Abstract
The link between HDL subclasses and the prognosis of cardiovascular diseases remains controversial. We thus evaluated the prognostic value of the HDL subclasses 3 and 2 cholesterol (HDL3-C, HDL2-C) as well as of total HDL-C for 3-month mortality in acute heart failure (AHF) patients. The serum levels of HDL3-C and total HDL-C were determined by detergent-based homogeneous assay. HDL2-C was computed by the difference between total HDL-C and HDL3-C. Out of the 132 analyzed patients, 35 (26.5%) died within three months after onset of AHF. Univariate logistic regression analyses revealed a significant inverse association of HDL3-C (odds ratio (OR) 0.46 per 1-SD increase, 95% confidence interval (CI) 0.27–0.72, p = 0.001) with 3-month mortality, whereas concentrations of total HDL-C and HDL2-C showed no significant association. After adjustment for various laboratory and clinical parameters known to be associated with mortality in heart failure patients, HDL3-C concentrations remained significantly associated with 3-month mortality (OR 0.34 per 1-SD increase, 95% CI 0.15–0.74, p =0.010). We conclude that low admission serum levels of HDL3-C are associated with an increased 3-month mortality in AHF patients, whereas total HDL-C and HDL2-C showed no association. HDL3-C might thus be useful as a prognostic parameter in AHF.
Keywords: HDL3 cholesterol, HDL particles, Homogeneous assays, Outcome
1. Introduction
Heart failure (HF) is a frequent cause of morbidity and mortality worldwide [1]. Being a final stage of various cardiovascular diseases, HF is defined by the European Society of Cardiology (ESC) as an abnormality of the cardiac structure and function, resulting in a diminished ability of the heart to maintain optimal perfusion of metabolizing tissues [2,3]. Acute heart failure (AHF) denotes the rapid onset of, or changes in, symptoms and signs of HF [3].
Previous studies have demonstrated the capacity of HDL to inhibit mechanical stress-induced cardiomyocyte autophagy and cardiac hypertrophy [4], improve cell survival, preserve mitochondrial function, attenuate oxidative stress [5,6], and promote glucose uptake by cardiomyocytes [7]. HDL is a potent inducer of endothelial NO production [8], which promotes relaxation of the coronary arteries and, in turn, perfusion of the failing heart. Additionally, HDL-induced NO might contribute to the maintenance of the physiological titin phosphorylation and normal cardiomyocyte tension [9].
It is important to note that HDL particles comprise two major subclasses, namely large buoyant HDL2 particles and smaller, denser HDL3 particles. Accordingly, quantity of the total HDL-C is dominated by the contribution of the larger, cholesterol-rich HDL2. This results in an inadequately low contribution of the smaller, denser, cholesterol-poor HDL3, whose cardioprotective activities are superior to that of HDL2 [10,11]. Indeed, recent studies revealed that serum levels of cholesterol in the HDL3 subclass (HDL3-C) are predictive of cardiovascular events and that the HDL3 subclass may be primarily responsible for the inverse association of HDL-C and cardiovascular disease [12–15]. Clinical studies have shown that assessing circulating concentrations of HDL particles (HDL-P) by NMR spectroscopy is superior to HDL-cholesterol in predicting cardiovascular risk [16].
2. Theory
We have shown in our previous study that low serum concentrations of small but not large HDL-P, quantified by NMR spectroscopy, are associated with increased 3-month mortality in AHF patients [17]. Considering that small HDL-P may primarily represent the HDL3 subclass, we hypothesized that the serum levels of HDL3-C might have a prognostic capacity for mortality in AHF. In contrast to the quantification of HDL-P by NMR spectroscopy, the HDL subclasses can be measured by a simple and convenient method on automated analyzers in routine clinical laboratories. We therefore aimed to assess the associations of the concentrations of the HDL subclasses with 3-month mortality in AHF.
3. Material and methods
3.1. Study design and patients
Study design, inclusion and exclusion criteria as well as patient characteristics for our AHF cohort have been described in our previous reports [17–20]. In brief, we performed a prospective observational study including consecutive hospitalized AHF patients. Written informed consent was obtained from each patient and the study was conducted in adherence to the ethical guidelines of the Declaration of Helsinki [21]. The study was approved by the Ethics Committees of the University Hospital Centre Sisters of Charity, Zagreb, Croatia and the Medical University of Graz, Austria. All patients were treated according to the European Society of Cardiology (ESC) Guidelines for AHF [3,22].
3.2. Laboratory assays
The collection of the blood samples, the standard laboratory methods and measurements of small, large and total HDL-P have been described in previous reports on our AHF cohort [17–20,23]. The levels of total HDL cholesterol (HDL-C) and HDL3-C were measured using homogeneous assays from Denka Seiken Co., Ltd. (Tokyo, Japan) [24] on an Olympus AU680 automated analyzer. HDL2-C was estimated by subtracting HDL3-C from total HDL-C.
