Abstract
Objective:
Few large animal models exist for the study of aortic aneurysms. β-aminopropionitrile (BAPN) is a compound known to cause aortic aneurysms by inhibiting lysyl oxidase, a collagen cross-linking enzyme. It is hypothesized that BAPN plus aneurysm induction surgery would result in significant aneurysm formation in swine with biologic properties similar to human disease.
Methods:
Initial experiments were performed in uncastrated male swine not treated with BAPN (surgery alone). Subsequently, uncastrated male swine were fed BAPN (0.15 grams/kilogram) for 7 days before undergoing surgery: the infrarenal aorta was circumferentially dissected and measured, balloon dilated, perfused with elastase (500 units) and Type 1 collagenase (8000 units), with extraluminal elastase application. In the BAPN groups, daily BAPN feedings continued until swine harvest at postoperative days 7, 14, and 28.
Results:
Swine undergoing surgery alone (n=12) had significantly less dilation at 28 days compared with BAPN + surgery swine (51.9±29.2% (0–100%) vs. 113.5±30.2% (52.9–146.2%); p<0.0003). Mean aortic dilation in animals undergoing treatment with surgery and BAPN was 86.9%±47.4% (range, 55.6–157.1%), 105.4%±58.1% (50– 133.3%), and 113.5%±30.2% (52.9–146.2%) at 7, 14, and 28 days, respectively. In the BAPN + surgery group, significant elastolysis was present at all time points, while aortic wall collagen content was not significantly different. Smooth muscle cells were significantly depleted at 14 and 28 days, and M1 macrophages were increased at 14 and 28 days (p<0.05, all). Matrix metalloproteinase 2 was elevated at 7 days (p<0.05, all). Multiple pro-inflammatory cytokines were elevated within the aortic wall of BAPN + surgery swine.
Conclusions:
BAPN plus Surgery resulted in significantly larger aortic aneurysms than surgery alone and was critical to aneurysm formation in this novel swine model. Hallmarks of human disease, such as elastin fragmentation, smooth muscle cell depletion, macrophage infiltration, matrix metalloproteinase activation, and pro-inflammatory cytokine expression were observed in BAPN-treated swine. This model better parallels many of the characteristics of human AAAs, and may be suitable for pre-human drug trials.
Keywords: Cardiovascular Diseases, Aortic Aneurysm, Cytokines, Immunohistochemistry, Macrophages, Inflammation
Here is the edited TOC summary:
A combination of balloon dilatation, elastase/collagenase perfusion, extra luminal elastase application, and oral supplementation with β-aminopropionitrile (BAPN) resulted in a reproducible pig model of abdominal aortic aneurysm that will likely allow studying endovascular device deployment and aneurysm biology.
Introduction
Abdominal aortic aneurysms (AAAs) are responsible for over 150,000 deaths worldwide.1 In the United States, AAAs remain a leading cause of death and as the population ages the disease is projected to become more prevalent.2 Much of the research accomplished to date has been performed in rodents. However, the clinical applicability and relevance of murine models is limited and therefore translation has lagged.
β-aminopropionitrile (BAPN) is a naturally occurring compound found in the plant genus Lathyrus that has been shown to cause aortic aneurysms in humans.3 BAPN inhibits the enzyme lysyl oxidase, which is responsible for collagen cross-linking in the extracellular matrix of the aortic wall.4 Disruption of this cross-linking is seen in human AAAs.5 Previous work from our laboratory using BAPN in both mice and rats has documented that the AAAs are larger, contain thrombus, rupture, and form aneurysms remote from the isolated, treated segments.6,7
Large animals, such as swine, have a circulatory system that better correlates with human biology.8 However, few large animal models exist to study the biological mechanisms involved in AAA formation.9 A large animal model for AAA is critical for transition to humans, especially with the potential application of novel technologies. We sought to build upon our success in the BAPN rodent models, and hypothesized that a biologically relevant and reproducible swine AAA model could be developed using a combination of mechanical forces (balloon dilatation), enzymatic degradation, and inhibition of lysyl oxidase (BAPN).
Materials and Methods
Animals
Experiments were conducted with the approval of the University of Virginia Institutional Animal Care and Use Committee (Protocol #3848). Uncastrated domestic male swine weighing 20–30 kg were used for the experiments. The swine were fed usual chow and unlimited water. A group of 12 swine did not receive BAPN and underwent surgery alone. In a separate group of animals (n=21), weights were obtained weekly and a weight-based dosing of BAPN (0.15 grams/kilogram) was fed to the pigs once daily mixed in whole milk plain yogurt for one week prior to surgery and daily postoperatively. The swine were fasted the night prior to surgery and imaging studies.
Creation of New Swine AAA Model
The surgical procedure is a modified protocol described by Hynecek et al.9 Induction of general anesthesia was performed using Telazol (6mg/kg), Xylazine (2mg/kg), and atropine sulfate (0.04 mg/kg) via intramuscular injections. After intubation, general anesthesia was maintained using inhaled isoflurane (0.2 mg/kg). Vital signs are monitored intraoperatively using electrocardiography, pulse oximetry, and oral temperature. A blood sample was taken prior to incision.
