Abstract
Aims
Heart failure (HF) is a major public health issue currently affecting more than 23 million patients world-wide. Hyponatraemia has been shown to be a predictor of poor outcome in patients with acute and chronic HF. Therefore, we aimed at finding a marker for early detection of patients at risk for developing hyponatraemia. To this end, the present study investigated the relationship between initial serum chloride and follow-up sodium levels in acute heart failure (AHF) patients.
Methods and results
The present study was performed as a prospective, single-centre, observational research with a total of 152 hospitalised AHF patients. Compared to patients with initial normochloraemia, patients with initial hypochloraemia had a statistically significantly higher incidence of hyponatraemia after a 3-month follow-up [P < 0.001; odds ratio (OR) = 27.08, CI: 4.3–170.7]. A similar finding was obtained upon exclusion of patients with initial hyponatraemia with Fishers test [P = 0.034; odds ratio (OR) = 15.5, CI:1.7–140.6]. Binary logistic regression revealed a significantly increased in-hospital mortality in the hypochloraemic/normonatriaemic (OR = 4.08, CI 1.08–15.43, P = 0.039), but not in the hypochloraemic/hyponatraemic, normochloraemic/hyponatraemic or normonatriaemic/normochloraemic patients. Ejection fraction (EF) at admission was significantly higher in hypochloraemic/normonatriaemic, compared to normonatriaemic/normochloraemic patients, but similar to EF in both hypochloraemic/hyponatraemic and normochloraemic/hyponatraemic patients. The N-terminal precursor Brain Natriuretic Peptide (Nt-proBNP) levels at admission were significantly lower in hypochloraemic/normonatriaemic compared to hypochloraemic/hyponatraemic and normonatriaemic/normochloraemic patients, respectively.
Conclusion
The data show that initial low serum chloride concentration is predictive of developing hyponatraemia and associated with increased in-hospital mortality in AHF patients.
Keywords: Acute heart failure, Hyponatraemia, Hypochloraemia, Survival
1. Introduction
Due to longer life expectancy, heart failure (HF) has become a growing health issue currently affecting more than 23 million patients worldwide, and, consequently, emerging as the topic of many interesting and intriguing studies [1]. For the last ten years, it has been a well-known and established fact that hyponatraemia in chronic and AHF acute heart failure is a predictor of poor outcome [2–4]. Due to its importance, the level of serum sodium has been incorporated in predictive models for heart failure such as the Heart Failure Survival Score and the Seattle Heart Failure Model [5,6]. Hyponatraemia is not only a nemesis in heart failure, but also a predictor of poor outcome in pneumonia [7], cirrhosis [8,9] and neurological disorders [10]. The prevalence of dilutional (hypervolaemic) hyponatraemia, defined as a sodium value lower than 136 mmol/L, in AHF is 15–20% [3,11]. Numerous studies have shown that during HF exacerbation patients with hyponatraemia have higher in-hospital and post-discharge mortality, longer hospital stay and higher incidence of rehospitalisation due to recurrence of heart failure [2–4,12,13]. Interestingly, this indicator is independent of ejection fraction (EF) [2]. Understandably, researchers have directed their focus on finding an optimal treatment for correcting this independent prognostic indicator of morbidity and mortality in patients with AHF. Although one would presume, that the correction of sodium levels in AHF patients with hyponatraemia would benefit the patient, studies have shown the opposite [14]. Since 1992, several non-peptide vasopressin receptor antagonists (vaptans) have been developed in an attempt to treat hyponatraemia [15,16]. In Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Congestive Heart Failure (ACTIV in CHF) [11] and Effects of Oral Tolvaptan in Patients Hospitalised for Worsening Heart Failure (EVEREST) [17], studies performed on patients with AHF, vaptans did provide some clinical benefit in terms of the form of shorter hospital stay, but did not lower the incidence of total and cardiovascular mortality. Although it is still unknown whether hyponatraemia is just a marker or the core of the problem of poor outcome in patients with HF, one is certain: early detection of patients at risk for developing hyponatraemia could help to initiate earlier intervention and thereby reduce mortality in hyponatraemic AHF patients. Therefore, the present study investigated whether initial low serum chloride concentration is predictive of developing hyponatraemia in AHF patients.
