Abstract
Pig liver xenotransplantation appears to be more perplexing comparable to heart or kidney transplant, albeit great progress has already been achieved. The relevant molecular mechanisms involving in xenogeneic rejection, coagulopathy, and particularly thrombocytopenia, are extremely messy, and need to be systematically illustrated. Briefly, the deletion of expression of Gal antigens in liver graft highlights the injurious impacts of nonGal antigens that induce severe humoral rejection. The innate immunity, particularly mediated by macrophages and natural killer cells, complexly interplays with inflammation and coagulation disorders. Kupffer cells and liver sinusoidal endothelial cells (LSECs) together mediate leukocyte, erythrocyte, and platelet sequestration, which can be exacerbated by increased cytokine production, cell desialylation, and interspecies incompatibilities. Coagulation cascade is activated by TF release which can be dependent or independent of xenoreactive immune response. Depletion of endothelial anticoagulant and anti-platelet capacity amplify coagulation activation, and interspecies incompatibilities of coagulation-regulatory proteins facilitate dysregulation. LSECs involved in platelet phagocytosis and transcytosis, coupled with hepatocyte-mediated degradation, are responsible for thrombocytopenia. The adaptive immunity could be problematic in future long-term liver graft survival. Currently, relevant evidences and study results of various genetic modifications to the pig donor need to be fully discussed, aiming to identify the ideal transgene combination for pig liver xenotransplantation. In the end, we believe that clinical trials of pig liver xenotransplantation should be initially considered as a bridge to allotransplantation.
Keywords: Coagulation dysregulation, liver, pig, genetically-engineered, xenotransplantation
Introduction
Thousands of acute or chronic liver failure patients die on liver transplant waiting lists each year, owing to the fact that the demand for donor livers far outpaces supply, and despite the fact that bioartificial liver support systems have already been approved to serve as temporary life-saving devices 1, 2. Today, much effort has been directed to developing limitless replacement of organs, e.g., by producing human stem cell-derived chimeric organs 3, or gene-edited pig organs, which could be available whenever required without limitation 4.
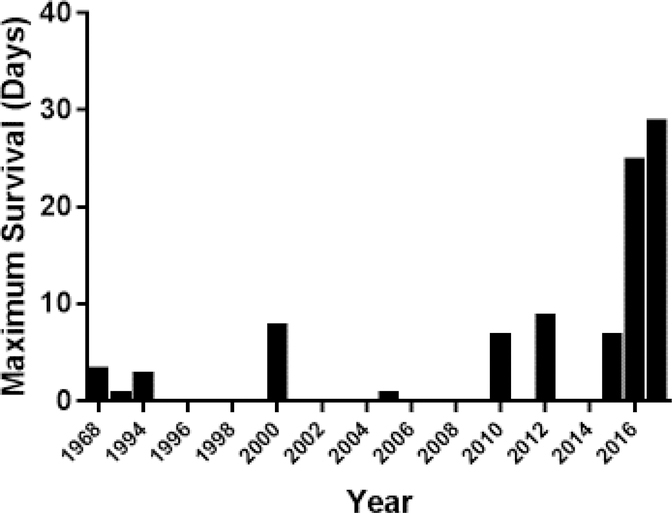
Recently, significant progress has been achieved in the results of pig organ xenotransplantation, evidenced by ground-breaking records of 945 days 5 and 435 days6, respectively, for heterotopic (non-life-supporting) pig heart and life-supporting kidney xenotransplantation. Nevertheless, pig liver xenograft survival is limited to less than a month 7 (Figure 1) due to major unresolved problems, particularly relating to coagulation dysregulation 8, 9. In particular, profound platelet loss results in lethal hemorrhage 10, 11.
Figure 1:
In pig liver xenotransplantation, maximum survival has been 29 days.
We here provide a systematic review of the present status of pig liver xenotransplantation, and summarize relevant molecular mechanisms related to xenogeneic immunity, coagulation dysregulation, and thrombocytopenia. Our purpose is to consider strategies to overcome the current barriers to further advance pig liver transplantation towards the clinic.
Experimental models
In vivo transplantation models using nonhuman primates (NHPs) as recipients are particularly informative, but extremely intensive in resources, and difficult to perform. Notably, orthotopic liver transplantation is a life-supporting procedure, although auxiliary or heterotopic liver transplantation can be performed. Orthotopic life-supporting liver transplantation provides definitive answers to the questions we ask, but non-life-sustaining models may provide equally valuable information 12–14, and may simulate clinical ‘bridging’ procedures.
Ex vivo perfusion models have proved to be cost-efficient and convenient substitutes for in vivo transplantation experiments, albeit long-term survival of the graft (and recipient) cannot be determined 15. Pig liver perfusion with human blood could effectively reduce usage of NHPs, and accurately simulate interspecies molecular incompatibilities, xenogeneic immunity, and metabolic function of the pig liver 16. Furthermore, perfusion with human blood may be particularly valuable to study specific situations where the antigenicity between humans and pigs is not accurately modeled in NHPs, e.g., human antibodies against N-glycolylneuraminic acid (Neu5Gc), which are not present in NHPs 16, 17.
In vitro models, e.g., represented by interspecies cell cultures, are also helpful. Porcine endothelium in combination with human blood components allows measurement of xenoreactive responses 18. There are also microfluidic channels pre-coated with pig cellular monolayers or ligands, and then perfused with human blood or blood components under pre-set shear stress conditions, that can be used to investigate platelet adhesion, activation, or xenogeneic injury 19.
Progress in pig-to-NHP liver transplantation
Experience of in vivo pig liver transplantation in NHPs is limited (Table 1). Immunosuppressive regimens were ineffective in sustaining liver graft survival until α1,3-galactosyltransferase gene knockout (GalT-KO) pigs, with or without multiple other genetic modifications (e.g., deletion of other porcine genes or insertion of human transgenes), became available. These resulted in attenuation of xenogeneic immunity and prolonged liver graft outcome. Nonetheless, severe coagulation dysregulation, represented as thrombotic microangiopathy (TMA) and systemic consumptive coagulopathy, negatively impacted graft survival.
Table 1:
Major in vivo experiments of pig liver transplantation in NHPs
| Year | Donor pig | Recipient | Immunosuppressive regimen | Max. survival. (days) | Type of Tx | First author | Ref. |
|---|---|---|---|---|---|---|---|
| 1968 | WT | Baboon | Aza, Cs | <1–3 | Orthotopic | Calne | 85 |
| 1970 | WT | Rhesus | ALG | <1 | Orthotopic | Calne | 87 |
| 1970 | WT | Chimpanzee | ALG | <1 | Orthotopic | Calne | 86 |
| 1994 | WT | Cynomolgus | Gal-depletion, WBI, ATG, pig BMTx, | <1–3 | Orthotopic | Powelson | 88 |
| 1998 | WT | Rhesus | CsA, CTX, Cs | <1 | Orthotopic | Luo | 89 |
| 1998 | WT | Baboon | sCR1 | <1 | Auxiliary orthotopic | Hayashi | 12 |
| 2000 | hCD55 | Baboon | CsA, CyP, Cs | 8 | Orthotopic | Ramirez | 66 |
| 2005 | hCD55.hCD59.HT | Baboon | CyP, Dacluzimab, Cs, Rituximab, CsA, MMF. | 1 | Orthotopic | Ramirez | 67 |
| 2010 | GTKO | Baboon | ATG, Tac, MMF, Cs | <1 | Orthotopic | Ekser | 84 |
| 2010 | GTKO.hCD46 | Baboon | CyP, Tac, MMF, Cs | 7 | Orthotopic | Ekser | 65 |
| 2012 | GTKO | Baboon | ATG, CVF, Tac, Aza, Cs, aCD154mAb, LoCD2b | 9 | Orthotopic | Kim | 20 |
| 2014 | GTKO | Baboon | ATG, CVF, Tac, Cs | 15 | Auxiliary heterotopic | Yeh | 13 |
| 2014 | GTKO | Tibetan macaque | ATG, CVF, Cs, Tac, MMF, aCD154mAb | 14 | Auxiliary heterotopic | Ji | 14 |
| 2015 | GTKO | Baboon | ATG, CVF, Tac, Cs | 7 | Orthotopic | Navarro-Alvarez | 30 |
| 2016 | GTKO | Baboon | ATG, CVF, Belatacept, Tac, Cs | 25 | Orthotopic | Shah | 21 |
| 2017 | GTKO | Baboon | ATG, CVF, Tac, Cs, aCD40mAb | 29 | Orthotopic | Shah | 11 |
Abbreviations: ALG/ATG=anti-thymocyte globulin; Aza=azathioprine; BMTx=bone marrow transplantation; CS=corticosteroids; CsA=cyclosporine; CTX=cytoxan; CyP=cyclophosphamide; CVF=cobra-venom factor; Gal=galactose-α1,3-galactose; GTKO=α-1,3-galactosyltransferase knock out; mAb=monoclonal antibody; MMF=mycophenolate mofetil; sCR1=soluble complement-receptor-1; Tac=tacrolimus; WBI=whole body irradiation.
