Abstract
Numerous aspects of mammalian physiology exhibit cyclic daily patterns, known as circadian rhythms. However, studies in aged humans and animals indicate that these physiological rhythms are not consistent throughout the life span. The simultaneous development of disrupted circadian rhythms and age-related impairments suggests a shared mechanism which may be amenable to therapeutic intervention. Recently, the endocannabinoid system has emerged as a complex signaling network which regulates numerous aspects of circadian physiology relevant to the neurobiology of aging. Agonists of cannabinoid receptor-1 (CB1) have consistently been shown to decrease neuronal activity, core body temperature, locomotion, and cognitive function. Paradoxically, several lines of evidence now suggest that very low doses of cannabinoids are beneficial in advanced age. One potential explanation for this phenomenon is that these drugs exhibit hormesis - a biphasic dose-response wherein low doses produce the opposite effects of higher doses. Therefore, it is important to determine the dose-, age-, and time-dependent effects of these substances on the regulation of circadian rhythms and other processes dysregulated in aging. This review highlights three fields - biological aging, circadian rhythms, and endocannabinoid signaling - to critically assess the therapeutic potential of endocannabinoid modulation in aged individuals. If the hormetic properties of exogenous cannabinoids are confirmed, we conclude that precise administration of these compounds may bidirectionally entrain central and peripheral circadian clocks and benefit multiple aspects of aging physiology.
Keywords: Advanced Age, Circadian Rhythm, Cannabis, Cognition, Chronopharmacology, Chronobiotic, Endocannabinoid, Hormesis
1. Biological Aging
In humans, it is well accepted that the end of life is wrought with numerous concomitant disease states which impose immense personal and social burden. As such, the study of core biological processes involved in aging has become an increasingly prominent topic. Interestingly, there is a clear dichotomy among individuals regarding measures of age-related performance (Rowe and Kahn, 2015). This phenotypic heterogeneity is particularly important when considering cognitive ability, since the deterioration of mental functions can greatly influence a person’s quality of life. Though many aspects of cognition decline with age, spatial orientation and speed of processing appear particularly susceptible to age-related dysfunction (Hedden and Gabrieli, 2004). Physiologically, the loss of neuronal synapses, chronic inflammation, oxidative stress, and impaired neurovascular coupling all contribute to declining cognitive performance with age (Ekdahl et al., 2009; Mariani et al., 2005; Morrison and Baxter, 2012). Fortunately, several studies indicate age-related cognitive impairment can be partially prevented or delayed using targeted pharmacological or hormonal interventions (Benedict et al., 2004; Cardinali et al., 2012; Lee and Silva, 2009; Li et al., 2011; Lichtenwalner et al., 2001).
One of the earliest reported symptoms of aging is disturbed sleep (Musiek et al., 2015; Rauchs et al., 2013). Sleep/wake cycles are one example of the biologic phenomenon known as circadian rhythms. Though sleep requirements change throughout the lifespan, sleep quality and consistency are known to markedly deteriorate with age (Bushey et al., 2010). This is relevant to cognitive decline since sleep is an integral factor in the consolidation of memory, and age-related sleep disruptions are often concomitant with cognitive impairment and/or neurodegenerative diseases (Dijk et al., 1999; Espiritu, 2008; Harand et al., 2012; Herculano-Houzel, 2013; Stickgold, 2012; Van Cauter et al., 2000). Despite the necessity of sleep for survival, defining the physiological purpose of sleep has been exceedingly difficult. However, relatively recent discovery of the glymphatic system and the sleep-dependent regulation of metabolite clearance from the brain indicates that sleep is vital to the maintenance of proteostasis in the central nervous system (Xie et al., 2013). Since proteostatic dysfunction is common in many pathologies of aging, disturbed sleep may play a causal role in age-related cognitive disorders. Although it remains unclear how age-dependent changes in the physiologic regulation of sleep are controlled, the maintenance of healthy sleep patterns in advanced age seems undeniably important to cognitive function.
2. Circadian Rhythms and Aging
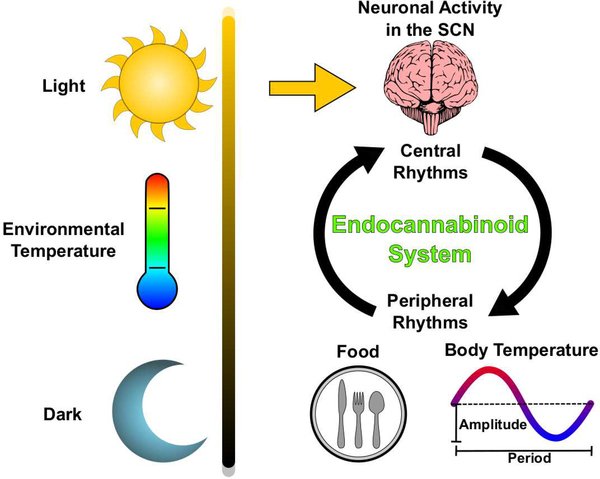
Circadian rhythms are scale-invariant biological patterns that correlate to the cyclic relationship of the Sun and Earth (Pittendrigh, 1993; Refinetti, 2016). Extrinsic cues such as light and environmental temperature are known as zeitgebers (time-givers) which entrain organisms’ behaviors to particular times of day and improve evolutionary fitness Figure 1 (Aschoff, 1965; Pittendrigh, 1960). Though it has long been observed that animals behave in a manner inherently tied to the time of day, only recently have the molecular and physiological underpinnings of these processes been elucidated (Hardin et al., 1990; Konopka and Benzer, 1971; Liu et al., 1997). Importantly, changes in these clock genes are attributed to both the process of aging and the pathogenesis of age-related diseases (Kondratova and Kondratov, 2012; Kress et al., 2018; Musiek et al., 2015).
Figure 1. Environmental inputs and physiological outputs of mammalian circadian rhythms.
Routine daily exposure to light entrains the SCN to a 24-hour period via the retinohypothalamic tract. Neurons of the SCN rhythmically alter their rates of firing in response to changing environmental conditions and humoral signals. Outputs from SCN neurons drive central rhythms in hormone production, locomotor activity, feeding behavior, and body temperature. Peripherally, cellular rhythms are entrained by the daily oscillation of body temperature and food-intake. Since endocannabinoid activity is known to regulate SCN neurons, body temperature, and food-intake, evidence suggests that the endocannabinoid system is a key component of physiological circadian rhythms.
Seminal work in hamsters and mice revealed that when aged animals are housed in complete darkness, their free-running (intrinsic) circadian rhythms of locomotion are significantly different from younger animals (Nakamura et al., 2011; Nakamura et al., 2016; Pittendrigh and Daan, 1974). Additionally, studies of molecular clocks indicate that both rhythm amplitude and regularity deteriorate with age (Yamazaki et al., 2002). More recent studies have confirmed these age-related changes in circadian rhythm amplitude and period are exacerbated in the absence of environmental cues (Nakamura et al., 2015). Though these findings suggest deteriorating rhythms can be masked or compensated by environmental cues, aberrant sleep/wake cycles have also been observed in several species of aged subjects under normal lighting conditions (Bushey et al., 2010; Espiritu, 2008; Saper et al., 2005a; Van Cauter et al., 2000).
In addition to impaired sleep, the amplitude of circadian locomotor activity and body temperature are known to decline in aging humans and rodents (Hu et al., 2013; Huang et al., 2002; Kramer et al., 2001). The circadian range of rectal temperature in mice is ~2.0°C, which declines to 0.5–1.0°C in advanced age; a similar reduction in range has been reported in humans (Koster-van Hoffen et al., 1993; Satinoff, 1998; Weitzman et al., 1982). Daily locomotion also goes down in mice, with average daily running wheel counts declining by ~50% (Valentinuzzi et al., 1997). Furthermore, sleep-dependent production of the pleiotropic humoral factor Growth Hormone declines with age, and it is reported that these disruptions precede or are comorbid with cognitive dysfunction (Michael et al., 1980; Sonntag et al., 2013). A recent study suggests that age-related changes in the epigenetic regulation of the clock gene Per1 underlies some aspects of cognitive decline (Kwapis et al., 2018). Whether circadian dysfunction is causal in age-related cognitive decline remains to be known, however the striking overlap of these observations warrants further investigation. Taken together, these observations suggest that both molecular and behavioral circadian rhythms might be responsive to- and responsible for- many aspects of biological aging.