The surgical area is prepped with betadine and 70% isopropyl alcohol and then draped in the usual sterile fashion. A midline abdominal incision is made and dissection carried out using the Bovie Electrocautery (Bovie Medical Corporation, Purchase, NY). The abdominal viscera is displaced cranially and laterally in order to expose the retroperitoneum. The retroperitoneum is incised and the aorta circumferentially dissected from the renal arteries to the aortic trifurcation. The external diameter of the aorta is measured using a caliper (Roboz Surgical Instrument Co., Gaithersburg, MD). At this time, 5,000 units of unfractionated heparin sulfate is administered intravenously.
The caudal mesenteric artery is identified and clamped. Once no threat to bowel viability is evident, the artery is cannulated using a 0.018” stainless steel wire guide from a Micropuncture® Introducer Set (Cook Incorporated, Bloomington, IN). A 5.0 French introducer from the same kit is then used to dilate the artery over the wire, followed by a 7.0 French introducer. The 0.018” wire guide is removed and a 0.03 guide-wire is inserted through the introducer into the aortic lumen. The introducer is removed and an Atlas® PTA dilation catheter sizes 14 or 16 millimeter (BARD Peripheral Vascular, Tempe, AZ) is exchanged over the wire into the infrarenal aorta. The balloon is gradually inflated to its profile to dilate the aorta for 10 minutes at a constant 2 atmospheric pressure. When fully inflated, the balloon is 2 centimeters in length. The balloon is then deflated and withdrawn, leaving the guide-wire within the aortic lumen. Circulation is resumed.
After 10 minutes, the aorta is cross-clamped distal to the renal arteries and proximal to the terminal aortic trifurcation. Lumbar branches are identified and clamped to isolate the infrarenal aorta from systemic circulation. The caudal mesenteric artery is again cannulated using the introducer sheath over the wire. The wire is now withdrawn and a mixture of 8,000 units of Type I collagenase (Sigma-Aldrich, St. Louis, MO) and 500 units of elastase (Sigma-Aldrich, St. Louis, MO) in a 30 mL solution is infused via the sheath under pressure until the solution begins weeping from the adventitial surface. This is maintained for 10 minutes, and then the solution is irrigated from the aortic lumen. All clamps are removed, the aorta is again perfused, and the caudal mesenteric artery stump is ligated. Clamp time was restricted to no more than 10 minutes in order to prevent spinal cord ischemia.
Next, a piece of surgical sponge is cut to the size of the infrarenal aorta and placed on top of the aorta and soaked with 20 mL of undiluted elastase (27u/mL) for 10 minutes. The aorta is again measured with a caliper. The retroperitoneum is irrigated, all instruments withdrawn, and the abdomen closed in three layers.
Postoperative Care
A transdermal fentanyl patch (75 mcg) and intramuscular buprenorphine (0.01–0.02 mg/kg) were administered at the end of the operation. Cephalexin (1 gram) was administered for 3 days postoperatively. The fentanyl patch was removed on postoperative day 3 and the pigs then socially housed.
Harvest Procedure
At time of harvest, the pigs were sedated with Telazol® , intubated, and maintained under general anesthesia with isoflurane. The intestines were displaced from the abdomen and the retroperitoneum was incised and dissected until the aorta was encountered. The external aortic diameter was measured using a caliper. Non-BAPN-treated swine (surgery alone) infrarenal aortic diameters were measured at day 28. The infrarenal aortas of BAPN-treated swine were measured at 7, 14, and 28 days. Concurrent suprarenal aortas were used as controls in these experiments. A lethal dose of Euthasol was administered via the vena cava. The aorta was then dissected from the trifurcation up to the suprarenal aorta and explanted. Samples of the distal suprarenal aorta and proximal-to-mid infrarenal aorta of 20 millimeters in size were flash-frozen with liquid nitrogen or fixed formalin for histology.
Specimen Care
The flash-frozen tissue was thawed over ice and a transmural section approximately 3 millimeters in size was cut from the sample and placed in a FastPrep® Matrix Lysing Tube D (MP Biomedical, Santa Ana, CA) along with 1 milliliter of cell lysis buffer (BioVision Inc., Milpitas, CA). The tubes were then placed in the FastPrep® −24 (MP Biomedical, Santa Ana, CA) and lysed for 4 30-second cycles with 5 minutes of rest between each cycle. The isolated protein liquid was then extracted from the lysing tubes, placed in Eppendorf tubes, and frozen to −80°C. This homogenate was then used for cytokine array, gelatin zymography, and enzyme-linked immunosorbent assay (ELISA). For histology, a 3–4 mm (height) cross-section piece of suprarenal and aortic tissue was fixed in 4% paraformaldehyde for 24 hours, rinsed with water, and transferred to 70% ethanol. Further processing included imbedding in paraffin and sectioning at 5 µm.
Aortic Comparisons
Size comparison was performed at harvest time point day 28 between non-BAPN treated swine (surgery alone) infrarenal aorta and BAPN-treated swine (BAPN + surgery) infrarenal aorta. In the BAPN + surgery animals, the suprarenal sample from an individual animal was used as a self-control for the infrarenal treated aorta from the same animal for biomolecular analyses. Similarly, histologic and cytokine analyses were performed on both infrarenal and suprarenal aorta samples and compared.
