Abstract
Objective:
Inhaled pollutants can contact vocal fold tissue and induce detrimental voice changes. Acrolein is a pollutant in cigarette smoke and can also be inhaled during the combustion of fossil fuels, animal fats, and plastics in the environment. However, the vocal fold pathological changes induced by acrolein and the underlying inflammatory pathways are not well understood. These biologic data are needed to understand why voice problems may result from pollutant exposure.
Study Design:
In vivo prospective design with experimental and control groups
Methods:
Sprague-Dawley male rats (N=36) were exposed to acrolein (3 ppm) or filtered air (control) through a whole-body exposure system for 5 hour/day, for 5 day/week, over 4 weeks. Histopathological changes, presence of edema, expression of proinflammatory cytokines and markers, and the phosphorylation of NF-kB were investigated.
Results:
Histological evaluation and quatification demonstrated that subacute acrolein exposure induced significant vocal fold edema. Acrolein exposure also induced epithelial sloughing and cell death. Quantitative PCR showed a significant upregulation of genes encoding Irf-5, and Chi3l3. Western blot revealed a 76.8% increase in phosphorylation of NF-kB P65 after subacute acrolein exposure.
Conclusions:
These findings suggest that 4 week exposures to 3 ppm acrolein induce vocal fold inflammation manifested as edema, related to the activation of NF-κB signaling. The edema may underlie the voice changes reported in speakers exposed to pollutants.
Keywords: acrolein, pollutants, vocal folds, edema, inflammation, voice problems
INTRODUCTION
Acrolein exists abundantly in primary cigarette smoke and is considered a major respiratory toxicant.1,2 It is also found at high concentrations in the environment during combustion of fossil fuels, animal fats, plastics, and forest fires.3 Chronic exposure to pollutants is associated with respiratory diseases.4 Voice problems have also been reported anecdotally in speakers exposed to pollutants such as primary cigarette smoke. Cigarette smoke can result in vocal fold edema and hoarseness.5–8 It is important to understand the inflammatory mechanisms underlying vocal fold edema so that treatments can be better targeted for this condition.9
Acrolein is a common envrionmental pollutant that induces airway toxicity. Exposure to 4 ppm acrolein for 62 days induced epithelial necrosis with edema and hemorrhage in rat lung.10,11 Nasal pathological alterations including hyperplasia, squamous metaplasia of epithelium covering nasal cavity and infiltration of neutrophils were observed following exposure to 3 ppm acrolein for 3 weeks.12 These toxic effects have been reported across species.13 The vocal folds have a different structure from other tissues of the proximal and distal airway. It is therefore possible that pollutant exposure can induce distinct pathological changes in the vocal folds as compared to the rest of the airway tissue, and therefore the effects of polluntants such as acrolein on the vocal fold tissue should be quantified rather than extrapolated from airway literature.
There is some research on the effects of acrolein exposure to the larynx. In excised porcine vocal folds, acute acrolein exposure reduced epithelial sodium ion transport, induced lipid peroxidation, and impaired epithelial integrity and barrier function.14,15 But in vivo studies are needed to fully understand the mechanisms underlying pathological changes from specific pollutant exposures such as acrolein. The underlying mechanisms for acrolein-induced inflammation in the distal airway have been investigated. One mechanism is the activation of the NF-κB pathway.16–17 NF-κB is a transcription factor and is critical in regulating the expression of genes encoding proinflammatory cytokines.18 It is important to understand if this pathway is also implicated in vocal fold pathological changes.
The objective of this study was to investigate pathological changes to the rodent vocal folds following subacute exposure to 3 ppm acrolein. We also investigated the mechanistic pathways underlying these changes. We hypothesized that subacute acrolein exposure would induce vocal fold inflammatory response manifested as edema. We further hypothesized that the inflammation was caused by increased cytokine expression related with NF-κB signaling pathway.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats, 4-month of age at purchase, were obtained from Envigo RMS, Inc., (Indianapolis, IN). All rats were maintained in a temperature and humidity controlled room with a 12 hrs light/12 hrs dark cycle and allowed to acclimate for 6 days prior to exposures in the animal facility at Purdue University. The study was approved by the Institutional Animal Care and Use Committee at Purdue University.