Initial meaningful life-supporting GalT-KO pig liver transplantation into baboons was performed in 2012, with recipient survival extending up to 9 days 20. Whereas, recipients previously developed lethal hemorrhage, aminocaproic acid was administered to sustain platelet count >30,000/μl throughout the experiment. Histology showed TMA within the liver graft, but without features of acute humoral or cellular rejection 20. Red blood cell and platelet debris were found in the hepatic sinusoidal endothelial cells (LSECs) and hepatocytes 20.
Two years later, our group 14 and Yeh et al. 13 performed auxiliary heterotopic GalT-KO pig liver xenotransplantation. Both recipients obtained transfusion-free survival for up to approximately 2 weeks. Confusingly, recipients in the studies by Yeh’s group developed severe thrombocytopenia (compared to our group) that was even lower than that following conventional orthotopic liver transplantation (possibly associated with different NHP species, surgical procedures, and therapeutic strategies). Notably, relatively stable blood counts, without any clinical signs of bleeding, were recorded. Nevertheless, the recipients ultimately succumbed to TMA within the grafts. These results indicated that the native liver synthesized clotting components, which were beneficial to maintain hemostasis in the recipient.
Progress was made in 2016 21. A GalT-KO pig liver was orthotopically transplanted into a baboon that survived for 25 days, with anti-thymocyte globulin (ATG), cobra-venom factor (CVF), belatacept, tacrolimus, and corticosteroids as immunosuppressive therapy 21. In the following year, the same group achieved 29-days’ pig liver survival, using an anti-CD40mAb as a substitute for belatacept 11. Significantly, a continuous human prothrombin complex concentrate (hPCC) was infused in both cases. Histology revealed a preserved hepatic architecture, mild inflammation, and the absence of necrosis or TMA, but thrombosis occurred within the portal vein in the 29-days’ surviving liver graft.
Xenogeneic liver graft injury
In both ex vivo perfusion systems and in vivo transplant models, a complex interplay of inflammation, tissue injury, and coagulation disorders may account for the relatively rapid loss of the liver graft, similarly to the rapid loss of other pig organ xenografts. Currently, hyperacute rejection has been largely prevented by the deletion of expression of Gal antigens in the graft (in a GalT-KO pig), but residual innate immunity (mediated by preformed anti-nonGal antibodies, and monocytes, macrophages, NK cells, etc.), and adaptive immunity (mediated by T and B cells) remain problematic in liver transplantation and need to be overcome 22.
The innate immune response
Complement cascade activation has been proven to be triggered at least in part by preformed anti-nonGal antibodies 23, and the absence of expression of human complement-regulatory proteins 24. These results additionally hinted that humoral immunological barriers might contribute significantly to the rejection of GalT-KO pig livers within a few days or weeks. The nonGal antigens include carbohydrate, glycoprotein, and glycolipid structures. The known carbohydrate nonGal xenoantigens are N-glycolylneuraminic acid (Neu5Gc) 25, 26 and the blood group Sda/Cad (a product of the enzyme, β−1,4-N-acetyl-galactosaminyltransferase (β4GalNT2)) 27, 28.
Previous study has demonstrated that specific depletion of anti-α-galactosyl antibodies alone incompletely protected xenograft from hyperacute rejection 23. In our experience, one GalT-KO pig liver failed within 24 hours post-transplant, and a biopsy revealed histopathological appearances compatible with hyperacute rejection, strongly suggesting that nonGal antigen-mediated GalT-KO liver injury remains problematic (data not shown).
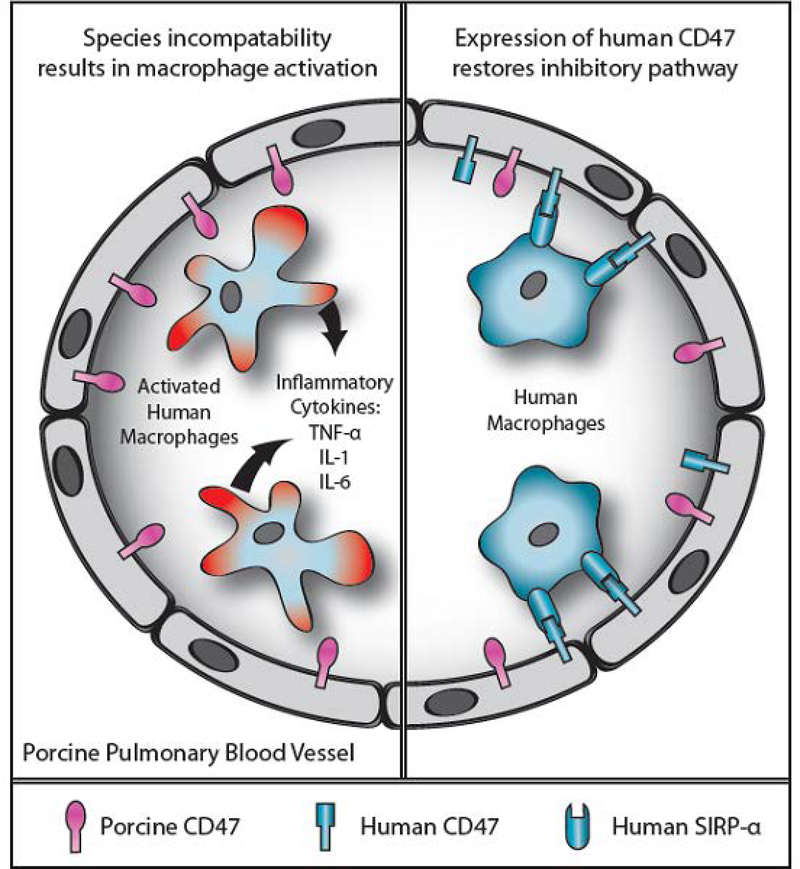
Leukocyte, erythrocyte, and platelet sequestration can occur either during ex vivo perfusion 29 or after in vivo transplantation 10, 30, associated with adhesion and capture mechanisms, and these could be exacerbated by increased cytokine production 10, cell desialylation 31, and interspecies incompatibilities 32. Kupffer cells in the pig liver and circulating macrophages release proinflammatory and procoagulant factors that augment graft injury 33. Pig Kupffer cells destroy human erythrocytes through a sialic acid-dependent mechanism 34, 35, and also recognize desialylated β-glucans (specifically N-acetyl D glucosamine [β-GlcNac]) through heterodimer CD11b/CD18 (macrophage antigen complex 1 [Mac1 or αmβ2 integrin]) to phagocytose platelets 36. Cross-species incompatibility between CD47 (in the donor liver) and signal regulatory protein-α (SIRP-α) (in the recipient) contributes to phagocytosis of recipient blood cells 37 (Figure 2). Species discordance of negative regulatory proteins, such as HLA-E on porcine endothelial cells, facilitates NK cell-mediated cytotoxicity in an antibody-dependent or independent manner 38.
