Abstract
Cancer and its treatment are associated with neurotoxic side effects, including cognitive dysfunction, altered functional connectivity in the brain and structural abnormalities in white matter. There is evidence that cancer and its treatment can accelerate aging. Tau is a microtubule associated protein that contributes to microtubule stability thereby playing a key role in neuronal function. Clustering of tau is commonly observed in the aged brain and is related to cognitive decline. We hypothesized that chemotherapy-induced cognitive impairment is associated with accelerated development of tau clustering in the brain as a sign of accelerated aging.
We show for the first time that treatment of adult (7–8 month-old) male C57/Bl6 mice with cisplatin results in reduced cognitive function and a marked increase in the number of large endogenous tau clusters in the hippocampus when assessed 4 months later. In contrast, we detected only few small tau clusters in the hippocampus of age-matched 11–12 month-old control mice. Astrocyte GFAP expression was increased in close vicinity to the tau clusters in cisplatin-treated mice. We did not detect changes in the microglial marker IBA-1 in the brain of mice treated with cisplatin. The accelerated formation of Tau-1 clusters in cisplatin-treated mice was associated with a decrease in the levels of the post-synaptic marker PSD95 and of the presynaptic marker synaptophysin in the hippocampus.
We demonstrate here for the first time that chemotherapy markedly accelerates development of signs of tauopathy and loss of synaptic integrity in the hippocampus. These findings provide a mechanistic link between chemotherapy cognitive decline and accelerated aging in cancer survivors.
INTRODUCTION:
More than 74% of the 15.5 million cancer survivors in the United States are 60 years old or older (Miller et al., 2016). Various reports suggest that 35%‒85% of patients treated for cancer suffer from long term reductions in cognitive function, which include attention deficits, decreased executive functioning and multitasking, and decreased memory function (Ahles et al., 2008; Argyriou et al., 2011; Ferguson et al., 2007; Fung and Vaughn, 2011; Kannarkat et al., 2007; Kesler et al., 2013). Neuroimaging data obtained in patients treated for cancer indicate that cognitive deficits in patients treated for cancer are associated with changes in the functional connectome and in structure of the white matter (Correa et al., 2004; Simó et al., 2013). In at least a subset of cancer survivors, there is evidence for accelerated biological aging (Henderson et al., 2014). Recently, a study by Carroll et al. identified a relation between measures of biological aging and cognitive performance in breast cancer survivors (Carroll et al., 2019). Moreover, functional neuroimaging studies identified signs of accelerated aging of the brain in cancer survivors (Kesler et al., 2013, 2017). It remains to be determined whether the accelerated aging observed in patients is the result of cancer and/or its treatment.
Aging increases neuronal vulnerability and is associated with buildup of damaged proteins that perturb neuronal circuits. Tau proteins are a group of axonal microtubule-associated proteins that contribute to neuronal health by regulating microtubule assembly, dynamic behavior, spatial organization, and axonal transport of organelles (Drubin and Kirschner, 1986; Terwel et al., 2002). During aging, conformational changes and post-translational modifications of tau protein, such as phosphorylation, result in dissociation of tau from axonal microtubules (Buée et al., 2000; Dickson, 1999). These changes in tau lead to missorting and clustering of the protein, a process known as age-related tauopathy (Franzmeier et al., n.d.; Tseng et al., 2017; Zhou et al., 2017). Tauopathy is associated with the synapse loss and neuroinflammation that occur during aging, and is exaggerated in human AD patients and in animal models of the disease (Buée et al., 2000; Dickson, 1999; Terwel et al., 2002; Tseng et al., 2017).
Most studies on chemotherapy-induced cognitive impairment have been done in patients undergoing treatment for breast cancer (Deprez et al., 2011; Li et al., 2018). However, there is accumulating evidence that patients treated with platinum-based compounds for solid tumors including testicular, lung, bladder, and head and neck cancer also frequently develop cognitive deficiencies and structural abnormalities in the brain (Amidi et al., 2017; Bromis et al., 2017; Simó et al., 2015; Stouten-Kemperman et al., 2018). Preclinical studies have shown that administration of chemotherapeutic agents, including cisplatin and doxorubicin to mice increases expression of cellular senescence markers (Demaria et al., 2017). We and others showed that treatment of young adult rats or mice with these chemotherapeutics reduces their performance in cognitive function tasks and induces structural changes in the brain (Cheng et al., 2017; Chiu et al., 2018, 2017; Lomeli et al., 2017; Ma et al., 2018; Seigers et al., 2015; Zhou et al., 2016).
Here, we tested the hypothesis that treatment of mice that have fully reached adulthood, with cisplatin accelerates brain aging as evidenced by development of signs of tauopathy and loss of synaptic integrity. We treated 7–8 month-old male mice (i.e. at 25–30% of their life span) with cisplatin and assessed the development of Tau-1 clusters, levels of phosphorylated tau, expression of the astrocyte marker GFAP and the microglial marker IBA-1, and of markers of synaptic integrity 4 months later, when the mice were 11–12 months old. This time point was selected because the first evidence for tau clustering in the brain of wild type mice is detected at this age (Tseng et al., 2017).
MATERIALS AND METHODS:
Mice
Male C57BL/6J mice (Jackson Laboratory) of 7–8 months of age at start of the study were used. All experiments were approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center in Houston, TX.