Chemicals and reagents
Primary antibodies included rabbit anti-rat phospho-NF-κB p65 (Ser 536) antibody, mouse anti-rat NF-κB p65 antibody (3033, 6956; Cell Signaling, Danvers, MA); secondary antibodies included: anti-rabbit IgG-HRP (goat) and anti-mouse IgG-HRP (goat) (sc-2004, sc-2005, Santa Cruz Biotechnology, Dallas, TX). cDNA synthesis kit and iTaq Universal SYBR Green Supermix were from Bio-Rad (Hercules, CA). Primers for qPCR analysis were obtained from Integrated DNA Technologies (Coralville, Iowa). Acrolein gas cylinders were ordered from Praxair (Danbury, CT).
Acrolein administration and exposure
A whole-body 50 L exposure chamber (EZ-179, World Precision Instruments, Sarasota, FL) was utilized. 350 ppm acrolein in nitrogen (Praxair gas cylinder, CT) was mixed with filtered air to achieve a final concentration of 3 ppm acrolein. The flow rates of 350 ppm acrolein and filtered air were monitored and controlled using two mass flow meter & controllers separately (41695K, 41945K, McMaster-Carr, Chicago, IL). The two air flows were then mixed in a “T” tube to achieve a final concentration of 3 ppm of acrolein with a total flow of 8 L/min. The final flow then went into the chamber through the inlet on one side of the chamber. Expired air was passed through the outlet on the other side of the chamber and through a charcoal filter (World Precision Instruments, FL) into a chemical ventilation hood. The actual chamber concentration was monitored by a calibrated Volatile Organic Compounds (VOC) detector (Mocon Baseline, CO). The acrolein concentration of the whole exposure chamber was tested to be stable for 5 hours before the experiment. Rats were exposed to 3 ppm acrolein for 5 hours every day, 5 days per week for four weeks. For the control group, the rats were exposed to filtered air in the chamber. All rats were given filtered water and standard rat chow ad libitum during the exposure.
Euthanasia
Rats were euthanized at 4 hours after the last dose by I.V. administration of overdosed sodium pentobarbital (Beuthanasia®; Intervet Inc., Merck, Madison, NJ ) at 100 mg/kg. Exsanguination was administrated after cessation of heartbeat. Larynges were dissected immediately after death.
Histopathological evaluation and quantification of edema
Each larynx was fixed in 10% neutral buffered formalin, dehydrated and embedded in paraffin. Each block was sliced into 4μm coronal sections. One section from every 6 sections in the mid-membranous region was stained with hematoxylin and eosin (HE). A veterinary pathologist (author AD) who was blinded to animal assignment to the exposure conditions assessed histopathological changes (N = 4/group). Epithelial hyperplasia, epithelial sloughing, epithelial cell death, and presence of edema were graded as normal or altered.
For quantification of edema, virtual slides of rat larynges were created using a Leica Biosystems Aperio CS2 scanner (Aperio Technologies, Vista, CA). Images of the vocal fold mid-membranous region were analyzed using Aperio ImageScope software (v12.3.2.5030) algorithms, optimized for our laboratory. Because edema is accumulated fluid and staining fluid is challenging, we used the ratio of non-staining area to total area as a means to compare edema in the acrolein and control groups. A positive pixel count (PPC) algorithm detected stained areas of mid-membranous true vocal folds, normalized to the area analyzed. Because we used the same microscope objective and digital scanner for slides scanning in both groups, the pixel density was the same for both group. Thus, the smaller the index, the higher the ratio of non-stained (edematous) area out of total area it represents.
Quantitative Polymerase Chain Reaction (qPCR) detection for gene expression at mRNA level
The mRNA level of genes encoding TNF-α, IL-1α, IL-1β, IL-6, IL-10, IL-12B, IFN-γ, GM-CSF, interferon regulatory factor (Irf-5), Arg1, Retnla, chitinase-3-like protein 3 (Chi3l3) were quantified using qPCR. For this, each larynx was microdissected under an operating microscope (Stemi DV4, Zeiss, Germany). The upper margin of tissue was the top of the thyroid cartilage and the lower margin was the first tracheal ring. No cartilage was included in the sample. Total RNA was isolated and purified using TRIzol reagent (Life Teconologies). The RNA was reverse transcribed to cDNA using the Bio-Rad iScript cDNA Synthesis Kit according to manufacturer’s instructions. The qPCR analyses were performed in duplicate using CFX connect Real-Time PCR Detection System (Bio-Rad). iTaq Universal SYBR Green Supermix kit (Bio-Rad) was used for the qPCR detection. The qPCR was performed using a protocol described previously.15 Relative expressions of the target genes were assessed using ΔΔCt.. The Ct values in the target genes were normalized with β-actin in the same sample. The forward and reverse primers for genes being tested in qPCR were designed using Primer3.19 The sequence of each primer is listed in table 1.