3.3. Statistical analysis
Differences in the serum levels of HDL3-C, HDL2-C, and total HDL-C between groups according to various patient characteristics were tested with the Mann-Whitney U test. Correlations between HDL3-C, HDL2-C, and total HDL-C and various laboratory and clinical parameters were determined by the Spearman correlation coefficient due to the skewed distribution of many of the laboratory parameters. Because of multiple comparisons for these analyses, we applied a Bonferroni correction to the significance level: for the correlation analyses (18 parameters) a p-value of < 0.003 and for group differences (6 parameters) a p-value of < 0.008 was considered significant. Furthermore, univariable and multivariable logistic regression analyses were used to examine the impact of HDL3-C, HDL2-C, and total HDL-C on 3-month mortality. In the multivariable analyses, we adjusted for age, sex, N-terminal probrain natriuretic peptide (NT-proBNP), glomerular filtration rate (GFR), mean arterial pressure (MAP), low-density lipoprotein (LDL)-cholesterol, log(triglycerides), type 2 diabetes (T2D), and C-reactive protein (CRP). Results are presented as odds ratio (OR) and the respective 95% confidence interval (CI) per standard deviation (SD) increase. R version 3.4.4 was used for these analyses.
4. Results
4.1. Patients' clinical characteristics, medication and laboratory parameters
The baseline characteristics, comorbidities, medication, laboratory results, and outcome of our 152 AHF patients have been described elsewhere [17–20]. Serum samples of 132 patients [65 (49.2%) were female and median and range for age were 77.3 (45.5–92.4) years], were available for the analyses presented here. Of these, 35 (26.5%) died within three months of onset of AHF. Median and range for serum levels of HDL3-C, HDL2-C, and total HDL-C were 0.49 (0.23–0.85) mmol/L, 0.62 (0.19–1.67) mmol/L and 1.13 (0.42–2.46) mmol/L, respectively. Clinical characteristics, medication and laboratory parameters of patients who died compared to those who were alive the first three months after onset of AHF are shown in supplemental Tables S1 and S2.
4.2. Correlation of HDL3-C, HDL2-C and total HDL-C concentrations with laboratory and clinical variables
The serum levels of HDL3-C and total HDL-C, but not of HDL2-C, were significantly positively correlated with MAP and negatively with urea and CRP after the Bonferroni correction (Table 1). Furthermore, HDL3-C was significantly negatively correlated with systolic pulmonary artery pressure (SPAP) and NT-proBNP and positively with platelets. HDL-C was solely significantly negatively correlated with creatinine (Table 1).
Table 1. Correlation analyses of HDL3-C, HDL2-C, and total HDL-C with clinical and laboratory parameters.
| HDL3-C (mmol/L) | HDL2-C (mmol/L) | Total HDL-C (mmol/L) | |||||
|---|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | n | |
| Age (years) | –0.12 | 0.166 | 0.11 | 0.189 | 0.02 | 0.843 | 132 |
| BMI (kg/m2) | 0.03 | 0.728 | –0.24 | 0.005 | –0.15 | 0.091 | 132 |
| MAP (mm Hg) | 0.44 | < 0.001 | 0.25 | 0.003 | 0.33 | < 0.001 | 132 |
| SPAP (mm Hg) | –0.36 | 0.002 | –0.13 | 0.282 | –0.21 | 0.063 | 76 |
| Heart rate (beats/min) | 0.22 | 0.011 | 0.15 | 0.082 | 0.18 | 0.036 | 132 |
| NT-proBNP (pg/ml) | –0.28 | 0.001 | –0.03 | 0.781 | –0.13 | 0.162 | 126 |
| GFR (ml/min/1.73 m2) | 0.23 | 0.007 | 0.13 | 0.137 | 0.19 | 0.029 | 131 |
| Urea (mmol/L) | –0.31 | < 0.001 | –0.21 | 0.014 | –0.27 | 0.002 | 131 |
| Creatinine (μmol/L) | –0.25 | 0.004 | –0.24 | 0.005 | –0.27 | 0.002 | 131 |
| ALT (U/L) | 0.07 | 0.443 | 0.03 | 0.719 | 0.03 | 0.772 | 128 |
| AST (U/L) | –0.08 | 0.386 | 0.08 | 0.351 | 0.03 | 0.760 | 129 |
| Protein (g/L) | 0.26 | 0.003 | 0.05 | 0.556 | 0.13 | 0.146 | 129 |
| Fibrinogen (g/L) | 0.20 | 0.021 | –0.01 | 0.873 | 0.08 | 0.394 | 127 |
| Albumin (g/L) | 0.17 | 0.058 | –0.01 | 0.902 | 0.04 | 0.679 | 129 |
| IL-6 (pg/mL) | –0.21 | 0.016 | –0.04 | 0.617 | –0.09 | 0.312 | 132 |
| CRP (μg/mL) | –0.31 | < 0.001 | –0.22 | 0.011 | –0.27 | 0.002 | 130 |
| Leukocytes (×109/L) | 0.22 | 0.010 | 0.10 | 0.278 | 0.16 | 0.074 | 131 |
| Platelets (×1012/L) | 0.32 | < 0.001 | 0.16 | 0.072 | 0.24 | 0.006 | 132 |
Data presented are the Spearman correlation coefficient r, the corresponding p-values, and the number of available samples (n);
P-values of < 0.003 are considered significant after a Bonferroni correction for multiple comparison and are depicted in bold.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CRP, C-reactive protein; GFR, glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; IL-6, interleukin 6; LDL, low-density lipoprotein; MAP, mean arterial pressure; NT-proBNP, N-terminal pro brain natriuretic peptide; SPAP, systolic pulmonary artery pressure.