Chemotherapy administration
7–8 month old mice received cisplatin (Fresenius Kabi USA; cumulative dose 23 mg/kg; equivalent to 70 mg/m2 in humans http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm078932.pdf) or phosphate-buffered saline (PBS). Cisplatin was administered intraperitoneally (i.p.) in 2 rounds of 5 daily doses of 2.3 mg/kg with 5 days of rest in between to prevent excessive body weight loss(Chiu et al., 2017; Mao-Ying et al., 2014; Ta et al., 2009).
Immunohistochemistry
Four months after cisplatin treatment, mice were perfused intracardially with ice-cold PBS followed by 4% paraformaldehyde (PFA) in PBS. Brains were post-fixed in 4% PFA for 6 hours, cryoprotected in sucrose and frozen in optimal cutting temperature compound (O.C.T., Sakura Finetek, Torrance, CA) and cut into 8 μm in the coronal plane. Slides were incubated with the following antibodies: mouse anti-Tau-1 (1:200, Millipore), mouse anti-phospho-Tau (AT8) (1:100, Thermo Scientific), rabbit anti-GFAP (1:1000, Acris Antibodies), rabbit anti-Iba-1 (1:500, Wako), rabbit anti-synaptophysin (1:1000, Millipore), rabbit anti-PSD95 (1:1000, Abcam), followed by secondary antibody incubation in Alexa-488 goat-anti-rabbit (1:1000, Invitrogen, Grand Island, NY) for synaptophysin, Alexa-488 goat anti-mouse (1:500, Invitrogen, Grand Island, NY) for Tau-1 and AT8, and Alexa-594 goat anti-rabbit (1:500, Invitrogen, Grand Island, NY) for GFAP, Iba-1, and PSD95. As a negative control, primary antibody was omitted. Sections were visualized using a Leica fluorescence microscope. The entire section was examined for signs of Tau-1 clustering and the number of clusters in the hippocampus was quantified. Expression of each synaptic marker was quantified in the CA1 and dentate gyrus of the hippocampus using 3–4 sections that were cut 100 μm apart per mouse and 4–5 mice per group. The mean intensity of fluorescence was calculated using ImageJ software.
Behavior
Novel Object Place Recognition Test (NOPRT)
The novel object-place recognition test (NOPRT), a validated test for recognition memory, relies on a rodent’s innate preference for novelty. This test was performed as previously described (Chiu et al., 2017). Briefly, each animal is allowed to freely explore an area containing two identical objects for 5 minutes during the familiarization phase. After that, animals are returned to their home cage for 30 minutes. Subsequently, mice are returned to the area, where one object is replaced by a novel object of a different shape, and placed in a difference location and mice freely explore for another 5 minutes. The exploration time for the familiar and new object during the 5 minutes of the test phase was analyzed using EthoVision XT 10.1 video tracking software (Noldus Information Technology., Leesburg, VA). The discrimination index measure calculated as (T Novel – T Familiar)/(T Novel + T Familiar) is used as an indicator of memory function.
Puzzle Box Test
The puzzle box test exploits the preference of mice for the dark. Mice are placed in a brightly lit arena (55 cm × 28 cm) connected to a dark area (15 cm × 28 cm) by an underpass (4 cm × 2.5 cm). In the easy trials (trials 1–4), the underpass is open. During intermediate trials (trials 5–7), mice the underpass is blocked by bedding and in the difficult trials (trials 8–11), the underpass is covered by a lid. The time elapsed before animal enters the dark compartment is recorded as a measure of executive function.
Statistical analysis
Data are expressed as Mean ± SEM. Statistical analyses were performed using two-way ANOVA followed by Tukey test or using student t-test where appropriate in GraphPad Prism 7.01.
RESULTS:
Cisplatin treatment induces cognitive impairment
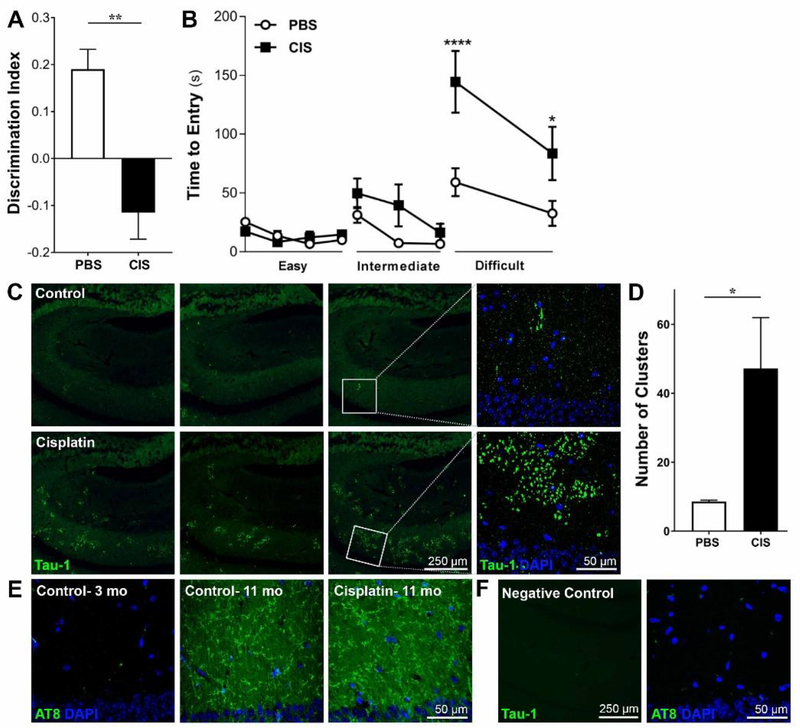
To assess the effect of cisplatin on cognitive function, 7–8 months old male mice were treated with cisplatin (cumulative dose 23 mg/kg) and performance in two different tasks of cognitive function was examined 3–4 months later. In the NOPRT, cisplatin-treated mice did not show a preference for novelty while control mice clearly preferred the novel object/place (Figure 1A). These findings indicate a decrease in spatial and working memory in cisplatin-treated mice. Total interaction times with the objects did not significantly differ between the two groups (90.10 ± 8.542 for PBS and 73.13 ± 7.011 for Cisplatin; unpaired t-test p = 0.6152), indicating that deficits in spatial and working memory were not due to a difference in motivation or interest in the objects.