Table 1.
Primers for Quantitative PCR Analysis
| Tnf |
CTCTGACCCCCATTACTCTGAC TACTTCAGCGTCTCGTGTGTTT |
| Il1a |
TCCTTAAATCCTCTGAGCTTGC ACAGATTGGTGATCATGACTGC |
| Il1b |
AGTGTGGATCCCAAACAATACC AACTGTGCAGACTCAAACTCCA |
| Il6 |
GTCAACTCCATCTGCCCTTCAG GGCAGTGGCTGTCAACAACAT |
| Il10 |
CAACTGCATAGAAGCCTACGTG GGTACAAACGAGGTTTTCCAAG |
| Il12b |
AGAACTCTCAGGTGGAGGTCAG TCCTTCGTCTTTTCTTTCTTGC |
| Ifng |
GCACAAAGCTGTCAATGAACTC CTCTCTACCCCAGAATCAGCAC |
| Csf2 |
GAGAACGAAAAGAACGAAGACG CCCGTAGACCCTGCTTGTATAG |
| Irf5 |
GGAGAAGAGGAGGAAGAGGAAG TTGGGTAAGGAATAGGGTGCTA |
| Arg1 |
AGTATGGCAATTGGAAGCATCT GGGAACTTTCCTTTCAGTTCCT |
| Retnla |
GCCAACTGTCCTAAGAATGGAG ATACATCAAATGCGCATGAGTC |
| Chi3l3 |
TATCAATCTCCATCCGACACTG TGTTAGAGGGGTCACTCAGGAT |
| Actb |
GGCACCACACTTTCTACAATGA CATGATCTGGGTCATCTTTTCA |
Western blot analysis of phosphorylation of NF-κB
Laryngeal tissue was dissected, snap-frozen, and stored in −80 °C. Total protein of larynges was extracted using homogenization buffer. The concentration of total protein of each sample was quantified using a Bovine Serum Albumin (BSA) assay kit. Isolated protein samples were mixed with 2xLeammli at the ratio of 1:1 and boiled for 5 min. Samples were concentrated using a CentriVap Concentrator (Labconco, MO) and loaded to the SDS-polyacrylamide gel. A polyvinylidene difluoride (PVDF) membrane was used to transfer the proteins and blocked by 5% milk for 1 hour. The membrane was then moved to primary anti-rat phospho-NF-κB p65 antibody (1:500) at 4°C overnight. The membrane was rinsed and incubated in the secondary antibody (Goat anti-rabbit IgG-HRP, 1:3000) for 1 hour.. The bands of protein were visualized and imaged in the Bio-Rad Molecular Imager. The membrane was washed using the ECL stripping buffer and rinsed using TBST after imaging. The membrane was then incubated in the primary anti-rat NF-κB p65 antibody (1:1000) followed by the secondary antibody (Goat anti-mouse IgG-HRP, 1:5000). The image of bands was taken in the Bio-Rad Molecular Imager as described above. The intensities of bands for phospho- NF-κB and total NF-κB were quantified using ImageJ software (NIH, Bethesda, Maryland). Relative phosphorylation of NF-κB was normalized to the total NF-κB.
Statistical analyses
All data are presented as mean ± SD. All statistical analyses were performed using IBM SPSS (version 20, Chicago, Illinois). Analysis for histological assessment was performed using the Fisher exact test. Comparisons of acrolein and control group for the index of stained tissue, gene expression, and phosphorylation of NF-κB were carried out with t-tests. Differences were considered significant when p values ≤ 0.05.
RESULTS
Concentration of acrolein in the exposure chamber
The stability of the whole-body exposure system was monitored. The average acrolein concentrations in the chamber at one hour after the beginning of exposure as measured by the VOC detector was 3.1 ± 0.9 ppm.