2. Methods
2.1. Study design and patients
The study was performed as a prospective, single-centre, observational research study including consecutive adult hospitalised AHF patients. The study was approved by the local Ethics Committee of the University Hospital Centre Sisters of Charity, Zagreb, Croatia (UHC SC). Written informed consent was obtained from each patient, and the investigation conformed with the principles outlined in the Declaration of Helsinki [18].
2.2. Data collection
In total, 152 patients were recruited from the Emergency Department of University Hospital Centre Sisters of Charity from November 2013 to February 2015. The patients were defined and categorised according to the European Society of Cardiology (ESC), the American College of Cardiology Foundation and the American Heart Association Guidelines (ACCF/AHA) classification for AHF, the initial values of chloride, the final clinical presentation and the EF. All patients were treated in accordance with the standard ESC guidelines for AHF [19]. Patients with renal replacement therapy, hepatic cirrhosis, malignancy, trauma, surgical diseases, pregnancy, patients younger than 18 years and those who had major systemic diseases were not included. For each patient, the collected data included: demographic profile, past medical history and vital signs and symptoms at admission. Blood samples for routine laboratory assays with blood gas analysis were obtained at admission. Electrocardiography, chest radiography and echocardiography were performed in the first 24 h of hospitalisation. Patients were followed up on the 2nd, 3rd and 7th days of hospitalisation, as well as 3 months post-hospitalisation. Follow-up included collecting data about vital signs, symptoms, daily diuresis, electrocardiography, serum electrolytes, creatinine, urea and blood gas analysis.
2.3. Laboratory assays
Whole blood samples were drawn by venepuncture, and blood analysis (including complete blood count, blood gas analysis, metabolic and electrolyte panel, urea nitrogen, creatinine, lipid profile, C-reactive protein, urinalysis and NT-proBNP) was done at the time of admission. Serum electrolyte, creatinine, urea, total plasma cholesterol, LDL cholesterol, HDL cholesterol and triglycerides were measured using a Beckman Coulter instrument AU 2700, 2007 (Brea, CA, US) and Architect c8000, Abbott 2013 (Chicago, IL, US). Total blood count was automatically counted by Coulter:Counter S plus junior (Coulter Electronics Limited, Luton, England). Nt-proBNP was determined by electrochemiluminescence immunoassay (ECLIA) with Elecsys e411 (Roche Diagnostics GmbH, Mannheim, Germany). The results were shown in pg/mL.
2.4. Statistical analysis
The type of distribution for quantitative data was assessed by the Kolmogorov–Smirnov test and according to the results, appropriate, mainly nonparametric, statistical tests were used. Quantitative data are presented by the median and interquartile ranges. Categorical variables are presented in absolute frequencies and related interests. The difference in quantitative data between the groups was assessed by the Mann–Whitney U or Kruskal–Wallis tests for assessing data among more than three groups. The difference in categorical data was analysed by the χ2 test. Spearman's coefficient of rank correlation was calculated for the difference between clinical features of patients. Binary logistic regression was used to assess the prediction of in-hospital and 3-month mortality. The differences were considered significant if the P value was <0.05. The statistical analysis was done with MedCalc statistical software for Windows, version 15.1 (MedCalc Software, Ostend, Belgium).
3. Results
From November 2013 to February 2015, a total of 152 patients were hospitalised for AHF, 52% of which were female. The median age was 77 years (70–82). 14% of the studied patients had hyponatraemia at admission. Worsening of chronic HF was present in the majority (69%) of the patients. The most frequently recorded comorbidities were hypertension (89.5%), metabolic syndrome (55.9%) and type 2 diabetes mellitus (51.7%). The patients' baseline characteristics are presented in Table 1. After a 3-month follow-up, 7 patients from 152 in our study were alive, but not reachable.