Figure 2:
Schematic representation of CD47-SIRP-α interaction in relation to natural expression of SIRP-α on human macrophages. Left: After transplantation of an unmodified pig lung into a human, the expression of pig CD47 on the endothelial cells of the pulmonary blood vessels will not be recognized by human SIRP-α-expressing macrophages, which will therefore not be inhibited but will become activated; inflammatory cytokines will be produced and graft injury will occur. Right: When a lung from a pig transgenic for human CD47 is transplanted, the human SIRP-α-expressing macrophages will recognize the pig tissues as ‘self’, and activation will be inhibited; cytokine production and graft injury will not occur. (Reproduced with permission from Cooper DKC et al, Xenotransplantation 2012;19:144–158)
The adaptive immune response
Possibly related to the limited survival of pig liver xenografts to date, classical acute cellular rejection (predominantly mediated by lymphocytes) has not been reported, which might explain why the major focus has been directed at the inflammatory response and coagulation disorders. Nevertheless, suppression of the adaptive immune response by T cell signal 2 costimulation blockade (first introduced into xenotransplantation by Buhler and his colleagues 39) has proved very successful after pig heart 5, 40 and kidney 6, 41 xenotransplantation. In in vitro experiments, thrombin-activated pig endothelial cells (ECs) increased human T cell proliferation via upregulation of CD86 on the pig ECs 42. Recent in vivo pig liver xenotransplantation studies suggested that cellular rejection could be effectively inhibited by a combination of conventional immunosuppressive therapy and anti-CD40mAb 11, though the costimulation blockade contribution to this regimen is probably more important.
Coagulation dysregulation
Thrombotic microangiopathy in the graft and systemic consumptive coagulopathy appear to be more severe after pig liver xenotransplantation when compared with pig heart or kidney transplantation 43, and ultimately result in lethal hemorrhage. Both recipient- and pig donor-derived tissue factor (TF) initiate the coagulation cascade 44,45 which is intricately associated with inflammation and innate immunity 46. Depletion of endothelial anticoagulant and anti-platelet capacity amplify this activation 47, and the absence of expression of effective coagulation-regulatory proteins, e.g., human tissue factor pathway inhibitor (TFPI) or thrombomodulin, eventually result in hemorrhagic complications 14. In addition, thrombin has a wide-ranging proinflammatory effect by activating protease-activated receptors, and facilitates complement activation via cleaving C3 and C5. C5a then reciprocally induces TF upregulation on neutrophils, and further exacerbates the problem 48.
The coagulation cascade initiated by TF (extrinsic pathway) or negatively-charged surface contact (intrinsic pathway) is integral to the normal hemostatic response to vascular injury. This response appears to be over-activated after pig organ xenotransplantation, and exacerbates the complex network of inflammation, xenogeneic immunity, and coagulation. TF is expressed on the vascular sub-endothelial cells, and is the recognized trigger for coagulation dysfunction. Notably, inactivated ECs, monocytes, or platelets do not express TF until exposed to inflammatory molecules. Studies have revealed that a xenogeneic insult can activate ECs to expose sub-endothelial TF, which eventually initiates the coagulation cascade 44. Furthermore, peripheral blood mononuclear cells (PBMCs) couple with platelets expressing TF within 2h after pig liver xenotransplantation, and aggregate within the graft 44. Importantly, this may be independent of the xenoreactive immune response.
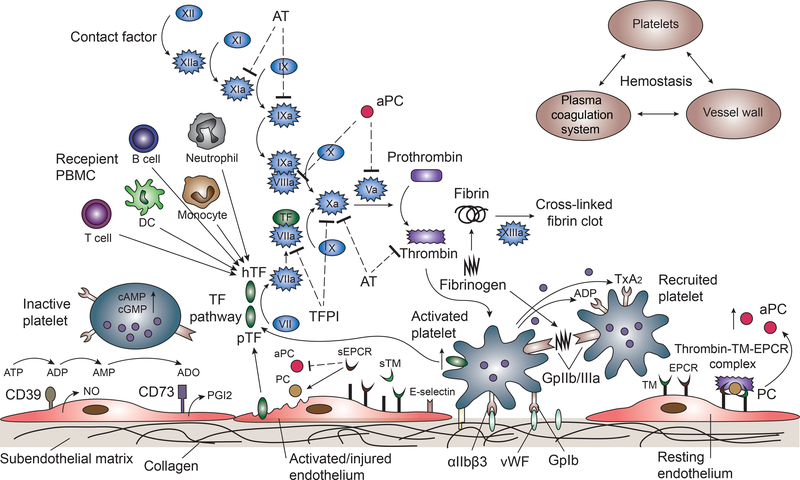
These data indicate that both donor- and recipient-derived TF contribute to activation of the extrinsic coagulation cascade 49 (Figure 3). In addition to the effect of TF, activated platelets release inorganic polyphosphate to cleave factor XII, and promote thrombin generation and fibrin formation 50. Inducible fibrinogen-like protein 2 (fgl2/fibroleukin) expressed on porcine endothelium directly activates human prothrombin to thrombin in the absence of other coagulation factors 51.
Figure 3: The coagulation cascade relating to pig liver xenotransplantation is illustrated.
The extrinsic pathway is initiated by tissue factor (TF), which forms complexes with factor VIIa, activating factor X, which in turn activates thrombin downstream. Thrombin converts factor XI to XIa, VIII to VIIIa, V to Va, fibrinogen to fibrin, and XIII to XIIIa, and promotes platelet activation and aggregation. Glycoprotein 1b (GpIb) is a component of the GPIb-V-IX complex on platelets, which binds von Willebrand factor (vWF), allowing platelet adhesion. ADP activates platelets to change conformation of GpIIb/IIIa receptors, which bind to fibrinogen to mediate platelet aggregation and endothelial adherence. Protein C is activated by the thrombin-thrombomodulin (TM)-endothelial protein C receptor (EPCR) complex. (TM serves as a cofactor in the thrombin-induced activation of protein C. EPCR is a receptor for protein C that enhances its activation). Activated protein C and its cofactor, protein S, deactivate factor Va and VIIIa. Tissue factor pathway inhibitor (TFPI) rapidly inhibits the TF/VIIa complex, and deactivates factor Xa. Anti-thrombin (AT) inhibits factors IXa, Xa, XIa, and thrombin via a complex formation with its active site. CD39 hydrolyzes ATP and ADP to AMP, and CD73 converts AMP to adenosine (ADO), to inactivate platelets.
(Other abbreviations: hTF=human TF; pTF=pig TF; sTM=soluble TM; sEPCR=soluble EPCR; PGI2= prostaglandin I2; TxA2=thromboxane A2; PBMC=peripheral blood monocular cells.)
Although TMA and consumptive coagulopathy can occur in the presence or absence of features of acute humoral xenograft rejection 44, 48, dysregulated coagulation is associated with a failure to prevent the anti-nonGal antibody response 52. Inefficient inhibition of activated clotting factors or other components contribute to the over-activated coagulation cascade, and interspecies molecular incompatibilities account for defective anticoagulant activity 9.
The special problem of thrombocytopenia
Once platelets come into contact with any thrombogenic surface, e.g., injured endothelium and/or sub-endothelium, or an artificial surface, such as a vascular graft, they become activated. In addition, physiological agonists, including collagen, thrombin, ADP, thromboxane A2 (TxA2), and platelet activation factor (PAF), can elicit a platelet response. Once platelets are activated, their granule contents are secreted, and procoagulant membrane phospholipids are exposed. This greatly accelerates intrinsic tenase complex (IXa/VIIIa) and prothrombinase (Xa/Va) reactions, resulting in the generation of thrombin 53.
In liver xenografts, the disruption of vascular walls, exposing thrombogenic sub-endothelium, results in exposure of collagen, vWF, and/or fibrin, activating platelets to adhere to the sub-endothelium through the interaction of GpIb-vWF and sub-endothelial collagen recognition (through numerous receptors, e.g., GPIa/IIa, GPIV (CD36), GPVI, and PECAM-1(CD31)). This in turn activates intracellular signaling pathways that leads to contraction and release of storage granules (e.g., ADP, TxA2). The receptors present on the platelet surface that can respond directly to the released agents (e.g., thrombin, ADP, and TxA2) contribute to platelet aggregation. Stimulation of ADP and TxA2 induces a conformational change in the fibrinogen receptor to increase the affinity of integrin GPIIb/IIIa on the platelets for fibrinogen, that can mediate further aggregation 53 (Figure 3).