Within the brain, the suprachiasmatic nuclei (SCN) of the mammalian hypothalamus are believed to be the primary neural sites of circadian integration (Saper, 2013). The SCN as a whole, through unknown mechanisms, integrates the oscillatory rhythm of each constituent neuron and collectively adopts a unified tone (Hastings et al., 2018; Liu et al., 1997; Welsh et al., 1995). Neuronal projections from the SCN transmit this coordinated rhythm to surrounding hypothalamic and brainstem structures responsible for basic physiological functions such as the sleep/wake cycle, locomotor activity, regulation of body temperature, and hormone production (Bass and Lazar, 2016; Hastings et al., 2003). At the molecular level, cellular circadian clocks consist of transcription-translation feedback loops which exhibit tightly coupled daily rhythms Figure 2. When exposed to a normal 24-hour light cycle, neurons of the SCN rhythmically express these clock genes that oscillate with periods of roughly 24 hours (Bass and Lazar, 2016; Menaker et al., 1978). Light, temperature, food, and pharmaceuticals can all act as zeitgebers, which modulate endogenous clock gene activity by extending or shortening the period of oscillation (Longo and Panda, 2016; Saper et al., 2005b). Such drugs, known as chronobiotics, influence circadian physiology by either directly impinging on core clock molecules or altering entrainment systems. Therapeutically, chronobiotics are used to shift or amplify endogenous circadian rhythms and reduce dissonance with environmental conditions (Redfern et al., 1994). Though circadian dysregulation is reported with chronic or uncontrolled use of exogenous substances such as caffeine, cannabis, and stimulant medications (Burke et al., 2015; Hasler et al., 2012; Stein et al., 2012; Whitehurst et al., 2015). Promising ongoing research suggests that chronobiotics may be beneficial in cases of jet-lag, shiftwork, and potentially aging, (Arendt and Skene, 2005; Potter et al., 2016).
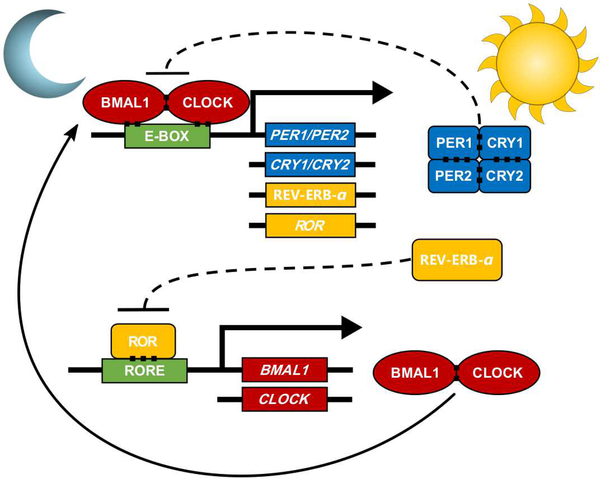
Figure 2: Molecular components of cellular circadian rhythms.
The core circadian molecular loop consists of two proteins, Brain and muscle arnt-like 1 (BMAL1) and Circadian Locomotor Output Cycles Kaput (CLOCK), which interact to form a transcriptional activator complex that stimulates transcription of the Period and Cryptochrome genes (PER1, PER2, CRY1, and CRY2). The Per and Cry proteins accumulate in the cytoplasm throughout the day and ultimately bind to the BMAL1:CLOCK complex. Sufficient binding of the PER:CRY complex to the BMAL1:CLOCK complex prohibits transcription of PER and CRY mRNA. As protein levels of PER and CRY diminish, the BMAL1:CLOCK complex is allowed to stimulate transcription once more.
The SCN is often considered the primary regulator of mammalian circadian rhythms, although cell-autonomous molecular feedback loops have been observed in nearly all tissues throughout the body (Schibler et al., 2015; Yamazaki et al., 2000). In peripheral tissues, the period of each cell’s rhythm is very near to 24 hours, although their exact rates are determined by body temperature, humoral factors, and nutritional state (Hattar et al., 2003; Longo and Panda, 2016; Pittendrigh, 1960; Pittendrigh and Minis, 1964; Rosenwasser and Turek, 2015; Welsh et al., 1995). Critically, in contrast to the clock gene rhythms of peripheral tissues, neurons of the SCN appear resistant to entrainment by body temperature (Buhr et al., 2010; Mohawk et al., 2012). Since the SCN is directly responsible for the rhythm of body temperature, this presents a potential mechanism through which the SCN can regulate clocks in peripheral tissues (Partch et al., 2014; Schibler et al., 2015). Core body temperature - a vital physiological output which exhibits circadian rhythmicity - has consistently been shown to decline with age and older subjects exhibit impaired thermogenesis (Balmagiya and Rozovski, 1983; Harper et al., 2005; Van Someren, 2007). If the circadian rhythms of peripheral tissues are functionally entrained by body temperature, then age-related impairments of thermoregulatory capacity could explain why the rhythms of some peripheral tissues are unable to be properly maintained. Furthermore, since neuronal activity in the SCN determines central clock rhythms, and peripheral cellular clocks are entrained by thermic signals, pharmacological interventions which influence both neuronal activity and body temperature are of particular interest.
Declining circadian function with age is not associated with changes in overall SCN volume, however several studies report altered physiological properties in this brain region (Roozendaal et al., 1987; Tsukahara et al., 2005). In vivo electrophysiological recordings of the SCN show reduced amplitude and “noisy” signals as animals age, suggesting that neuronal function is compromised (Nakamura et al., 2011). Additionally, increases in reactive astrocytes have been observed in the SCN of aged rodents (Roozendaal et al., 1987; Tsukahara et al., 2005). This is further emphasized by the concomitant age-related reduction in neural excitability within one downstream region of the hypothalamus innervated by the SCN, the subparaventricular zone. Mechanistically, reductions in several forms of potassium conductance have been shown to contribute to age-related alterations in SCN neural activity (Farajnia et al., 2015; Farajnia et al., 2012). More work is needed to understand the molecular mechanisms that precipitate these functional changes in the SCN and to determine their specific role age-related circadian dysfunction.
Even if targeting the SCN is not currently feasible, several studies demonstrate pharmacological manipulation of the peripheral clock network is possible (Balsalobre et al., 1998; Yamazaki et al., 2000). One of the most profound regulators of lifespan across species is caloric intake, and evidence in flies suggests that this effect on lifespan is mediated via peripheral circadian clock gene expression (Katewa et al., 2016). Pharmacologically, Dexamethasone was shown to alter clock gene expression in the liver, kidney, and heart via hormonal glucocorticoid signaling (Balsalobre et al., 2000). Peripheral circadian rhythms have also been modulated by drug-induced increases in cAMP levels (Yamazaki et al., 2000). These findings show that although some aged tissues are arrhythmic they can still be pharmacologically induced to oscillate. Such reports are promising, since they suggest the machinery governing clock gene expression in the periphery remains intact even when the system is desynchronized. Targeting cAMP receptors is particularly exciting, as G-protein coupled receptors (GPCRs) are often regulators of cAMP. GPCRs are commonly successful drug targets, and over 35% of currently approved drugs act on these receptors (Sriram and Insel, 2018). Collectively, this implies that a large number of proteins may be amenable to therapeutically regulating circadian signaling. Future studies aimed at restoring circadian function within the SCN or preventing peripheral dysregulation may simultaneously benefit multiple pathologies of aging.
3. The Endocannabinoid System in Advanced Age
One potential target for the pharmacological manipulation of circadian rhythms in advanced age is the endocannabinoid system (Howlett et al., 2002). Discovery of the endocannabinoid system occurred when searching for the receptors responsible for the psychotropic effects of plants from the genus Cannabis (Devane et al., 1988; Matsuda et al., 1990; Munro et al., 1993). Multiple cannabinoid receptors have been identified in mammals, including the canonical CB1 and CB2 as well as more-recently identified receptors like GPR55 and GPR18 (Console-Bram et al., 2014; Howlett et al., 2002; Irving et al., 2017; Jarai et al., 1999). These cannabinoid receptors are GPCRs which exhibit distinct binding affinities for various endogenous and exogenous ligands (Console-Bram et al., 2014; Henstridge et al., 2010; Howlett et al., 2002). Radiographic localization of these receptors revealed profound expression of CB1 in the brain and central nervous tissue, while CB2 is primarily located in peripheral immune cells (Herkenham et al., 1991; Herkenham et al., 1990; Howlett, 1995). The term cannabinoid refers to any compound which binds to these receptors, while the term endocannabinoid specifically refers to endogenously produced ligands (Howlett et al., 2002). The endocannabinoid system consists of these receptors and their endogenous ligands, which include N-arachidonoylethanolamine (Anandamide) and 2-Arachidonoyl glycerol (2-AG) among others (Pertwee, 2014; Wilson and Nicoll, 2002). Numerous studies of the endocannabinoid system demonstrate that these ligands and receptors collectively regulate sleep, hunger, body temperature, and cognition - several of the circadian behaviors disrupted in advanced age (Abel, 1970; Barratt and Adams, 1973; Carlini et al., 1970; Cone et al., 1988).