Figure 1. Reduced performance in cognitive tasks and accelerated development of Tau-1 clusters in response to cisplatin.
Male mice were treated with cisplatin or saline at an age of 7–8 months and cognitive function was tested 3.5 months later. A. Performance in the NOPRT; data represents discrimination index calculated as (Time Novel – Time Familiar) / (Time Novel + Time Familiar). Results are expressed as mean ± SEM; n = 8 mice per group; **p < 0.01. B. Performance in the puzzle box testing 3 levels of difficulty: easy (open underpass; trials 1–4), intermediate (bedding in the underpass; trials 5–7), and difficult (underpass closed by plug; trials 8–9). Time to escape into the dark compartment is recorded. Results are expressed as mean ± SEM; n = 8 mice/group; * p < 0.05; ****p < 0.0001. C. Four months after the last cisplatin injection, brains of 11–12 month old mice were collected and slices from control (top row) and cisplatin (bottom row) treated mice were stained with a Tau-1 antibody. Representative examples of Tau-1 clustering in 3 different mice from each treatment group and a larger magnification of the clusters in one mouse per group are presented. D. Quantification of the number of clusters per hippocampus for control and cisplatin-treated mice. Results are expressed as mean ± SEM; n = 5 mice per group; * p < 0.05. E. Representative examples of phospho-tau immunofluorescence using the AT8 antibody in young control mice and in 11 month-old control and cisplatin-treated mice F. Representative images of staining controls on sections incubated with secondary antibodies without the addition of primary antibodies.
To assess executive function, we used the puzzle box test in which mice escape from a light to a dark compartment through an underpass that is first open, then blocked by bedding and finally with a plug (see methods section for details). Control and cisplatin-treated mice both readily escaped to the dark compartment during the easy (open underpass) and intermediate (bedding in the underpass) trials, and there were no group differences. However, on the difficult task, mice treated with cisplatin required significantly more time to escape than control mice (Figure 1B), indicating reduced executive function.
Cisplatin treatment induces tauopathy
To determine whether the cisplatin-induced decrease in performance in the cognitive tasks were associated with signs of accelerated aging, we examined Tau-1 staining in brains collected after completion of behavioral analyses, when the mice were 11–12 months old. In control mice of this age, we detected few small clusters of Tau-1 in the hippocampus (Figure 1C, top row). This is consistent with a recent study showing endogenous mouse tau clustering can be first detected in untreated wild type mice of 11–12 months, and is limited to few and small clusters as compared to the appearance of abundant clusters at an age of 24 months (Tseng et al., 2017). Notably, cisplatin treatment at the age of 7–8 months strongly increased the number of Tau-1 clusters in the hippocampus when analyzed at 11–12 months. Moreover, the individual clusters were larger than the sparse clusters detected in the age-matched control mice (Figure 1C, bottom row). The increase in the number and size of Tau-1 clusters induced by cisplatin was limited to the hippocampus (Figure 1D). We did not detect Tau-1 clusters in other brain regions, including the cortex, cerebellum or brainstem. Interestingly, the accumulation of Tau-1 clusters in 11–12-month-old mice was not associated with an increase in phospho-tau levels (Figure 1E); staining with the AT8 antibody to detect phospho-tau was similar in the brains of control and cisplatin-treated mice. As a positive control we compared AT8 staining in brains of untreated young (3-month-old mice) and old (11–12 months) mice. The results in Figure 1E demonstrate that we clearly detected an age-related increase in phospho-tau when comparing the untreated young and old mice.
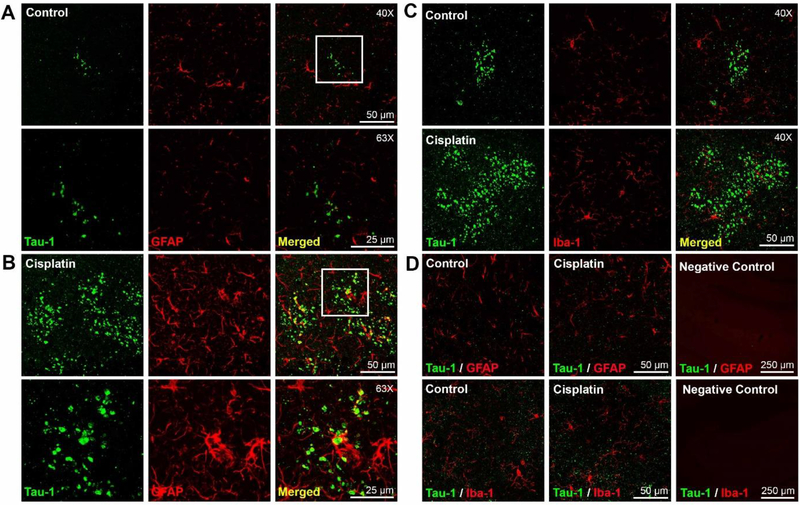
We detected an increase in glial fibrillary acidic protein (GFAP) staining in close proximity to the Tau-1 clusters, as compared to areas that do not contain Tau-1 clusters (Figure 2AB, D). The GFAP staining was most pronounced in the areas immediately surrounding the large Tau-1 clusters in the cisplatin-treated mice. However, we did not detect co-localization of GFAP and Tau-1, indicating that the clusters are not in the astrocytes themselves (Figure 2B). There was no evidence for increases in Iba-1 or changes in the morphology of the microglia in the cisplatin-treated mice as compared to aged matched control mice (Figure 2C).