4.3. Correlation of HDL3-C, HDL2-C and total HDL-C concentrations with small, large, and total HDL-P
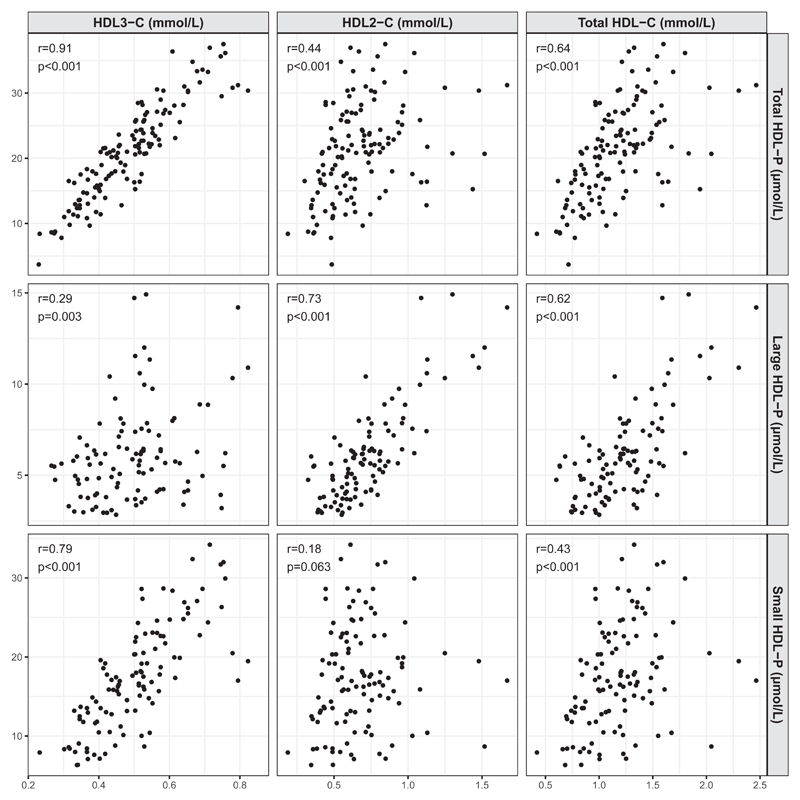
The serum levels of HDL3-C showed a strong positive correlation with total and small HDL-P, as well as a weak positive correlation with large HDL-P (Fig. 1). In contrast, concentrations of HDL2-C showed a strong positive correlation with large HDL-P, a moderate positive correlation with total HDL-P, and no significant correlation with small HDL-P. Finally, the serum levels of total HDL-C showed a moderate positive correlation with the total, and large HDL-P, and a moderate positive correlation with small HDL-P.
Fig. 1.
Correlation of HDL3-C, HDL2-C, and total HDL-C concentrations with small, large, and total HDL-P. Correlations of HDL3-C, HDL2-C, and total HDL-C with small, large, and total HDL-P were determined using the Spearman correlation coefficient. HDL-P, concentrations of HDL particles.
4.4. Levels of HDL3-C, HDL2-C, and total HDL-C compared between different subgroups of AHF patients
As shown in Table 2, after the Bonferroni correction the levels of HDL3-C, but not of total HDL-C and HDL2-C, were significantly lower in AHF patients with worsening of chronic HF compared to de novo AHF patients, in AHF patients with one or more signs implying venous volume overload (jugular venous distension, enlarged liver, peripheral edema or ascites), and in patients that died within three months after onset of AHF. The levels of HDL3-C, but not of HDL-C and HDL2-C, were higher in patients with hypercholesterolemia. In contrast, AHF patients with T2D had significantly lower total HDL-C and HDL2-C, but similar HDL3-C, levels when compared to AHF patients without T2D.