As with many other physiological processes, the endocannabinoid system varies markedly with age (Bilkei-Gorzo, 2012). There have been conflicting reports regarding age-related changes of CB1 in the brain. Some reports in rodents suggest that CB1 mRNA expression is reduced in advanced age (Canas et al., 2009; Romero et al., 1998), while others indicate there is no change or even region-specific increases in CB1 (Berrendero et al., 1998; Liu et al., 2003; Mailleux and Vanderhaeghen, 1992; Wang et al., 2003). These discrepancies are also observed in humans, with post-mortem analyses showing reductions in CB1 radiolabeling in some studies, while newer PET scans of living individuals showing sex-specific increases in CB1 reactivity within aged females (Mato and Pazos, 2004; Van Laere et al., 2008; Westlake et al., 1994). Despite the various reported changes in expression, studies have shown a reduction in CB1-stimulated GTPyS functional activity in rodents and humans (Mato and Pazos, 2004; Romero et al., 1998; Wang et al., 2003). In addition to potential changes in receptor expression and function, reductions in the endocannabinoid ligand 2-AG have been observed in advanced age (Piyanova et al., 2015). Considering the importance of this brain region to learning, memory, and pathologies of aging, it is likely that the changing endocannabinoid system impacts cognitive behaviors. In support of this idea, studies using CB1-deficient mice show that reducing these signals leads to the development of unique age-related behavioral disturbances earlier than wildtype mice (Bilkei-Gorzo et al., 2005). Curiously, young CB1-deficient mice outperform wildtype controls in social and object recognition tasks as well as operant learning paradigms, suggesting that the effects of the endocannabinoid system are influenced by the developmental age of the animals (Albayram et al., 2012; Bilkei-Gorzo et al., 2005; Reibaud et al., 1999). Although the exact mechanisms of these fluctuations are still under investigation, it appears that the preservation of endocannabinoid system function is vital to the aging brain.
Given the extensive activity of endocannabinoids throughout the central nervous system, there appears to be a substantial link between the endocannabinoid system and those physiological processes subject to age-related dysfunction. Additional evidence suggests that the endocannabinoid system plays a key role in circadian physiology. Several circadian behaviors are intricately linked to endocannabinoid signaling, namely: thermoregulation, nociception, locomotion, and food-intake (Abel, 1970; Barratt and Adams, 1973; Carlini et al., 1970; Cone et al., 1988). Centrally, neurons of the SCN express CB1 and have been shown to alter their firing rates in the presence of synthetic cannabinoid agonists and antagonists (Acuna-Goycolea et al., 2010; Sanford et al., 2008). A recent primate study also revealed a circadian rhythm of cannabinoid receptor transcription in both the central nervous system and peripheral tissues (Mure et al., 2018). Moreover, cannabinoids are powerful regulators of body temperature, which has been shown to entrain peripheral as previously mentioned. Taken together, these findings demonstrate a connection between the behavioral impairments observed in advanced age, disrupted circadian rhythms, and alterations in the endocannabinoid system.
4. Cannabinoid Behavioral Pharmacology
Although the endocannabinoid system is regulated by endogenous molecules like 2-AG and anandamide, exogenous cannabinoids such as those found in Cannabis are also known to modulate this system. Rigorous pharmacological study of Cannabis (also known as Marijuana, Marihuana) has a long and complex history (Farnsworth, 1969). Historical records of Cannabis-use have been documented for millennia, but the structures and potential functions of the chemicals synthesized within Cannabis are still being elucidated (Gaoni and Mechoulam, 1964; Matsuda et al., 1990; Munro et al., 1993; Russo, 2014). Two of the most-studied phytocannabinoids (plant-derived cannabinoids), are Δ−9-tetrahydrocannabinol (THC) and cannabidiol (CBD), although growing bodies of literature exist for cannabigerol (CBG), cannabivarin (CBV), and many others (Pertwee, 2014). THC is considered to be the primary psychoactive compound in Cannabis and is an agonist of both CB1 and CB2 (Gaoni and Mechoulam, 1964). The mechanism by which CBD exerts its physiological effects is unknown and widely disputed, as the Ki for CB1 and CB2 is over 100 fold lower than that of THC on these receptors (Bow and Rimoldi, 2016). Though most of the focus is on phytocannabinoids like THC, it is important to also note that Cannabis produces a wide variety of monoterpenoids and sesquiterpenoids - which may also act directly on cannabinoid receptors (Bahi et al., 2014; Russo, 2011). Extensive reviews of Cannabis, phytocannabinoids (Pertwee, 2014), cannabinoid receptors (Howlett et al., 2002), and endocannabinoid pharmacology (Howlett and Abood, 2017), have previously been published.
Human empirical and anecdotal evidence demonstrates that exogenous cannabinoids profoundly impact cognition and physiology (Farnsworth, 1969; Russo, 2014; Zuardi, 2006; Zuardi et al., 2012). However, studies of phytocannabinoids are somewhat difficult to interpret given the extreme diversity of compounds present in raw plant-matter or extracts. As such, knowledge of each cannabinoid’s specific pharmacological profile is crucial to consider any potential therapeutic applications for these substances. Receptor-subtype-specific compounds have been vital to delineating the shared and specific effects of CB1 and CB2 on physiological functions (Pertwee, 2006; Soethoudt et al., 2017). Many synthetic cannabinoids have been identified using the Tetrad Assay - a battery of behavioral tasks for assessing CB1 function in rodents (Howlett et al., 2002; Metna-Laurent et al., 2017). These tests measure locomotion, catalepsy, thermoregulation, and analgesia as endpoints of CB1 receptor activity (Metna-Laurent et al., 2017). Though the Tetrad Assay has proven useful as a drug screening mechanism, its relatively limited scope does not permit full characterization of an animal’s behavioral status, especially when one considers the behaviors altered in advanced age. In addition to nociception, locomotion, and thermoregulation, CB1 activity has been shown to regulate learning and memory, sleep/wake activity, food-intake, anxiety, attention, and cardiovascular function (Babson et al., 2017; Hlozek et al., 2017; Javadi-Paydar et al., 2017; Jensen et al., 2015; Long, L. E. et al., 2010; Maldonado et al., 2016; Rohleder et al., 2016; Tai et al., 2015).
In both animals and humans, memory impairment following acute or chronic administration of CB1 agonists has been repeatedly reported (Essman, 1984; Heyser et al., 1993; Lichtman et al., 1995; Nakamura et al., 1991; Taffe, 2012). However, alternative studies indicate that these memory-impairing effects are dose- and age-dependent (Amal et al., 2010; Bilkei-Gorzo et al., 2017; Bolla et al., 2002; Fishbein et al., 2012; Stark and Dews, 1980; Suliman et al., 2017). Recent evidence in rodents suggest that some these effects can be partially blocked by co-administration with CBD, a finding which may explain why anecdotes of whole-plant Cannabis-use often disagree with the receptor-specific effects seen in animal studies (Morgan et al., 2010; Mori et al., 2017). The diverse behavioral phenotypes elicited from modulation of the endocannabinoid system emphasize the importance of understanding this integral physiological system.
Despite the large volume of studies conducted on exogenously administered cannabinoids, synthesis of this data is difficult due to inconsistent compositions of phytocannabinoids, doses, and routes of administration (ROAs) (Boggs et al., 2018; Grotenhermen, 2003; McGilveray, 2005; McLaughlin, 2018). Additionally, there is a significant disparity between preclinical dosing regimens and those currently accepted for human consumption. Regarding THC, many rodent experiments use intraperitoneal doses ranging from 1mg/kg to 30mg/kg, however, current recreational and clinical amounts for humans are closer to 0.15mg/kg orally (Ahmed et al., 2014; Deiana et al., 2012; Killestein et al., 2002; Martin-Santos et al., 2012). While the pharmacokinetic profiles vary markedly between rodents and humans (Reagan-Shaw et al., 2008), several studies now show that injections of THC can alter animal behavior and molecular signaling at doses as low as 0.002mg/kg (Fishbein et al., 2012; Sarne et al., 2018). Recent attempts have been made to more adequately model the ROAs used by humans (Grella et al., 2014; Hlozek et al., 2017; Javadi-Paydar et al., 2017; Nguyen et al., 2016; Swortwood et al., 2017; Vandrey et al., 2017). The results of these studies indicate that cannabinoids administered orally have a delayed onset and longer duration of action than when they are inhaled (Hart et al., 2002; Hlozek et al., 2017; Vandrey et al., 2017). These route of administration-dependent effects are to be expected; however, preliminary evidence suggests that chronic Cannabis-use may also alter the gut microbiome (Panee et al., 2017). Given that recreational and medicinal cannabinoids are often administered orally, future studies of microbiome-mediated cannabinoid metabolism are of particular importance. Taken together, it is imperative that these discrepancies in dosing and route of administration are carefully considered and discussed in future studies.