Figure 2. Accelerated development of tau clusters in cisplatin treated mice is associated with increased GFAP expression.
(A and B): Brains of control and cisplatin treated mice were co-stained for Tau-1 and GFAP. Representative examples of GFAP staining in an area of minimal Tau-1 clusters in control mice (A) and overt Tau-1 pathology in cisplatin treated mice (B). (Top row; 40x objective; Bottom row; 63x objective). The higher magnification demonstrates that there is no overlap between Tau-1 clusters and GFAP. C. GFAP staining in areas without Tau-1 clusters (left and middle panel) and control staining with secondary antibodies only (right). C. Double immunofluorescence analysis of Tau-1 and Iba-1 in brain of control (top) and cisplatin-treated (bottom) mice in areas of Tau-1 clusters. D. GFAP (top) and Iba-1 (bottom) expression in brain areas without Tau-1 clusters in control (left) and cisplatintreated mice (middle) and control with secondary antibodies only (right).
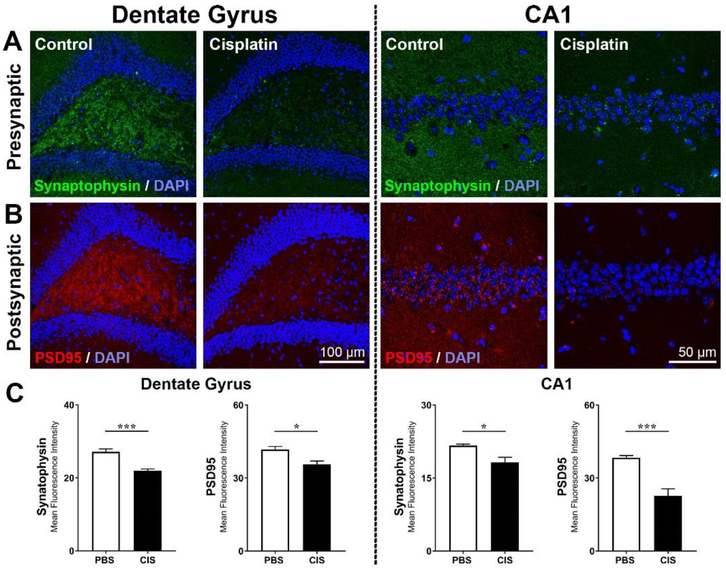
Formation of Tau-1 clusters interferes with synapse function and can contribute to loss of synapses. Therefore, we compared expression of the pre- and post-synaptic markers synaptophysin and PSD95 in the dentate gyrus and CA1 of the hippocampus (Figure 3A–B). Cisplatin-treated mice showed a clear decrease in both synaptophysin and PSD95 in the DG and in the CA1 areas of the hippocampus (Figure 3C).
Figure 3. Cisplatin- induces loss of synaptic integrity in the hippocampus.
Brains from control and cisplatin-treated mice were stained for markers of synaptic integrity: Expression of (A) pre-synaptic marker synaptophysin and (B) the post-synaptic marker PSD95 in the dentate gyrus and CA1 regions of the hippocampus C. Quantification of the mean fluorescence intensity of synaptophysin and PSD95 immunostaining. Results are expressed as mean ± SEM; n = 5 mice for each treatment; *p < 0.05; ***p < 0.001.
DISCUSSION:
Patients treated for cancer show signs of advanced aging (Armstrong et al., 2014; Henderson et al., 2014; Kesler et al., 2017), which can be due to cancer and its treatment. We aimed at determining whether the chemotherapeutic cisplatin results in development of signs of accelerated aging in the mouse brain. Our results demonstrate that treatment of adult male mice with cisplatin markedly accelerates brain aging as evidenced by extensive development of endogenous mouse tau clusters in the hippocampus at the age of 11–12 months. In contrast, only very few small tau clusters were detected in the hippocampus of age-matched control mice. The accelerated development of tauopathy in cisplatin-treated mice was associated with increased GFAP expression, indicating local astrocyte activation, and with loss of synaptic integrity in the hippocampus. The cisplatin-induced reduction in cognitive function is similar in male and female mice (Chiu et al., 2018), but it remains to be determined whether cisplatin also induces signs of tauopathy in female mice.
