Letter
T cell checkpoint inhibitors including those targeting the programmed cell death protein-1 (PD1) pathway have improved treatment outcomes for cutaneous melanoma; however, many patients do not respond favorably (Carretero-Gonzalez et al., 2018). An alternative and potentially complementary approach centers on modulating transcription in tumor cells by interfering with epigenetic modifiers. Such compounds include those targeting members of the Bromodomain and Extra Terminal domain (BET) family of epigenetic readers that influence transcription by binding to histones (Filippakopoulos et al., 2010). BET proteins promote malignancy through their effects on the expression of genes essential for tumor growth and survival. Conversely, BET inhibitors can arrest melanoma cell growth in vitro and in vivo (Segura et al., 2013).
A key question in melanoma is whether the efficacy of checkpoint blockade can be improved by inhibiting BET protein function. Emerging evidence suggests that BET inhibitors may have useful effects on tumor-responsive T cells. For instance, the BET inhibitor JQ1 enhanced CD8 T cell response in an adoptive immunotherapy model (Kagoya et al., 2016), and enhanced anti-tumor immunity by reducing programmed cell death-1 ligand (PD-L1) expression on B lymphoma and ovarian cancer cells (Hogg et al., 2017, Zhu et al., 2016). More recently, JQ1 rendered lung cancer cells receptive to PD1 blockade (Adeegbe et al., 2018), raising the possibility that dual inhibition of BET proteins and PD1 function may effectively constrain malignant cell growth for a variety of cancers. However, T cell function and response to immunotherapy and BET inhibition may vary within disparate types of solid tumors. Consistent with this, a greater clinical benefit to PD1 blockade is observed in patients with melanoma compared to lung cancer (Carretero-Gonzalez et al., 2018). Here we establish the capacity of the next-generation BET inhibitor PLX51107, a potent and structurally unique compound with optimized pharmacokinetic properties (Ozer et al., 2018), to modulate melanoma tumor growth, tumor infiltrating T cells, and the efficacy of PD1 blockade in melanoma.
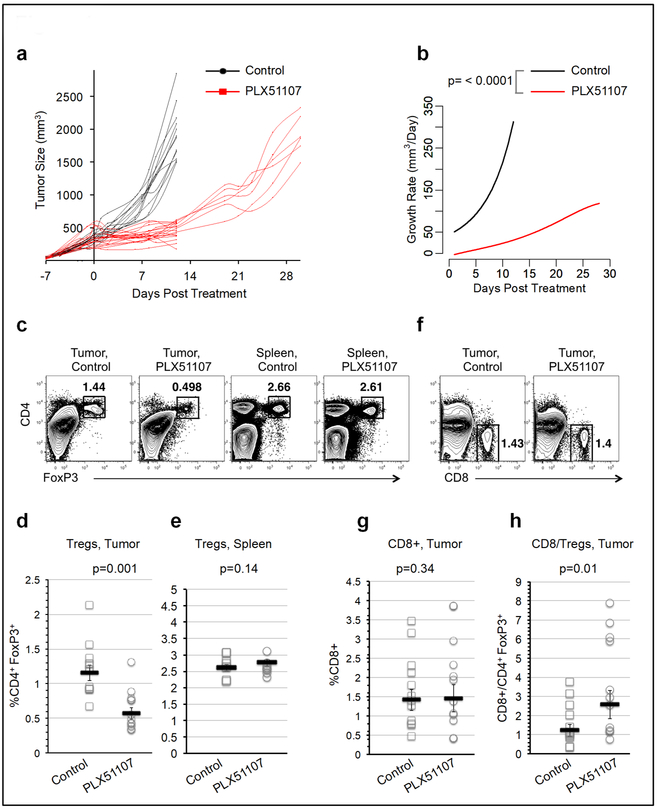
The effects of PLX51107 on melanoma were tested in the immune competent mice bearing BrafV600E, Ptennull, Cdkn2dnul1 YUMM1.7 tumors (Meeth et al., 2016). All animal experiments were performed according to the approved protocols of the Thomas Jefferson University Institutional Animal Care and Use Committee. YUMM1.7 cells expressed several members of the BET family with BRD2 and BRD3 detected (Figure S1). Melanoma tumors in mice receiving PLX51107 exhibited significantly delayed growth compared to controls (Figure 1a and b). During treatment, serum PLX51107 levels remained stable (557-1500 ng/ml, Figure S2a), and animals lost no more than 15% of their starting weight (Figure S2b). As expected (Baras et al., 2016), frequencies of FoxP3+ regulatory T cells (Tregs) were increased in YUMM1.7 tumors (Figure S3). The Treg frequency decreased in PLX51107 treated tumors but not in spleens (Figure 1c, d, and e). While no significant difference was found in frequencies of tumor infiltrating CD8 T cells with PLX51107 treatment (Figure 1f and g), the CD8 to Treg ratio was significantly higher in the treatment group (Figure 1h).
