follow-up visits, including by 24-h ambulatory blood pressure monitoring.(34) Thus, it is unclear whether an interaction between kidney donation and arteriosclerosis leads to hypertension, or arteriosclerosis alone leads to hypertension.
There were several potential limitations to this study. Only half of the donors returned for the follow-up visit as many either declined follow-up or chose to receive follow-up with local providers. Most of the data in our study precedes contemporary efforts to have more complete donor follow-up.(35) The kidney donors studied were predominantly white, and findings may differ in other ethnic groups. Longer-term follow-up is needed to determine whether these microstructural findings at the time of kidney donation are predictive of more clinically significant morbidity and mortality.
In conclusion, larger nephrons, low nephron number for age, and more nephrosclerosis than expected for age at the time of donation were modest predictors of mGFR, albuminuria, and hypertension. These findings provide insights into the temporal relationship between kidney microstructure and subsequent changes in kidney function. They further suggest an opportunity to improve the detection of subclinical pathology in kidney donors.
Supplementary Material
Acknowledgments
This study was supported with funding from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK090358). We thank Miloš Denić for assistance with computer algorithms for processing of biopsy annotations data.
Abbreviations
- BMI
Body mass index
- ESRD
End stage renal disease
- eGFR
Estimated glomerular filtration rate
- GFR
Glomerular filtration rate
- GSG
Globally sclerotic glomeruli
- IF/TA
Interstitial Fibrosis/Tubular Atrophy
- IQR
Interquartile range
- mGFR
Measured glomerular filtration rate
- SBP
Systolic blood pressure
Footnotes
Parts of these analyses were presented in a poster form at the Kidney Week of the American Society of Nephrology, October 31st to November 5th, 2017 in New Orleans, LA and as an oral presentation at American Transplant Congress June 2018 in Seattle, WA, USA.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Data Availability Statement
Due to privacy concerns, patient level data cannot be made publically available.
Supporting Information
Additional material may be found online in the Supporting Information section for this article.
References
- 1.Mjoen G, Hallan S, Hartmann A, Foss A, Midtvedt K, Oyen O et al. Long-term risks for kidney donors. Kidney Int 2014;86(1):162–167. [DOI] [PubMed] [Google Scholar]
- 2.Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL et al. Risk of end-stage renal disease following live kidney donation. Jama 2014;311(6):579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorenz EC, Vrtiska TJ, Lieske JC, Dillon JJ, Stegall MD, Li X et al. Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin J Am Soc Nephrol 2010;5(3):431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denic A, Alexander MP, Kaushik V, Lerman LO, Lieske JC, Stegall MD et al. Detection and Clinical Patterns of Nephron Hypertrophy and Nephrosclerosis Among Apparently Healthy Adults. Am J Kidney Dis 2016;68(1):58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denic A, Lieske JC, Chakkera HA, Poggio ED, Alexander MP, Singh P et al. The Substantial Loss of Nephrons in Healthy Human Kidneys with Aging. J Am Soc Nephrol 2017;28(1):313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 2010;152(9):561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kremers WK, Denic A, Lieske JC, Alexander MP, Kaushik V, Elsherbiny HE et al. Distinguishing age-related from disease-related glomerulosclerosis on kidney biopsy: the Aging Kidney Anatomy study. Nephrol Dial Transplant 2015;30(12):2034–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hommos MS, Zeng C, Liu Z, Troost JP, Rosenberg AZ, Palmer M et al. Global glomerulosclerosis with nephrotic syndrome; the clinical importance of age adjustment. Kidney Int 2018;93(5):1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivastava A, Palsson R, Kaze AD, Chen ME, Palacios P, Sabbisetti V et al. The Prognostic Value of Histopathologic Lesions in Native Kidney Biopsy Specimens: Results from the Boston Kidney Biopsy Cohort Study. J Am Soc Nephrol 2018;29(8):2213–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohashi Y, Thomas G, Nurko S, Stephany B, Fatica R, Chiesa A et al. Association of metabolic syndrome with kidney function and histology in living kidney donors. Am J Transplant 2013;13(9):2342–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chauhan A, Diwan TS, Franco Palacios CR, Dean PG, Heimbach JK, Chow GK et al. Using implantation biopsies as a surrogate to evaluate selection criteria for living kidney donors. Transplantation 2013;96(11):975–980. [DOI] [PubMed] [Google Scholar]
