Abstract
Transcranial Direct Current Stimulation (tDCS) has shown efficacy in augmenting the effects of language therapy in Primary Progressive Aphasia (PPA). The mechanism of action of tDCS is not understood but preliminary work in healthy adults suggests it modulates GABA levels to create an environment optimal for learning. It is unknown if this proposed mechanism translates to aging or neurodegenerative conditions. This study tested the hypothesis that tDCS reduces GABA at the stimulated tissue in primary progressive aphasia (PPA). We applied GABA-edited MRS to quantify GABA levels before and after a sham-controlled tDCS intervention with language therapy in PPA. All participants showed improvements but those receiving active tDCS showed significantly greater language improvements compared to sham both immediately after the intervention and at 2 months follow-up. GABA levels in the targeted tissue decreased from baseline after the intervention and remained decreased 2 months after the intervention. This work supports the hypothesis that tDCS modulates GABAergic inhibition to augment learning and is clinically useful for PPA combined with language therapy.
Keywords: Anodal tDCS, language therapy, neurodegenerative disorder, GABA-edited MRS, neural plasticity, learning
1. Introduction
Primary progressive aphasia (PPA) is a neurodegenerative condition, which primarily affects language (Gorno-Tempini, M. L. et al., 2011). Symptoms include difficulties with word finding, word usage, comprehension and sentence construction. The primary treatment tool is language therapy, which includes oral and written word generation cued by pictures, word association, writing words and spelling practice, has shown some therapeutic effects, though improvements are not sustained (Tippett et al., 2015).
Transcranial direct current stimulation (tDCS) passes a weak current between two electrodes and appears to modulate cortical excitability, increasing excitability at the anode and decreasing excitability at the cathode (Krause et al., 2013; Lefaucheur, 2016). We have previously shown in a small cohort of patients that 15 sessions of anodal tDCS over the left inferior frontal gyrus (IFG) improved spelling in PPA (Tsapkini et al., 2014), while Cotelli’s group showed that 10 sessions of anodal tDCS over the dorsolateral prefrontal cortex improved oral naming (Cotelli et al., 2014). Subsequent studies using similar designs have confirmed the augmentative role of anodal tDCS over the left IFG (Gervits et al., 2016; McConathey et al., 2017) as well as over the left inferior parietal gyrus (Roncero et al., 2017). These studies indicate that tDCS may: (A) provide greater therapeutic gains than language therapy alone (sham condition) and (B) sustain therapeutic benefits longer than language therapy alone. Despite the growing interest in using tDCS clinically, the mechanism by which tDCS effects are induced has not been adequately explored.
Mechanistic studies in young, healthy, control adults suggest that tDCS increases glutamate and/or decreases GABA at the anode (Clark et al., 2011; Kim et al., 2014; Stagg, 2014; Stagg et al., 2009; Stagg and Nitsche, 2011). These changes in glutamate and GABA and the associated changes in inhibition and excitation are proposed to provide an optimal neural environment for plasticity and learning (Krause et al., 2013). Reducing inhibitory tone would result in lower inhibition to synaptic signal transfer and therefore facilitate transmission of signal to the subsequent neuron(s). An increase in excitatory tone will have a similar effect. This conditional neuronal firing would result in neural learning for the behavioural task in Hebbian terms. Previous studies have provided evidence of a reduction in GABA following one application of tDCS (Stagg et al., 2011; Stagg et al., 2009), while others have shown a glutamatergic increase (glutamate or glutamate measured with glutamine as Glx) (Clark et al., 2011; Hunter et al., 2015; Stagg et al., 2009). These studies have served as foundational evidence of a GABA- and glutamate-mediated tDCS mechanism in healthy adult controls. For tDCS effects to have a significant clinical value, interventions likely need to be performed for multiple days, weeks or even months. Ries and colleagues (Reis et al., 2009) showed that 5 consecutive sessions of tDCS appears to result in longer and more robust behavioural effects than a single session. It is important to study repeated tDCS sessions because behavioural effects of one tDCS application that last for only a few hours post-stimulation have little clinical utility. Furthermore, understanding tDCS mechanisms in clinical populations is necessary to target and optimize therapy. To date, metabolite changes following tDCS applications for language therapy in clinical populations have not been investigated, thus there is a lack of direction for effectively applying and improving tDCS therapy in language rehabilitation in any clinical population.
The current study addresses this paucity of mechanistic evidence. Specifically, under the hypothesis that GABA plays a mechanistic role in learning and is modulated with tDCS, we examined change in GABA after a tDCS intervention targeting the left frontal operculum (inferior frontal gyrus, IFG) in combination with language therapy in a sham-controlled study. Secondary objectives included examining other metabolites (glutamate reported in combination with glutamine as Glx, creatine Cr, and N-acetyl aspartate, NAA), and confirming the local specificity of this intervention.