Histopathological evaluation
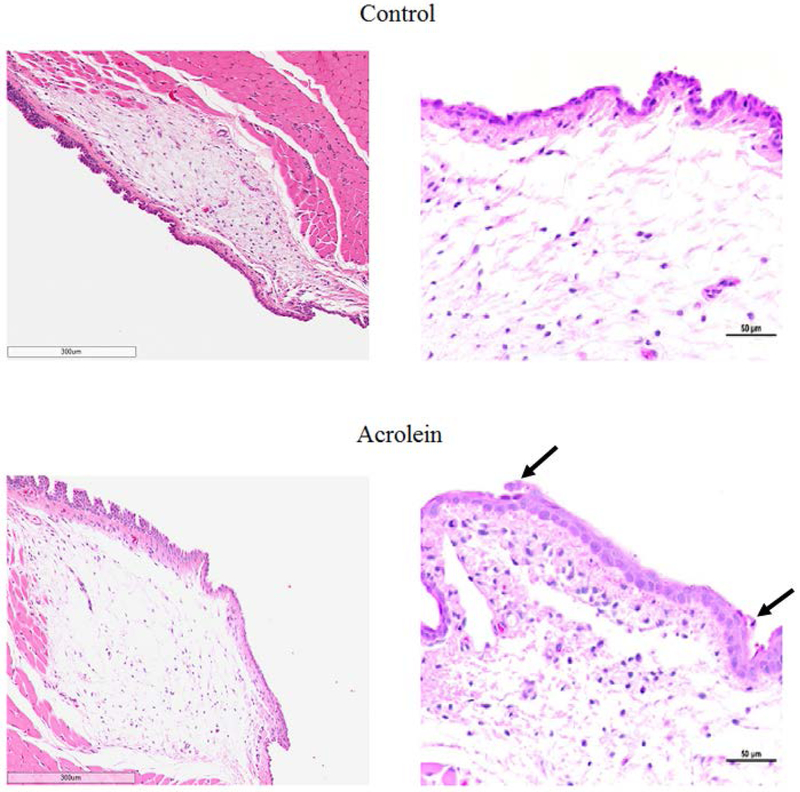
Three of four vocal folds from the acrolein exposure group showed epithelial sloughing and epithelial cell death. The desquamated epithelial cells were shrunken with pyknotic nuclei, which were suspected as potential apoptotic bodies. Conversely, none of the vocal folds from the control group showed any change in epithelial sloughing or epithelial cell death (Figure 1). These differences did not reach statistical significance (N=4, p >0.05)
Figure 1.
Representative HE stained image of vocal folds in acrolein and control groups. Left: Vocal folds in acrolein exposure group showed edema. Right: Vocal folds in acrolein exposure group showed epithelial sloughing and epithelial cell death. represents epithelial sloughing and epithelial cell death.
Presence of edema
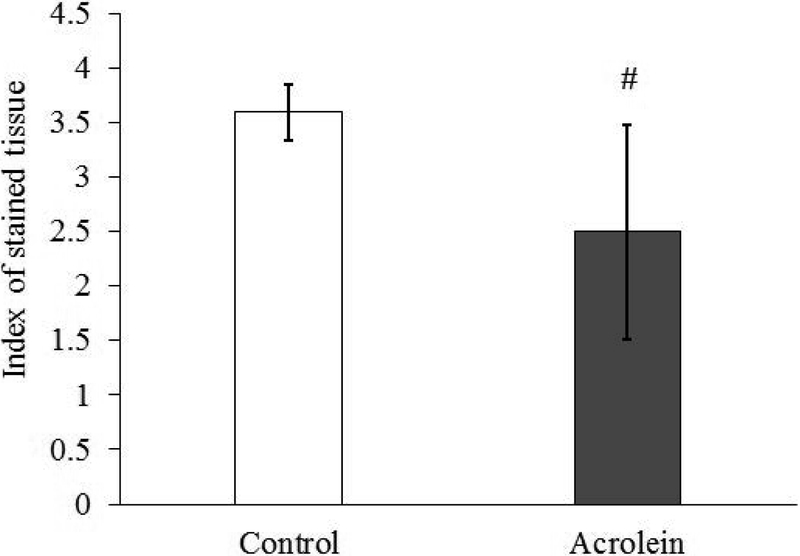
Blinded, qualitative evaluation of vocal folds revealed mild edema in the lamina propria of the acrolein group as compared to the control group (N = 4, p = 0.029, Figure 1). The index of stain was calculated as the ratio of positive staining pixel number to the area of the total region. The index of stained tissue was reduced in the acrolein group compared to control group suggesting edema after subacute acrolein exposure (N=9, p = 0.005, Figure 2).