Histology
Cross-section samples were prepared using Verhoeff-Van Gieson (VVG) Trichrome to stain for collagen and elastin. Two independent blinded reviewers scored elastin breaks using the following scale: 1=concentric heavy rings of elastin, 2=mild elastolysis, 3=moderate elastolysis, and 4=extensive elastolysis. These scores were averaged and compared. Immunohistochemical staining was also performed for anti-mouse α smooth muscle actin (α-SMA) (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), macrophages (anti-rat Mac-2; 1:10,000, Cedarlane Laboratories, Burlington, ON, Canada), M1 macrophages (IL-1β ligand; 1:200, Abcam), M2 macrophages (Arginine type 1; 1:200; Abcam), and CD3 for T cells (1:500; Santa Cruz Biotechnology). Visualization color development was completed using alkaline phosphatase (Dako, Glostrup, Denmark) for α-SMA, and diaminobenzidine for Mac-2, anti-neutrophil, and CD3.
Images of the sectioned aortas were acquired and analyzed as previously published.10 In short, images are obtained using AxioCam 4.6 software with 4x objective and an AxioCam MRc camera (Carl Zeiss GmBh). For quantification, the integrated optical density of the positive staining area in the cross sectional image of the aortic tissue sample was selected and measured using Image-Pro Plus 7.0 (Media Cybernetics, Bethesda, MD, USA).
Cytokine Array
Cytokine array was performed on aortic tissue to determine the concentrations of granulocyte macrophage colony stimulating factor (GM-CSF), interferon gamma (IFN- γ), tumor necrosis factor alpha (TNF-α), and interleukin (IL)-1α, −1β, −1RA, −2, −4, −6, −8, −10, −12, and - 18. Using the isolated protein homogenate, cytokine arrays (Millipore Sigma, Bellerica, MA) were completed according to the manufacturer instructions on pigs harvested at the respective time intervals. Samples from the groups were run in duplicate, pooled for analysis, and the mean value was used.
Gelatin Zymography
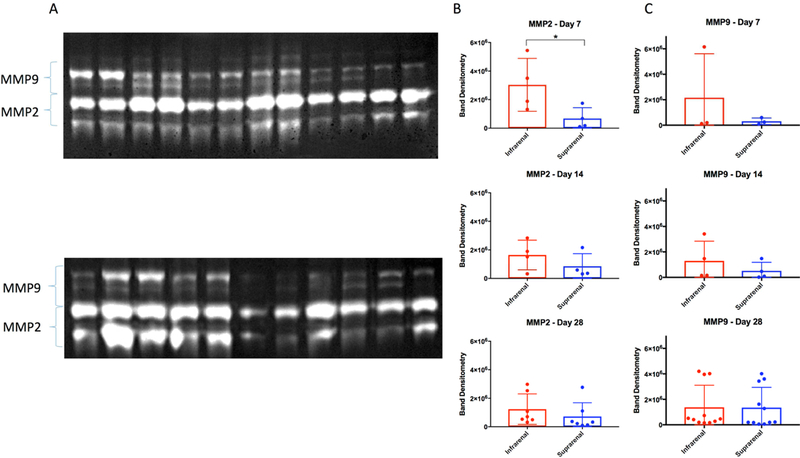
Gelatin zymography was performed using the isolated protein homogenate from the aortic samples to measure active matrix metalloproteinase (MMP) 2 and 9 activity. Precast zymography gels (10% Invitrogen, Carlsbad, CA) were loaded with 3 µg of isolate from each aortic sample. The samples were diluted into Tris-glycine SDS sample buffer and electrophoretically separated under non-reducing condition. The gels were then renatured for 30 minutes in renaturing buffer and incubated in developing buffer for 24 hours at 37°C while rocking. The gels were then stained in Simply Blue Safe Stain (Invitrogen, Carlsbad, CA) and the forms of MMP 2 and 9 appeared as clear bands against the blue gel. Image Lab Software version 4.0 (Bio-Rad, Hercules, CA) was used to quantify the optical density of the bands.
Statistical Analysis
All statistical analyses were performed with GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA) using Fisher’s exact test or chi-square test as appropriate. Aortic dilation (%) was calculated as follows: [(harvest infrarenal aortic diameter – initial operative infrarenal diameter) X 100)]. Data values are reported as mean ± standard deviation. Statistical significance was set to an alpha of less than 0.05. Confidence intervals were set at 95%. All assays were performed in duplicate. Blinding was performed as feasible.
Results
Twelve swine underwent AAA induction surgery without BAPN treatment (surgery alone), and 21 swine underwent AAA induction surgery with BAPN. One died from intraoperative aortic rupture during balloon dilatation and was not included in the study group. Another died in the early postoperative period due to a torsed bladder and also was not included. Two expired from aortic rupture at 7 days and are included. Data were collected from 19 swine that ultimately comprised the BAPN + surgery study group. Eight swine were harvested at 28 days, 5 at 14 days, and 6 at 7 days.