2. Materials and Methods
2.1. Patient recruitment
Patients diagnosed with PPA through consensus criteria (Gorno-Tempini, M. L. et al., 2011) (evaluated with neurological examination, cognitive and language testing and imaging results), were recruited to participate in this study. Expert clinicians who based their diagnosis on the established criteria at specialized hospital or university centers confirmed patients’ diagnoses. In all patients, the most robust symptom remained the progressive deterioration of language function(s) despite other preserved cognitive abilities. Additional inclusion criteria for study participation were: at least a 12th grade education, right-handedness and English as first language. Patients were excluded if they were: not pre-morbidly proficient spellers, in advanced stages of PPA or other dementia, over 90 years of age, or had contraindications to MR scanning such as severe claustrophobia. Participants with conditions such as previous stroke or any psychiatric or developmental disorder were also excluded. Patients were randomized to tDCS or sham therapy, to gain approximately equal groups matched for several demographic parameters (see Table 1 for demographic information). Twenty-two patients diagnosed with PPA were recruited. Of these patients, 6 were diagnosed with semantic variant PPA, 10 with non/fluent agrammatic variant PPA and 6 with logopenic variant PPA. In this paper we did not stratify by variants that may have different pathology since there is no literature showing any effect of brain pathology on GABA or other metabolites. The fronto-temporal dementia clinical dementia rate (FTD-CDR) scale was used to assess overall disease severity, including language functions and other function (memory, attention, independence) (Knopman et al., 2008).
Table 1.
Patient demographics. For age, years post onset, and severity, values shown are mean (standard deviation). P-values are from two-sample permutation tests for continuous outcomes and Fisher’s exact test for categorical outcomes. Language severity is based on the language subset from the FTD-CDR scale. Total severity refers to all language and behaviour assessments as per Knopman et al., (Knopman et al., 2008). The two groups (tDCS vs. sham) were matched in all measures.
| Combined (n = 22) | tDCS (n = 11) | Sham (n = 11) | P-value | |
|---|---|---|---|---|
| Sex | 11F, 11M | 6F, 5M | 5F, 6M | 1.000 |
| PPA variant | 6L, 10N, 6S | 4L, 4N, 3S | 2L, 6N, 3S | 0.857 |
| Age | 66.9 (7.5) | 64.1 (8.4) | 69.6 (5.7) | 0.090 |
| Years post onset | 5.0 (3.0) | 5.6 (3.4) | 4.5 (2.5) | 0.392 |
| Language severity (FTD-CDR) | 1.9 (0.8) | 1.9 (0.8) | 1.9 (0.8) | 1.000 |
| Total with language severity 0.5 | 2 | 1 | 1 | - |
| Total with language severity 1 | 4 | 2 | 2 | - |
| Total with language severity 2 | 12 | 6 | 6 | - |
| Total with language severity 3 | 4 | 2 | 2 | - |
| Total severity (FTD-CDR) | 7.4 (4.8) | 6.4 (3.6) | 8.3 (5.7) | 0.386 |
| Marital status (number single) | 4 | 3 | 1 | 0.587 |
2.2. Language therapy
We used an oral and written naming task with an adaptation of a spell-study-spell procedure described in previous studies (Beeson and Egnor, 2006; Rapp and Glucroft, 2009; Tsapkini et al., 2018). Briefly, each trial consisted of showing the patient a picture (presented on a computer). The patient named the object, first orally and then in writing. If the patient could not name the object, s/he was asked for 3 features of the object. If the patient made an error, s/he was told it was incorrect and given the chance to correctly name the object. If the patient orally named the object but wrote its name incorrectly, the clinician taught the correct spelling using a spell-study-spell procedure. Each letter was rehearsed individually and learning was reinforced with copying, as this repetition has been shown to have synergy with both oral and written naming (Beeson and Egnor, 2006). Scoring was quantified as a percentage of correct letter-to-sound correspondences in all words based on the accuracy of each letter, where each correct letter was given one point (0.5 points for correct identity and 0.5 points for correct position), and points (and half-points) were subtracted for letters deleted, added, substituted, or moved (Goodman and Caramazza, 1985). Two trained scorers followed this rule-based system independently and inter-rater reliability was 95%.
2.3. tDCS
An investigational double-blind tDCS system was used (Model 1500, Soterix Transcranial Direct Current Stimulator Clinical Trials). The anode was placed over the left inferior frontal (IFG) gyrus which corresponds to the F7 electrode in the electroencephalography, 10–20 system (Homan et al., 1987). Additionally, we co-registered the IFG electrode placement to the pre-treatment MRI scans using a fiducial marker. The reference electrode, the cathode, was placed on each participant’s right cheek, a montage that has previously successfully delivered electrical current in the left motor and premotor areas (Buch et al., 2017; Homan et al., 1987; Hummel et al., 2005; Reis et al., 2009). The current modeling of this particular montage has shown to be delivering the current mostly in the intended area and has produced beneficial behavioural effects in our previous studies in PPA (Tsapkini et al., 2014; Tsapkini et al., 2018). Saline soaked electrodes (2 inches by 2 inches) delivered 2mA of current (ramped up from 0 mA over the course of 30 sec), for 20 min. A previously validated sham condition consisting of the 30 s of stimulation (the ramp phase) was applied to mimic the skin sensations associated with active tDCS (Gandiga et al., 2006). Language therapy started at the beginning of stimulation and continued for a regular speech-language therapy session of 45–50 minutes, i.e., 25–30 minutes after the end of stimulation for both anodal tDCS and sham conditions for all therapeutic sessions. The participant, the therapist and the technician who performed the language and cognitive evaluations were blind to the stimulation condition.