After pig liver xenotransplantation, profound thrombocytopenia can occur within minutes to hours, which exacerbates the coagulation dysfunction, resulting in lethal hemorrhage 44. Ex vivo liver perfusion studies, with wild-type pig livers perfused with a purified human platelet perfusate, have to some extent elucidated the mechanism involved in the development of thrombocytopenia. In one report, 93% of the platelets contained in the perfusate were lost within 15min, and biopsies identified their phagocytosis by the LSECs. Asialoglycoprotein receptor 1 (ASGR1), expressed on the surface of LSECs, interacts with platelet-expressed ligands to affect the half-life of human platelets 54, 55. Particularly, human platelets contain increased amounts (4 times) of proposed ASGR1 ligands, such as galactose β1–4N-acetyl-D-glucosamine, comparable to fresh porcine platelets 56.
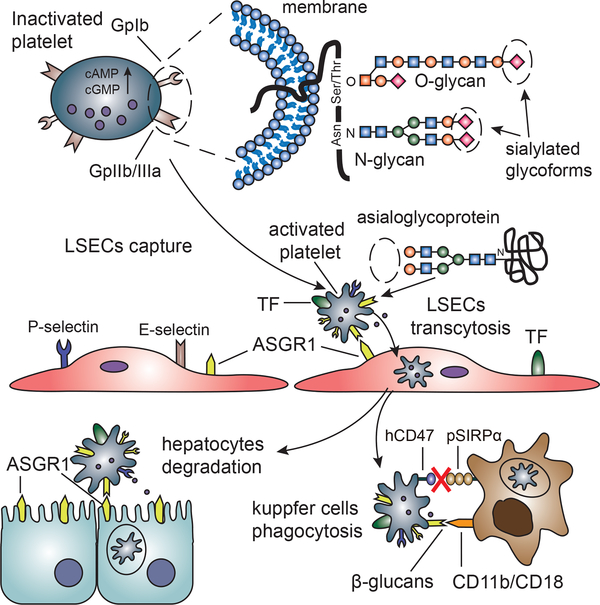
The human platelets are transferred from the LSECs to hepatocytes through a process known as transcytosis, evidenced by a later platelet degradation in the hepatocytes 57. Although the exact mechanism remains unclear, ASGR-1 and platelet glycosylation pattern-mediated phagocytosis in LSECs and hepatocytes can partly account for the dramatic loss of platelets 58 (Figure 4). In addition, evidence has been put forward to indicate that some platelets may be sequestered, and therefore effectively lost from the circulation 59.
Figure 4: Platelet transcytosis and phagocytosis in a pig liver graft are illustrated.
The asialoglycoprotein receptor 1 (ASGR1) expressed on liver sinusoidal endothelial cells (LSECs) and hepatocytes binds platelet asialoglycoprotein ligands from which sialic acid has been removed to expose galactose residues. Kupffer cells recognize exposed β-glucans, specifically β-GlcNac through heterodimer CD11b/CD18 (also named macrophage antigen complex 1, Mac1, and αmβ2 integrin) to phagocytose platelets. Incompatibility between pig signal regulatory protein-alpha (SIRPα) and human CD47 results in Kupffer cell-mediated phagocytic dysregulation.
In addition to LSECs and hepatocytes, pig Kupffer cells have also been implicated in platelet binding and phagocytosis (mediated by Mac1 (CD11b/CD18)), resulting in the removal of human platelets from the circulation 36. Blockade of the CD11b/CD18 receptor results in a reduction of human platelet binding and phagocytosis by porcine Kupffer cells 36. This anticoagulant function can be coordinated with galactose-type lectin-mediated clearance of vWF and factor VIII by macrophages from the circulation 60.
Although pig livers have been shown to phagocytose human platelets in the absence of immune-mediated graft injury (explained by ASGR1-platelet glycoprotein interplay), livers not expressing Gal or Neu5Gc (GalT-KO/CMAH-KO) demonstrate reduced consumption of human platelets - even less than that of ASGR1-KO pig livers 61. This suggests that an antibody-elicited xenogeneic response is exacerbating ASGR1-mediated human platelet phagocytosis.
In addition, liver sequestration (capturing of circulating platelets in the liver graft), resulting in thrombocytopenia, has been observed in other pig organs. For example, Bongoni and colleagues demonstrated that ASGR1-mediated platelet phagocytosis occurs through pig aortic and femoral arterial vascular endothelium 62.
Genetic modification of the organ-source pig
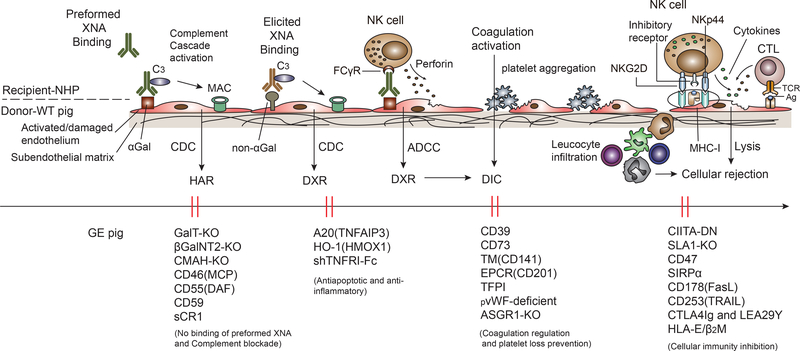
The interspecies incompatibility between pig and primate has been minimized through genome editing (e.g., CRISPR/Cas9), which is an important factor in recent progress in pig organ xenotransplantation. Numerous genetic modifications have been introduced63, with some pigs now expressing nine manipulations, all aimed at reducing the impact of the primate immune response and minimizing tissue injury (Figure 5).
Figure 5: The mechanisms involved in pig xenograft rejection are illustrated.
XNA= xenoreactive natural antibody; MAC=membrane attack complex; CDC=complement-dependent cytotoxicity; ADCC=antibody-dependent cell-mediated cytotoxicity; HAR=hyperacute rejection; DXR=delayed xenograft rejection; DIC=disseminated intravascular coagulation (consumptive coagulopathy).
Relevant genome-edited pigs were generated by:
1. Deletion of pig genes
Referred to as ‘knock out’ (-KO) in the case of (a) GalT=α-1,3-galactosyltransferase; (b) β4GalNT2=β−1,4 N-acetylgalactosaminyltransferase 2; (c) CMAH=cytidine monophospho-N-acetylneuraminic acid hydroxylase (Neu5Gc-KO); (d) pvWF=porcine von Willebrand factor; (e) ASGR1=asialoglycoprotein receptor 1.
2. Insertion of human transgenes
Referred to as ‘knock-in’ in the case of (a) MCP (CD46)=membrane cofactor protein; (b) DAF (CD55)=decay-accelerating factor; (c) CD59=membrane attack complex C5b-9 inhibitory protein (MAC-IP) or membrane inhibitor of reactive lysis (MIRL); (d) sCR1= soluble complement receptor 1; (e) A20=tumor necrosis factor alpha-induced protein-3; (f) HO-1=hemeoxygenase-1; (g) sTNFRI-Fc=soluble tumor necrosis factor alpha receptor inhibitor-Fc; (h) CD39=ectonucleoside triphosphate diphosphohydrolase-1 (NTPDase1); (i) CD73=5’-nucleotidase (5’-NT); (j) TM=thrombomodulin; (k) EPCR=endothelial protein C receptor; (l) TFPI=tissue factor pathway inhibitor; (m) CIITA-DN=class II trans-activator dominant negative; (n) CD47=integrin associated protein (IAP); (o) FasL=Fas ligand; (p) TRAIL=tumor necrosis factor-related apoptosis-inducing ligand; (q) CTLA4-Ig=cytotoxic T-lymphocyte-associated protein 4-immunoglobulin; (r) LEA29Y=variant of CTLA4-Ig; (s) HLA-E/β2m= MHC class I antigen E/β2 microglobulin.