Table 1. Baseline characteristics, biochemical laboratory parameters, comorbidities, classification and outcome of AHF patients (n = 152).
| Baseline characteristics | ||
| Age (years) | 77 (70–82) | |
| Sex | Male | 73 (48%) |
| Female | 79 (52%) | |
| NYHA 2 | 11 (7.2%) | |
| NYHA 3 | 83 (54.6%) | |
| NYHA 4 | 58 (38.2%) | |
| Classification of acute heart failure by time of onset | ||
| Worsening of chronic heart failure | 105 (69.1%) | |
| De novo | 47 (30.9%) | |
| Classification of acute heart failure by Ejection Fraction* | ||
| HFrEF | 83 (57.6%) | |
| HFpEF | 61 (42.4%) | |
| EF (%) | 45 (35–55) | |
| Outcome of hospitalisation | ||
| Improvement | 130 (85.5%) | |
| Death | ICU | 12 (7.9%) |
| Ward | 10 (6.6%) | |
| Vital signs and symptoms on admission | ||
| Systolic blood pressure (mmHg) | 140 (120–160) | |
| Diastolic blood pressure (mmHg) | 80 (80-100) | |
| Heart rate | 100 (80–120) | |
| Respiratory rate | 28 (21–32) | |
| Dyspnoea | 143 (94.1%) | |
| Peripheral oedema | 82 (54.7%) | |
| Syncope | 8 (5.3%) | |
| Biochemical laboratory parameters at admission | ||
| Sodium (mmol/L) | 140 (138–143) | |
| Potassium (mmol/L) | 4.4 (3.9–4.7) | |
| Chloride (mmol/L) | 104 (100–106) | |
| Creatinine (μmol/L) | 106 (87–138) | |
| Blood urea nitrogen (mmol/L) | 8 [6–13] | |
| Nt-proBNP (pg/mL)** | 9494 (3629–17,325) | |
AHF — Acute Heart Failure.
HFpEF — Heart Failure with preserved Ejection Fraction.
HFrEF — Heart Failure with reduced Ejection Fraction.
NYHA — New York Heart Association Functional Classification.
Nt-proBNP (pg/mL) - N-terminal pro-Brain Natriuretic Peptide.
Based on measurements in 144 patients.
Based on measurements in 147 patients.
After a 3-month follow-up, out of the 11 patients with hypochloraemia at admission, 5 patients (45.5%) developed hyponatraemia, whereas out of 67 patients without hypochloraemia at admission, only 2 patients (3%) developed hyponatraemia (Table 2). X2 test showed that hypochloraemia at admission and hyponatraemia after 3 months are statistically significantly associated (P < 0.001), with an odds ratio (OR) of 27.08 (95 CI: 4.3–170.7) (Table 2). To further investigate the relationship between sodium and chloride in normonatraemic patients, we excluded the initially hyponatraemic patients (19 patients, 13%) and calculated by Fisher exact test the chance to develop hyponatraemia, for the initially normochloraemic/normonatraemic as well as hypochloraemic/normonatraemic patients. While after a 3-month follow-up 2 patients (3.1%) out of 64 normochloraemic/normonatraemic patients developed hyponatraemia, out of 6 hypochloraemic/normonatraemic patients 2 patients (33.3%) developed hyponatraemia (P = 0.034, OR = 15.5; CI 1.7–140.6;) (Table 3).
Table 2. Association between patients with initial hypochloraemia and 3-month hyponatraemia.
| Without hyponatraemia after 3 months | Hyponatraemia after 3 months | P | OR | 95% CI | |
|---|---|---|---|---|---|
| Without hypochloraemia at admission | 65 (97.0%) | 2 (3.0%) | < 0.001 | 27.1 | 4.3–170.7 |
| Hypochloraemia at admission | 6 (54.5%) | 5 (45.5%) |
Association was calculated with X2 test. OR = odds ratio; CI = confidence interval.