2.4. MRS
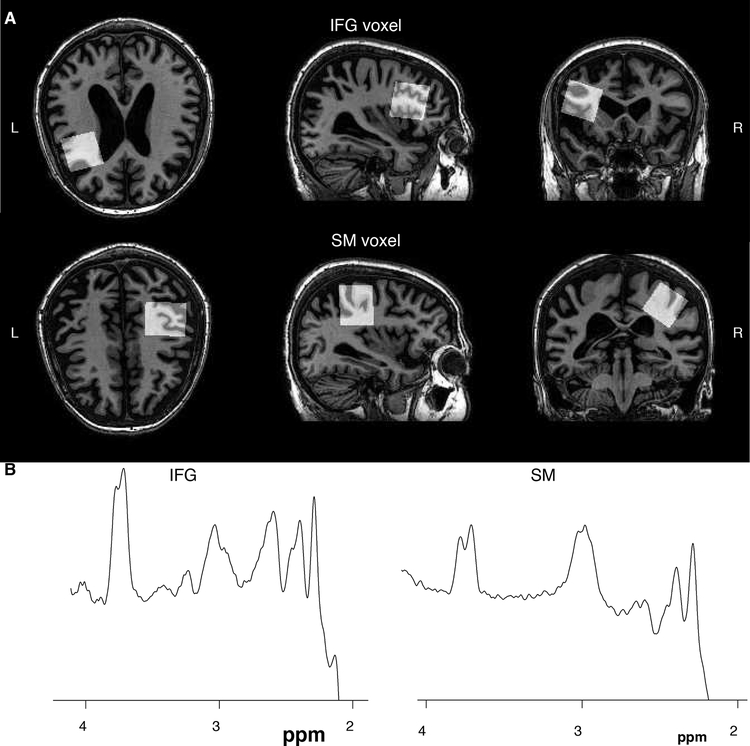
All imaging and spectroscopy were performed at 3T (Philips Achieva). Prior to scanning, a fiducial marker was placed on the left temple to assist with landmarking. For voxel localization and subsequent tissue segmentation, a whole-brain MP-RAGE sequence was acquired (TR/TE = 8/3.75 ms, 1mm3 isotropic voxels). MRS voxels (3 × 3 × 3 cm3) were centered on the left IFG using the fiducial marker and anatomical landmarks of the lateral ventricles and the insula (see Figure 1). As a control region, the right sensorimotor cortex was used, centering the voxel on the hand knob in the precentral gyrus. Voxels were placed to avoid the edge of the brain and the ventricles.
Figure 1.
Exemplar voxel placement and GABA spectra for the IFG voxel and the SM voxel.
Due to more abundant, overlapping peaks, a specialized MRS sequence is required to measure GABA, the most common being J-difference editing, such as MEGA-PRESS (Harris et al., 2017). Data were acquired using a GABA-edited MEGA-PRESS acquisition (TR/TE = 2s/68 ms, 14 ms editing pulses at 1.9 ppm and 7.46 ppm alternating every 2 averages across, 320 averages) to measure GABA and a standard PRESS acquisition (TR/TE = 2s/32 ms, 48 averages) to measure glutamate (reported in combination with glutamine as Glx), NAA, choline (Cho) and creatine (Cr). For both acquisitions, 8 unsuppressed water scans were acquired for quantification.
GABA data were analyzed using the Gannet pipeline (Edden et al., 2014; Harris et al., 2015), including tissue correction to correct for voxel tissue content (which may be impacted by atrophy) as well as differences in GABA between white and grey matter, assuming that the level of GABA in grey matter is twice that in white matter(Harris et al., 2015). Short-echo PRESS data to quantify NAA, Cr, Cho, and Glx were analyzed using LCModel (Provencher, 1993) and subsequently CSF-corrected (correction accounting for cerebrospinal fluid), using the same voxel fractions determined from the Gannet pipeline.
2.5. Statistical analysis
Changes in language scores were tested for time intervals “baseline-post intervention” and “baseline-2 month follow up” with paired t-tests in the tDCS and sham groups. Similarly, metabolite changes for time intervals “baseline-post intervention” and “baseline-2 month follow up” in the IFG and the SM were tested with paired t-tests within the tDCS and the sham groups separately. Dropouts on the 2-months follow-up were assumed to be completely at random. Welch two-sample t-tests with Satterthwaite degrees of freedom (DF) were applied for comparisons between the treatment groups due to the inequality of group variances or numbers of available observations (Ruxton, 2006).
Additionally, data were analyzed with non-parametric permutation testing due to the small sample size. Changes in the metabolite data from IFG and the SM were tested with paired (one-sample) permutation tests within the tDCS and the sham groups separately. The changes in language scores between baseline-post intervention and baseline-2month follow-up were also tested using the same approach. Two-sample permutation tests were applied for comparisons between the treatment groups. Dropouts on the 2-months follow-up were assumed to be completely at random. The null distributions of all permutation tests were approximated via Monte Carlo resampling (Good, 2000). P-values were calculated with two-sided tests. For one-sample permutation tests, at each iteration step, the signs of changes were resigned by 1 or −1 with equal probabilities, and the test statistic was computed as the mean of the resigned changes. For two-sample permutation tests, at each iteration step, the treatment group labels were randomly permuted, and the test statistic was computed as the difference between the means of the two groups after label permutation. The total iteration number of all permutation tests was 10,000.
3. Results
3.1. Patient demographics and tDCS tolerability
A summary of patient demographics is shown in Table 1. All patients completed at least 10 language therapy and tDCS sessions and most completed the full 15-session protocol (mean 13.5 sessions, standard deviation 1.7). Both the tDCS and sham group were almost identical in the number of sessions; the variance was due to unavoidable events in this aging population (flu, colds, other medical appointments, etc.). No serious adverse events occurred, no patients reported any side-effects past the stimulation period and no one discontinued the study due to pain complaints. Patients tolerated tDCS well in general, except for tingling or itching sensations reported at the beginning of the session. This was reported in both tDCS and sham conditions, and patients could not differentiate between conditions. The mean pain ratings for tDCS was 2.51 (standard deviation 3.50, range 0–10) and for sham it was 2.04 (standard deviation 2.08, range 0–10). All patients completed the full assessment at the end of the therapy intervention (time point “post”). Four patients did not have any language assessments and were not scanned at the 2-month follow-up, and one additional patient was not scanned at the 2-month follow-up. Table 2 summarizes the demographics of the patients in tDCS and sham groups at the 2-month follow-up time point illustrating no apparent bias from the dropouts.