(Modified from Le Bas-Bernardet S et al, Gene Ther 2008;15(18):1247–56.)
To reduce the injurious effect of natural preformed xenoreactive antibodies, which can initiate hyperacute or delayed antibody-mediated rejection (and also increase markedly in the presence of an adaptive immune response), the three known pig carbohydrate xenoantigens (Gal, Neu5Gc, and Sda) have been deleted successfully 64. With regard to pig liver xenotransplantation, absence of expression of Neu5Gc (in CMAH-KO pigs) is associated with attenuated loss of human (or NHP) erythrocytes, but not with attenuation of the profound early loss of primate platelets 29. Primate complement-mediated pig liver injury has been prevented or reduced by the introduction into the pig of transgenes for human complement-regulatory proteins, e.g., hCD46 (MCP) 65, hCD55 (DAF) 66, and/or hCD59 67. Alternatively, expression in the pig of soluble complement receptor-1 (sCR-1) has been proposed 68 and tested 12.
As the complex interaction of inflammation, tissue injury, and coagulation is a major problem in xenotransplantation, human A20 (TNFAIP3) 69, human hemeoxygenase-1 (HO-1) 70, and shTNFRI-Fc 71 transgenic pigs have been generated to inhibit inflammation and apoptosis. Attention has also been directed towards preventing the coagulation dysfunction by the transgenic expression of human coagulation-regulatory proteins, e.g., thrombomodulin (CD141), TFPI, EPCR (CD201), CD39, and CD73, which all may contribute to correcting the imbalance between procoagulant and anticoagulant activity. Although the importance of vWF as a procoagulant factor has been demonstrated through experiments involving vWF-deficient pigs, complete vWF-deficiency causes a bleeding phenotype that can be life-threatening to the pig, and so key segments of pig vWF have been replaced by analogous human vWF segments, coupled with ASGR1 knock-out 55, 61. Adhesion, aggregation, and phagocytosis of primate platelets exposed to livers from these pigs are greatly reduced.
Cellular rejection of a xenograft can be mediated by the innate (neutrophils, dendritic cells, natural killer (NK) cells, macrophages) and/or the adaptive (T and B cells) immune response. Genetically-engineered pigs with swine leukocyte antigen (SLA) class I knock-out (SLA-1 KO) 72, or expression of a mutant human class II transactivator gene (CIITA-DN) 73 have been produced to reduce pig antigen expression, and thus reduce the recipient’s immune response, but are not yet being used consistently in experiments in NHPs. Pigs with transgenic expression of human HLA-E/β2m 74, 75 and CD178 (FasL) have been produced to inhibit NK cell cytotoxicity, while the transgenic human CD47 gene contributes to regulate macrophage activation and phagocytosis 76. In addition, transgenic human CTLA4-Ig (CD152) 77 and CD253 (TRAIL) expression interrupt T cell activation and induce T cell apoptosis, respectively.
Pharmacologic interventions
Genome editing narrows interspecies differences, but may never eliminate the discrepancies completely. In contrast to allotransplantation, more effort will be required to reduce inflammation, coagulation dysregulation, and immune rejection. In addition to conventional immunosuppressive therapy (with agents such as tacrolimus, cyclosporine, mycophenolate mofetil (MMF), anti-thymocyte globulin, and corticosteroids), current attractive pharmacologic interventions include CTLA-4Ig (abatacept or belatacept) 21, anti-CD154 mAb 14, and anti-CD40 mAb 11. Of these, however, only CTLA-4Ig is currently approved for clinical use in the USA.
Several groups have reported excellent pig organ graft survival in NHPs receiving experimental agents that block the CD40-CD154 costimulation pathway 5, 6, 39, 78. Notably, the novel anti-CD154 domain antibody (BMS-986004) lacks the risk of platelet activation and resultant thromboembolism, but can effectively block CD40-CD154 interactions and may synergize with conventional immunosuppressive therapy 79. Anti-CD40mAb has shown ground-breaking efficacy after pig heart 5, kidney 80, and liver 11 xenotransplantation.
In addition, although complement fragment 1 esterase inhibition (C1-INH) did not prevent pig lung injury 81, it effectively inhibited coagulation and thrombus formation 82. Liposomal clodronate administration depleted pulmonary intravascular macrophages and successfully prevented hyperacute pulmonary xenograft dysfunction 83. However, when used in a pig-to-NHP liver xenotransplantation model, it proved problematic 84.
Wild-type pig liver xenotransplantation was carried out intermittently between 1968 85 and 1998 12, with various NHPs as recipients 86–89. The therapeutic strategies mainly focused on overcoming hyperacute rejection, but with disappointing and inadequate results. Even after the generation of GalT-KO pigs, coagulation disorders and delayed antibody-mediated rejection have remained major barriers, perhaps in part because NHPs have more hypercoagulable profiles than humans, represented by lower fibrinogen levels, but shorter clotting times and higher clot firmness 90. There is, however, some organ-specific variability, with evidence suggesting that recipients of pig heart transplants are less prone to developing platelet sequestration and consumptive coagulopathy than those with pig kidney grafts, owing to differential gene responses within the graft vasculature 43.
Traditional anticoagulant or antiplatelet agents, such as heparin or aspirin, have proved to be largely ineffective to prevent TMA and/or consumptive coagulopathy. The intravenous infusion of activated protein C provided encouraging results initially, but needed to be infused repeatedly and was associated with an increased risk of bleeding 91. Human soluble urinary thrombomodulin, functioning as a facilitator of activated protein C generation 92, improved hepatic microcirculation in the xeno-perfused porcine liver 93. The continuous intravenous infusion of a human prothrombin complex concentrate in recipients of pig livers inhibited clotting factor activity and prolonged recipient survival to 29 days 11.
Studies in pig lung xenotransplantation or ex vivo pig lung perfusion with human blood have provided information of relevance to pig liver xenotransplantation. For example, carbon monoxide therapy showed some beneficial effect in pig lung xenotransplantation by inhibiting abnormal platelet activation and impeding the development of intravascular thrombosis 94. The ectonucleotidase CD39 enzymatically degrades ATP and ADP to AMP, which can then be further degraded to adenosine by endothelially-expressed CD73 to exert antiplatelet activity 95. The fusion protein, GpVI-CD39, effectively delayed vascular thrombosis without a risk of bleeding 96. GPIb blockade proved insufficient to prevent platelet activation and sequestration in pig livers perfused with human blood 97, but a combination of GpIb and GpIIb/IIIa blockade successfully prevented platelet sequestration 98. Pre-infusion of 1-deamino-8-d-arginine vasopressin to the donor pig reduced vWF content in the lung graft to attenuate platelet activation and complement/coagulation activation 99. α-1-antitrypsin, a circulating anti-inflammatory glycoprotein, inhibited proteases released by inflammatory cells, especially neutrophils, to attenuate xenograft rejection 17.
Conclusions
The predominant lesions associated with pig liver failure are thrombotic microangiopathy and consumptive coagulopathy, with resulting ischemia and extensive hemorrhage. Although a continuous infusion of human prothrombin complex concentrate might be associated with prolonged graft and recipient survival, in our opinion genome editing remains the key to overcome the problems related to xenogeneic discordance.
As bioartificial liver devices are currently not as effective as ventricular assist devices or renal dialysis, patients with fulminant hepatic failure would benefit from short-term pig liver support as a ‘bridge’ to liver allotransplantation, which could be life-saving. In contrast to pig heart or kidney xenotransplantation, if transplanted as a ‘bridge’ to allotransplantation, survival of a transplanted pig liver may not need to be for more than a few days or weeks. Patients with fulminant hepatic failure spend an average of only 3 days on the waiting list prior to their demise 22, and so bridging by emergent pig liver xenotransplantation even for a few days might be life-saving. Chari et al. used an ex vivo pig liver (wild type) perfusion system to successfully ‘bridge’ a patient for 10 days until a subsequent liver allotransplantation100, and transgenic (human CD55/CD59) modifications further prolonged the duration of pig liver perfusion101.