BAPN treatment allows for a more robust and reproducible swine AAA
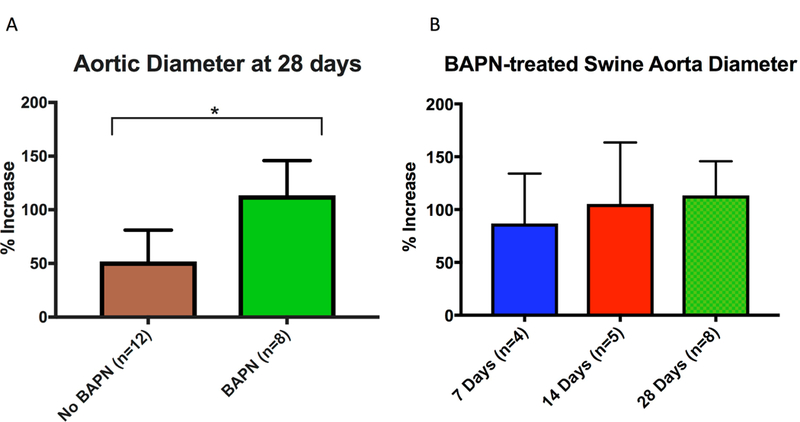
Size analysis was conducted on BAPN-treated swine at 28 days (n=8), 14 days (n=5), and 7 days (n=4), as well as non-BAPN-treated animals at 28 days (n=12). Two animals ruptured at day 7, so live measurements were not made. At 28 days, swine undergoing surgery alone, but not fed BAPN, had an infrarenal aneurysm size of only 51.9±29.2% (0–100%), and this differed significantly from BAPN-treated swine (51.9±29.2% (0–100%) vs. 113.5±30.2% (52.9–146.2%); p<0.0003) (Fig. 1A). Swine undergoing AAA surgery and fed a BAPN diet had an average increase of the infrarenal aorta of 86.9±47.4% (range, 55.6–157.1%), 105.4±58.1% (50–133.3%), and 113.5±30.2% (52.9–146.2%) at 7, 14, and 28 days, respectively (Fig. 1B).
Figure 1. BAPN treatment increases swine AAA size.
(A) BAPN + surgery swine had significantly higher mean aortic dilation compared with non BAPN-treated (surgery alone) swine at 28 days (113.5± 30.2% (52.9–146.2%) vs. 59.7±29.2% (0–86.7%); p<0.01). (B) BAPN-treated swine showed mean aortic dilation of 86.9±47.4% (55.6–157.1%), 105.4±58.1% (50–133.3%), and 113.5±30.2% ( 52.9–146.2%) at 7, 14, and 28-day harvest timepoints, respectively;
Swine with surgically induced AAA have histological changes similar to human AAA
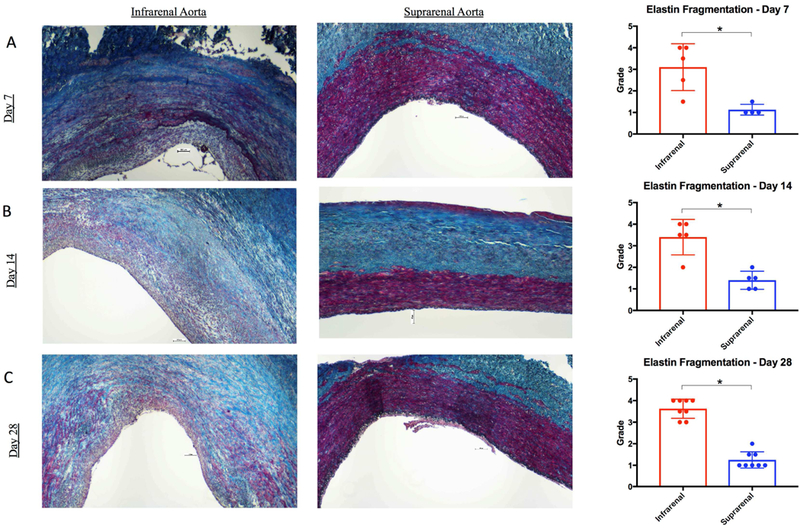
In BAPN treated swine, the infrarenal aorta had significantly more elastin fragmentation compared with the suprarenal aorta at all time points (Figure 2 A, B, C). Histologic staining with VVG demonstrated derangements in the collagen architecture of BAPN-treated swine. However, collagen content was not significantly different compared with unmanipulated suprarenal self-controls at all time points (Day 7: 0.2±0.1 vs. 0.3±0.2, p=0.09; Day 14: 0.2±0.2 vs. 0.3±0.1, p=0.40; Day 28: 0.2±0.1 vs. 0.2±0.1, p=0.29; Fig.3 A, B, C).
Figure 2. Elastin fragmentation is increased in BAPN-treated swine AAA.
Van Gieson staining in infrarenal aorta and suprarenal aorta at 7 days (A), 14 days (B), and 28 days (C). Elastin (black) fragmentation as measured by independent reviewers of infrarenal aorta vs. suprarenal aorta at 7, 14, and 28 days (Far Right A, B, and C, respectively). Scale bar, 500μ. 4x lens objective. * indicates p<0.05.
Figure 3. Collagen is altered in BAPN-treated swine AAA.