Table 2.
Dropout report for the 2-month follow-up visit. Dropouts only occurred at the 2- month follow-up. Four patients did not attend the appointment and one patient had the language assessment but was not scanned. For age, years post onset, severity and total treatment sessions, values shown are mean (standard deviation). Language severity is based on the language subset from the FTD-CDR scale. As above, total severity refers to the sum of boxes, including language and behavior as per Knopman et al., (Knopman et al., 2008).
| Combined (n = 22) | Complete cases (n = 17) | GABA dropouts (n = 5) | Language dropouts (n = 4) | |
|---|---|---|---|---|
| Treatment group | 11 tDCS, 11 Sham | 7 tDCS, 10 Sham | 4 tDCS, 1 Sham | 4 tDCS, 0 Sham |
| Sex | 11F, 11M | 7F, 10M | 4F, 1M | 3F, 1M |
| PPA variant (L = leukopenic, N = non-fluent, S = semantic) | 6L, 10N, 6S | 4L, 8N, 5S | 2L, 2N, 1S | 2L, 2N, 0S |
| Age | 66.9 (7.5) | 66.6 (6.7) | 67.6 (11.0) | 65.8 (11.8) |
| Years post onset | 5.0 (3.0) | 5.0 (3.0) | 5.0 (3.2) | 4.9 (3.6) |
| Language severity (FTD-CDR) | 1.9 (0.8) | 1.8 (0.8) | 2.0 (0.7) | 2.0 (0.8) |
| Total with language severity 0.5 | 2 | 2 | 0 | 0 |
| Total with language severity 1 | 4 | 3 | 1 | 1 |
| Total with language severity 2 | 12 | 9 | 3 | 2 |
| Total with language severity 3 | 4 | 3 | 1 | 1 |
| Total severity (FTD-CDR) | 7.4 (4.8) | 7.3 (5.3) | 7.6 (2.8) | 7.6 (3.2) |
| Marital status single | 4 | 2 | 2 | 2 |
3.2. Language scores changes
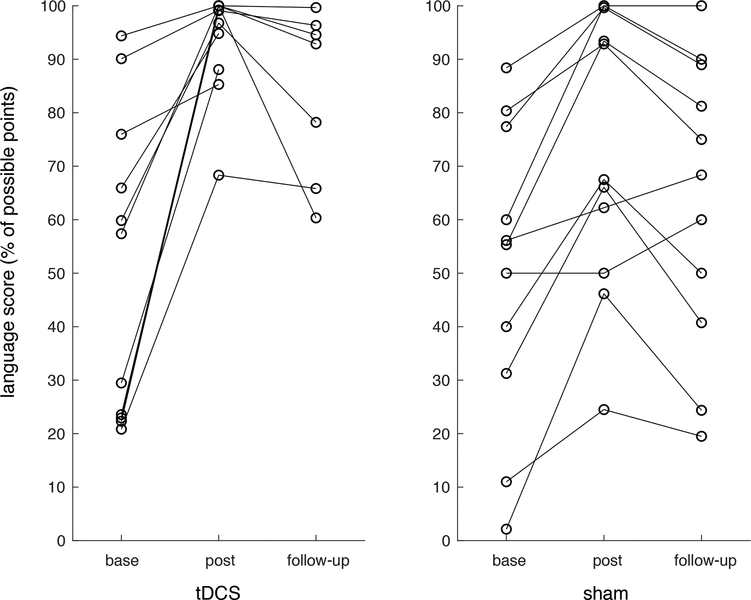
Post-treatment, all patients showed improvement with therapy on an individual and group level; tDCS group mean change in language score: 42.62, degrees of freedom (DF): 10, T-statistic = 5.13, p <0.001; sham group mean change in language score: 22.76, DF: 10, T-statistic = 5.02, p <0.001, see Table 3 and Figure 2. Patients who received anodal tDCS showed greater improvements compared to the sham group (mean difference in scores: 19.86, DF = 15.47, T-statistic = 2.10, p = 0.053). The permutation testing showed consistent results; significant improvements were seen in both the tDCS group (p < 0.0001) and sham group (p = 0.0021) and the tDCS showed significantly greater language improvements to sham (p = 0.048)
Table 3.
Reports of means, SDs, and numbers of observations for Language Scores on different time points within different treatment groups.
| Time point | Treatment group | Mean (SD) of Language Scores | Number of observations |
|---|---|---|---|
| Before | tDCS | 51.16 (28.48) | 11 |
| After | tDCS | 93.78 (9.87) | 11 |
| 2month | tDCS | 83.97 (15.87) | 7 |
| Before | Sham | 50.18 (27.52) | 11 |
| After | Sham | 72.94 (26.03) | 11 |
| 2month | Sham | 63.47 (27.08) | 11 |
Figure 2.
Language scores at pre-intervention (base), following the tDCS protocol (post) and at 2-months follow-up (follow-up). Scores are presented as a percentage of the possible points for each patient.