Table 2. HDL3-C, HDL2-C, and total HDL-C levels in various groups of AHF patients.
| HDL3-C (mmol/L) | HDL2-C (mmol/L) | Total HDL-C (mmol/L) | n | ||
|---|---|---|---|---|---|
| Sex | Male | 0.48 (0.23–0.85) | 0.59 (0.19–1.30) | 1.04 (0.42–1.83) | 67 |
| Female | 0.50 (0.27–0.82) | 0.69 (0.30–1.67) | 1.19 (0.57–2.46) | 65 | |
| p =0.733 | p =0.024 | p =0.095 | |||
| AHF type | De novo | 0.54 (0.32–0.85) | 0.64 (0.33–1.48) | 1.24 (0.70–2.30) | 40 |
| Worsening of CHF | 0.46 (0.23–0.79) | 0.61 (0.19–1.67) | 1.05 (0.42–2.46) | 92 | |
| p < 0.001 | p =0.115 | p =0.017 | |||
| Sign(s) | No | 0.57 (0.33–0.85) | 0.62 (0.33–1.25) | 1.22 (0.75–2.03) | 28 |
| Yes | 0.46 (0.23–0.82) | 0.62 (0.19–1.67) | 1.09 (0.42–2.46) | 104 | |
| p = 0.001 | p =0.555 | p =0.089 | |||
| T2D | No | 0.51 (0.23–0.82) | 0.70 (0.35–1.67) | 1.22 (0.63–2.46) | 60 |
| Yes | 0.47 (0.23–0.85) | 0.59 (0.19–1.09) | 1.03 (0.42–1.59) | 71 | |
| p =0.210 | p = 0.002 | p = 0.007 | |||
| HypChol | No | 0.46 (0.23–0.69) | 0.62 (0.19–1.44) | 1.09 (0.42–1.94) | 81 |
| Yes | 0.53 (0.23–0.85) | 0.61 (0.30–1.67) | 1.21 (0.57–2.46) | 51 | |
| p < 0.001 | p =0.516 | p =0.070 | |||
| Three months after onset of AHF | Alive | 0.51 (0.26–0.85) | 0.62 (0.20–1.48) | 1.17 (0.46–2.30) | 92 |
| Dead | 0.41 (0.23–0.79) | 0.55 (0.19–1.67) | 0.99 (0.42–2.46) | 35 | |
| p < 0.001 | p =0.215 | p =0.023 |
Data are presented as median and range (minimum to maximum). Differences in the serum levels of HDL3-C, HDL2-C, and HDL-C between the groups were tested with the Mann-Whitney U test.
P-values of < 0.008 are considered significant after a Bonferroni correction for multiple comparison and are depicted in bold.
AHF, acute heart failure; CHF, chronic heart failure; HDL-C, high-density lipoprotein cholesterol; HypChol, hypercholesterolemia; Signs include enlarged liver, peripheral edemas, ascites and jugular venous distension.
4.5. Logistic regression analyses
To study association of HDL subclasses with mortality logistic regression analyses were performed. Univariate analyses revealed a significant inverse association of 3-month mortality and the serum concentrations of HDL3-C, but showed no association with the total HDL-C and HDL2-C concentrations (Table 3). Importantly, the concentrations of HDL3-C remained significantly associated with 3-month mortality after adjusting for age, sex, NT-proBNP, GFR, MAP, LDL cholesterol, log (triglycerides), T2D, and CRP the clinical and laboratory parameters that are known to be associated with mortality in HF patients (Table 3).
Table 3. Univariable and multivariable logistic regression analyses of 3-month mortality for HDL3-C, HDL2-C, and total HDL-C.
| Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| HDL3-C (mmol/L) | 0.46 (0.27–0.72) | 0.001 | 0.34 (0.15–0.74) | 0.010 | |
| HDL2-C (mmol/L) | 0.89 (0.57–1.33) | 0.528 | 0.72 (0.39–1.30) | 0.283 | |
| Total HDL-C (mmol/L) | 0.70 (0.44–1.05) | 0.099 | 0.59 (0.30–1.09) | 0.101 | |
OR and CI are presented on the SD scale (increase per 1 SD); SDs for HDL3-C, HDL2-C, and total HDL-C are 0.13, 0.26, and 0.36 mmol/L, respectively.
Data of 127 patients (35 events) were available for the unadjusted analyses and data of 118 patients (33 events) for the adjusted analyses.
Significant results are depicted in bold.
CI, confidence interval; CRP, C-reactive protein; GFR, glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL, low-density lipoprotein; MAP, mean arterial pressure; NT-proBNP, N-terminal pro brain natriuretic peptide; OR, odds ratio; SD, standard deviation; T2D, type 2 diabetes.
The model was adjusted for age, sex, NT-proBNP, GFR, MAP, LDL-cholesterol, log(triglycerides), T2D, and CRP.