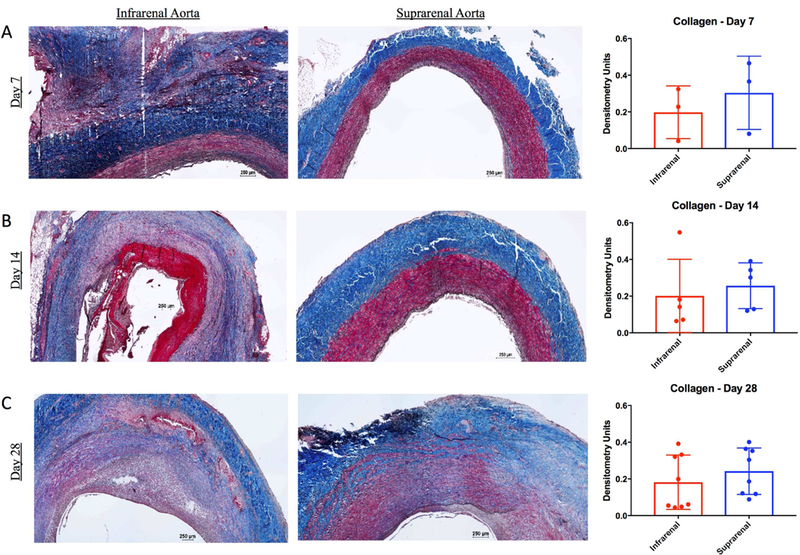
Masson’s trichrome and Van Gieson staining in infrarenal aorta and suprarenal aorta at 7 days (A), 14 days (B), and 28 days (C). Collagen (blue) content within the wall of infrarenal versus suprarenal aorta as measured by densitometry units at 7, 14, and 28 days (Far Right A, B, and C, respectively). Scale bar, 250μ. 4x lens objective.
T lymphocytes did not differ significantly in infrarenal BAPN treated aneurysm segments versus suprarenal controls at 7 days or 28 days, but did differ significantly at 14 days, with more in the infrarenal aortas (p=0.03). Smooth muscle cells were not significantly depleted from the swine BAPN-treated AAA wall at day 7 (Fig. 4A), but were significantly depleted compared with suprarenal aortic controls at 14 (p=0.04) and 28 days (p =0.03) (Fig. 4B and C, respectively).
Figure 4. Smooth muscle cells are diminished in BAPN-treated swine AAA.
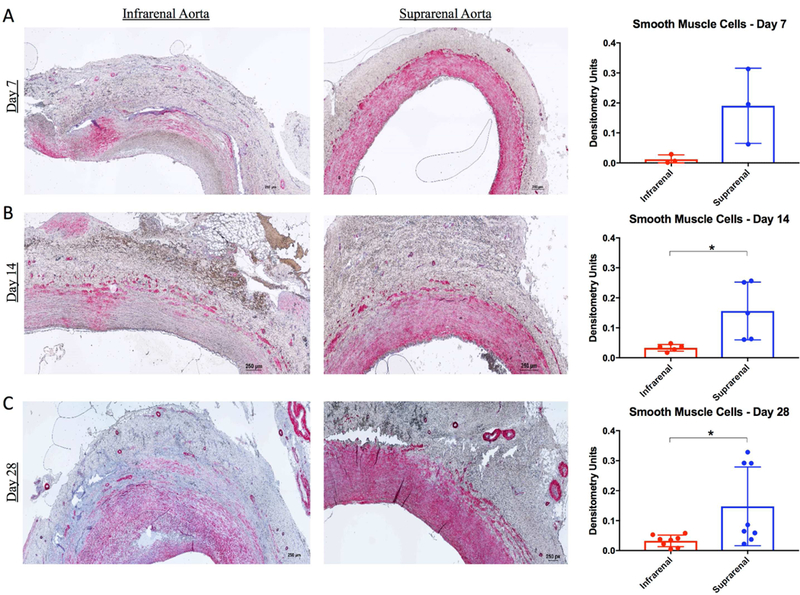
Alpha smooth muscle actin staining within the wall of infrarenal and suprarenal aorta at 7 days (A), 14 days (B), and 28 days (C). Smooth muscle cell (pink) content within the wall of infrarenal versus suprarenal aorta as measured by densitometry units at 7, 14, and 28 days (Far Right A, B, and C, respectively). Scale bar, 250μ. 4X lens objective. * indicates p<0.05.
Macrophages were polarized to the pro-inflammatory M1 phenotype
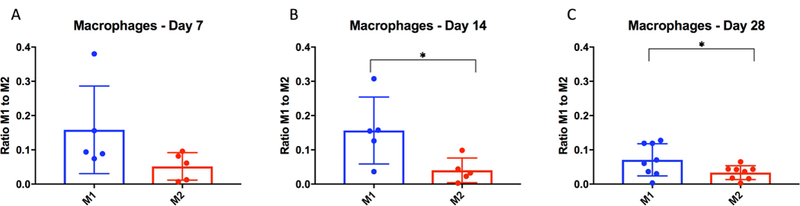
Figure 5 demonstrates macrophage phenotype polarization within the wall of the BAPN-treated swine infrarenal AAA segment. While not significantly different at day 7 (Fig. 5A), macrophages were more significantly polarized to the pro-inflammatory M1 phenotype compared with the pro-resolving M2 phenotype in the infrarenal segments at 14 days (Fig. 5B; p=0.03). M1 macrophages still predominated over the M2 phenotype at 28 days (Fig. 5C; p=0.03).
Figure 5. M1 macrophages are increased in BAPN-treated swine AAA.
M1 vs. M2 macrophage polarization in swine infrarenal aorta as measured by densitometry units at 7, 14 and 28 days. * indicates p<0.05.
BAPN+ surgery swine have increased MMPs in the infrarenal aorta
Figure 6A depicts gel zymograms used to detect MMP2 and MMP9 isoforms. Gel zymography detected an upregulation in overall MMP2 activity in the infrarenal aortic of BAPN treated infrarenal aortic segments compared with the suprarenal aortic segment at day 7, (2,426,677±2,101,152 densitometry units vs. 676,305±757,812 densitometry units; p= 0.06; Fig 6B). This increase and resultant degradation of extracellular matrix proteins was seen at all time points. While not statistically significant, MMP9 activity was increased at all time points in the infrarenal segment compared with suprarenal aorta, but this did not reach significance (Fig. 6C).