Heterotopic auxiliary pig liver xenotransplantation could reduce the risks associated with the subsequent definitive liver allotransplantation. Furthermore, as the current evidence is that sensitization to pig xenoantigens is not associated with any increase in anti-HLA antibodies (i.e., does not sensitize the patient to HLA) 102, a pig xenograft would not be detrimental to obtaining or maintaining a subsequent allograft.
We conclude that, if the remaining pathobiological problems can be overcome, there are several good reasons why a clinical trial of pig liver xenotransplantation should be considered as a bridge to allotransplantation in patients with life-threatening hepatic failure. Experience gained from clinical trials of bridging will almost certainly lead to subsequent successful destination therapy using genetically-engineered pig liver xenografts.
Acknowledgements
This work was supported by grant from the National Basic Research Program of China (973 Program, 2015CB554100), National Key R&D Plan (2017YFC1103703), the Natural Science Foundation of China (81870446, 81270549, 81470873, 81671838, 81670593), Shaanxi Natural Science Foundation Research Project (2017JM8014). Work on xenotransplantation at the University of Alabama at Birmingham is supported in part by NIH NIAID U19 grant AI090959.
Abbreviations
- ASGR1
asialoglycoprotein receptor 1
- C1-INH
complement fragment 1 esterase inhibition
- ECs
endothelial cells
- EPCR
endothelial protein C receptor
- Gal
galactose-α1,3-galactose
- GalT-KO
α-1,3-galactosyltransferase knock out
- GpIb
glycoprotein Ib
- LSECs
liver sinusoidal endothelial cells
- Mac1
macrophage antigen complex 1
- Neu5Gc
N-glycolylneuraminic acid
- NHPs
nonhuman primates
- TF
tissue factor
- TFPI
tissue factor pathway inhibitor
- TMA
thrombotic microangiopathy
- TxA2
thromboxane A2
- vWF
von Willebrand factor
- β4GalNT2
beta-1,4-N-acety1-galactosaminyltransferase 2
- β-GlcNAc
N-Acetyl-D-glucosamine
Footnotes
Conflict of Interest Statement
The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Banares R, Nevens F, Larsen FS, et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57:1153–1162. [DOI] [PubMed] [Google Scholar]
- 2.Gerth HU, Pohlen M, Tholking G, et al. Molecular adsorbent recirculating system (MARS) in acute liver injury and graft dysfunction: Results from a case-control study. PLoS One. 2017;12:e0175529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J, Platero-Luengo A, Sakurai M, et al. Interspecies Chimerism with Mammalian Pluripotent Stem Cells. Cell. 2017;168:473–486 e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer K, Kind A, Schnieke A. Assembling multiple xenoprotective transgenes in pigs. Xenotransplantation. 2018:e12431. [DOI] [PubMed] [Google Scholar]
- 5.Mohiuddin MM, Singh AK, Corcoran PC, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun. 2016;7:11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams AB, Kim SC, Martens GR, et al. Xenoantigen Deletion and Chemical Immunosuppression Can Prolong Renal Xenograft Survival. Ann Surg. 2018;268:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meier RPH, Muller YD, Balaphas A, et al. Xenotransplantation: back to the future? Transpl Int. 2018;31:465–477. [DOI] [PubMed] [Google Scholar]
- 8.Cowan PJ, Robson SC. Progress towards overcoming coagulopathy and hemostatic dysfunction associated with xenotransplantation. Int J Surg. 2015;23:296–300. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Cooper DKC, Burdorf L, et al. Overcoming Coagulation Dysregulation in Pig Solid Organ Transplantation in Nonhuman Primates: Recent Progress. Transplantation. 2018;102:1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, Li X, Zhang H, et al. Cytokine profiles in Tibetan macaques following alpha-1,3-galactosyltransferase-knockout pig liver xenotransplantation. Xenotransplantation. 2017;24. [DOI] [PubMed] [Google Scholar]
- 11.Shah JA, Patel MS, Elias N, et al. Prolonged Survival Following Pig-to-Primate Liver Xenotransplantation Utilizing Exogenous Coagulation Factors and Costimulation Blockade. Am J Transplant. 2017;17:2178–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi S, Lin MG, Katayama A, et al. Auxiliary orthotopic xenogeneic liver transplantation using pigs for fulminant hepatic failure: with special reference to the technique and immunosuppression. Transplant Proc. 1998;30:3836. [DOI] [PubMed] [Google Scholar]
- 13.Yeh H, Machaidze Z, Wamala I, et al. Increased transfusion-free survival following auxiliary pig liver xenotransplantation. Xenotransplantation. 2014;21:454–464. [DOI] [PubMed] [Google Scholar]
- 14.Ji H, Li X, Yue S, et al. Pig BMSCs Transfected with Human TFPI Combat Species Incompatibility and Regulate the Human TF Pathway in Vitro and in a Rodent Model. Cell Physiol Biochem. 2015;36:233–249. [DOI] [PubMed] [Google Scholar]
- 15.Kumar R, Chung WY, Dennison AR, et al. Ex Vivo Porcine Organ Perfusion Models as a Suitable Platform for Translational Transplant Research. Artif Organs. 2017;41:E69–E79. [DOI] [PubMed] [Google Scholar]
- 16.Cimeno A, French BM, Powell JM, et al. Synthetic liver function is detectable in transgenic porcine livers perfused with human blood. Xenotransplantation. 2018;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laird C, Burdorf L, Pierson RN 3rd. Lung xenotransplantation: a review. Curr Opin Organ Transplant. 2016;21:272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao H, Chen P, Wei L, et al. Angiopoietin-1 and angiopoietin-2 protect porcine iliac endothelial cells from human antibody-mediated complement-dependent cytotoxicity through phosphatidylinositide 3-kinase/AKT pathway activation. Xenotransplantation. 2017;24. [DOI] [PubMed] [Google Scholar]
- 19.Harris DG, Benipal PK, Cheng X, et al. Four-dimensional characterization of thrombosis in a live-cell, shear-flow assay: development and application to xenotransplantation. PLoS One. 2015;10:e0123015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K, Schuetz C, Elias N, et al. Up to 9-day survival and control of thrombocytopenia following alpha1,3-galactosyl transferase knockout swine liver xenotransplantation in baboons. Xenotransplantation. 2012;19:256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah JA, Navarro-Alvarez N, DeFazio M, et al. A Bridge to Somewhere: 25-day Survival After Pig-to-Baboon Liver Xenotransplantation. Ann Surg. 2016;263:1069–1071. [DOI] [PubMed] [Google Scholar]
- 22.Patel MS, Louras N, Vagefi PA. Liver xenotransplantation. Curr Opin Organ Transplant. 2017;22:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macchiarini P, Oriol R, Azimzadeh A, et al. Evidence of human non-alpha-galactosyl antibodies involved in the hyperacute rejection of pig lungs and their removal by pig organ perfusion. J Thorac Cardiovasc Surg. 1998;116:831–843. [DOI] [PubMed] [Google Scholar]
- 24.Byrne GW, McCurry KR, Martin MJ, et al. Transgenic pigs expressing human CD59 and decay-accelerating factor produce an intrinsic barrier to complement-mediated damage. Transplantation. 1997;63:149–155. [DOI] [PubMed] [Google Scholar]
- 25.Bouhours D, Pourcel C, Bouhours JE. Simultaneous expression by porcine aorta endothelial cells of glycosphingolipids bearing the major epitope for human xenoreactive antibodies (Gal alpha 1–3Gal), blood group H determinant and N-glycolylneuraminic acid. Glycoconj J. 1996;13:947–953. [DOI] [PubMed] [Google Scholar]
- 26.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–381. [DOI] [PubMed] [Google Scholar]
- 27.Zhao C, Cooper DKC, Dai Y, et al. The Sda and Cad glycan antigens and their glycosyltransferase, beta1,4GalNAcT-II, in xenotransplantation. Xenotransplantation. 2018;25:e12386. [DOI] [PubMed] [Google Scholar]