At the 2-month follow up, both groups had significantly improved language scores compared to baseline. The tDCS group showed an increase in language score of 40.94, DF: 6, T-statistics = 4.34, p = 0.005 and the sham group showed a language score increase of 13.29, DF: 10, T-statistic = 4.57, p = 0.001. The tDCS group showed significantly higher language scores compared to the sham group at the 2-month follow up (difference in improved language score: 27.65, DF: 7.15, T-statistic = 4.57, p = 0.001). Similarly, the non-parametric permutation testing showed significant language improvements at 2-month follow up for tDCS (p = 0.014) and sham (p = 0.0019) and that tDCS showed significantly greater language improvements to sham (p = 0.0019).
3.3. Metabolite changes
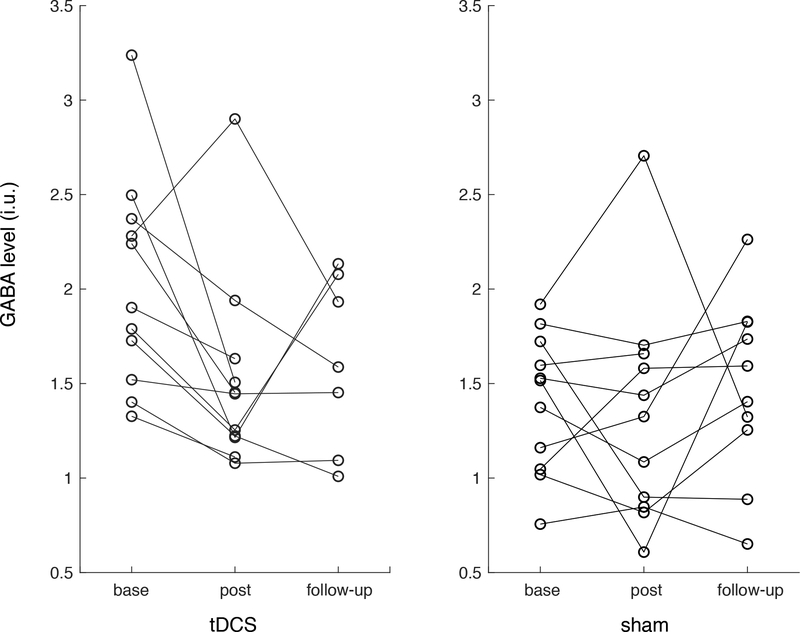
GABA in the IFG significantly decreased from baseline in the tDCS group directly after the tDCS intervention (change: −0.50 i.u., DF: 10, T statistic: −2.70, P-value: 0.022) and was decreased at trend level at 2-months follow-up time point (change: −0.33 i.u., DF: 6, T statistic: −2.37, P-value: 0.056, see Figure 3). GABA did not change in the IFG of the sham group after the intervention (estimated change: −0.07, DF: 10, T statistic: −0.47, P-value: 0.65) or at 2-months follow up (estimated change: −0.09, DF: 9, T statistic: 0.65, P-value: 0.61). No changes were seen in the SM voxel of either group at either time point (Figure 3) and no changes were seen in any other metabolite (Glx, NAA, Cr or Cho). For validation, our permutation testing showed generally consistent results; from baseline to post-intervention, there was a significant decrease in GABA in the tDCS group (p = 0.021) but not in the sham group. However, in the permutation testing, GABA was significantly decreased at 2-months follow up (p = 0.047) compared to the trend-level decrease detected with the parametric statistics. No changes in GABA were detected in the SM voxel with the permutation testing.
Figure 3.
GABA levels in the IFG voxel for each patient at pre-intervention (base), following the tDCS protocol (post) and at 2-months follow-up (follow-up).
4. Discussion
The IFG has previously been shown to be a hub for oral and written naming and spelling as it is an area recruited in numerous studies in recent reviews and meta-analyses (Price, 2010; Purcell et al., 2011; Roux et al., 2014). Furthermore, the left IFG has been shown to be involved in semantic selection of the appropriate word (Thompson-Schill et al., 1999) and active retrieval (Owen et al., 1996), thus is crucial for earlier and later stages of word production. Targeting the IFG with anodal tDCS has been shown to be an effective way to improve the results of a language intervention for PPA (Cotelli et al., 2014; Tsapkini et al., 2014).
The present study tested the hypothesis that a reduction in the inhibitory neurometabolite GABA is modulated by anodal tDCS in PPA. Patients with PPA who participated in a language intervention (written naming/spelling) were randomized to anodal tDCS or sham tDCS targeting the left IFG for ~15 sessions. GABA-edited MRS and conventional MRS were performed in two locations in the brain – the left IFG, the tDCS target, and the right SM cortex as a control location to detect regional changes in GABA and glutamate following a language and tDCS intervention. After the intervention, all patients (both anodal and sham tDCS groups) showed improved language scores. However, we show that repeated, consecutive tDCS applications resulted in larger improvements in language outcomes than sham and there was a decrease in GABA in the tissue targeted by the tDCS anode. Furthermore, the additional behavioural improvements as well as GABA reduction were sustained at 2 months and were significantly greater than sham effects. To our knowledge, this is the first time GABA reductions following tDCS have been demonstrated long (weeks) after the intervention. None of the other metabolites examined (Glx, NAA, Cr, Cho) showed a significant change post-stimulation indicating a GABA-specific mechanism. Moreover, GABA concentrations did not change in the sham condition or at the control area, the right SM cortex. These results indicate the localized effects of tDCS on the metabolite GABA. To our knowledge, this is also the first study examining GABA reduction as a mechanism of tDCS in a neurodegenerative condition with consecutive tDCS sessions.