5. Hormesis and Cannabinoid Chronopharmacology
There is little debate regarding the deleterious effects of cannabinoids in high doses, however the reported effects of more modest amounts are somewhat conflicting. High doses of THC (≥3mg/kg in rodents, ≥0.15mg/kg in humans) are consistently reported to disrupt cognitive function in rodents and produce psychoactive effects in humans (Abel, 1971, 1975; Bolla et al., 2002; Essman, 1984; Filbey et al., 2014; Heyser et al., 1993). Despite the consistent inhibitory or soporific effects of cannabinoids at higher doses, studies which have examined lower amounts often report stimulatory effects at the lowest doses tested (Crawley et al., 1993; Grisham and Ferraro, 1972; Katsidoni et al., 2013; Long, Leonora E et al., 2010; Rey et al., 2012; Sulcova et al., 1998; Suliman et al., 2017; Taylor and Fennessy, 1977). Though exogenous cannabinoids are known to induce hypothermia and hypolocomotion, several investigations have reported increased body temperature and locomotion following treatment at low doses (Sofia, 1972; Taylor and Fennessy, 1977; Tselnicker et al., 2007). A similar biphasic effect of THC on intracranial self-stimulation was reported in rats treated with 0.1 or 1.0mg/kg (Katsidoni et al., 2013). Based on this study, the authors concluded that an acute intraperitoneal injection of 0.1mg/kg THC induced reward-seeking behavior while the higher dose elicited anhedonia. Mechanistically, the effects of higher doses appear to be mediated in-part by CB1 signaling in GABAergic neurons (Rey et al., 2012). A growing body of literature now suggests that low doses (≤3mg/kg) of THC and other synthetic cannabinoids may prevent certain aspects of age-related cognitive decline in rodents (Bilkei-Gorzo et al., 2017; Marchalant et al., 2008; Sarne et al., 2018; Suliman et al., 2017).
Within the context of exogenously administered cannabinoids and cognition, the hormetic dose-response of this compound may rectify these disparate reports (Calabrese and Rubio-Casillas, 2018). Hormesis describes a biphasic dose-response where low amounts of a substance produce opposite effects of higher doses (Calabrese and Baldwin, 2002). Great efforts have been made in recent years to catalog and characterize reports of dose-response experiments which cannot be explained by traditional, linear models (Calabrese, 2013). Continued work in this field now suggests that one explanation for this biphasic response is through preconditioning (Calabrese, 2016; Calabrese, 2018; Sarne et al., 2011). This is in line with work previously presented by Sarne et al. which indicates that exposure to low doses of exogenous cannabinoids blunt the negative impacts of subsequent insults (Sarne, 2018; Sarne et al., 2011). These findings have intriguing implications for the study of aging, since preconditioning biological systems during critical developmental windows may bolster resilience to age-related dysfunction (Calabrese and Mattson, 2017; Gidday, 2015).
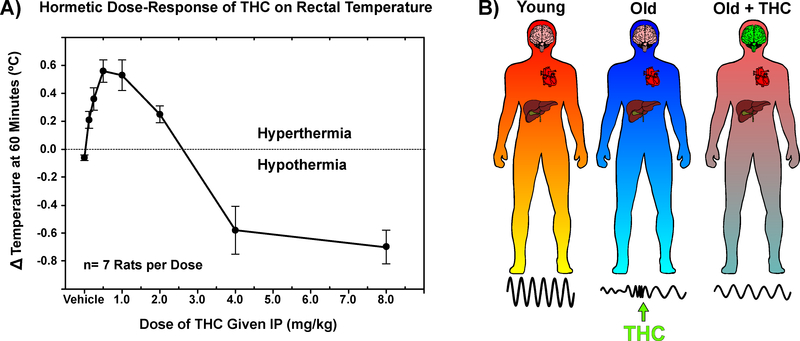
Interestingly, the hormetic dose-response of THC on body temperature has been reported for many years Figure 3A (Sarne et al., 2011; Sofia, 1972; Taylor and Fennessy, 1977). Additionally, exogenous cannabinoids alter anxiety-related behaviors in dose-dependent, bi-directional manner - although the nature of this relationship is poorly understood (Jenniches et al., 2016; Witkin et al., 2005; Wotjak, 2005; Zlebnik and Cheer, 2016). Early observations regarding the time- and temperature-dependence of THC’s effects may also shed light on these seemingly incongruent findings. A classic study by Ernest Abel revealed that the time of day in which THC is administered drastically affects the physiological response, a phenomenon now referred to as chronopharmacology (Abel, 1973). Similarly, an elegant study by Pertwee and Tavendale in 1977 revealed that the ambient temperature markedly altered rates of oxygen consumption and body temperature changes induced by THC administration (Pertwee and Tavendale, 1979). Furthermore, sex-specific responses to cannabinoids may present a confounding factor when interpreting these results, as previous reports have indicated that doses of THC that impaired cognition in males actually improved measures in females (Craft et al., 2013; Makela et al., 2006).
Figure 3: Hormesis and the chronobiotic potential of THC in aged subjects.
A) Hormetic dose-response of THC on rectal temperature in rats. Data redrawn from: Sofia, R.D., 1972. A paradoxical effect for 1-tetrahydrocannabinol on rectal temperature in rats. Research communications in chemical pathology and pharmacology, 4(2), pp.281–288.
B) Declining amplitude and increasing lability of central and peripheral rhythms with age may be amenable to therapeutics which simultaneously alter neuronal activity in the SCN and body temperature. Using the chronopharmacological properties and hormetic dose-response of THC, low-dose exposure may help to rescue dysfunctional clocks in aging systems.
Given the current evidence, it is difficult to discern whether the cognitive-enhancing effects of low-dose cannabinoids are due to ‘true’ hormesis, age-dependent changes in endocannabinoid function, or both. The work by Sarne and colleagues demonstrates that exceptionally small amounts of THC (0.002mg/kg) are sufficient to influence neurobiology. These studies reported that a single dose of 0.002mg/kg, IP produced neuroprotection in young male mice and lasting cognitive enhancement in old females (Fishbein et al., 2012; Sarne et al., 2018; Senn et al., 2008; Tselnicker et al., 2007). Critically, pilot studies at this dose were reported to increase body temperature and stimulate locomotion - a finding which supports the hormetic stimulatory response (Sarne et al., 2011). While the evidence presented by Suliman also supports that the effects of cannabinoids are age-dependent, the data from this study indicate that there is a ‘window’ in which cannabinoids may improve function (Suliman et al., 2017). Although there are not enough doses in these studies to clearly demonstrate hormesis, we feel that this evidence is supportive nonetheless.
The range of doses studied by Sarne et al. indicate that doses which improve cognitive performance in old animals (0.002mg/kg) cause impairments in young animals, and the studies by Bilkei Gorzo et al. (3mg/kg THC) in old animals also support this. These findings, and others, indicate a clear effect of aging on response to exogenous cannabinoid administration. The possibility remains, however, that the lowest dose of THC reported by Sarne et al. (0.0005mg/kg THC, IP) in young animals, may still lie above the stimulatory hormetic range for this age group. This hypothesis is supported by their biochemical studies which report the highest activation of P-ERK in the cerebella of young animals at this dose (0.0005mg/kg) (Amal 2010). Since we are unaware of a study which.
To date, we are unaware of a modern study specifically designed to determine if cannabinoids exhibit hormesis, and whether this hormetic dose-range is altered with age. Even if preliminary evidence suggests there is cannabinoid hormesis in young animals, it remains to be seen if this same hormetic curve persists with age or if the stimulatory range might change. Though there are several potential mechanistic explanations for these age-dependent effects, recent studies support a desensitization of endocannabinoid machinery with age. Ultimately, additional studies testing multiple doses in young and old animals are required to directly compare the hormetic range of exogenous cannabinoids.
Despite the importance of these pharmacological considerations across different compounds and systems, hormesis and chronopharmacology remain understudied components of many biomedical studies - particularly those in aged animals (Dallmann et al., 2014). These two distinct properties have intriguing implications for the potential use of cannabinoids as therapeutics. Namely, that it may be possible to attain opposing physiological effects based solely on the dose- and time-of-administration. To this end, application of these pharmacological properties to a highly dynamic and heterogenous condition such as age-dependent circadian dysfunction, may provide a wide range of therapeutic potential Figure 3B.
6. Conclusions
Taken together, the findings discussed here suggest that altered circadian rhythms are a potential biomarker of aging, and restoration or preservation of these rhythms in aged individuals might benefit certain age-related pathologies. The endocannabinoid system is a promising target in the treatment of age-related disease, given the diverse physiological processes it regulates. Centrally, modulation of SCN activity by cannabinoids supports their classification as a chronobiotics, and careful, therapeutic application use of these compounds may serve to restore abnormal behavioral rhythms in aged subjects. Moreover, the biphasic effects of cannabinoids on body temperature may allow for the “tuning” of peripheral molecular clocks. Many additional experiments are necessary to fully characterize the hormetic dose-response of exogenous cannabinoids such as THC and examine their potential efficacy in the amelioration of age-related circadian dysfunction. As societal opinions of ‘aging as a disease’ and ‘cannabinoids as medicine’ shift, further inquiry of these previously intractable topics may prove greatly beneficial to human health.
Highlights.
Altered circadian rhythms are concomitant with numerous age-related disease states.
The endocannabinoid system is a relatively novel target in aging research.
Cannabinoids are capable of modifying both central and peripheral circadian clocks.
Cannabinoids reportedly stimulate at low doses, suggesting they exhibit hormesis.
Modulation of the endocannabinoid system may have therapeutic value in aging.