Table 3. Association between initial hypochloraemia and 3-month hyponatraemia in initially normonatriaemic patients.a*.
| Without hyponatraemia after 3 months * | Hyponatraemia after 3 months* | P | OR | 95% CI | |
|---|---|---|---|---|---|
| Without hypochloraemia with normonatraemia | 62 (96.9%) | 2 (3.1%) | 0.034 | 15.5 | 1.7–140.6 |
| Hypochloraemia with normonatraemia at admission | 4 (66.6%) | 2 (33.3%) |
Association was calculated with Fisher exact test. OR = odds ratio; CI = confidence interval.
Without patients with initial hyponatraemia.
Binary logistic regression of in-hospital outcome demonstrated that in contrast to hypochloraemic/hyponatraemic as well as normochloraemic/hyponatraemic patients, the patients hypochloraemic/normonatraemic at admission exhibited statistically significantly higher in-hospital mortality (OR 4.08, CI 1.08–15.43, P = 0.039) (Table 4).
Table 4. Association of in-hospital mortality with sodium/chloride status at admission.
| Death/Total (N) |
OR | 95% CI | P | |
|---|---|---|---|---|
| Normonatraemia and normochloraemia (ref.) | 13/119 | 0.130 | ||
| Hypochloraemia and hyponatraemia | 3/13 | 2.45 | 0.60–10.05 | 0.215 |
| Hypochloraemia and normonatraemia | 4/12 | 4.08 | 1.08–15.43 | 0.039 |
| Normochloraemia and hyponatraemia | 2/8 | 2.72 | 0.50–14.89 | 0.249 |
Association of in-hospital mortality was calculated with binary logistic regression. N = total number of patients in category; OR = odds ratio; CI = confidence interval.
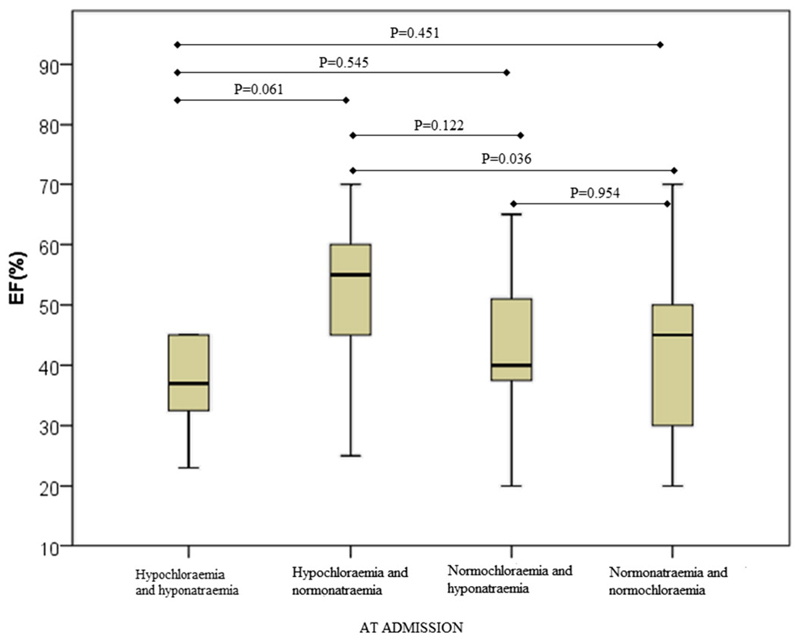
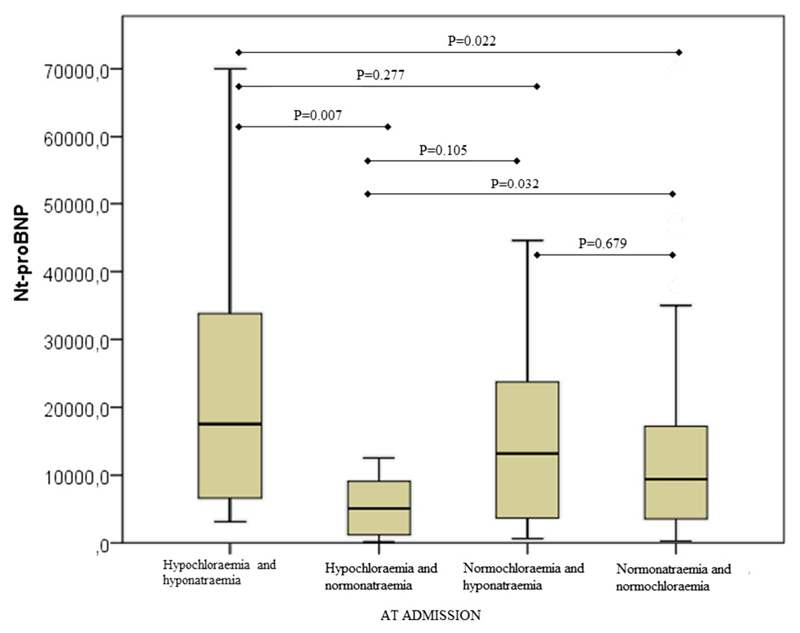
Investigation of EF in various groups showed that EF was statistically significantly different only between hypochloraemic/normonatraemic and normochloraemic/normonatraemic patients (test and control groups) (Fig. 1). Next, we examined the differences in Nt-proBNP plasma concentrations. As shown in Fig. 2 the Nt-proBNP levels were significantly higher in hypochloraemic/hyponatraemic (median 17,527 pg/mL; IQR = 6081–39,045 pg/mL) compared to both hypochloraemic/normonatraemic (median = 5101 pg/mL; IQR = 1151–9965 pg/mL) and normonatraemic/normochloraemic patients (median = 9410,50 pg/mL; IQR = 3501–17,238 pg/mL). Moreover, the Nt-proBNP levels were significantly lower in hypochloraemic/normonatraemic (median = 5101 pg/mL; IQR = 1151–9965 pg/mL) compared to normonatraemic/normochloraemic patients (median = 9410,50 pg/mL; IQR = 3501–17,238 pg/mL).