These behavioural results are consistent with previous behavioural studies in neurodegenerative diseases, including ours (Cotelli et al., 2014; Tippett et al., 2015; Tsapkini et al., 2014; Tsapkini et al., 2018); patients who received anodal tDCS showed significantly greater improvements compared to those who received sham treatments. Little is known about the brain mechanisms of tDCS. Previous studies, mainly from Stagg’s group, have shown GABA decreases at the stimulation site after one application of anodal tDCS over the primary motor cortex (M1) (Bachtiar et al., 2015; Stagg et al., 2014). There is evidence that resting state connectivity is also altered following tDCS (Bachtiar et al., 2015; Ficek et al., 2018), though it is not clear whether alterations in functional connectivity cause improvements in task performance or if task improvements result in changes in functional connectivity.
Several studies have shown that GABA decreases during protocols designed to induce cortical plasticity and there is increasing evidence that it is necessary for GABA to decrease for long-term-potentiation-induced plasticity to occur (Stagg et al., 2011). Building on this premise, modulating GABA levels with non-invasive neurostimulation during training may provide a mechanism to improve learning and/or therapeutic effects of behavioural interventions. tDCS has been shown to induce long-term language and motor improvements after several consecutive applications, possibly through the mechanism of long-term-potentiation (LTP) (Fritsch et al., 2010; Monte-Silva et al., 2013). The present study additionally showed that the decrease in GABA immediately post-treatment was sustained for two months. This may indicate that changes in GABA persist longer than anticipated through these LTP-based mechanisms or it may indicate that in this population, learning persists beyond the intervention. As patients are motivated to maintain their language improvements following this intense language therapy intervention, it is may be that these patients actively engage in activities to maintain their language skills. This may result in the brain maintaining a more plastic state with lower GABA for on-going learning. These activities may be overt, such as practicing and repeating exercises learning during the language intervention or through less formalized events such as actively engaging in challenging conversations to have the same effect of maintaining language skills.
Studies in young and healthy control populations have shown increased glutamate or GLX local to anodal tDCS (Clark et al., 2011; Kim et al., 2014; Stagg et al., 2009), which may be related to increases in network connectivity (Hunter et al., 2015). However, in aging or clinical populations, such as PPA, these observations may not be seen. In a very recent study of elderly control subjects (Antonenko et al., 2017) only changes in GABA were observed, no changes in GLX were seen. This may indicate that the cortical response to tDCS may change with age (or the ability to detect metabolite changes is impacted with age). This indicates that further studies are warranted to better delineate the mechanisms of tDCS across the lifespan, as it appears incorrect to assume that results from young adult populations will translate (Antonenko et al., 2017). Age may also modulate the impact of tDCS on learning as reported through changes in task performance. In a study comparing young and elderly populations receiving tDCS during a language-learning task, the elderly population showed significant learning after anodal tDCS compared to sham while the young population did not show the same effects (Fiori et al., 2017).
Another effect of age that we need to consider, because it may influence effectiveness of tDCS, is the loss of grey matter volume itself. This drives the majority of the age-related decline in GABA (Gao et al., 2013; Porges et al., 2017; Puri et al., 2015). In healthy populations, decreases in grey matter drive observed decreases in GABA concentration; the concentration of GABA in grey matter itself (i.e., the grey matter remaining) is not decreasing (Porges et al., 2017). It is unknown if this is also true in PPA. In a recent study, baseline grey matter density was correlated with behavioural improvements after 15 days of language therapy and tDCS of the DLPFC (Cotelli et al., 2016). Similarly, tissue volumes of specific areas of the language network and the hippocampus predict therapy scores immediately after and two months post-therapy (Tsapkini, 2017). In the present study we explicitly correct for the tissue composition of the voxel but age may interact with tDCS effects in ways we do not completely understand.
Limitations of the present study
A potential limitation of our study is that initial GABA levels were higher in the tDCS group despite the fact that the participants were randomized and the groups did not differ in language and dementia severity or in initial naming and spelling performance. While this appears to be a random occurrence, it may be that GABA levels can only decrease during a learning if they are initially high enough i.e., if GABA levels are too low, they cannot further decrease. If this is the case, it is possible that had the GABA levels in the sham group been higher at baseline, they may have also shown decreases; this is, however, highly speculative.
Second, there were four drop-outs in our anodal tDCS group. These dropouts were due to unrelated issues (e.g., falls, cancer). Our demographic data analysis showed no bias in drop-out so to the best of our ability we have shown these drop-outs were random and did not impact the results.
Finally, we did not find any relationship between behavioural measures and metabolite levels. Future studies with larger sample sizes are needed to evaluate whether the tDCS effects seen in behavioural measures correlate with measures of GABA concentrations at the stimulated site.
Table 4.
Reports of means, SDs, and numbers of observations for GABA in the IFG and the SM areas on different time points within different treatment groups.
| Time point | Treatment group | Mean (SD) of IFG GABA | Mean (SD) of SM GABA | Number of observations |
|---|---|---|---|---|
| Before | tDCS | 2.03 (0.57) | 2.09 (0.29) | 11 |
| After | tDCS | 1.52 (0.52) | 2.24 (0.29) | 11 |
| 2month | tDCS | 1.61 (0.46) | 2.19 (0.45) | 7 |
| Before | Sham | 1.40 (0.37) | 1.93 (0.47) | 11 |
| After | Sham | 1.33 (0.59) | 2.23 (0.38) | 11 |
| 2month | Sham | 1.48 (0.48) | 2.05 (0.50) | 10 |
Highlights.