- 12.Elsherbiny HE, Alexander MP, Kremers WK, Park WD, Poggio ED, Prieto M et al. Nephron hypertrophy and glomerulosclerosis and their association with kidney function and risk factors among living kidney donors. Clin J Am Soc Nephrol 2014;9(11):1892–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi KH, Yang SC, Joo DJ, Yoon YE, Kim KH, Lee K et al. Do the abnormal results of an implantation renal biopsy affect the donor renal function? Transplant Proc 2014;46(2):359–362. [DOI] [PubMed] [Google Scholar]
- 14.Fahmy LM, Massie AB, Muzaale AD, Bagnasco SM, Orandi BJ, Alejo JL et al. Long-term Renal Function in Living Kidney Donors Who Had Histological Abnormalities at Donation. Transplantation 2016;100(6):1294–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poggio ED, Rule AD, Tanchanco R, Arrigain S, Butler RS, Srinivas T et al. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int 2009;75(10):1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matas AJ, Ibrahim HN. The unjustified classification of kidney donors as patients with CKD: critique and recommendations. Clin J Am Soc Nephrol 2013;8(8):1406–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chong J, Fotheringham J, Tomson C, Ellam T. Renal albumin excretion in healthy young adults and its association with mortality risk in the US population. Nephrol Dial Transplant 2018. [DOI] [PubMed] [Google Scholar]
- 18.Sumida K, Molnar MZ, Potukuchi PK, George K, Thomas F, Lu JL et al. Changes in Albuminuria and Subsequent Risk of Incident Kidney Disease. Clin J Am Soc Nephrol 2017;12(12):1941–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieb W, Mayer B, Stritzke J, Doering A, Hense HW, Loewel H et al. Association of low-grade urinary albumin excretion with left ventricular hypertrophy in the general population: the MONICA/KORA Augsburg Echocardiographic Substudy. Nephrol Dial Transplant 2006;21(10):2780–2787. [DOI] [PubMed] [Google Scholar]
- 20.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004;110(1):32–35. [DOI] [PubMed] [Google Scholar]
- 21.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001;286(4):421–426. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Lieske JC, Alexander MP, Jayachandran M, Denic A, Mathew J et al. Tubulointerstitial Fibrosis of Living Donor Kidneys Associates with Urinary Monocyte Chemoattractant Protein 1. Am J Nephrol 2016;43(6):454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mischak H Pro: urine proteomics as a liquid kidney biopsy: no more kidney punctures! Nephrol Dial Transplant 2015;30(4):532–537. [DOI] [PubMed] [Google Scholar]
- 24.Berger JC, Muzaale AD, James N, Hoque M, Wang JM, Montgomery RA et al. Living kidney donors ages 70 and older: recipient and donor outcomes. Clin J Am Soc Nephrol 2011;6(12):2887–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grams ME, Sang Y, Levey AS, Matsushita K, Ballew S, Chang AR et al. Kidney-Failure Risk Projection for the Living Kidney-Donor Candidate. N Engl J Med 2016;374(5):411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matas AJ, Vock DM, Ibrahim HN. GFR </=25 years postdonation in living kidney donors with (vs. without) a first-degree relative with ESRD. Am J Transplant 2018;18(3):625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denic A, Glassock RJ, Rule AD. Single-Nephron Glomerular Filtration Rate in Healthy Adults. N Engl J Med 2017;377(12):1203–1204. [DOI] [PubMed] [Google Scholar]
- 28.Hodgin JB, Bitzer M, Wickman L, Afshinnia F, Wang SQ, O’Connor C et al. Glomerular Aging and Focal Global Glomerulosclerosis: A Podometric Perspective. J Am Soc Nephrol 2015;26(12):3162–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bohle A, Wehrmann M, Greschniok A, Junghans R. Renal morphology in essential hypertension: analysis of 1177 unselected cases. Kidney Int Suppl 1998;67:S205–206. [DOI] [PubMed] [Google Scholar]
- 30.Kopp JB. Rethinking hypertensive kidney disease: arterionephrosclerosis as a genetic, metabolic, and inflammatory disorder. Curr Opin Nephrol Hypertens 2013;22(3):266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez OA, Ferrara LK, Rein S, Berglund D, Matas AJ, Ibrahim HN. Hypertension after kidney donation: Incidence, predictors, and correlates. Am J Transplant 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibrahim HN, Foley RN, Reule SA, Spong R, Kukla A, Issa N et al. Renal Function Profile in White Kidney Donors: The First 4 Decades. J Am Soc Nephrol 2016;27(9):2885–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boudville N, Prasad GV, Knoll G, Muirhead N, Thiessen-Philbrook H, Yang RC et al. Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med 2006;145(3):185–196. [DOI] [PubMed] [Google Scholar]
- 34.Kasiske BL, Anderson-Haag T, Israni AK, Kalil RS, Kimmel PL, Kraus ES et al. A prospective controlled study of living kidney donors: three-year follow-up. Am J Kidney Dis 2015;66(1):114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Organ P, Transplantation Network O. New OPTN requirements and resources for the living donor kidney transplant programs. Prog Transplant 2013;23(2):117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.