5. Discussion
Although several studies examined the association between the outcome and prognosis in HF and HDL-cholesterol [25–27] our study is the first to address the association of the HDL subclasses with mortality in AHF.
Here we show for the first time that a low concentration of cholesterol in circulating HDL3 particles, but not in HDL2 or total HDL, is associated with increased 3-month mortality of AHF patients. Considering that HDL3-C may reflect the cholesterol content of small HDL-P and HDL2-C that of large HDL-P, the present finding is fully in line with our previous results showing that only the concentrations of small HDL-P were associated with mortality, whereas the concentrations of large HDL-P and total HDL-C were not [17]. Indeed, in the present study we found a strong correlation between HDL3-C and small HDL-P as well as between HDL2-C and large HDL-P. Due to its simplicity and operability on automated analyzers that are available in routine clinical laboratories, measurement of HDL3-C is applicable in daily clinical practice. This is not the case with NMR-based measurements of HDL particle concentrations, a method requiring special instrumentation that is available only in some routine clinical laboratories.
Our results appear to be in line with recent studies showing that the serum levels of HDL3-C are predictive of cardiovascular events and that the HDL3 subclass may be primarily responsible for the inverse association between HDL-C and cardiovascular disease [12–15].
Several studies examined the association of the outcome and prognosis in HF with HDL-C [25–27]. However, as mentioned above, no study has examined the prognostic capacity of HDL3-C concentrations for mortality in AHF so far. In the present study, we observed a strong independent association of low levels of the HDL subclass 3 but not of HDL2 with mortality, suggesting cardiovascular protective activities of the former subclass. Our results are in good agreement with previous studies showing that anti-oxidative enzymes as well as particular lipid species entailing cardiovascular protective activities are enriched in HDL3 subclass [10,11].
Interestingly, the serum concentrations of HDL3-C, but not of HDL2-C or total HDL-C, were negatively correlated with NT-proBNP suggesting a sensitivity of HDL3-C to the severity of the AHF pathophysiology. Furthermore, only HDL3-C, but not HDL2-C or total HDL-C, was negatively correlated with SPAP, whose elevation reflects left ventricular dysfunction, heart congestion, and elevated left-sided filling pressure accompanied by increased NT-proBNP [28].
A more pronounced negative impact of the AHF pathophysiology on HDL3-C, compared to HDL2-C, is also illustrated by the lower levels of HDL3-C, but not of HDL2-C, in AHF patients with a more severe state of the disease, namely worsening of chronic HF, compared to de novo AHF patients [20]. Additionally, the levels of HDL3-C were lower in AHF patients with sign(s) implying venous congestion, a consequence of right-sided heart failure, as compared to patients without these signs.
In the present study, the serum levels of HDL3-C, but not of HDL2-C or total HDL-C, were (borderline significantly; p =0.003) positively correlated with the serum levels of total proteins, the markers of nutritional status and liver biosynthetic capacity. Accordingly, our data suggest that only the serum levels of HDL3-C are affected by alterations in the biosynthetic capacity of the liver, which in HF is frequently decreased due to hypoperfusion and congestion [29]. This may, at least in part, explain why the serum levels of HDL3-C, but not of HDL2-C or total HDL-C, were lower in AHF patients with venous congestion, as compared to patients without.
It is well established that the augmented translocation of bacterial endotoxins from the edematous intestine into the circulation [30], as well as the pro-inflammatory activation of venous endothelial cells due to congestion, contribute to the chronic inflammation in HF [31], resulting in increased formation and clearance of leukocyte - platelet complexes [32]. Considering the role of circulating lipoproteins in the binding and neutralization of endotoxines [33], a negative correlation of HDL3-C with CRP, as well as a positive correlation with platelets, might reflect the counteraction of an inflammatory response by HDL3. Alternatively, the observed correlations between HDL3-C and the inflammatory markers might be a consequence of the opposite regulation of HDL3-C and the inflammatory molecules by the AHF pathophysiology. A similar effect might also explain the negative correlations of HDL3-C, HDL2-C, and total HDL-C with the markers of kidney function, which is frequently impaired in HF [34].
The positive correlation of HDL3-C, HDL2-C (borderline significant; p =0.003), and total HDL-C with MAP might reflect their negative regulation by the AHF pathophysiology. Alternatively, a better tissue perfusion in patients with higher MAP might conceivably, similar as endurance training [35], raise serum HDL levels.
In the present study, we observed that the concentrations of total HDL-C and HDL2-C, but not of HDL3-C, were lower in AHF patients with T2D compared to those without. This is in line with results of a previous study demonstrating that insulin resistance and adiposity affect lipoprotein size and the subclass concentrations in the general population [36].