Figure 2.
Index of staining showing the presence of edema after acrolein exposure. The lower the index, the higher the percentage of non-stained area out of total area it represents.
Gene expression of proteins participating in immune response
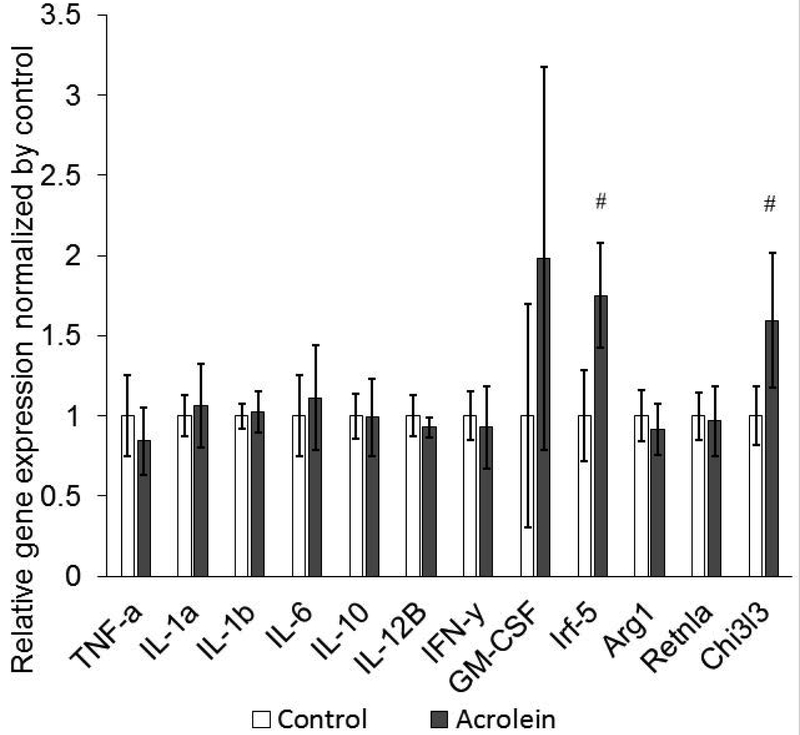
Expressions of genes encoding Irf-5 and Chi3l3 increased significantly in the acrolein group as compared to the control group (N = 6, p = 0.002, p = 0.009 respectively). The expression levels of all the other genes were not significantly different between two groups. (Figure 3)
Figure 3.
Expression of mRNAs encoding proteins in proinflammatory reaction using quantitative PCR. Expressions of Irf-5 and Chi3l3 increased following acrolein exposure.
Activation of NF-κB pathway
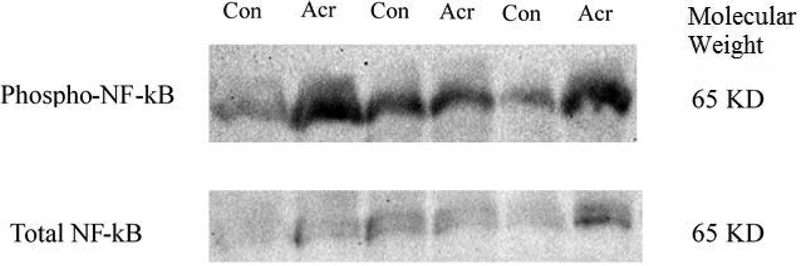
Phosphorylation of NF-κB increased significantly in the acrolein group. (N=3, p = 0.042, Figure 4).
Figure 4.
Increased phosphorylation of NF-κB p65 following acrolein exposure assessed by Western Blot.
DISCUSSION
The current study investigated the proinflammatory reaction of subacute acrolein exposure on vocal fold gene expression of proteins participating in the immune response and the regulating pathway. Subacute acrolein exposure induced vocal fold inflammation manifested as edema, an upregulation in gene expression associated with macrophage regulation, and activation of the NF-κB pathway. Other pathological changes observed after subacute acrolein exposure included epithelial cell death and epithelial sloughing.