7. Acknowledgements
We are grateful to Jordan Cleveland, Alberto Del Arco, Jason Paris, Albert Orock, Noa Valcarcel-Ares, Disha Prabhu, Jessica Posey, Gabriella Hartman, and Sariya Khan for their insight and editorial suggestions. Additionally, we would like to thank Samantha Gulley for her artistic assistance when creating the figures.
Funding: This work is partially funded by NIH 1P30GM122733 to NMA
Footnotes
Declarations of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL, 1970. Marijuana and memory. Nature 227(5263), 1151–1152. [DOI] [PubMed] [Google Scholar]
- Abel EL, 1971. Marihuana and memory: acquisition or retrieval? Science 173(4001), 1038–1040. [DOI] [PubMed] [Google Scholar]
- Abel EL, 1973. Chronopharmacology of delta9-tetrahydrocannabinol hypothermia in mice. Experientia 29(12), 1528–1529. [DOI] [PubMed] [Google Scholar]
- Abel EL, 1975. Marihuana, learning, and memory. Int Rev Neurobiol 18, 329–356. [DOI] [PubMed] [Google Scholar]
- Acuna-Goycolea C, Obrietan K, van den Pol AN, 2010. Cannabinoids excite circadian clock neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 30(30), 10061–10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed AI, van den Elsen GA, Colbers A, van der Marck MA, Burger DM, Feuth TB, Rikkert MG, Kramers C, 2014. Safety and pharmacokinetics of oral delta-9-tetrahydrocannabinol in healthy older subjects: a randomized controlled trial. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 24(9), 1475–1482. [DOI] [PubMed] [Google Scholar]
- Albayram O, Bilkei-Gorzo A, Zimmer A, 2012. Loss of CB1 receptors leads to differential age-related changes in reward-driven learning and memory. Frontiers in aging neuroscience 4, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amal H, Fridman-Rozevich L, Senn R, Strelnikov A, Gafni M, Keren O, Sarne Y, 2010. Long-term consequences of a single treatment of mice with an ultra-low dose of Delta9-tetrahydrocannabinol (THC). Behavioural brain research 206(2), 245–253. [DOI] [PubMed] [Google Scholar]
- Arendt J, Skene DJ, 2005. Melatonin as a chronobiotic. Sleep medicine reviews 9(1), 25–39. [DOI] [PubMed] [Google Scholar]
- Aschoff J, 1965. Circadian Rhythms in Man. Science 148(3676), 1427–1432. [DOI] [PubMed] [Google Scholar]
- Babson KA, Sottile J, Morabito D, 2017. Cannabis, Cannabinoids, and Sleep: a Review of the Literature. Curr Psychiatry Rep 19(4), 23. [DOI] [PubMed] [Google Scholar]
- Bahi A, Al Mansouri S, Al Memari E, Al Ameri M, Nurulain SM, Ojha S, 2014. β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiology & behavior 135, 119–124. [DOI] [PubMed] [Google Scholar]
- Balmagiya T, Rozovski SJ, 1983. Age-related changes in thermoregulation in male albino rats. Experimental gerontology 18(3), 199–210. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U, 2000. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289(5488), 2344–2347. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U, 1998. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93(6), 929–937. [DOI] [PubMed] [Google Scholar]
- Barratt ES, Adams PM, 1973. Chronic marijuana usage and sleep-wakefulness cycles in cats. Biol Psychiatry 6(3), 207–214. [PubMed] [Google Scholar]
- Bass J, Lazar MA, 2016. Circadian time signatures of fitness and disease. Science 354(6315), 994–999. [DOI] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W, 2004. Intranasal insulin improves memory in humans. Psychoneuroendocrinology 29(10), 1326–1334. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Romero J, García-Gil L, Suarez I, De la Cruz P, Ramos J, Fernandez-Ruiz J, 1998. Changes in cannabinoid receptor binding and mRNA levels in several brain regions of aged rats. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1407(3), 205–214. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, 2012. The endocannabinoid system in normal and pathological brain ageing. Philos Trans R Soc Lond B Biol Sci 367(1607), 3326–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Albayram O, Draffehn A, Michel K, Piyanova A, Oppenheimer H, Dvir-Ginzberg M, Racz I, Ulas T, Imbeault S, Bab I, Schultze JL, Zimmer A, 2017. A chronic low dose of Delta(9)-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nature medicine 23(6), 782–787. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Valverde O, Otto M, Michel K, Sastre M, Zimmer A, 2005. Early age-related cognitive impairment in mice lacking cannabinoid CB1 receptors. Proceedings of the National Academy of Sciences of the United States of America 102(43), 15670–15675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs DL, Nguyen JD, Morgenson D, Taffe MA, Ranganathan M, 2018. Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Delta(9)- Tetrahydrocannabinol. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43(1), 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL, 2002. Dose-related neurocognitive effects of marijuana use. Neurology 59(9), 1337–1343. [DOI] [PubMed] [Google Scholar]
- Bow EW, Rimoldi JM, 2016. The structure-function relationships of classical cannabinoids: CB1/CB2 modulation. Perspectives in medicinal chemistry 8, PMC. S32171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr ED, Yoo SH, Takahashi JS, 2010. Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330(6002), 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke TM, Markwald RR, McHill AW, Chinoy ED, Snider JA, Bessman SC, Jung CM, O’neill JS, Wright KP, 2015. Effects of caffeine on the human circadian clock in vivo and in vitro. Science translational medicine 7(305), 305ra146–305ra146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey D, Hughes KA, Tononi G, Cirelli C, 2010. Sleep, aging, and lifespan in Drosophila. BMC neuroscience 11, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, 2013. Hormetic mechanisms. Crit Rev Toxicol 43(7), 580–606. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, 2016. Preconditioning is hormesis part I: Documentation, dose-response features and mechanistic foundations. Pharmacological Research 110, 242–264. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, 2018. Hormesis: Path and Progression to Significance. International journal of molecular sciences 19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA, 2002. Defining hormesis. Human & experimental toxicology 21(2), 91–97. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Mattson MP, 2017. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech Dis 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Rubio-Casillas A, 2018. Biphasic effects of THC in memory and cognition. European journal of clinical investigation 48(5), e12920. [DOI] [PubMed] [Google Scholar]
- Canas PM, Duarte JM, Rodrigues RJ, Kofalvi A, Cunha RA, 2009. Modification upon aging of the density of presynaptic modulation systems in the hippocampus. Neurobiology of aging 30(11), 1877–1884. [DOI] [PubMed] [Google Scholar]
- Cardinali DP, Vigo DE, Olivar N, Vidal MF, Furio AM, Brusco LI, 2012. Therapeutic application of melatonin in mild cognitive impairment. American journal of neurodegenerative disease 1(3), 280–291. [PMC free article] [PubMed] [Google Scholar]
- Carlini EA, Hamaoui A, Bieniek D, Korte F, 1970. Effects of (--) delta-9-trans-tetrahydrocannabinol and a synthetic derivative on maze performance of rats. Pharmacology 4(6), 359–368. [DOI] [PubMed] [Google Scholar]
- Cone EJ, Johnson RE, Paul BD, Mell LD, Mitchell J, 1988. Marijuana-laced brownies: behavioral effects, physiologic effects, and urinalysis in humans following ingestion. J Anal Toxicol 12(4), 169–175. [DOI] [PubMed] [Google Scholar]
- Console-Bram L, Brailoiu E, Brailoiu GC, Sharir H, Abood ME, 2014. Activation of GPR18 by cannabinoid compounds: a tale of biased agonism. British journal of pharmacology 171(16), 3908–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Marusich JA, Wiley JL, 2013. Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life sciences 92(8–9), 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Corwin RL, Robinson JK, Felder CC, Devane WA, Axelrod J, 1993. Anandamide, an endogenous ligand of the cannabinoid receptor, induces hypomotility and hypothermia in vivo in rodents. Pharmacology, biochemistry, and behavior 46(4), 967–972. [DOI] [PubMed] [Google Scholar]
- Dallmann R, Brown SA, Gachon F, 2014. Chronopharmacology: new insights and therapeutic implications. Annual review of pharmacology and toxicology 54, 339–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiana S, Watanabe A, Yamasaki Y, Amada N, Arthur M, Fleming S, Woodcock H, Dorward P, Pigliacampo B, Close S, 2012. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology 219(3), 859–873. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC, 1988. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34(5), 605–613. [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA, 1999. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. The Journal of physiology 516 ( Pt 2), 611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl C, Kokaia Z, Lindvall O, 2009. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience 158(3), 1021–1029. [DOI] [PubMed] [Google Scholar]
- Espiritu JR, 2008. Aging-related sleep changes. Clinics in geriatric medicine 24(1), 1–14, v. [DOI] [PubMed] [Google Scholar]
- Essman EJ, 1984. Marijuana intoxication in rats: interruption of recent memory and effect on brain concentration of delta 9-tetrahydrocannabinol. Psychol Rep 55(2), 563–567. [DOI] [PubMed] [Google Scholar]
- Farajnia S, Meijer JH, Michel S, 2015. Age-related changes in large-conductance calcium-activated potassium channels in mammalian circadian clock neurons. Neurobiology of aging 36(6), 2176–2183. [DOI] [PubMed] [Google Scholar]