Figure 6. MMPs are active in BAPN-treated swine AAA.
(A) Gelatin zymograms of active MMP2 and MMP9. (B) MMP2 activity within the wall of infrarenal versus suprarenal aorta at 7-, 14-, 28-days, and (C) MMP9 activity at 7-, 14-, and 28-days. * indicates p<0.05.
Cytokine levels in surgically induced BAPN-treated swine AAA
Cytokine array was performed on swine aorta at harvest time points of 7 days (n = 3), 14 days (n = 5), and 28 days (n = 9). Supplementary Figures 1 and 2 show cytokine levels of BAPN treated animals as measured by densitometry units over time. IL-6 was significantly elevated in infrarenal aorta compared with suprarenal segment at 7 days (p=0.04). At 14 days, there were significantly higher levels of IL-1α, IL-1β, IL-2, IL-10, and IL-18 in BAPN + surgery treated infrarenal aortic segments compared with the suprarenal segment (p<0.05, all). BAPN + surgery swine harvested at 28 days showed significantly increased levels of IL-1α, IL-1β, IL-4, IL-6, and IL-18 in the infrarenal aortic segment compared with the suprarenal segment (p<0.05, all).
Discussion
In the present study, a novel infrarenal AAA model was created in which aortic dilation is reproducibly greater than 100% with histochemical features, as well as MMP and cytokine changes that parallel human AAA disease. This occurred by using a combination of mechanical dilatation, elastase/collagenase perfusion, topical elastase application, and daily BAPN feedings.
Marinov and colleagues investigated whether a AAA model could be developed in swine using elastase perfusion alone.11 Their work demonstrated that perfusion using elastase concentrations ranging from 300U to 937.5U created histologic changes in the infrarenal aorta of swine, namely elastin disruption and smooth muscle cell attrition. However, aneurysm dilatation of the perfused segment to the requisite 50% increase in size was not achieved. Ten years later, Hynecek et al published their results combining elastase and collagenase perfusion with mechanical balloon dilatation.9 This work demonstrated that an infrarenal swine aneurysm model was possible. Using a perfusion solution consisting of 30 units of elastase and 8000 units of collagenase, mean aortic diameters were increased by 73% (range 33–117%). This group found histologic changes of endothelial loss, neutrophil infiltrate, and elastin disruption at early time points of 1, 3, and 7 days, with significant smooth muscle cell attrition seen at 1 week. The lack of smooth muscle cell population persisted at longer time points (3 and 6 weeks).
We sought to build upon the work by Hynecek and colleagues, and focused on increased elastase and collagenase concentrations, as well as the addition of BAPN. Prior investigation in our lab using a model of topical elastase application to the infrarenal aorta in conjunction with daily BAPN feeding via water source allowed for successful AAA formation in mice that persisted with chronic features.6 BAPN treatment alone was tested in swine in the 1970s, but no dilatation of the aorta was observed, even with massive daily dosages.4 The surgical procedure in the present study was similar to the Hynecek group9, but differed in several distinct ways: (1) use of domestic versus Yorkshire swine, (2) increased elastase and collagenase perfusion concentration, (3) the addition of topical elastase application to the aorta, and (4) daily BAPN administration via diet. In the present model, the infrarenal aortic segment was perfused with 500 units of elastase and 8000 units of collagenase compared with 30 units of elastase and 8000 units of collagenase used by Hynecek. This modified technique allowed for a much more robust and consistent increase in AAA size compared with the surgical technique alone.
Histologic alterations in human AAA have been extensively investigated. AAAs are characterized by structural remodeling involving elastin fiber destruction, collagen turnover, smooth muscle cell depletion, and inflammatory cell infiltration.12,13 It has been reported that elastin is increased in the wall of human AAA14–17 , while others have found a decrease.18–20 These discrepancies are possibly due to different quantification methods: a change in “content” refers to an increase or decrease in the actual amount of something, while a change in “concentration” means an increase or decrease in th e actual amount of the variable of interest or a change in the amount of surrounding components with no net alteration in the amount of the variable. The present study found significant elastolysis at all time points in swine infrarenal aortic segments compared with the non-aneurysmal suprarenal segment. The method used to quantify this has been used in prior publications from our lab and refers to elastin content.6,10,21 Importantly, when performing our analysis, the elastin content in the infrarenal aorta was compared to elastin content in the suprarenal aorta. Elastin content in the human aorta differs depending on location, and elastin content has been shown to decrease 49–58% from the suprarenal aorta to the mid-infrarenal aorta.22,23 Nevertheless, the destruction of elastin fibers seen in the present analysis is consistent with other published results in human AAA.14,16–19,24,25
Similar to the contrasting amounts of elastin, collagen is increased in human AAAs compared to controls according to some studies15,26, decreased in others20, and unchanged in another report.16 Swine treated with BAPN and surgery showed collagen content to be less, but not significantly, in the infrarenal segments compared with suprarenal controls. Similar to elastin, while collagen content has been shown to not significantly differ between the suprarenal and infrarenal aorta in humans27, this has not been investigated in swine.