Figure 1: PLX51107 treatment effect on murine melanoma tumor growth and tumor infiltrating T cells.
(a) YUMM1.7 murine melanoma tumors were generated in C57BL/6 hosts. When tumor sizes reached 200-500 mm3, recipients were placed on either control chow (black) or PLX51107-laced chow (red). Tumor volumes were monitored at the indicated time points. Each line corresponds to an individual mouse. (b) The corresponding tumor growth rate functions are shown. (c) Mice were sacrificed after 12 days of treatment. Spleens and tumor cells were homogenized and analyzed by flow cytometry. CD4+, FoxP3+ Treg frequencies within the (d) bulk tumor or (e) spleen cells were compared in control versus treated mice. (f) CD8+ cells were gated in tumors and (g) the frequencies of CD8+ cells and (h) the CD8+/Tregs ratios in tumors were compared. Each symbol represents an individual mouse.
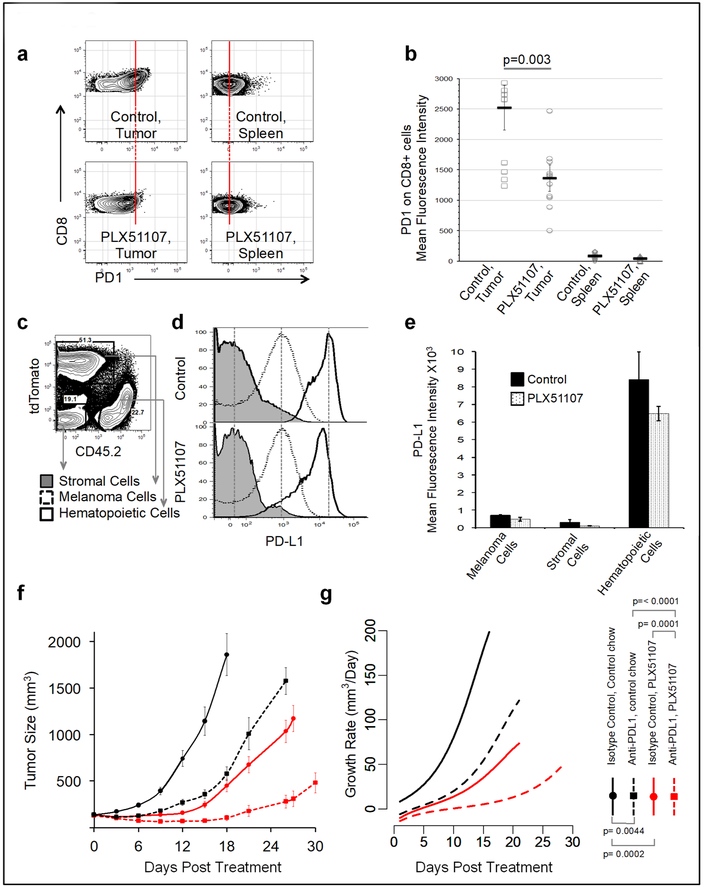
We examined PD1 expression, an indicator of T cell dysfunction, on tumor infiltrating CD8 T cells. While in both control and treated mice, CD8 cell PD1 expression was higher in tumors compared to spleens, CD8 cells in PLX51107-treated tumors exhibited lower surface PD1 compared to untreated controls (Figure 2a and b). PD1 ligand PD-L1 is expressed on a wide variety of cells. To distinguish PD-L1 expressing cellular subsets within tumors, we generated a tdTomato expressing YUMM1.7 line though a potential for introducing novel antigenicity needs to be considered in this model. From tumors of tdTomato-expressing YUMM1.7 cells, we resolved three distinct cellular populations based on differential expression of CD45.2 and tdTomato (Figure 2c). PD-L1 was not detected on host stromal cells, but was detected on tumor cells and CD45+ hematopoietic cells and remained unchanged during PLX51107 treatment (Figure 2d and e).