- Farajnia S, Michel S, Deboer T, vanderLeest HT, Houben T, Rohling JH, Ramkisoensing A, Yasenkov R, Meijer JH, 2012. Evidence for neuronal desynchrony in the aged suprachiasmatic nucleus clock. The Journal of neuroscience : the official journal of the Society for Neuroscience 32(17), 5891–5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth NR, 1969. Pharmacognosy and chemistry of “cannabis sativa”. J Am Pharm Assoc 9(8), 410–414 passim. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, Segall J, 2014. Long-term effects of marijuana use on the brain. Proceedings of the National Academy of Sciences 111 (47), 16913–16918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein M, Gov S, Assaf F, Gafni M, Keren O, Sarne Y, 2012. Long-term behavioral and biochemical effects of an ultra-low dose of Delta9-tetrahydrocannabinol (THC): neuroprotection and ERK signaling. Experimental brain research 221(4), 437–448. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R, 1964. Isolation, structure, and partial synthesis of an active constituent of hashish. Journal of the American chemical society 86(8), 1646–1647. [Google Scholar]
- Gidday JM, 2015. Extending injury-and disease-resistant CNS phenotypes by repetitive epigenetic conditioning. Frontiers in neurology 6, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grella CE, Rodriguez L, Kim T, 2014. Patterns of medical marijuana use among individuals sampled from medical marijuana dispensaries in Los Angeles. Journal of psychoactive drugs 46(4), 263–272. [DOI] [PubMed] [Google Scholar]
- Grisham MG, Ferraro DP, 1972. Biphasic effects of 9 -tetrahydrocannabinol on variable interval schedule performance in rats. Psychopharmacologia 27(2), 163–169. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F, 2003. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 42(4), 327–360. [DOI] [PubMed] [Google Scholar]
- Harand C, Bertran F, Doidy F, Guenole F, Desgranges B, Eustache F, Rauchs G, 2012. How aging affects sleep-dependent memory consolidation? Frontiers in neurology 3, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin PE, Hall JC, Rosbash M, 1990. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 343(6258), 536–540. [DOI] [PubMed] [Google Scholar]
- Harper DG, Volicer L, Stopa EG, McKee AC, Nitta M, Satlin A, 2005. Disturbance of endogenous circadian rhythm in aging and Alzheimer disease. Am J Geriatr Psychiatry 13(5), 359–368. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW, 2002. Comparison of smoked marijuana and oral Delta(9)-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 164(4), 407–415. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Smith LJ, Cousins JC, Bootzin RR, 2012. Circadian rhythms, sleep, and substance abuse. Sleep medicine reviews 16(1), 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, Brancaccio M, 2018. Generation of circadian rhythms in the suprachiasmatic nucleus. Nature Reviews Neuroscience, 1. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, Maywood ES, 2003. A clockwork web: circadian timing in brain and periphery, in health and disease. Nature reviews. Neuroscience 4(8), 649–661. [DOI] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW, 2003. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424(6944), 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD, 2004. Insights into the ageing mind: a view from cognitive neuroscience. Nature reviews neuroscience 5(2), 87. [DOI] [PubMed] [Google Scholar]
- Henstridge CM, Balenga NA, Schröder R, Kargl JK, Platzer W, Martini L, Arthur S, Penman J, Whistler JL, Kostenis E, 2010. GPR55 ligands promote receptor coupling to multiple signalling pathways. British journal of pharmacology 160(3), 604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, 2013. Neuroscience. Sleep it out. Science 342(6156), 316–317. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC, 1991. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. The Journal of neuroscience : the official journal of the Society for Neuroscience 11(2), 563–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC, 1990. Cannabinoid receptor localization in brain. Proceedings of the National Academy of Sciences of the United States of America 87(5), 1932–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Hampson RE, Deadwyler SA, 1993. Effects of delta-9-tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short-term memory associated with changes in task specific firing of hippocampal cells. The Journal of pharmacology and experimental therapeutics 264(1), 294–307. [PubMed] [Google Scholar]
- Hlozek T, Uttl L, Kaderabek L, Balikova M, Lhotkova E, Horsley RR, Novakova P, Sichova K, Stefkova K, Tyls F, Kuchar M, Palenicek T, 2017. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 27(12), 1223–1237. [DOI] [PubMed] [Google Scholar]
- Howlett AC, 1995. Pharmacology of cannabinoid receptors. Annu Rev Pharmacol Toxicol 35, 607–634. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Abood ME, 2017. CB1 and CB2 Receptor Pharmacology. Adv Pharmacol 80, 169–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG, 2002. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54(2), 161–202. [DOI] [PubMed] [Google Scholar]
- Hu K, Harper DG, Shea SA, Stopa EG, Scheer FA, 2013. Noninvasive fractal biomarker of clock neurotransmitter disturbance in humans with dementia. Scientific reports 3, 2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-L, Liu R-Y, Wang Q-S, Van Someren EJ, Xu H, Zhou J-N, 2002. Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiology & behavior 76(4–5), 597–603. [DOI] [PubMed] [Google Scholar]
- Irving A, Abdulrazzaq G, Chan SLF, Penman J, Harvey J, Alexander SPH, 2017. Cannabinoid Receptor-Related Orphan G Protein-Coupled Receptors. Adv Pharmacol 80, 223–247. [DOI] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G, 1999. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proceedings of the National Academy of Sciences of the United States of America 96(24), 14136–14141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi-Paydar M, Nguyen JD, Grant Y, Vandewater SA, Cole M, Taffe MA, 2017. Effects Of A9-THC And Cannabidiol Vapor Inhalation In Male And Female Rats. bioRxiv, 128173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenniches I, Ternes S, Albayram O, Otte DM, Bach K, Bindila L, Michel K, Lutz B, Bilkei-Gorzo A, Zimmer A, 2016. Anxiety, Stress, and Fear Response in Mice With Reduced Endocannabinoid Levels. Biol Psychiatry 79(10), 858–868. [DOI] [PubMed] [Google Scholar]
- Jensen B, Chen J, Furnish T, Wallace M, 2015. Medical Marijuana and Chronic Pain: a Review of Basic Science and Clinical Evidence. Curr Pain Headache Rep 19(10), 50. [DOI] [PubMed] [Google Scholar]
- Katewa SD, Akagi K, Bose N, Rakshit K, Camarella T, Zheng X, Hall D, Davis S, Nelson CS, Brem RB, 2016. Peripheral circadian clocks mediate dietary restriction-dependent changes in lifespan and fat metabolism in Drosophila. Cell metabolism 23(1), 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsidoni V, Kastellakis A, Panagis G, 2013. Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity. International journal of neuropsychopharmacology 16(10), 2273–2284. [DOI] [PubMed] [Google Scholar]
- Killestein J, Hoogervorst EL, Reif M, Kalkers NF, Van Loenen AC, Staats PG, Gorter RW, Uitdehaag BM, Polman CH, 2002. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology 58(9), 1404–1407. [DOI] [PubMed] [Google Scholar]
- Kondratova AA, Kondratov RV, 2012. The circadian clock and pathology of the ageing brain. Nature reviews. Neuroscience 13(5), 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka RJ, Benzer S, 1971. Clock mutants of Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America 68(9), 2112–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster-van Hoffen G, Mirmiran M, Bos N, Witting W, Delagrange P, Guardiola- Lemaitre B, 1993. Effects of a novel melatonin analog on circadian rhythms of body temperature and activity in young, middle-aged, and old rats. Neurobiology of aging 14(6), 565–569. [DOI] [PubMed] [Google Scholar]
- Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC, Weitz CJ, 2001. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science 294(5551), 2511–2515. [DOI] [PubMed] [Google Scholar]
- Kress GJ, Liao F, Dimitry J, Cedeno MR, FitzGerald GA, Holtzman DM, Musiek ES, 2018. Regulation of amyloid-β dynamics and pathology by the circadian clock. Journal of Experimental Medicine, jem. 20172347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Alaghband Y, Kramár EA, López AJ, Ciernia AV, White AO, Shu G, Rhee D, Michael CM, Montellier E, 2018. Epigenetic regulation of the circadian gene Per1 contributes to age-related changes in hippocampal memory. Nature communications 9(1), 3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Silva AJ, 2009. The molecular and cellular biology of enhanced cognition. Nature reviews. Neuroscience 10(2), 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RC, Guo SZ, Raccurt M, Moudilou E, Morel G, Brittian KR, Gozal D, 2011. Exogenous growth hormone attenuates cognitive deficits induced by intermittent hypoxia in rats. Neuroscience 196, 237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR, 2001. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience 107(4), 603–613. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR, 1995. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology (Berl) 119(3), 282–290. [DOI] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Strogatz SH, Reppert SM, 1997. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell 91(6), 855–860. [DOI] [PubMed] [Google Scholar]