- 28.Byrne G, Ahmad-Villiers S, Du Z, et al. B4GALNT2 and xenotransplantation: A newly appreciated xenogeneic antigen. Xenotransplantation. 2018;25:e12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cimeno A, Hassanein W, French BM, et al. N-glycolylneuraminic acid knockout reduces erythrocyte sequestration and thromboxane elaboration in an ex vivo pig-to-human xenoperfusion model. Xenotransplantation. 2017;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navarro-Alvarez N, Shah JA, Zhu A, et al. The Effects of Exogenous Administration of Human Coagulation Factors Following Pig-to-Baboon Liver Xenotransplantation. Am J Transplant. 2016;16:1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.French BM, Sendil S, Pierson RN, 3rd, et al. The role of sialic acids in the immune recognition of xenografts. Xenotransplantation. 2017;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25–50. [DOI] [PubMed] [Google Scholar]
- 33.Lu TF, Yang TH, Zhong CP, et al. Dual Effect of Hepatic Macrophages on Liver Ischemia and Reperfusion Injury during Liver Transplantation. Immune Netw. 2018;18:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brock LG, Delputte PL, Waldman JP, et al. Porcine sialoadhesin: a newly identified xenogeneic innate immune receptor. Am J Transplant. 2012;12:3272–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waldman JP, Brock LG, Rees MA. A human-specific mutation limits nonhuman primate efficacy in preclinical xenotransplantation studies. Transplantation. 2014;97:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chihara RK, Paris LL, Reyes LM, et al. Primary porcine Kupffer cell phagocytosis of human platelets involves the CD18 receptor. Transplantation. 2011;92:739–744. [DOI] [PubMed] [Google Scholar]
- 37.Ide K, Wang H, Tahara H, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007;104:5062–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lilienfeld BG, Crew MD, Forte P, et al. Transgenic expression of HLA-E single chain trimer protects porcine endothelial cells against human natural killer cell-mediated cytotoxicity. Xenotransplantation. 2007;14:126–134. [DOI] [PubMed] [Google Scholar]
- 39.Buhler L, Awwad M, Basker M, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69:2296–2304. [DOI] [PubMed] [Google Scholar]
- 40.Mohiuddin MM, Singh AK, Corcoran PC, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014;14:488–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higginbotham L, Mathews D, Breeden CA, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ezzelarab C, Ayares D, Cooper DK, et al. Human T-cell proliferation in response to thrombin-activated GTKO pig endothelial cells. Xenotransplantation. 2012;19:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knosalla C, Yazawa K, Behdad A, et al. Renal and cardiac endothelial heterogeneity impact acute vascular rejection in pig-to-baboon xenotransplantation. Am J Transplant. 2009;9:1006–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ekser B, Lin CC, Long C, et al. Potential factors influencing the development of thrombocytopenia and consumptive coagulopathy after genetically modified pig liver xenotransplantation. Transpl Int. 2012;25:882–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zelaya H, Rothmeier AS, Ruf W. Tissue factor at the crossroad of coagulation and cell signaling. J Thromb Haemost. 2018;16:1941–1952. [DOI] [PubMed] [Google Scholar]
- 46.Long AT, Kenne E, Jung R, et al. Contact system revisited: an interface between inflammation, coagulation, and innate immunity. J Thromb Haemost. 2016;14:427–437. [DOI] [PubMed] [Google Scholar]
- 47.Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cowan PJ, Robson SC, d’Apice AJ. Controlling coagulation dysregulation in xenotransplantation. Curr Opin Organ Transplant. 2011;16:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ekser B, Ezzelarab M, Hara H, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379:672–683. [DOI] [PubMed] [Google Scholar]
- 50.Muller F, Mutch NJ, Schenk WA, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghanekar A, Mendicino M, Liu H, et al. Endothelial induction of fgl2 contributes to thrombosis during acute vascular xenograft rejection. J Immunol. 2004;172:5693–5701. [DOI] [PubMed] [Google Scholar]
- 52.Lin CC, Ezzelarab M, Hara H, et al. Atorvastatin or transgenic expression of TFPI inhibits coagulation initiated by anti-nonGal IgG binding to porcine aortic endothelial cells. J Thromb Haemost. 2010;8:2001–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gremmel T, Frelinger AL 3rd, Michelson AD. Platelet Physiology. Semin Thromb Hemost. 2016;42:191–204. [DOI] [PubMed] [Google Scholar]
- 54.Paris LL, Chihara RK, Reyes LM, et al. ASGR1 expressed by porcine enriched liver sinusoidal endothelial cells mediates human platelet phagocytosis in vitro. Xenotransplantation. 2011;18:245–251. [DOI] [PubMed] [Google Scholar]
- 55.Paris LL, Estrada JL, Li P, et al. Reduced human platelet uptake by pig livers deficient in the asialoglycoprotein receptor 1 protein. Xenotransplantation. 2015;22:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paris LL, Chihara RK, Sidner RA, et al. Differences in human and porcine platelet oligosaccharides may influence phagocytosis by liver sinusoidal cells in vitro. Xenotransplantation. 2012;19:31–39. [DOI] [PubMed] [Google Scholar]
- 57.Burlak C, Paris LL, Chihara RK, et al. The fate of human platelets perfused through the pig liver: implications for xenotransplantation. Xenotransplantation. 2010;17:350–361. [DOI] [PubMed] [Google Scholar]
- 58.Tanowitz M, Hettrick L, Revenko A, et al. Asialoglycoprotein receptor 1 mediates productive uptake of N-acetylgalactosamine-conjugated and unconjugated phosphorothioate antisense oligonucleotides into liver hepatocytes. Nucleic Acids Res. 2017;45:12388–12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ekser B, Burlak C, Waldman JP, et al. Immunobiology of liver xenotransplantation. Expert Rev Clin Immunol. 2012;8:621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ward SE, O’Sullivan JM, Drakeford C, et al. A novel role for the macrophage galactose-type lectin receptor in mediating von Willebrand factor clearance. Blood. 2018;131:911–916. [DOI] [PubMed] [Google Scholar]
- 61.Butler JR, Paris LL, Blankenship RL, et al. Silencing Porcine CMAH and GGTA1 Genes Significantly Reduces Xenogeneic Consumption of Human Platelets by Porcine Livers. Transplantation. 2016;100:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bongoni AK, Kiermeir D, Denoyelle J, et al. Porcine extrahepatic vascular endothelial asialoglycoprotein receptor 1 mediates xenogeneic platelet phagocytosis in vitro and in human-to-pig ex vivo xenoperfusion. Transplantation. 2015;99:693–701. [DOI] [PubMed] [Google Scholar]
- 63.Cooper DK, Ekser B, Ramsoondar J, et al. The role of genetically engineered pigs in xenotransplantation research. J Pathol. 2016;238:288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Estrada JL, Martens G, Li P, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation. 2015;22:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ekser B, Echeverri GJ, Hassett AC, et al. Hepatic function after genetically engineered pig liver transplantation in baboons. Transplantation. 2010;90:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramirez P, Chavez R, Majado M, et al. Life-supporting human complement regulator decay accelerating factor transgenic pig liver xenograft maintains the metabolic function and coagulation in the nonhuman primate for up to 8 days. Transplantation. 2000;70:989–998. [DOI] [PubMed] [Google Scholar]