Figure 2: PD1 and PD-L1 expression in YUMM1.7 tumors and effect of combined BET inhibition and anti-PD-L1 on YUMM1.7 tumor growth.
T cells were isolated from YUMM1.7 tumors and spleens as described in Fig1c. (a) CD8+ cells were analyzed for PD1 surface expression. Lines are extended through the population centers in control samples. (b) Surface PD1 expression on CD8+ cells of treated versus control mice is depicted. (c) tdTomato-expressing YUMM1.7 tumors were analyzed on day 12 post-treatment. Distinct populations are resolved based on CD45.2 and tdTomato expression. (d) Representative histogram and (e) mean surface PD-L1 expression on populations resolved in c are shown. (f) When YUMM1.7 tumor sizes reached approximately 50 mm3, recipients were placed on either control (dashed) or PLX51107-laced chow (solid) while receiving either anti-PD-L1 antibody (red) or isotype control (black). Tumor volumes overtime and (g) the corresponding growth rate functions are shown.
BET inhibition shifted tumor infiltrating CD8 T cells from high to intermediate surface PD1 expression (Figure 2a and b). This finding is informative, as exhausted T cells expressing high levels of surface PD1 are generally resistant to revitalization via PD-L1 blockade, whereas T cells with intermediate PD1 levels are more easily rescued with this approach (Blackburn et al., 2008). In line with this, other epigenetic modifiers have been demonstrated to directly ameliorate T cell exhaustion (Zhang et al., 2014). These observations suggest that PLX51107 may render melanoma infiltrating exhausted T cells more responsive to PD-L1 blockade by causing decreases in PD-1 expression. Thus, we examined if anti-PD-L1 therapy would improve PLX51107 efficacy. Indeed, PLX51107 combined with anti-PD-L1 significantly constrained melanoma growth compared to either anti-PD-L1 or PLX51107 alone (Figure 2f-g and S4).
We found an increased ratio of effector to suppressive Tregs in BET inhibitor-treated tumors. While increased effector CD8+ T cells in tumors have been reported to be associated with improved clinical outcomes (Ali et al., 2014), presence of suppressive Tregs in tumors clearly contribute to immune suppressive environments (Viguier et al. 2004). Furthermore, the CD8 to Treg ratio has been implicated as a positive factor in predicting response to immunotherapy (Baras et al., 2016). Reduced Tregs and an increased CD8/Treg ratio in tumors suggest a shift towards a less immune suppressive tumor microenvironment during PLX51107 treatment.
Our data also indicate that the capacity of BET inhibitors to modulate tumor-specific T cell responses in melanoma may not reflect a universal direct effect on PD-L1 expression on tumor cells. In recent work in ovarian cancer and B cell lymphoma, BET inhibition caused the down-regulation of PD-L1 expression (Hogg et al., 2017, Zhu et al., 2016). We did not observe substantial amounts of surface PD-L1 on YUMM1.7 cells in vitro unless these cells were stimulated with IFNγ (Figure S5). Modulation of PD-L1 expression on solid tumor cells may depend greatly on local concentrations of factors such as IFNγ within the tumor microenvironment, as well as intrinsic tumor responsiveness to these factors.
In summary, optimizing therapies for metastatic melanoma is critical. This study determines that the next generation BET inhibitor, PLX51107, can be effectively combined with anti-PD-L1 to lower PD-1 expression on CD8+ T cells and delay melanoma tumor growth. PLX51107 is currently being tested in a Phase 1 study as single agent in solid tumors (NCT02683395) and provides an opportunity to show that the combination of PLX51107 and anti-PD-L1 is a promising treatment option for patients with metastatic melanoma.