Most mouse studies on tauopathy rely on transgenic mice overexpressing human tau. To our knowledge there is only one study in which age-related changes in the distribution of endogenous mouse tau accumulation in wild type mice has been examined (Tseng et al., 2017). Consistent with our findings, very few and only small clusters of Tau-1 were detected by Tseng et al. at an age of 11 months. Further aging was associated with a stark increase in the number and size of the clusters, with abundant and large clusters towards the end of the 2–2.5 year life span of the mouse. We show here for the first time that cisplatin treatment markedly accelerates the development of Tau-1 clusters in the murine brain, with abundant and large clusters in the brain already by the age of 11–12 months. Notably, the specific localization of Tau-1 clusters in the hippocampus of cisplatin-treated 11-month old mice is similar to the localization that has been reported in 19–27-month old control mice (Tseng et al., 2017). The Tau-1 clusters in the brain of cisplatin-treated mice did not co-label with the AT8 antibody that detects phosphorylated tau, consistent with the previous report that AT8 staining was negative in neuritic tau beads in the brain of aged mice (Tseng et al., 2017). We did detect an age-dependent increase in phospho-tau expression from 12 week old untreated mice compared to 11–12 month old untreated mice. Taken together, our data indicates that although we detect age-related changes in phospho-tau levels in mice, the cisplatin-induced Tau-1 clusters do not contain phospho-tau. Recently, we have shown that cisplatin treatment induces an increase in phospho-tau (without detectable Tau-1 clusters) at 3–4 weeks after administration of cisplatin in younger (8–10 week-old) mice (Ma et al., 2018). It remains to be determined whether the increase in phospho-tau in mice treated with cisplatin precedes the formation of the Tau-1 clusters or is an independent phenomenon. The accelerated development of tau clusters in the hippocampus may be part of a more maladaptive aging profile in the brain of cisplatin-treated mice. In summary, at an age (11–12 months) when endogenous mouse Tau-1 clusters are barely detectable in control mice, mice treated with cisplatin have developed Tau-1 clusters that are similar to those in aged (19–27 month old) control mice with respect to localization, morphology, abundance, and lack of co labeling with phospho-tau. Therefore, we propose that the cisplatin-induced increase in Tau-1 clustering in the brain is a sign of accelerated aging. Our current work focuses on the question whether the presence of a tumor enhances the expression of Tau-1 clusters.
Neuroinflammation is another prominent feature of the aging brain (Ownby, 2010). Several lines of research suggest an interplay between neuroinflammatory responses such as gliosis and tau pathology in neurodegenerative disorders including AD (Cook et al., 2015; Verkhratsky et al., 2010). In mouse models of AD, accumulation of activated microglia and astrocytes in affected brain areas has been shown, and the severity of inflammation correlates with neuronal death and the rate of disease progression (González-Reyes et al., 2017; Millington et al., 2014). After cisplatin treatment in the younger 10–12 weeks old mice, we never observed changes in GFAP or IBA-1 expression or in cytokine production in the brain (Chiu et al., 2017; Zhou et al., 2016). However, in older 7–8 month old mice, cisplatin treatment increased GFAP expression, but only within the regions that display Tau-1 clustering. While we detected an increase in GFAP expression within close vicinity to the Tau-1 clusters, there was no evidence of co-localization, which is in line with the notion that we are dealing with axonal Tau-1 beading. It remains to be determined whether the local increase in GFAP expression in the brain of cisplatin treated mice is associated with increased secretion of pro-inflammatory factors. Such a neuroinflammatory response could contribute to tauopathy and to loss of synaptic integrity and persistence of cognitive dysfunction in these aged mice (Chun et al., 2018; Colombo and Farina, 2016).
We recently demonstrated that cisplatin-induced cognitive impairment in young adult mice is caused by damage to mitochondria without inducing signs of apoptosis (Chiu et al., 2017; Maj et al., 2017). We proposed that this mitochondrial damage is initiated by cisplatin entering the brain, because we detect a rapid increase in mitochondrial p53 in brain mitochondria following a single dose of cisplatin. Moreover, protecting the mitochondria by co-administration of the small compound pifithrin-μ, which prevents the early cisplatin-induced accumulation of p53 at brain mitochondria, prevented both the downstream reduction in mitochondrial bioenergetics and cognitive function (Chiu et al., 2017). This is interesting in the context of the present study, because there is evidence that mitochondrial stress can promote abnormal distribution of Tau-1 (Escobar-Khondiker et al., 2007; Greenwood et al., 2007; Melov et al., 2007; Takeuchi et al., 2005). Conversely, tauopathy has been suggested as a driver of mitochondrial dysfunction as well. Moreover, aggregation of tau leads to disassembly of microtubules thereby impairing axonal mitochondrial and protein transport (Eckert et al., 2014; Kopeikina et al., 2011; Reddy, 2011). In support of this model, we showed that inhibition of HDAC6, which is known to restore axonal transport, reverses the increase in phospho-tau in the brain of mice treated with cisplatin (Ma et al., 2018). HDAC6 inhibition also normalized mitochondrial function in synaptosomes of cisplatin-treated mice (Ma et al., 2018). The latter would indicate that de-acetylation of tau could play a role in the post-translational modification of tau ultimately resulting in Tau-1 clustering (Esteves et al., 2018). Mitochondrial dysfunction also leads to the production of oxygen radicals which could be another driver of tau pathology (Alam and Sharma, 2019). Future studies should examine whether the early disturbance of neuronal bioenergetics (shortly after cisplatin treatment), drives the accelerated tau clustering we describe here in mice treated during the adult phase of their life.