One manifestation of inflammatory response in the current study was the presence of edema following subacute acrolein exposure. The edema observed was mild and diffuse in the subepithelial region of the vocal folds. Cigarette smoking causes vocal fold edema and the edema alters the biomechanical properties of the vocal folds leading to voice problems.6,20 Acrolein is a significant toxic component of cigarette smoke and other environmental pollutants.2,3,21 Our study demonstrates a possible mechanism for cigarette smoke and other pollutant-induced inflammatory edema. While edema can result from disruption in fluid homeostasis often due to disease affecting the heart or kidneys, the presence of these underlying conditions was not observed in the experimental animals. Thus it appears likely that subacute acrolein exposure induced vocal fold inflammatory edema. This finding is supported by the increased gene expression of interferon regulatory factor (Irf-5) and chitinase-3-like protein 3 (Chi3l3). Irf-5 mediates macrophage activation and promotes proinflammatory reactions.22,23 Chi3l3 is a type of lectin that is also expressed in macrophages.24,25
Results from Western blotting provide further support for a proinflammatory response by showing activation of the NF-κB pathway. This pathway is considered to play key role in regulating immune response during inflammation.18 One mechanism of NF-κB activation is via ubiquitination of IκB.18 Another important mechanism to activate NF-κB is to phosphorylate the dimer itself.26 A previous cigarette smoke study identified inflammation in the rat lung with activation of NF-κB pathway without degradation of IκB.27 Further, an acrolein exposure study from mice supported the phosphorylation of NF-κB p65 at the site of Ser536 in mice lung.17 Thus, we hypothesized phosphorylation of NF-κB as a mechanism for activation of the pathway.
The dose of acrolein we used in this study was 3 ppm, which is much lower than the highest level of acrolein in a polluted environment (up to 50–70 ppm in primary cigarette smoke, and 50–60 ppm during the combustion of wood and cutton.1,3 Pilot data (not shown) also suggested that 4 ppm acrolein induced significant distress in the animal. Hence 3 ppm acrolein was used in this study. It is therefore noteworthy that we observed edema in the vocal folds at this dose. Additionally, exposure only occurred over 4 weeks. A 4-week subacute exposure duration can induce inflammation and edema in the respiratory system and was therefore selected to represent a subacute timeline.17,28
CONCLUSION
Our study is the first to demonstrate the pathological effects of subacute acrolein exposure on vocal fold tissue. We identified edema and an inflammatory response characterized by upregulation of genes related with macrophages activation and activation of NF-κB pathway. The data lay the groundwork for future mechanistic studies targetting the NF-κB pathway to reduce inflammation in addition to exploring the toxic effects of chronic acrolein exposure on vocal fold tissue pathobiology and voice production.
Acknowledgements
This work was supported by R01DC011759 and R01DC015545 (National Institutes of Health/National Institute on Deafness and other Communication Disorders). We thank Dr. Jae Hong Park and David McMillan for assisting us with designing and constructing the whole-body exposure system. We acknowledge the contributions of Ruth Anderson and Nathan Garrison with the exposure studies.
Funding: Funding was provided from R01DC011759 and R01DC015545 (National Institutes of Health/National Institute on Deafness and other Communication Disorders).