Interestingly, there appeared to be a correlation in the temporal expression of cytokines and MMP with cellular populations. Pro-inflammatory M1 macrophages were significantly increased compared with anti-inflammatory M2 macrophages at 14 and 28 days. The macrophage-secreted cytokines IL-1 and IL-18 were significantly upregulated at both time points, and IL-6 was significantly increased at 28 days. At 14 days, IL-2 was up-regulated which correlates with the increased mural presence of T lymphocytes at that time point. Overall, the inflammatory milieu of the model must be investigated further as there appears to be upregulation of pro- and anti-inflammatory markers at similar times, indicating a complex process of cellular crosstalk.
Macrophages have been shown to play an important role in the inflammatory milieu present in AAA.28 The present study found macrophages were predominantly polarized to the pro-inflammatory M1 phenotype at days 14 and 28 in the present swine AAA model. The sustained population of M1 macrophages is indicative of ongoing inflammation, and a feature of the model that reflects human disease.
A main limitation of the current study is the uncertainty as to which of the multiple interventions used in the operation is most critical to achieve aneurysm dilatation and histochemical changes. The work by Hynecek et al lacked BAPN, but still had an overall only 50% aortic dilatation. The current model’s addition of topical elastase application may also confound results. Its application alone is successful in rodent models, but the swine aorta is more robust. Additionally, success of the perfusion aspect of the procedure varied even though an attempt to maintain a constant pressure was made. Importantly, it was difficult to standardize perfusion pressures, as aortic volumes varied based on number of factors, including pig size. This could be an area of improvement for future studies.
Conclusion
The present study describes a novel model of AAA in the swine infrarenal aorta. A modified surgical technique for AAA induction and concurrent daily BAPN administration allowed for a much more robust aneurysm compared with prior studies.9,11 The observed histologic changes in the swine AAA are analogous to those found in rodent AAA models and human AAAs. In addition, cytokine and MMP expression closely mirrored those seen in human AAA. This novel model in a large animal will hopefully assist with further elucidation of AAA biology, as well as device and drug development.
Supplementary Material
Type of Research:
Experimental study of aortic aneurysms in pigs.
Key Findings:
Aneurysms were produced in a consistent fashion using a combination of balloon dilatation, elastase/collagenase perfusion, extra luminal elastase application, and oral supplementation with β-aminopropionitrile (BAPN)
Take Home Message:
This reproducible pig model of aneurysms will allow future mechanistic studies and may allow endovascular device development.
Translation of AAA models from small animals to large animals is critical for device development and further elucidation of AAA pathogenesis mechanisms. We have developed a reproducible large animal model of AAA disease with over 100% dilatation in swine. The swine AAA have a histology and inflammatory milieu similar to human and further work using this model will hopefully expand our understanding of this disease.
Acknowledgments
We would like to expressly thank Anthony Herring and Cindy Dodson for their dedication to this project by contributing their expertise in the perioperative and postoperative care of the study animals. Nothing in these pages would have been possible without your tireless and selfless commitment to science, as well as your expertise and care of these animals.
Sources of Funding:
The National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL007849.
National Institutes of Health / The National Heart, Lung, and Blood Institute Grant Number R01HL081629–07 (Upchurch, 2014–2018) Gender differences in experimental aortic aneurysms.
National Institutes of Health / The National Heart, Lung, and Blood Institute Grant Number R01HL124131–01 (Upchurch, 2014–2018) Role of Neutrophil Extracellular Traps in AAA Pathogenesis.
National Institutes of Health / The National Heart, Lung, and Blood Institute Grant Number R01HL126668–01 (Ailawadi, 2014–2018) The Role of IL-1 in cell transitions in Aortic Aneurysm Pathogenesis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: There are no actual or potential perceived conflicts of interest.
References
- 1.Mokdad AH, Forouzanfar MH, Daoud F, Mokdad AA, El Bcheraoui C, Moradi-Lakeh M, et al. Global burden of diseases, injuries, and risk factors for young people’s health during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2016;387(10036):2383–401. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, Chang AR, Cheng S, Chiuve SE, et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation 2018;137(12):e67–e492. [DOI] [PubMed] [Google Scholar]
- 3.Barrow MV, Simpson CF, Miller EJ. Lathyrism: a Review. Q Rev Biol 1974;49(2):101–28. [DOI] [PubMed] [Google Scholar]
- 4.Coulson WL, Linker A, Bottcher E. Lathyrism in Swine. Arch Pathol 1969;87(4):411–7. [PubMed] [Google Scholar]