Fig. 1.
EF in AHF patients with different sodium/chloride status at admission. EF was measured by echocardiography and data analysed by post-hoc Mann–Whitney U test and shown with Box and Whiskers plots.
Fig. 2.
Nt-proBNP levels in AHF patients with different sodium/chloride status at admission. Nt-proBNP concentrations were measured by ECLIA and data analysed by post-hoc Mann–Whitney U test and shown with Box and Whiskers plots.
4. Discussion
During the 1990s, chloride was called the “queen of electrolytes “due to its role in the then relatively new approach to acid–base pathophysiology, the Stewart approach [20,21]. Although chloride was the first electrolyte in our bodies that could be quantified, its importance had always been overshadowed by other serum electrolytes like sodium, potassium and calcium. This was emphasised in an article written by Yunos et al. In 2010, when those authors searched PubMed for the term ‘hyperchloraemia’, it generated only 181 citations, while ‘hypernatraemia’ and ‘hypercalcaemia’ generated 2481 and 15,518 citations, respectively [22].
Searching the relevant literature related to AHF with hyponatraemia, we could not find results that included concentrations of chloride and their statistical interpretation. Nevertheless, we did find an article by Tani, Morimatsu, Takatsu and Morita [23], who investigated the incidence and prognostic value of hypochloraemia in critically ill patients. They found that patients hospitalised in ICU with hypochloraemia had statistically significantly greater in-hospital mortality and a longer hospital stay. While hyponatraemia was associated with hypochloraemic patients, remarkably, not all patients with hypochloraemia had hyponatraemia, as found in dilatational hypochloraemia [24]. Using the Stewart approach, the authors revealed that in all patients with alkalosis, the alkalosis was caused by hypochloraemia. This study showed that hyponatraemia is not always accompanied by hypochloraemia and that chloride, although undeniably involved in acid–base and sodium disorders, has its own physiology that has not been completely elucidated yet.