Anodal tDCS augments language therapy in patients with PPA compared to sham-tDCS
tDCS decreases GABA in the left IFG, the tissue local to the tDCS anode following the intervention
Improvements in language scores are better maintained with active tDCS at 2 months follow-up
GABA remains lower in the targeted tissue (left IFG) in the anodal tDCS group at 2 months follow-up
5. Acknowledgments
We are grateful to our participants for their unfailing commitment and interest in our study. We also thank referring physicians.
Funding
This work was supported by grants from the Science of Learning Institute at Johns Hopkins University and by National Institutes of Health (National Institute of Deafness and Communication Disorders) through award R01 DC014475 to KT. ADH was supported through funding from the Science of Learning Institute Grant to KT and is currently funded by the Canada Research Chairs Program, the Alberta Children’s Hospital Research Institute and the Hotchkiss Brain Institute. RAE was supported through R01 EB016089, R01 023693, and P41 EB015909.
Abbreviations:
- Cho
choline
- Cr
creatine
- DF
degrees of freedom
- EEG
electroencephalography
- GABA
γ-aminobutyric acid
- Glx
glutamate+glutamine
- IFG
inferior frontal gyrus
- LTP
long-term potentiation
- MEGA
Mescher-Garword editing
- MRS
magnetic resonance spectroscopy
- NAA
N-acetyl aspartate
- PPA
Primary progressive aphasia
- PRESS
point-resolved single voxel spectroscopy
- SM
sensorimotor cortex
- tDCS
transcranial direct current stimulation
- TE
echo time
- TR
repetition time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no conflicts of interest relevant to the subject matter of this manuscript.
7. References
- Antonenko D, Schubert F, Bohm F, Ittermann B, Aydin S, Hayek D, Grittner U, Floel A, 2017. tDCS-Induced Modulation of GABA Levels and Resting-State Functional Connectivity in Older Adults. J Neurosci 37(15), 4065–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtiar V, Near J, Johansen-Berg H, Stagg CJ, 2015. Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. Elife 4, e08789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson PM, Egnor H, 2006. Combining treatment for written and spoken naminig. J Int Neuropsychol Soc 12(6), 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch ER, Santarnecchi E, Antal A, Born J, Celnik PA, Classen J, Gerloff C, Hallett M, Hummel FC, Nitsche MA, Pascual-Leone A, Paulus WJ, Reis J, Robertson EM, Rothwell JC, Sandrini M, Schambra HM, Wassermann EM, Ziemann U, Cohen LG, 2017. Effects of tDCS on motor learning and memory formation: A consensus and critical position paper. Clin Neurophysiol 128(4), 589–603. [DOI] [PubMed] [Google Scholar]
- Clark VP, Coffman BA, Trumbo MC, Gasparovic C, 2011. Transcranial direct current stimulation (tDCS) produces localized and specific alterations in neurochemistry: a (1)H magnetic resonance spectroscopy study. Neurosci Lett 500(1), 67–71. [DOI] [PubMed] [Google Scholar]
- Cotelli M, Manenti R, Paternico D, Cosseddu M, Brambilla M, Petesi M, Premi E, Gasparotti R, Zanetti O, Padovani A, Borroni B, 2016. Grey Matter Density Predicts the Improvement of Naming Abilities After tDCS Intervention in Agrammatic Variant of Primary Progressive Aphasia. Brain Topogr 29(5), 738–751. [DOI] [PubMed] [Google Scholar]
- Cotelli M, Manenti R, Petesi M, Brambilla M, Cosseddu M, Zanetti O, Miniussi C, Padovani A, Borroni B, 2014. Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. J Alzheimers Dis 39(4), 799–808. [DOI] [PubMed] [Google Scholar]
- Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ, 2014. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging 40(6), 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficek BN, Wang Z, Zhao Y, Webster KT, Desmond JE, Hillis AE, Frangakis C, Vasconcellos Faria A, Caffo B, Tsapkini K, 2018. The effect of tDCS on functional connectivity in primary progressive aphasia. Neuroimage Clin 19, 703–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori V, Nitsche M, Iasevoli L, Cucuzza G, Caltagirone C, Marangolo P, 2017. Differential effects of bihemispheric and unihemispheric transcranial direct current stimulation in young and elderly adults in verbal learning. Behav Brain Res 321, 170–175. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B, 2010. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66(2), 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG, 2006. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 117(4), 845–850. [DOI] [PubMed] [Google Scholar]
- Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, Zhao B, Wang H, Bai X, Zhao C, Wang X, Barker PB, 2013. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 78, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervits F, Ash S, Coslett HB, Rascovsky K, Grossman M, Hamilton R, 2016. Transcranial direct current stimulation for the treatment of primary progressive aphasia: An open-label pilot study. Brain Lang 162, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good P, 2000. Permutation Tests Springer, New York. [Google Scholar]
- Goodman RA, Caramazza A, 1985. The Johns Hopkins University Dysgraphia Battery. Johns Hopkins University, Baltimore, MD. [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M, 2011. Classification of primary progressive aphasia and its variants. Neurology 76(11), 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Puts NA, Edden RA, 2015. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn Reson Imaging 42(5), 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Saleh MG, Edden RA, 2017. Edited (1) H magnetic resonance spectroscopy in vivo: Methods and metabolites. Magn Reson Med 77(4), 1377–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan RW, Herman J, Purdy P, 1987. Cerebral location of international 10–20 system electrode placement. Electroencephalogr Clin Neurophysiol 66(4), 376–382. [DOI] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG, 2005. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 128(Pt 3), 490–499. [DOI] [PubMed] [Google Scholar]