- 67.Ramirez P, Montoya MJ, Rios A, et al. Prevention of hyperacute rejection in a model of orthotopic liver xenotransplantation from pig to baboon using polytransgenic pig livers (CD55, CD59, and H-transferase). Transplant Proc. 2005;37:4103–4106. [DOI] [PubMed] [Google Scholar]
- 68.Ruggieri EV, Bugelski PJ, Kaplan JM, et al. Relationships between antibodies against human soluble complement receptor 1 (hsCR1) from various species. J Clin Immunol. 1996;16:97–106. [DOI] [PubMed] [Google Scholar]
- 69.Ahrens HE, Petersen B, Ramackers W, et al. Kidneys From alpha1,3-Galactosyltransferase Knockout/Human Heme Oxygenase-1/Human A20 Transgenic Pigs Are Protected From Rejection During Ex Vivo Perfusion With Human Blood. Transplant Direct. 2015;1:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petersen B, Ramackers W, Lucas-Hahn A, et al. Transgenic expression of human heme oxygenase-1 in pigs confers resistance against xenograft rejection during ex vivo perfusion of porcine kidneys. Xenotransplantation. 2011;18:355–368. [DOI] [PubMed] [Google Scholar]
- 71.Kim GA, Lee EM, Jin JX, et al. Generation of CMAHKO/GTKO/shTNFRI-Fc/HO-1 quadruple gene modified pigs. Transgenic Res. 2017;26:435–445. [DOI] [PubMed] [Google Scholar]
- 72.Reyes LM, Estrada JL, Wang ZY, et al. Creating class I MHC-null pigs using guide RNA and the Cas9 endonuclease. J Immunol. 2014;193:5751–5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iwase H, Ekser B, Satyananda V, et al. Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs. Transpl Immunol. 2015;32:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laird CT, Burdorf L, French BM, et al. Transgenic expression of human leukocyte antigen-E attenuates GalKO.hCD46 porcine lung xenograft injury. Xenotransplantation. 2017;24. [DOI] [PubMed] [Google Scholar]
- 75.Abicht JM, Sfriso R, Reichart B, et al. Multiple genetically modified GTKO/hCD46/HLA-E/hbeta2-mg porcine hearts are protected from complement activation and natural killer cell infiltration during ex vivo perfusion with human blood. Xenotransplantation. 2018;25:e12390. [DOI] [PubMed] [Google Scholar]
- 76.Tena AA, Sachs DH, Mallard C, et al. Prolonged Survival of Pig Skin on Baboons After Administration of Pig Cells Expressing Human CD47. Transplantation. 2017;101:316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, Yang HQ, Jiang W, et al. Transgenic expression of human cytoxic T-lymphocyte associated antigen4-immunoglobulin (hCTLA4Ig) by porcine skin for xenogeneic skin grafting. Transgenic Res. 2015;24:199–211. [DOI] [PubMed] [Google Scholar]
- 78.Iwase H, Hara H, Ezzelarab M, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation. 2017;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim SC, Wakwe W, Higginbotham LB, et al. Fc-Silent Anti-CD154 Domain Antibody Effectively Prevents Nonhuman Primate Renal Allograft Rejection. Am J Transplant. 2017;17:1182–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iwase H, Kobayashi T. Current status of pig kidney xenotransplantation. Int J Surg. 2015;23:229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schroder C, Pfeiffer S, Wu G, et al. Effect of complement fragment 1 esterase inhibition on survival of human decay-accelerating factor pig lungs perfused with human blood. J Heart Lung Transplant. 2003;22:1365–1375. [DOI] [PubMed] [Google Scholar]
- 82.Schurmann D, Herzog E, Raquet E, et al. C1-esterase inhibitor treatment: preclinical safety aspects on the potential prothrombotic risk. Thromb Haemost. 2014;112:960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cantu E, Gaca JG, Palestrant D, et al. Depletion of pulmonary intravascular macrophages prevents hyperacute pulmonary xenograft dysfunction. Transplantation. 2006;81:1157–1164. [DOI] [PubMed] [Google Scholar]
- 84.Ekser B, Long C, Echeverri GJ, et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10:273–285. [DOI] [PubMed] [Google Scholar]
- 85.Calne RY, White HJ, Herbertson BM, et al. Pig-to-baboon liver xenografts. Lancet. 1968;1:1176–1178. [DOI] [PubMed] [Google Scholar]
- 86.Calne RY. Organ transplantation between widely disparate species. Transplant Proc. 1970;2:550–556. [PubMed] [Google Scholar]
- 87.Calne RY, Davis DR, Pena JR, et al. Hepatic allografts and xenografts in primates. Lancet. 1970;1:103–106. [DOI] [PubMed] [Google Scholar]
- 88.Powelson J, Cosimi AB, Austen W Jr., et al. Porcine-to-primate orthotopic liver transplantation. Transplant Proc. 1994;26:1353–1354. [PubMed] [Google Scholar]
- 89.Luo Y, Kosanke S, Mieles L, et al. Comparative histopathology of hepatic allografts and xenografts in the nonhuman primate. Xenotransplantation. 1998;5:197–206. [DOI] [PubMed] [Google Scholar]
- 90.Spiezia L, Bertini D, Boldrin M, et al. Reference values for thromboelastometry (ROTEM(R)) in cynomolgus monkeys (Macaca fascicularis). Thromb Res. 2010;126:e294–297. [DOI] [PubMed] [Google Scholar]
- 91.Feistritzer C, Schuepbach RA, Mosnier LO, et al. Protective signaling by activated protein C is mechanistically linked to protein C activation on endothelial cells. J Biol Chem. 2006;281:20077–20084. [DOI] [PubMed] [Google Scholar]
- 92.Miwa Y, Yazaki S, Iwamoto M, et al. Functional difference between membrane-bound and soluble human thrombomodulin. Transplantation. 2015;99:702–709. [DOI] [PubMed] [Google Scholar]
- 93.Shiraishi M, Oshiro T, Taira K, et al. Improved hepatic microcirculation by human soluble urinary thrombomodulin in the xeno-perfused porcine liver. Transplantation. 2001;71:1046–1050. [DOI] [PubMed] [Google Scholar]
- 94.Sahara H, Sekijima M, Ariyoshi Y, et al. Effects of carbon monoxide on early dysfunction and microangiopathy following GalT-KO porcine pulmonary xenotransplantation in cynomolgus monkeys. Xenotransplantation. 2018;25. [DOI] [PubMed] [Google Scholar]
- 95.Hohmann JD, Peter K. Activated-platelet targeting of CD39 as a potential way forward. The quest for efficient antithrombotic therapy without associated bleeding complications. Hamostaseologie. 2016;36:17–25. [DOI] [PubMed] [Google Scholar]
- 96.Degen H, Borst O, Ziegler M, et al. ADPase CD39 Fused to Glycoprotein VI-Fc Boosts Local Antithrombotic Effects at Vascular Lesions. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.LaMattina JC, Burdorf L, Zhang T, et al. Pig-to-baboon liver xenoperfusion utilizing GalTKO.hCD46 pigs and glycoprotein Ib blockade. Xenotransplantation. 2014;21:274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burdorf L, Riner A, Rybak E, et al. Platelet sequestration and activation during GalTKO.hCD46 pig lung perfusion by human blood is primarily mediated by GPIb, GPIIb/IIIa, and von Willebrand Factor. Xenotransplantation. 2016;23:222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim YT, Lee HJ, Lee SW, et al. Pre-treatment of porcine pulmonary xenograft with desmopressin: a novel strategy to attenuate platelet activation and systemic intravascular coagulation in an ex-vivo model of swine-to-human pulmonary xenotransplantation. Xenotransplantation. 2008;15:27–35. [DOI] [PubMed] [Google Scholar]
- 100.Chari RS, Collins BH, Magee JC, et al. Brief report: treatment of hepatic failure with ex vivo pig-liver perfusion followed by liver transplantation. N Engl J Med. 1994;331:234–237. [DOI] [PubMed] [Google Scholar]
- 101.Levy MF, Crippin J, Sutton S, et al. Liver allotransplantation after extracorporeal hepatic support with transgenic (hCD55/hCD59) porcine livers: clinical results and lack of pig-to-human transmission of the porcine endogenous retrovirus. Transplantation. 2000;69:272–280. [DOI] [PubMed] [Google Scholar]
- 102.Li Q, Hara H, Zhang Z, et al. Is sensitization to pig antigens detrimental to subsequent allotransplantation? Xenotransplantation. 2018;25:e12393. [DOI] [PMC free article] [PubMed] [Google Scholar]