- Liu P, Bilkey DK, Darlington CL, Smith PF, 2003. Cannabinoid CB1 receptor protein expression in the rat hippocampus and entorhinal, perirhinal, postrhinal and temporal cortices: regional variations and age-related changes. Brain research 979(1–2), 235–239. [DOI] [PubMed] [Google Scholar]
- Long LE, Chesworth R, Huang X-F, McGregor IS, Arnold JC, Karl T, 2010. A behavioural comparison of acute and chronic Δ9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. International Journal of Neuropsychopharmacology 13(7), 861–876. [DOI] [PubMed] [Google Scholar]
- Long LE, Chesworth R, Huang XF, McGregor IS, Arnold JC, Karl T, 2010. A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int J Neuropsychopharmacol 13(7), 861–876. [DOI] [PubMed] [Google Scholar]
- Longo VD, Panda S, 2016. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell metabolism 23(6), 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ, 1992. Age-related loss of cannabinoid receptor binding sites and mRNA in the rat striatum. Neuroscience letters 147(2), 179–181. [DOI] [PubMed] [Google Scholar]
- Makela P, Wakeley J, Gijsman H, Robson PJ, Bhagwagar Z, Rogers RD, 2006. Low doses of Δ−9 tetrahydrocannabinol (THC) have divergent effects on short-term spatial memory in young, healthy adults. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 31(2), 462. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Banos JE, Cabanero D, 2016. The endocannabinoid system and neuropathic pain. Pain 157 Suppl 1, S23–32. [DOI] [PubMed] [Google Scholar]
- Marchalant Y, Cerbai F, Brothers HM, Wenk GL, 2008. Cannabinoid receptor stimulation is anti-inflammatory and improves memory in old rats. Neurobiology of aging 29(12), 1894–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani E, Polidori M, Cherubini A, Mecocci P, 2005. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. Journal of Chromatography B 827(1), 65–75. [DOI] [PubMed] [Google Scholar]
- Martin-Santos R, Crippa JA, Batalla A, Bhattacharyya S, Atakan Z, Borgwardt S, Allen P, Seal M, Langohr K, Farre M, Zuardi AW, McGuire PK, 2012. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Des 18(32), 4966–4979. [DOI] [PubMed] [Google Scholar]
- Mato S, Pazos A, 2004. Influence of age, postmortem delay and freezing storage period on cannabinoid receptor density and functionality in human brain. Neuropharmacology 46(5), 716–726. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI, 1990. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346(6284), 561–564. [DOI] [PubMed] [Google Scholar]
- McGilveray IJ, 2005. Pharmacokinetics of cannabinoids. Pain Res Manag 10 Suppl A, 15A–22A. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, 2018. Toward a Translationally Relevant Preclinical Model of Cannabis Use. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43(1), 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menaker M, Takahashi JS, Eskin A, 1978. The physiology of circadian pacemakers. Annu Rev Physiol 40, 501–526. [DOI] [PubMed] [Google Scholar]
- Metna-Laurent M, Mondesir M, Grel A, Vallee M, Piazza PV, 2017. Cannabinoid-Induced Tetrad in Mice. Curr Protoc Neurosci 80, 9 59 51–59 59 10. [DOI] [PubMed] [Google Scholar]
- Michael SD, Kaplan SB, Macmillan BT, 1980. Peripheral plasma concentrations of LH, FSH, prolactin and GH from birth to puberty in male and female mice. Journal of reproduction and fertility 59(1), 217–222. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS, 2012. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35, 445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Schafer G, Freeman TP, Curran HV, 2010. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected]. Br J Psychiatry 197(4), 285–290. [DOI] [PubMed] [Google Scholar]
- Mori MA, Meyer E, Soares LM, Milani H, Guimaraes FS, de Oliveira RM, 2017. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog Neuropsychopharmacol Biol Psychiatry 75, 94–105. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG, 2012. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nature Reviews Neuroscience 13(4), 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M, 1993. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365(6441), 61–65. [DOI] [PubMed] [Google Scholar]
- Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi-Benyahya O, Cooper HM, 2018. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359(6381), eaao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Xiong DD, Holtzman DM, 2015. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Experimental & molecular medicine 47(3), e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura EM, da Silva EA, Concilio GV, Wilkinson DA, Masur J, 1991. Reversible effects of acute and long-term administration of delta-9-tetrahydrocannabinol (THC) on memory in the rat. Drug Alcohol Depend 28(2), 167–175. [DOI] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Tokuda IT, Ishikawa T, Kudo T, Colwell CS, Block GD, 2015. Age-Related Changes in the Circadian System Unmasked by Constant Conditions(1,2,3). eNeuro 2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS, Block GD, 2011. Age-related decline in circadian output. The Journal of neuroscience : the official journal of the Society for Neuroscience 31(28), 10201–10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Takasu NN, Nakamura W, 2016. The suprachiasmatic nucleus: age-related decline in biological rhythms. J Physiol Sci 66(5), 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Aarde SM, Vandewater SA, Grant Y, Stouffer DG, Parsons LH, Cole M, Taffe MA, 2016. Inhaled delivery of Delta(9)-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology 109, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panee J, Gerschenson M, Chang L, 2017. Associations Between Microbiota, Mitochondrial Function, and Cognition in Chronic Marijuana Users. Journal of Neuroimmune Pharmacology, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partch CL, Green CB, Takahashi JS, 2014. Molecular architecture of the mammalian circadian clock. Trends Cell Biol 24(2), 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee R, 2006. The pharmacology of cannabinoid receptors and their ligands: an overview. International journal of obesity 30, S13–S18. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, 2014. Handbook of cannabis. Oxford University Press, USA. [Google Scholar]
- Pertwee RG, Tavendale R, 1979. EFFECTS OF Δ9-TETRAHYDROCANNABINOL, 2.4-DINITROPHENOL AND PENTOLINIUM TARTRATE ON BEHAVIOURAL THERMOREGULATION IN MICE. British journal of pharmacology 66(1), 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS, 1960. Circadian rhythms and the circadian organization of living systems. Cold Spring Harbor symposia on quantitative biology 25, 159–184. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, 1993. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol 55, 16–54. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S, 1974. Circadian oscillations in rodents: a systematic increase of their frequency with age. Science 186(4163), 548–550. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Minis DH, 1964. The Entrainment of Circadian Oscillations by Light and Their Role as Photoperiodic Clocks. The American naturalist 98(902), 261–294. [Google Scholar]
- Piyanova A, Lomazzo E, Bindila L, Lerner R, Albayram O, Ruhl T, Lutz B, Zimmer A, Bilkei-Gorzo A, 2015. Age-related changes in the endocannabinoid system in the mouse hippocampus. Mechanisms of ageing and development 150, 55–64. [DOI] [PubMed] [Google Scholar]
- Potter GD, Skene DJ, Arendt J, Cade JE, Grant PJ, Hardie LJ, 2016. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocrine reviews 37(6), 584–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchs G, Carrier J, Peigneux P, 2013. Sleep and cognition in the elderly. Frontiers in neurology 4, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N, 2008. Dose translation from animal to human studies revisited. The FASEB journal 22(3), 659–661. [DOI] [PubMed] [Google Scholar]
- Redfern P, Minors D, Waterhouse J, 1994. Circadian rhythms, jet lag, and chronobiotics: an overview. Chronobiology international 11(4), 253–265. [DOI] [PubMed] [Google Scholar]
- Refinetti R, 2016. Circadian physiology. CRC press. [Google Scholar]
- Reibaud M, Obinu MC, Ledent C, Parmentier M, Bohme GA, Imperato A, 1999. Enhancement of memory in cannabinoid CB1 receptor knock-out mice. European journal of pharmacology 379(1), R1–2. [DOI] [PubMed] [Google Scholar]
- Rey AA, Purrio M, Viveros M-P, Lutz B, 2012. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA B receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 37(12), 2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder C, Muller JK, Lange B, Leweke FM, 2016. Cannabidiol as a Potential New Type of an Antipsychotic. A Critical Review of the Evidence. Front Pharmacol 7, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J, Berrendero F, Manzanares J, Pérez A, Corchero J, Fuentes JA, Fernández-Ruiz JJ, Ramos JA, 1998. Time-course of the cannabinoid receptor down-regulation in the adult rat brain caused by repeated exposure to Δ9-tetrahydrocannabinol. Synapse 30(3), 298–308. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, van Gool WA, Swaab DF, Hoogendijk JE, Mirmiran M, 1987. Changes in vasopressin cells of the rat suprachiasmatic nucleus with aging. Brain research 409(2), 259–264. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Turek FW, 2015. Neurobiology of circadian rhythm regulation. Sleep medicine clinics 10(4), 403–412. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL, 2015. Successful Aging 2.0: Conceptual Expansions for the 21st Century. J Gerontol B Psychol Sci Soc Sci 70(4), 593–596. [DOI] [PubMed] [Google Scholar]