The association of decreased HDL3-C serum levels with an increased mortality in our AHF cohort raises the question of whether HDL3-C is only a marker of the disease severity or an active counteractor of the detrimental underlying pathophysiology. Considering the numerous beneficial effects of HDL on the cardiovascular system, several potential HDL3-dependent mechanisms might contribute to the improvement of the patients' overall status and thus to a decreased mortality in AHF. Circulating HDL3 particles may exert a direct positive effect on the failing heart, such as improving cell survival, preserving mitochondrial function, attenuating oxidative stress [5,6], as well as promoting glucose uptake by cardiomyocytes [7], and stimulating endothelial NO production [8]. Indeed, it has been shown recently that raising HDL serum levels by overexpression of apoA-I, a major protein constituent of HDL, reduces cardiac hypertrophy and myocardial fibrosis, and improves cardiac function in mice with chronic pressure overload [37].
A previous study has shown that the cholesterol content per HDL-P, calculated as the ratio of HDL-C to HDL-P concentration, is independently associated with progression of carotid atherosclerosis in a cardiovascular disease-free population [38]. In the present study logistic regression analyses revealed a significant positive association of the ratio of total HDL-C to total HDL-P and HDL3-C to small HDL-P, but a significant negative association of the ratio of HDL2-C to large HDL-P with 3-month mortality (Supplemental Table S3). The associations did not remain significant upon adjustment for parameters associated with mortality in HF (Supplemental Table S3). Nevertheless, these data suggest that in AHF, cholesterol overload of small HDL-P renders the particles detrimental and therefore associated with increased mortality, whereas the opposite is the case with large HDL-P.
It remains to be determined whether an HDL raising therapy with niacin or cholesterol ester transfer protein inhibitors would affect the incidence of HF and the mortality rate in AHF patients.
There are several limitation to our present study: The design precludes drawing conclusions about cause and effect for the relationship between HDL and other laboratory and clinical parameters. Accordingly, we can only suggest a link between the serum levels of cholesterol in the HDL subclasses and the pathophysiologic processes in our patients. Therefore, we cannot provide a mechanistic explanation on how HDL3-C levels might affect mortality. Furthermore, in the present study HDL3-C was more strongly correlated with total than with small HDL-P. This, together with the limited specificity of homogenous assays, in particular in dyslipidemic samples, precludes the interpretation that HDL3-C reflects exclusively small HDL particles. In addition, having measured the cholesterol content in the HDL subclasses only at admission we are unable to address the relationship of changes in these parameters with the outcome. Finally, the moderate number of available serum samples (n = 132) in this monocentric study influences the statistical power of our analyses. Therefore, further and larger studies are needed to confirm our results.
6. Conclusions
We conclude that low admission serum levels of HDL3-C are associated with an increased 3-month mortality in AHF patients and that HDL3-C might thus be useful as a prognostic parameter in AHF.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cca.2018.12.020.
Acknowledgements
The authors thank Sabine Paulitsch and Lusik Balayan for their expert technical assistance. The authors also thank Denka Seiken Co., Ltd. (Tokyo, Japan) for the donation of reagents for the determination of the total HDL-C and HDL3-C. Denka Seiken Co., Ltd. (Tokyo, Japan) had no roles in the design of the study, data collection, analysis, and interpretation, report writing or article submission.
Funding
This research was supported by the Austrian Science Fund (P27166-B23 to SF), and the Jubilee Foundation of the Austrian National Bank (15858 to SF). The funders had no roles in the design of the study, data collection, analysis, and interpretation, report writing or article submission.
Abbreviations
- HDL-C
HDL cholesterol
- HDL-P
HDL particle concentrations
- AHF
acute heart failure
- HF
heart failure
- CI
confidence interval
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- GFR
glomerular filtration rate
- ESC
European Society of Cardiology
- MAP
mean arterial pressure
- SPAP
systolic pulmonary artery pressure
- OR
odds ratio
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CRP
C-reactive protein
- IL-6
interleukin-6
Footnotes
Author contributions statement
Conception and designed by: VD SF. Acquisition of data: IP MG HS TS BT. Analysis and interpretation of data: VD GP AB GM SF. Drafting the manuscript: VD GM SF. Revising manuscript for important intellectual content: VD MG IP GP AB HS TS BT GM SF. Final approval of the version to be submitted: VD MG IP GP AB HS TS BT GM SF.
Conflict of interest
None.
Declarations of interest
None.