Footnotes
Conflict of Interest: None
Level of Evidence: NA
References
- 1.Ayer HE, Yeager DW. Irritants in cigarette smoke plumes. Am J Public Health 1982;72:1283–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeager RP, Kushman M, Chemerynski S, Weil R, Fu X, White M, Callahan-Lyon P, Rosenfeldt H. Proposed Mode of Action for Acrolein Respiratory Toxicity Associated with Inhaled Tobacco Smoke. Toxicol Sci 2016;151:347–364. [DOI] [PubMed] [Google Scholar]
- 3.Faroon O, Roney N, Taylor J, Ashizawa A, Lumpkin MH, Plewak DJ. Acrolein environmental levels and potential for human exposure. Toxicol Ind Health 2008;24:543–564. [DOI] [PubMed] [Google Scholar]
- 4.Jiang XQ, Mei XD, Feng D. Air pollution and chronic airway diseases: what should people know and do? J Thorac Dis 2016;8:E31–E40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yonekawa H A clinical study of Reinke’s edema. Auris Nasus Larynx 1988;15:57–78. [DOI] [PubMed] [Google Scholar]
- 6.Marcotullio D, Magliulo G, Pezone T. Reinke’s edema and risk factors: Clinical and histopathologic aspects. Am J Otolaryngol 2002;23:81–84. [DOI] [PubMed] [Google Scholar]
- 7.Sorensen D, Horii Y. Cigarette smoking and voice fundamental frequency. J Commun Disord 1982;15:135–144. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez J, Carpi A. Early effects of smoking on the voice: a multidimensional study. Med Sci Monit 2004;10:Cr649–656. [PubMed] [Google Scholar]
- 9.Jetté ME, Dill-McFarland KA, Hanshew AS, Suen G, Thibeault SL. The human laryngeal microbiome: effects of cigarette smoke and reflux. Sci Rep 2016;6:35882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutzman RS, Popenoe EA, Schmaeler M, Drew RT. Changes in rat lung structure and composition as a result of subchronic exposure to acrolein. Toxicology 1985;34:139–151. [DOI] [PubMed] [Google Scholar]
- 11.Kutzman RS, Wehner RW, Haber SB. Selected responses of hypertension-sensitive and resistant rats to inhaled acrolein. Toxicology 1984;31:53–65. [DOI] [PubMed] [Google Scholar]
- 12.Leach CL, Hatoum NS, Ratajczak HV, Gerhart JM. The pathologic and immunologic effects of inhaled acrolein in rats. Toxicol Lett 1987;39:189–198. [DOI] [PubMed] [Google Scholar]
- 13.Feron VJ, Kruysse A, Til HP, Immel HR. Repeated exposure to acrolein vapor - subacute studies in hamsters, rats and rabbits. Toxicology 1978;9:47–57. [DOI] [PubMed] [Google Scholar]
- 14.Levendoski EE, Sivasankar MP. Vocal fold ion transport and mucin expression following acrolein exposure. J Membr Biol. 2014;247:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Zheng W, Sivasankar MP. Acute Acrolein Exposure Induces Impairment of Vocal Fold Epithelial Barrier Function. PLoS One. 2016;11:e0163237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarkar P, Hayes BE. Induction of COX-2 by acrolein in rat lung epithelial cells. Mol Cell Biochem 2007;301:191–9 [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Ito S, Nishio N, Tanaka Y, Chen N, Isobe K. Acrolein induced both pulmonary inflammation and the death of lung epithelial cells. Toxicol Lett 2014;229:384–392. [DOI] [PubMed] [Google Scholar]
- 18.Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Investig 2001;107: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 2000;132:365–386. [DOI] [PubMed] [Google Scholar]
- 20.Zeitels SM, Hillman RE, Bunting GW, Vaughn T. Reinke’s edema: phonatory mechanisms and management strategies. Ann Otol Rhinol Laryngol 1997;106:533–543. [DOI] [PubMed] [Google Scholar]
- 21.Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control 2003;12:424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss M, Blazek K, Byrne AJ, Perocheau DP, Udalova IA. IRF5 is a specific marker of inflammatory macrophages in vivo. Mediators Inflamm 2013;2013:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss M, Byrne AJ, Blazek K, Saliba DG, Pease JE, Perocheau D, Feldmann M, Udalova IA. IRF5 controls both acute and chronic inflammation. Proc Natl Acad Sci U.S.A. 2015;112:11001–11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair MG, Gallagher IJ, Taylor MD, Loke P, Coulson PS, Wilson RA, Maizels RM, Allen JE, Chitinase and Fizz Family Members Are a Generalized Feature of Nematode Infection with Selective Upregulation of Ym1 and Fizz1 by Antigen-Presenting Cells. Infect Immun 2005;73:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryszer T Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm 2015;2015:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci 2005;30:43–52. [DOI] [PubMed] [Google Scholar]
- 27.Marwick JA, Kirkham PA, Stevenson CS, Danahay H, Giddings J, Butler K, Donaldson K, Macnee W, Rahman I. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol 2004;31:633–642. [DOI] [PubMed] [Google Scholar]
- 28.Dorman DC, Struve MF, Wong BA, Marshall MW, Gross EA, Willson GA. Respiratory tract responses in male rats following subchronic acrolein inhalation. Inhal Toxicol 2008;20: 205–216. [DOI] [PubMed] [Google Scholar]