- 5.Tilson MD III, Kuivaniemi H, Upchurch G. The abdominal aortic aneurysm. Genetics, pathophysiology, and molecular biology. Proceedings of a conference. April 3–5, 2006 New York, New York, USA New York: Annals of the New York Academy of Sciences; 2006. [DOI] [PubMed] [Google Scholar]
- 6.Lu G, Su G, Davis JP, Schaheen B, Downs E, Roy RJ, et al. A novel chronic advanced stage abdominal aortic aneurysm murine model. J Vasc Surg 2017;66(1):232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.English SJ, Piert MR, Diaz JA, Gordon D, Ghosh A, D’Alecy LG, et al. Increased 18f-fdg uptake is predictive of rupture in a novel rat abdominal aortic aneurysm rupture model. Ann Surg 2015;261(2):395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crisóstomo V, Sun F, Maynar M, Báez-Díaz C, Blanco V, Garcia-Lindo M, et al. Common swine models of cardiovascular disease for research and training. Lab Anim 2016;45(2):67–74. [DOI] [PubMed] [Google Scholar]
- 9.Hynecek RL, DeRubertis BG, Trocciola SM, Zhang H, Prince MR, Ennis TL, et al. The creation of an infrarenal aneurysm within the native abdominal aorta of swine. Surgery 2007;142(2):143–9. [DOI] [PubMed] [Google Scholar]
- 10.Pope NH, Salmon M, Davis JP, Chatterjee A, Su G, Conte MS, et al. D-series resolvins inhibit murine abdominal aortic aneurysm formation and increase M2 macrophage polarization. FASEB J 2016;30(12):4192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marinov GR, Marois Y, Paris E, Roby P, Formichi M, Douville Y, et al. Can the infusion of elastase in the abdominal aorta of the Yucatan miniature swine consistently produce experimental aneurysms? J Investig Surg 1997;10(3):129–50. [DOI] [PubMed] [Google Scholar]
- 12.Tsamis A, Krawiec JT, Vorp DA. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J R Soc Interface 2013;10(83):20121004–20121004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wassef M, Baxter BT, Chisholm RL, Dalman RL, Fillinger MF, Heinecke J, et al. Pathogenesis of abdominal aortic aneurysms: A multidisciplinary research program supported by the National Heart, Lung, and Blood Institute. J Vasc Surg 2001;34(4):730–8. [DOI] [PubMed] [Google Scholar]
- 14.Baxter BT, McGee GS, Shively VP, Drummond IA, Dixit SN, Yamauchi M, et al. Elastin content, cross-links, and mRNA in normal and aneurysmal human aorta. J Vasc Surg 1992;16:192–200. [PubMed] [Google Scholar]
- 15.Minion DJ, Davis VA, Nejezchleb PA, Wang Y, McManus BM, Baxter BT. Elastin Is Increased in Abdominal Aortic Aneurysms. J Surg Res 1994;57(4):443–6. [DOI] [PubMed] [Google Scholar]
- 16.Gandhi RH, Irizarry E, Cantor JO, Keller S, B Nackman GB, Halpern VJ, et al. Analysis of elastin cross-linking and the connective tissue matrix of abdominal aortic aneurysms. Surgery 1994;15:617–620. [PubMed] [Google Scholar]
- 17.Krettek A, Sukhova GK, Libby P. Elastogenesis in human arterial disease: A role for macrophages in disordered elastin synthesis. Arterioscler Thromb Vasc Biol 2003;23(4):582–7. [DOI] [PubMed] [Google Scholar]
- 18.He CM, Roach MR. The composition and mechanical properties of abdominal aortic aneurysms. J Vasc Surg 1994;20(1):6–13. [DOI] [PubMed] [Google Scholar]
- 19.Humphrey JD, Holzapfel GA. Mechanics, mechanobiology, and modeling of human abdominal aorta and aneurysms. J Biomech 2012;45(5):805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmo M, Colombo L, Bruno A, Corsi FR, Roncoroni L, Cuttin MS, et al. Alteration of elastin, collagen and their cross-links in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2002;23(6):543–9. [DOI] [PubMed] [Google Scholar]
- 21.Johnston WF, Salmon M, Su G, Lu G, Stone ML, Zhao Y, et al. Genetic and pharmacologic disruption of interleukin-1β signaling inhibits experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol 2013;33(2):294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheuk BL, Cheng SW. Expression of Integrin α 5 β 1 and the Relationship to Collagen and Elastin Content in Human Suprarenal and Infrarenal Aortas. Vasc Endovasc Surg 2005;39(3):245–51. [DOI] [PubMed] [Google Scholar]
- 23.Maurel E, Shuttleworth CA, Bouissou H. Interstitial collagens and ageing in human aorta. Virchows Arch A 1987;410(5):383–90. [DOI] [PubMed] [Google Scholar]
- 24.Thompson RW, Geraghty PJ, Lee JK. Abdominal Aortic Aneurysms: Basic Mechanisms and Clinical Implications. Curr Probl Surg 2002;39(2):110–230. [DOI] [PubMed] [Google Scholar]
- 25.Henderson EL, Geng Y-J, Sukhova GK, Whittemore AD, Knox J, Libby P. Death of Smooth Muscle Cells and Expression of Mediators of Apoptosis by T Lymphocytes in Human Abdominal Aortic Aneurysms. Circulation 1999;99(1):96–104. [DOI] [PubMed] [Google Scholar]
- 26.Rizzo RJ, McCarthy WJ, Dixit SN, Lilly MP, Shively VP, Flinn WR, et al. Collagen types and matrix protein content in human abdominal aortic aneurysms. J Vasc Surg 1989;10(4):365–73. [DOI] [PubMed] [Google Scholar]
- 27.Halloran BG, Davis VA, McManus BM, Lynch TG, Baxter BT. Localization of Aortic Disease Is Associated with Intrinsic Differences in Aortic Structure. J Surg Res 1995;59(1):17–22. [DOI] [PubMed] [Google Scholar]
- 28.Wang KC, Li YH, Shi GY, Tsai HW, Luo CY, Cheng MH, et al. Membrane-Bound Thrombomodulin Regulates Macrophage Inflammation in Abdominal Aortic Aneurysm. Arterioscler Thromb Vasc Biol 2015;35(11):2412–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