References
- [1].Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- [2].Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European society of cardiology. Developed in collaboration with the heart failure association of the ESC (HFA) and endorsed by the European society of intensive care medicine (ESICM) Eur Heart J. 2008;29(19):2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- [3].McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European society of cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- [4].Lin L, Liu X, Xu J, Weng L, Ren J, Ge J, Zou Y. High-density lipoprotein inhibits mechanical stress-induced cardiomyocyte autophagy and cardiac hypertrophy through angiotensin II type 1 receptor-mediated PI3K/Akt pathway. J Cell Mol Med. 2015;19(8):1929–1938. doi: 10.1111/jcmm.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gomaraschi M, Calabresi L, Franceschini G. Protective effects of HDL against ischemia/reperfusion injury. Front Pharmacol. 2016;7:2. doi: 10.3389/fphar.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Van Linthout S, Frias M, Singh N, De Geest B. Therapeutic potential of HDL in cardioprotection and tissue repair. Handb Exp Pharmacol. 2015;224:527–565. doi: 10.1007/978-3-319-09665-0_17. [DOI] [PubMed] [Google Scholar]
- [7].Siebel AL, Heywood SE, Kingwell BA. HDL and glucose metabolism: current evidence and therapeutic potential. Front Pharmacol. 2015;6:258. doi: 10.3389/fphar.2015.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, Marcel YL, Anderson RG, Mendelsohn ME, Hobbs HH, Shaul PW. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med. 2001;7(7):853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- [9].Hopf AE, Andresen C, Kotter S, Isic M, Ulrich K, Sahin S, Bongardt S, Roll W, Drove F, Scheerer N, Vandekerckhove L, et al. Diabetes-induced cardiomyocyte passive stiffening is caused by impaired insulin-dependent titin modification and can be modulated by neuregulin-1. Circ Res. 2018;123(3):342–355. doi: 10.1161/CIRCRESAHA.117.312166. [DOI] [PubMed] [Google Scholar]
- [10].Du XM, Kim MJ, Hou L, Le Goff W, Chapman MJ, Van Eck M, Curtiss LK, Burnett JR, Cartland SP, Quinn CM, Kockx M, et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116(7):1133–1142. doi: 10.1161/CIRCRESAHA.116.305485. [DOI] [PubMed] [Google Scholar]
- [11].Camont L, Lhomme M, Rached F, Le Goff W, Negre-Salvayre A, Salvayre R, Calzada C, Lagarde M, Chapman MJ, Kontush A. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities, Arterioscler. Thromb Vasc Biol. 2013;33(12):2715–2723. doi: 10.1161/ATVBAHA.113.301468. [DOI] [PubMed] [Google Scholar]
- [12].Albers JJ, Slee A, Fleg JL, O'Brien KD, Marcovina SM. Relationship of baseline HDL subclasses, small dense LDL and LDL triglyceride to cardiovascular events in the AIM-HIGH clinical trial. Atherosclerosis. 2016;251:454–459. doi: 10.1016/j.atherosclerosis.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Joshi PH, Toth PP, Lirette ST, Griswold ME, Massaro JM, Martin SS, Blaha MJ, Kulkarni KR, Khokhar AA, Correa A, D'Agustino RB, Sr, et al. Lipoprotein Investigators Collaborative Study, Association of high-density lipoprotein subclasses and incident coronary heart disease: the Jackson heart and framingham offspring cohort studies. Eur J Prev Cardiol. 2016;23(1):41–49. doi: 10.1177/2047487314543890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim DS, Burt AA, Rosenthal EA, Ranchalis JE, Eintracht JF, Hatsukami TS, Furlong CE, Marcovina S, Albers JJ, Jarvik GP. HDL-3 is a superior predictor of carotid artery disease in a case-control cohort of 1725 participants. J Am Heart Assoc. 2014;3(3):e000902. doi: 10.1161/JAHA.114.000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Martin SS, Khokhar AA, May HT, Kulkarni KR, Blaha MJ, Joshi PH, Toth PP, Muhlestein JB, Anderson JL, Knight S, Li Y, et al. Lipoprotein Investigators, HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: the Lipoprotein Investigators Collaborative. Eur Heart J. 2015;36(1):22–30. doi: 10.1093/eurheartj/ehu264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Santos-Gallego CG. HDL: Quality or quantity? Atherosclerosis. 2015;243(1):121–123. doi: 10.1016/j.atherosclerosis.2015.08.027. [DOI] [PubMed] [Google Scholar]