Our current findings indicate that the chemotherapeutic cisplatin accelerates development of age-related tauopathy, identifying chemotherapy as one of the possible causes for the accelerated aging in cancer patients. Further studies should include additional chemotherapeutics and also investigate ways to prevent the development of tauopathy after chemotherapy in order to mitigate accelerated brain aging in patients treated for cancer.
Highlights:
Cisplatin treatment of adult wild type mice markedly accelerates development of tau pathology
Cisplatin-induced tau clusters are accompanied by astrocyte activation
Cisplatin induces loss of synaptic integrity in the hippocampus and cognitive deficits
Acknowledgements
This research was supported by R01CA208371, and R01CA227064 (C.J.H and A.K) from the National Cancer Institute of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors report no conflicts of interest in this work.
References
- Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA, 2008. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res. Treat 110, 143–152. 10.1007/s10549-007-9686-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam J, Sharma L, 2019. Potential Enzymatic Targets in Alzheimer’s: A Comprehensive Review. Curr. Drug Targets 20, 316–339. 10.2174/1389450119666180820104723 [DOI] [PubMed] [Google Scholar]
- Amidi A, Hosseini SMH, Leemans A, Kesler SR, Agerbæk M, Wu LM, Zachariae R, 2017. Changes in Brain Structural Networks and Cognitive Functions in Testicular Cancer Patients Receiving Cisplatin-Based Chemotherapy. J. Natl. Cancer Inst 109 10.1093/jnci/djx085 [DOI] [PubMed] [Google Scholar]
- Argyriou AA, Assimakopoulos K, Iconomou G, Giannakopoulou F, Kalofonos HP, 2011. Either called “chemobrain” or “chemofog,” the long-term chemotherapy-induced cognitive decline in cancer survivors is real. J. Pain Symptom Manage 41, 126–139. 10.1016/j.jpainsymman.2010.04.021 [DOI] [PubMed] [Google Scholar]
- Armstrong GT, Kawashima T, Leisenring W, Stratton K, Stovall M, Hudson MM, Sklar CA, Robison LL, Oeffinger KC, 2014. Aging and Risk of Severe, Disabling, Life-Threatening, and Fatal Events in the Childhood Cancer Survivor Study. J. Clin. Oncol 32, 1218–1227. 10.1200/JCO.2013.51.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromis K, Gkiatis K, Karanasiou I, Matsopoulos G, Karavasilis E, Papathanasiou M, Efstathopoulos E, Kelekis N, Kouloulias V, 2017. Altered Brain Functional Connectivity in Small-Cell Lung Cancer Patients after Chemotherapy Treatment: A Resting-State fMRI Study. Comput. Math. Methods Med. 2017. 10.1155/2017/1403940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR, 2000. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders11These authors contributed equally to this work. Brain Res. Rev 33, 95–130. 10.1016/S0165-0173(00)00019-9 [DOI] [PubMed] [Google Scholar]
- Carroll JE, Dyk KV, Bower JE, Scuric Z, Petersen L, Schiestl R, Irwin MR, Ganz PA, 2019. Cognitive performance in survivors of breast cancer and markers of biological aging. Cancer 125, 298–306. 10.1002/cncr.31777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Liu X, Cao L, Zhang T, Li H, Lin W, 2017. Neo-adjuvant chemotherapy with cisplatin induces low expression of NMDA receptors and postoperative cognitive impairment. Neurosci. Lett 637, 168–174. 10.1016/j.neulet.2016.11.028 [DOI] [PubMed] [Google Scholar]
- Chiu GS, Boukelmoune N, Chiang ACA, Peng B, Rao V, Kingsley C, Liu H-L, Kavelaars A, Kesler SR, Heijnen CJ, 2018. Nasal administration of mesenchymal stem cells restores cisplatin-induced cognitive impairment and brain damage in mice. Oncotarget 9, 35581–35597. 10.18632/oncotarget.26272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu GS, Maj MA, Rizvi S, Dantzer R, Vichaya EG, Laumet G, Kavelaars A, Heijnen CJ, 2017. Pifithrin-μ Prevents Cisplatin-Induced Chemobrain by Preserving Neuronal Mitochondrial Function. Cancer Res. 77, 742–752. 10.1158/0008-5472.CAN-16-1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun H, Marriott I, Lee CJ, Cho H, 2018. Elucidating the Interactive Roles of Glia in Alzheimer’s Disease Using Established and Newly Developed Experimental Models. Front. Neurol 9 10.3389/fneur.2018.00797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo E, Farina C, 2016. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 37, 608–620. 10.1016/j.it.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Cook C, Kang SS, Carlomagno Y, Lin W-L, Yue M, Kurti A, Shinohara M, Jansen-West K, Perkerson E, Castanedes-Casey M, Rousseau L, Phillips V, Bu G, Dickson DW, Petrucelli L, Fryer JD, 2015. Tau deposition drives neuropathological, inflammatory and behavioral abnormalities independently of neuronal loss in a novel mouse model. Hum. Mol. Genet 24, 6198–6212. 10.1093/hmg/ddv336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DD, DeAngelis LM, Shi W, Thaler H, Glass A, Abrey LE, 2004. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology 62, 548–555. [DOI] [PubMed] [Google Scholar]