- Russo EB, 2011. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. British journal of pharmacology 163(7), 1344–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB, 2014. The pharmacological history of cannabis Handbook of cannabis. Oxford University Press, Oxford, 23–43. [Google Scholar]
- Sanford AE, Castillo E, Gannon RL, 2008. Cannabinoids and hamster circadian activity rhythms. Brain research 1222, 141–148. [DOI] [PubMed] [Google Scholar]
- Saper CB, 2013. The central circadian timing system. Current opinion in neurobiology 23(5), 747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Cano G, Scammell TE, 2005a. Homeostatic, circadian, and emotional regulation of sleep. The Journal of comparative neurology 493(1), 92–98. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J, 2005b. Hypothalamic regulation of sleep and circadian rhythms. Nature 437(7063), 1257–1263. [DOI] [PubMed] [Google Scholar]
- Sarne Y, 2018. THC for age-related cognitive decline? Aging 10(12), 3628–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarne Y, Asaf F, Fishbein M, Gafni M, Keren O, 2011. The dual neuroprotective-neurotoxic profile of cannabinoid drugs. British journal of pharmacology 163(7), 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarne Y, Toledano R, Rachmany L, Sasson E, Doron R, 2018. Reversal of age-related cognitive impairments in mice by an extremely low dose of tetrahydrocannabinol. Neurobiology of aging 61, 177–186. [DOI] [PubMed] [Google Scholar]
- Satinoff E, 1998. Patterns of circadian body temperature rhythms in aged rats. Clinical and experimental pharmacology and physiology 25(2), 135–140. [DOI] [PubMed] [Google Scholar]
- Schibler U, Gotic I, Saini C, Gos P, Curie T, Emmenegger Y, Sinturel F, Gosselin P, Gerber A, Fleury-Olela F, 2015. Clock-talk: interactions between central and peripheral circadian oscillators in mammals, Cold Spring Harbor symposia on quantitative biology. Cold Spring Harbor Laboratory Press, pp. 223–232. [DOI] [PubMed] [Google Scholar]
- Senn R, Keren O, Hefetz A, Sarne Y, 2008. Long-term cognitive deficits induced by a single, extremely low dose of tetrahydrocannabinol (THC): behavioral, pharmacological and biochemical studies in mice. Pharmacology, biochemistry, and behavior 88(3), 230–237. [DOI] [PubMed] [Google Scholar]
- Soethoudt M, Grether U, Fingerle J, Grim TW, Fezza F, de Petrocellis L, Ullmer C, Rothenhausler B, Perret C, van Gils N, Finlay D, MacDonald C, Chicca A, Gens MD, Stuart J, de Vries H, Mastrangelo N, Xia L, Alachouzos G, Baggelaar MP, Martella A, Mock ED, Deng H, Heitman LH, Connor M, Di Marzo V, Gertsch J, Lichtman AH, Maccarrone M, Pacher P, Glass M, van der Stelt M, 2017. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nature communications 8, 13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia RD, 1972. A paradoxical effect for 1 -tetrahydrocannabinol on rectal temperature in rats. Res Commun Chem Pathol Pharmacol 4(2), 281–288. [PubMed] [Google Scholar]
- Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z, 2013. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Frontiers in aging neuroscience 5, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Insel PA, 2018. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol Pharmacol 93(4), 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark P, Dews PB, 1980. Cannabinoids. I. Behavioral effects. The Journal of pharmacology and experimental therapeutics 214(1), 124–130. [PubMed] [Google Scholar]
- Stein MA, Weiss M, Hlavaty L, 2012. ADHD treatments, sleep, and sleep problems: complex associations. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 9(3), 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, 2012. To sleep: perchance to learn. Nature neuroscience 15(10), 1322–1323. [DOI] [PubMed] [Google Scholar]
- Sulcova E, Mechoulam R, Fride E, 1998. Biphasic effects of anandamide. Pharmacology, biochemistry, and behavior 59(2), 347–352. [DOI] [PubMed] [Google Scholar]
- Suliman NA, Taib CNM, Moklas MAM, Basir R, 2017. Delta-9-Tetrahydrocannabinol ((9)-THC) Induce Neurogenesis and Improve Cognitive Performances of Male Sprague Dawley Rats. Neurotox Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swortwood MJ, Newmeyer MN, Andersson M, Abulseoud OA, Scheidweiler KB, Huestis MA, 2017. Cannabinoid disposition in oral fluid after controlled smoked, vaporized, and oral cannabis administration. Drug Test Anal 9(6), 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, 2012. Delta(9)Tetrahydrocannabinol impairs visuo-spatial associative learning and spatial working memory in rhesus macaques. J Psychopharmacol 26(10), 1299–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai S, Hyatt WS, Gu C, Franks LN, Vasiljevik T, Brents LK, Prather PL, Fantegrossi WE, 2015. Repeated administration of phytocannabinoid Delta(9)-THC or synthetic cannabinoids JWH-018 and JWH-073 induces tolerance to hypothermia but not locomotor suppression in mice, and reduces CB1 receptor expression and function in a brain region-specific manner. Pharmacological research : the official journal of the Italian Pharmacological Society 102, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DA, Fennessy MR, 1977. Biphasic nature of the effects of delta9-tetrahydrocannabinol on body temperature and brain amines of the rat. European journal of pharmacology 46(2), 93–99. [DOI] [PubMed] [Google Scholar]
- Tselnicker I, Keren O, Hefetz A, Pick CG, Sarne Y, 2007. A single low dose of tetrahydrocannabinol induces long-term cognitive deficits. Neuroscience letters 411(2), 108–111. [DOI] [PubMed] [Google Scholar]
- Tsukahara S, Tanaka S, Ishida K, Hoshi N, Kitagawa H, 2005. Age-related change and its sex differences in histoarchitecture of the hypothalamic suprachiasmatic nucleus of F344/N rats. Experimental gerontology 40(3), 147–155. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW, 1997. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. The American journal of physiology 273(6 Pt 2), R1957–1964. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Plat L, 2000. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA : the journal of the American Medical Association 284(7), 861–868. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Goffin K, Casteels C, Dupont P, Mortelmans L, de Hoon J, Bormans G, 2008. Gender-dependent increases with healthy aging of the human cerebral cannabinoid-type 1 receptor binding using [18F] MK-9470 PET. NeuroImage 39(4), 1533–1541. [DOI] [PubMed] [Google Scholar]
- Van Someren EJ, 2007. Thermoregulation and aging. American journal of physiology. Regulatory, integrative and comparative physiology 292(1), R99–102. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Herrmann ES, Mitchell JM, Bigelow GE, Flegel R, LoDico C, Cone EJ, 2017. Pharmacokinetic Profile of Oral Cannabis in Humans: Blood and Oral Fluid Disposition and Relation to Pharmacodynamic Outcomes. J Anal Toxicol 41(2), 83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G, 2003. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proceedings of the National Academy of Sciences 100(3), 1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman ED, Moline ML, Czeisler CA, Zimmerman JC, 1982. Chronobiology of aging: temperature, sleep-wake rhythms and entrainment. Neurobiology of aging 3(4), 299–309. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM, 1995. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14(4), 697–706. [DOI] [PubMed] [Google Scholar]
- Westlake T, Howlett A, Bonner T, Matsuda L, Herkenham M, 1994. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer’s brains. Neuroscience 63(3), 637–652. [DOI] [PubMed] [Google Scholar]
- Whitehurst LN, Fogler K, Hall K, Hartmann M, Dyche J, 2015. The effects of chronic marijuana use on circadian entrainment. Chronobiology international 32(4), 561–567. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA, 2002. Endocannabinoid signaling in the brain. Science 296(5568), 678–682. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Tzavara ET, Nomikos GG, 2005. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol 16(5–6), 315–331. [DOI] [PubMed] [Google Scholar]
- Wotjak CT, 2005. Role of endogenous cannabinoids in cognition and emotionality. Mini Rev Med Chem 5(7), 659–670. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Niff JJ, Takano T, Deane R, Nedergaard M, 2013. Sleep drives metabolite clearance from the adult brain. Science 342(6156), 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R. i., Ueda M, Block GD, Sakaki Y, Menaker M, Tei H, 2000. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288(5466), 682–685. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD, 2002. Effects of aging on central and peripheral mammalian clocks. Proceedings of the National Academy of Sciences of the United States of America 99(16), 10801–10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Cheer JF, 2016. Beyond the CB1 Receptor: Is Cannabidiol the Answer for Disorders of Motivation? Annu Rev Neurosci 39, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, 2006. History of cannabis as a medicine: a review. Rev Bras Psiquiatr 28(2), 153–157. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Crippa JA, Hallak JE, Bhattacharyya S, Atakan Z, Martin-Santos R, McGuire PK, Guimaraes FS, 2012. A critical review of the antipsychotic effects of cannabidiol: 30 years of a translational investigation. Curr Pharm Des 18(32), 5131–5140. [DOI] [PubMed] [Google Scholar]