Supplementary Material
Acknowledgments
We acknowledge Plexxikon Inc. (Berkeley, CA) for generously providing the mouse diets and Dr. Marcus Bosenberg (Yale University) for the YUMM1.7 cell line. We also thank Dr. David Allman (University of Pennsylvania), and Dr. Timothy Manser (Thomas Jefferson University) for their comments on this manuscript.
Financial Support: Neda Nikbakht is supported by Dermatology Foundation Research Fellowship Award, Skin Cancer Foundation Todd Nagel Memorial Award and an American Cancer Society IRG award. This work is supported by National Institutes of Health (NIH) R01 grants, CA196278 and CA160495 to A.E. Aplin. The Sidney Kimmel Cancer Center Flow Cytometry facilities are supported by NIH/National Cancer Institute Support Grant (P30 CA056036).
Footnotes
City, state and country in which the work was done: Philadelphia, Pennsylvania, United States
Conflict of Interest
Dr. Andrew E. Aplin discloses a previous grant from the Melanoma Research Alliance/Pfizer Inc. Partnership Award and has ownership interest in patent number 9880150.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adeegbe DO, Liu S, Hattersley MM, Bowden M, Zhou CW, Li S, et al. BET Bromodomain Inhibition Cooperates with PD-1 Blockade to Facilitate Antitumor Response in Kras-Mutant Non-Small Cell Lung Cancer. Cancer Immunol Res 2018;6(10):1234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol 2014;25(8):1536–43. [DOI] [PubMed] [Google Scholar]
- Baras AS, Drake C, Liu JJ, Gandhi N, Kates M, Hoque MO, et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. Oncoimmunology 2016;5(5):e1134412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A 2008;105(39):15016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretero-Gonzalez A, Lora D, Ghanem I, Zugazagoitia J, Castellano D, Sepulveda JM, et al. Analysis of response rate with ANTI PD1/PD-L1 monoclonal antibodies in advanced solid tumors: a meta-analysis of randomized clinical trials. Oncotarget 2018;9(9):8706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of BET bromodomains. Nature 2010;468(7327):1067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg SJ, Vervoort SJ, Deswal S, Ott CJ, Li J, Cluse LA, et al. BET-Bromodomain Inhibitors Engage the Host Immune System and Regulate Expression of the Immune Checkpoint Ligand PD-L1. Cell Rep 2017;18(9):2162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagoya Y, Nakatsugawa M, Yamashita Y, Ochi T, Guo T, Anczurowski M, et al. BET bromodomain inhibition enhances T cell persistence and function in adoptive immunotherapy models. J Clin Invest 2016;126(9):3479–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeth K, Wang JX, Micevic G, Damsky W, Bosenberg MW. The YUMM lines: a series of congenic mouse melanoma cell lines with defined genetic alterations. Pigment Cell Melanoma Res 2016;29(5):590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer HG, El-Gamal D, Powell B, Hing ZA, Blachly JS, Harrington B, et al. BRD4 Profiling Identifies Critical Chronic Lymphocytic Leukemia Oncogenic Circuits and Reveals Sensitivity to PLX51107, a Novel Structurally Distinct BET Inhibitor. Cancer Discov 2018;8(4):458–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura MF, Fontanals-Cirera B, Gaziel-Sovran A, Guijarro MV, Hanniford D, Zhang G, et al. BRD4 sustains melanoma proliferation and represents a new target for epigenetic therapy. Cancer Res 2013;73(20):6264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol 2004;173(2):1444–53. [DOI] [PubMed] [Google Scholar]
- Zhang F, Zhou X, DiSpirito JR, Wang C, Wang Y, Shen H. Epigenetic manipulation restores functions of defective CD8(+) T cells from chronic viral infection. Mol Ther 2014;22(9):1698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Bengsch F, Svoronos N, Rutkowski MR, Bitler BG, Allegrezza MJ, et al. BET Bromodomain Inhibition Promotes Anti-tumor Immunity by Suppressing PD-L1 Expression. Cell Rep 2016;16(11):2829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.