- Demaria M, O’Leary MN, Chang J, Shao L, Liu S, Alimirah F, Koenig K, Le C, Mitin N, Deal AM, Alston S, Academia EC, Kilmarx S, Valdovinos A, Wang B, de Bruin A, Kennedy BK, Melov S, Zhou D, Sharpless NE, Muss H, Campisi J, 2017. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 7, 165–176. 10.1158/2159-8290.CD-16-0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, Van den Stock J, Smeets A, Christiaens M-R, Leemans A, Van Hecke W, Vandenberghe J, Vandenbulcke M, Sunaert S, 2011. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum. Brain Mapp 32, 480–493. 10.1002/hbm.21033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, 1999. Tau and synuclein and their role in neuropathology. Brain Pathol. Zurich Switz 9, 657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin DG, Kirschner MW, 1986. Tau protein function in living cells. J. Cell Biol 103, 2739–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert A, Nisbet R, Grimm A, Götz J, 2014. March separate, strike together--role of phosphorylated TAU in mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. cta 1842, 1258–1266. 10.1016/j.bbadis.2013.08.013 [DOI] [PubMed] [Google Scholar]
- Escobar-Khondiker M, Höllerhage M, Muriel M-P, Champy P, Bach A, Depienne C, Respondek G, Yamada ES, Lannuzel A, Yagi T, Hirsch EC, Oertel WH, Jacob R, Michel PP, Ruberg M, Höglinger GU, 2007. Annonacin, a natural mitochondrial complex I inhibitor, causes tau pathology in cultured neurons. J. Neurosci. Off. J. Soc. Neurosci 27, 7827–7837. 10.1523/JNEUROSCI.1644-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves AR, Palma AM, Gomes R, Santos D, Silva DF, Cardoso SM, 2018. Acetylation as a major determinant to microtubule-dependent autophagy: Relevance to Alzheimer’s and Parkinson disease pathology. Biochim. Biophys. Acta BBA - Mol. Basis Dis 10.1016/j.bbadis.2018.11.014 [DOI] [PubMed] [Google Scholar]
- Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA, 2007. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 25, 3866–3870. 10.1200/JCO.2007.10.8639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzmeier N, Rubinski A, Neitzel J, Kim Y, Damm A, Na DL, Kim HJ, Lyoo CH, Cho H, Finsterwalder S, Duering M, Seo SW, Ewers M, n.d. Functional connectivity associated with tau levels in ageing, Alzheimer’s, and small vessel disease. Brain. 10.1093/brain/awz026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung C, Vaughn DJ, 2011. Complications associated with chemotherapy in testicular cancer management. Nat. Rev. Urol 8, 213–222. 10.1038/nrurol.2011.26 [DOI] [PubMed] [Google Scholar]
- González-Reyes RE, Nava-Mesa MO, Vargas-Sánchez K, Ariza-Salamanca D, Mora-Muñoz L, 2017. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci 10 10.3389/fnmol.2017.00427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood SM, Mizielinska SM, Frenguelli BG, Harvey J, Connolly CN, 2007. Mitochondrial Dysfunction and Dendritic Beading during Neuronal Toxicity. J. Biol. Chem 282, 26235–26244. 10.1074/jbc.M704488200 [DOI] [PubMed] [Google Scholar]
- Henderson TO, Ness KK, Cohen HJ, 2014. Accelerated aging among cancer survivors: from pediatrics to geriatrics. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet e423–430. 10.14694/EdBook_AM.2014.34.e423 [DOI] [PubMed] [Google Scholar]
- Kannarkat G, Lasher EE, Schiff D, 2007. Neurologic complications of chemotherapy agents. Curr. Opin. Neurol 20, 719–725. 10.1097/WCO.0b013e3282f1a06e [DOI] [PubMed] [Google Scholar]
- Kesler SR, Rao V, Ray WJ, Rao A, Alzheimer’s Disease Neuroimaging Initiative, 2017. Probability of Alzheimer’s disease in breast cancer survivors based on gray-matter structural network efficiency. Alzheimers Dement. Amst. Neth 9, 67–75. 10.1016/j.dadm.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Wefel JS, Hosseini SMH, Cheung M, Watson CL, Hoeft F, 2013. Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc. Natl. Acad. Sci. U. S. A 110, 11600–11605. 10.1073/pnas.1214551110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopeikina KJ, Carlson GA, Pitstick R, Ludvigson AE, Peters A, Luebke JI, Koffie RM, Frosch MP, Hyman BT, Spires-Jones TL, 2011. Tau Accumulation Causes Mitochondrial Distribution Deficits in Neurons in a Mouse Model of Tauopathy and in Human Alzheimer’s Disease Brain. Am. J. Pathol 179, 2071–2082. 10.1016/j.ajpath.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chen H, Lv Y, Chao HH, Gong L, Li C-SR, Cheng H, 2018. Diminished gray matter density mediates chemotherapy dosage-related cognitive impairment in breast cancer patients. Sci. Rep 8, 13801 10.1038/s41598-018-32257-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomeli N, Di K, Czerniawski J, Guzowski JF, Bota DA, 2017. Cisplatin-induced mitochondrial dysfunction is associated with impaired cognitive function in rats. Free Radic. Biol. Med 102, 274–286. 