- Hunter MA, Coffman BA, Gasparovic C, Calhoun VD, Trumbo MC, Clark VP, 2015. Baseline effects of transcranial direct current stimulation on glutamatergic neurotransmission and large-scale network connectivity. Brain Res 1594, 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Stephenson MC, Morris PG, Jackson SR, 2014. tDCS-induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: a 7 T magnetic resonance spectroscopy study. Neuroimage 99, 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, Miller BL, Mercaldo N, 2008. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 131(Pt 11), 2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause B, Marquez-Ruiz J, Cohen Kadosh R, 2013. The effect of transcranial direct current stimulation: a role for cortical excitation/inhibition balance? Front Hum Neurosci 7, 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, 2016. A comprehensive database of published tDCS clinical trials (2005–2016). Neurophysiol Clin 46(6), 319–398. [DOI] [PubMed] [Google Scholar]
- McConathey EM, White NC, Gervits F, Ash S, Coslett HB, Grossman M, Hamilton RH, 2017. Baseline Performance Predicts tDCS-Mediated Improvements in Language Symptoms in Primary Progressive Aphasia. Front Hum Neurosci 11, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte-Silva K, Kuo MF, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, Nitsche MA, 2013. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul 6(3), 424–432. [DOI] [PubMed] [Google Scholar]
- Owen AM, Evans AC, Petrides M, 1996. Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: A positron emission tomography study. Cerebral Cortex 6(1), 31–38. [DOI] [PubMed] [Google Scholar]
- Porges EC, Woods AJ, Lamb DG, Williamson JB, Cohen RA, Edden RAE, Harris AD, 2017. Impact of tissue correction strategy on GABA-edited MRS findings. Neuroimage 162, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, 2010. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci 1191, 62–88. [DOI] [PubMed] [Google Scholar]
- Provencher SW, 1993. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30(6), 672–679. [DOI] [PubMed] [Google Scholar]
- Purcell JJ, Napoliello EM, Eden GF, 2011. A combined fMRI study of typed spelling and reading. Neuroimage 55(2), 750–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri R, Hinder MR, Fujiyama H, Gomez R, Carson RG, Summers JJ, 2015. Duration-dependent effects of the BDNF Val66Met polymorphism on anodal tDCS induced motor cortex plasticity in older adults: a group and individual perspective. Front Aging Neurosci 7, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B, Glucroft B, 2009. The benefits and protective effects of behavioural treatment for dysgraphia in a case of primary progressive aphasia. Aphasiology 23(2), 236–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Schambra HM C. LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Wrakauer JK, 2009. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA 106(5), 1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncero C, Kniefel H, Service E, Thiel A, Probst S, Chertkow H, 2017. Inferior parietal transcranial direct current stimulation with training improves cognition in anomic Alzheimer’s disease and frontotemporal dementia. Alzheimers Dement (N Y) 3(2), 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux FE, Durand JB, Rehault E, Planton S, Draper L, Demonet JF, 2014. The neural basis for writing from dictation in the temporoparietal cortex. Cortex 50, 64–75. [DOI] [PubMed] [Google Scholar]
- Ruxton GD, 2006. The unequal variance t-test is an underused alternative to Student’s t-test and the Mann–Whitney U test. Behavioral Ecology 17(4), 688–690. [Google Scholar]
- Stagg CJ, 2014. Magnetic Resonance Spectroscopy as a tool to study the role of GABA in motor-cortical plasticity. Neuroimage 86, 19–27. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Amadi U, Gudberg CA, Ilie AS, Sampaio-Baptista C, O’Shea J, Woolrich M, Smith SM, Filippini N, Near J, Johansen-Berg H, 2014. Local GABA concentration is related to network-level resting functional connectivity. Elife 3, e01465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H, 2011. The role of GABA in human motor learning. Curr Biol 21(6), 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, Stephenson MC, O’Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, Johansen-Berg H, 2009. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci 29(16), 5202–5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA, 2011. Physiological basis of transcranial direct current stimulation. Neuroscientist 17(1), 37–53. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Aguirre GK, D’Esposito M, Farah MJ, 1999. A neural basis for category and modality specificity of semantic knowledge. Neuropsychologia 37(6), 671–676. [DOI] [PubMed] [Google Scholar]
- Tippett DC, Hillis AE, Tsapkini K, 2015. Treatment of Primary Progressive Aphasia. Curr Treat Options Neurol 17(8), 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, 2017. Transcranial direct current stimulation in primary progressive aphasia: Whom does it help?, Front. Hum. Neurosci. Conference Abstract: Academy of Aphasia 55th Annual Meeting. [Google Scholar]
- Tsapkini K, Frangakis C, Gomez Y, Davis C, Hillis AE, 2014. Augmentation of spelling therapy with transcranial direct current stimulation in primary progressive aphasia: Preliminary results and challenges. Aphasiology 28(8–9), 1112–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, Webster KT, Ficek BN, Desmond JE, Onyike CU, Rapp B, Frangakis CE, Hillis AE, 2018. Electrical brain stimulation in different variants of primary progressive aphasia: A randomized clinical trial. Alzheimers Dement (N Y) 4, 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]