- [17].Potocnjak I, Degoricija V, Trbusic M, Pregartner G, Berghold A, Marsche G, Frank S. Serum concentration of HDL particles predicts mortality in acute heart failure patients. Sci Rep. 2017;7 doi: 10.1038/srep46642. 46642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Radulovic B, Potocnjak I, Dokoza Teresak S, Trbusic M, Vrkic N, Malogorski D, Starcevic N, Milosevic M, Frank S, Degoricija V. Hypochloraemia as a predictor of developing hyponatraemia and poor outcome in acute heart failure patients. Int J Cardiol. 2016;212:237–241. doi: 10.1016/j.ijcard.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Potocnjak I, Radulovic B, Degoricija V, Trbusic M, Pregartner G, Berghold A, Meinitzer A, Frank S. Serum concentrations of asymmetric and symmetric dimethylarginine are associated with mortality in acute heart failure patients. Int J Cardiol. 2018;261:109–113. doi: 10.1016/j.ijcard.2018.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Degoricija V, Trbusic M, Potocnjak I, Radulovic B, Teresak SD, Pregartner G, Berghold A, Tiran B, Frank S. Acute heart failure developed as worsening of chronic heart failure is associated with increased mortality compared to de novo cases. Sci Rep. 2018;8(1):9587. doi: 10.1038/s41598-018-28027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].A World Medical. World Medical Association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- [22].Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- [23].Potocnjak I, Degoricija V, Trbusic M, Teresak SD, Radulovic B, Pregartner G, Berghold A, Tiran B, Marsche G, Frank S. Metrics of high-density lipoprotein function and hospital mortality in acute heart failure patients. PLoS One. 2016;11(6):e0157507. doi: 10.1371/journal.pone.0157507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ito Y, Satoh N, Ishii T, Kumakura J, Hirano T. Development of a homogeneous assay for measurement of high-density lipoprotein-subclass cholesterol. Clin Chim Acta. 2014;427:86–93. doi: 10.1016/j.cca.2013.09.009. [DOI] [PubMed] [Google Scholar]
- [25].Freitas HF, Barbosa EA, Rosa FH, Lima AC, Mansur AJ. Association of HDL cholesterol and triglycerides with mortality in patients with heart failure. Braz J Med Biol Res. 2009;42(5):420–425. doi: 10.1590/s0100-879x2009000500004. [DOI] [PubMed] [Google Scholar]
- [26].Mehra MR, Uber PA, Lavie CJ, Milani RV, Park MH, Ventura HO. High-density lipoprotein cholesterol levels and prognosis in advanced heart failure. J Heart Lung Transplant. 2009;28(9):876–880. doi: 10.1016/j.healun.2009.04.026. [DOI] [PubMed] [Google Scholar]
- [27].Cai A, Li X, Zhong Q, Li M, Wang R, Liang Y, Chen W, Huang T, Li X, Zhou Y, Li L. Associations of high HDL cholesterol level with all-cause mortality in patients with heart failure complicating coronary heart disease. Medicine. 2016;95(28):e3974. doi: 10.1097/MD.0000000000003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC, Jr, Grinfeld L, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34(11):835–843. doi: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
- [29].Cagli K, Basar FN, Tok D, Turak O, Basar O. How to interpret liver function tests in heart failure patients? Turk J Gastroenterol. 2015;26(3):197–203. doi: 10.5152/tjg.2015.0086. [DOI] [PubMed] [Google Scholar]
- [30].Anker SD, Chua TP, Ponikowski P, Harrington D, Swan JW, Kox WJ, Poole-Wilson PA, Coats AJ. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96(2):526–534. doi: 10.1161/01.cir.96.2.526. [DOI] [PubMed] [Google Scholar]
- [31].Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52(19):1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- [32].Glezeva N, Gilmer JF, Watson CJ, Ledwidge M. A central role for monocyteplatelet interactions in heart failure. J Cardiovasc Pharmacol Ther. 2016;21(3):245–261. doi: 10.1177/1074248415609436. [DOI] [PubMed] [Google Scholar]
- [33].Rauchhaus M, Coats AJ, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet. 2000;356(9233):930–933. doi: 10.1016/S0140-6736(00)02690-8. [DOI] [PubMed] [Google Scholar]
- [34].McCullough PA. Cardiorenal risk: an important clinical intersection. Rev Cardiovasc Med. 2002;3(2):71–76. [PubMed] [Google Scholar]
- [35].Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, Suzuki E, Shimano H, Yamamoto S, Kondo K, Ohashi Y. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Intern Med. 2007;167(10):999–1008. doi: 10.1001/archinte.167.10.999. [DOI] [PubMed] [Google Scholar]
- [36].Goff DC, Jr, D'Agostino RB, Jr, Haffner SM, Otvos JD. Insulin resistance and adiposity influence lipoprotein size and subclass concentrations. Results from the insulin resistance atherosclerosis study. Metab Clin Exp. 2005;54(2):264–270. doi: 10.1016/j.metabol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- [37].Amin R, Muthuramu I, Aboumsallem JP, Mishra M, Jacobs F, De Geest B. Selective HDL-raising human apo A-I gene therapy counteracts cardiac hypertrophy, reduces myocardial fibrosis, and improves cardiac function in mice with chronic pressure overload. Int J Mol Sci. 2017;9:18. doi: 10.3390/ijms18092012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Qi Y, Fan J, Liu J, Wang W, Wang M, Sun J, Liu J, Xie W, Zhao F, Li Y, Zhao D. Cholesterol-overloaded HDL particles are independently associated with progression of carotid atherosclerosis in a cardiovascular disease-free population: a community-based cohort study. J Am Coll Cardiol. 2015;65(4):355–363. doi: 10.1016/j.jacc.2014.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.