10.1016/j.freeradbiomed.2016.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Huo X, Jarpe MB, Kavelaars A, Heijnen CJ, 2018. Pharmacological inhibition of HDAC6 reverses cognitive impairment and tau pathology as a result of cisplatin treatment. Acta Neuropathol. Commun 6, 103 10.1186/s40478-018-0604-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj MA, Ma J, Krukowski KN, Kavelaars A, Heijnen CJ, 2017. Inhibition of Mitochondrial p53 Accumulation by PFT-μ Prevents Cisplatin-Induced Peripheral Neuropathy. Front. Mol. Neurosci 10 10.3389/fnmol.2017.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao-Ying Q-L, Kavelaars A, Krukowski K, Huo X-J, Zhou W, Price TJ, Cleeland C, Heijnen CJ, 2014. The Anti-Diabetic Drug Metformin Protects against Chemotherapy-Induced Peripheral Neuropathy in a Mouse Model. PLoS ONE 9 10.1371/journal.pone.0100701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S, Adlard PA, Morten K, Johnson F, Golden TR, Hinerfeld D, Schilling B, Mavros C, Masters CL, Volitakis I, Li Q-X, Laughton K, Hubbard A, Cherny RA, Gibson B, Bush AI, 2007. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PloS One 2, e536 10.1371/journal.pone.0000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A, 2016. Cancer treatment and survivorship statistics, 2016. CA. Cancer J. Clin 66, 271–289. 10.3322/caac.21349 [DOI] [PubMed] [Google Scholar]
- Millington C, Sonego S, Karunaweera N, Rangel A, Aldrich-Wright JR, Campbell IL, Gyengesi E, Münch G, 2014. Chronic Neuroinflammation in Alzheimer’s Disease: New Perspectives on Animal Models and Promising Candidate Drugs. BioMed Res. Int. 2014 10.1155/2014/309129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby RL, 2010. Neuroinflammation and Cognitive Aging. Curr. Psychiatry Rep 12, 39–45. 10.1007/s11920-009-0082-1 [DOI] [PubMed] [Google Scholar]
- Reddy PH, 2011. Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer’s disease. Brain Res. 1415, 136–148. 10.1016/j.brainres.2011.07.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigers R, Loos M, Van Tellingen O, Boogerd W, Smit AB, Schagen SB, 2015. Cognitive impact of cytotoxic agents in mice. Psychopharmacology (Berl.) 232, 17–37. 10.1007/s00213-014-3636-9 [DOI] [PubMed] [Google Scholar]
- Simó M, Rifà-Ros X, Rodriguez-Fornells A, Bruna J, 2013. Chemobrain: A systematic review of structural and functional neuroimaging studies. Neurosci. Biobehav. Rev 37, 1311–1321. 10.1016/j.neubiorev.2013.04.015 [DOI] [PubMed] [Google Scholar]
- Simó M, Root JC, Vaquero L, Ripollés P, Jové J, Ahles T, Navarro A, Cardenal F, Bruna J, Rodríguez-Fornells A, 2015. Cognitive and Brain Structural Changes in a Lung Cancer Population. J. Thorac. Oncol 10, 38–45. 10.1097/JTO.0000000000000345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouten-Kemperman MM, de Ruiter MB, Boogerd W, Kerst JM, Kirschbaum C, Reneman L, Schagen SB, 2018. Brain Hyperconnectivity >10 Years After Cisplatin-Based Chemotherapy for Testicular Cancer. Brain Connect. 8, 398–406. 10.1089/brain.2017.0569 [DOI] [PubMed] [Google Scholar]
- Ta LE, Low PA, Windebank AJ, 2009. Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Mol. Pain 5, 9 10.1186/1744-8069-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Mizuno T, Zhang G, Wang J, Kawanokuchi J, Kuno R, Suzumura A, 2005. Neuritic Beading Induced by Activated Microglia Is an Early Feature of Neuronal Dysfunction Toward Neuronal Death by Inhibition of Mitochondrial Respiration and Axonal Transport. J. Biol. Chem 280, 10444–10454. 10.1074/jbc.M413863200 [DOI] [PubMed] [Google Scholar]
- Terwel D, Dewachter I, Van Leuven F, 2002. Axonal transport, tau protein, and neurodegeneration in Alzheimer’s disease. Neuromolecular Med. 2, 151–165. 10.1385/NMM:2:2:151 [DOI] [PubMed] [Google Scholar]
- Tseng J-H, Xie L, Song S, Xie Y, Allen L, Ajit D, Hong J-S, Chen X, Meeker RB, Cohen TJ, 2017. The Deacetylase HDAC6 Mediates Endogenous Neuritic Tau Pathology. Cell Rep. 20, 2169–2183. 10.1016/j.celrep.2017.07.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Olabarria M, Noristani HN, Yeh C-Y, Rodriguez JJ, 2010. Astrocytes in Alzheimer’s disease. Neurother. J. Am. Soc. Exp. Neurother 7, 399–412. 10.1016/j.nurt.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, McInnes J, Wierda K, Holt M, Herrmann AG, Jackson RJ, Wang Y-C, Swerts J, Beyens J, Miskiewicz K, Vilain S, Dewachter I, Moechars D, Strooper BD, Spires-Jones TL, Wit JD, Verstreken P, 2017. Tau association with synaptic vesicles causes presynaptic dysfunction. Nat. Commun 8, 15295 10.1038/ncomms15295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kavelaars A, Heijnen CJ, 2016. Metformin Prevents Cisplatin-Induced Cognitive Impairment and Brain Damage in Mice. PLOS ONE 11, e0151890 10.1371/journal.pone.0151890 [DOI] [PMC free article] [PubMed] [Google Scholar]