In the present study we examined whether chloride is relevant as a predictor for patients developing hyponatraemia in HF. The data showed that patients who were initially hypochloraemic had a 27-fold increased chance of developing hyponatraemia after 3 months. As one can argue that patients with low serum chloride develop hyponatraemia more frequently than patients without hypochloraemia, we selected only patients who had hypochloraemia with normal sodium levels at admission and compared that group with a group of patients who had normal levels of sodium and chloride. It turned out that after a 3-month follow-up, the patients in the hypochloraemic group with initially normal sodium levels also developed hyponatraemia more frequently than normochloraemic/normonatraemic group. The results show that chloride could be a predictor for increased incidence of hyponatraemia in AHF, opening a whole range of possibilities for physicians to react timely and favourably. In the article by Yoo et al., the outcome of patients after hospitalisation with improved hyponatraemia did not differ from the outcome in those with persistent hyponatraemia [25]. Lee et al. reported similar results showing no difference in mortality or rehospitalisation between patients with improved hyponatraemia during hospitalisation and those without [14]. According to these results, although hyponatraemia is a predictor of greater in-hospital and post-discharge mortality and rehospitalisation, short-term improvement of already evolved hyponatraemia seems to be ineffective in term of improving outcome. However, it is tempting to assume that the efficacy of therapeutic intervention might be improved if applied before the development of hyponatraemia.
In our study, in-hospital mortality was higher in the group with hypochloraemia and normal sodium, however we found no impact of hypochloraemia on 3-month mortality. We could argue that patients with hypochloraemia are obviously a high-risk group with higher short-term mortality and a long-term predisposition of developing hyponatraemia. Interestingly, compared to the control group of patients with both normal sodium and chloride levels, our test group, namely hypocloraemic patients with normonatraemia had lower initial Nt-proBNP plasma levels and higher EF, but a higher in-hospital mortality. Based on this observation it is tempting to speculate that the treatment with vaptans could be used in those patients to prevent hyponatraemia and thereby associated poor outcome.
To our knowledge, this is the first study that investigated the relationship between chloride- and sodium levels in HF. We propose a novel use of chloride as a predictor for the development of hyponatraemia and of poor outcome in patients with AHF.
4.1. Study strengths and limitations
The study strengths lie in a structured and uniformed protocol with well-defined data collection and statistical analysis. The subject has never been discussed and investigated so far. However, the overall sample size was modest in the term of studies with acute heart failure patients. The main problem lies in the inability of most patients to maintain a proper follow-up, due to the overall poor general condition of patients with HF.
5. Conclusion
Chloride, a long forgotten electrolyte, emerges as a possible predictor for the development of hyponatraemia in patients with AHF. The optimal window of opportunity to treat patients with HF would be before the onset of hyponatraemia. The present study shows that chloride could be a useful predictor for developing hyponatraemia and could therefore be used to start appropriate therapy early enough to prevent development of hyponatraemia and thereby associated complications and poor outcome. The main advantage of that chloride-status based diagnostics is its wide availability, practicability and low cost. Considering our modest sample size, further larger studies should be done to confirm our findings.
Acknowledgements
We thank Aleksandra Žmegač Horvat, University of Zagreb School of Medicine, for language editing the text.
Funding
This work was supported by the Austrian Science Foundation [P27166-B23 to S.F., and the Jubilee Foundation of the Austrian National Bank [15858 to S.F.].
Abbreviations
- HF
heart failure
- AHF
acute heart failure
- NYHA
New York Heart Association
- EF
ejection fraction
- ESC
European Society of Cardiology
- ACCF/AHA
American College of Cardiology Foundation/American Heart Association
- NT-proBNP
N-terminal pro-Brain Natriuretic Peptide.
Footnotes
Conflicts of interest
None declared. The authors declare that they have no financial or any other conflict of interest. The authors declare no external financial support in producing this article. The authors and investigators declare no potential conflict of interest relevant to this article.
References
- [1].Shahar E, Lee S, Kim J, Duval S, Barber C, Luepker RV. Hospitalized heart failure: rates and long-term mortality. J Card Fail. 2004;10:374–379. doi: 10.1016/j.cardfail.2004.02.003. [DOI] [PubMed] [Google Scholar]
- [2].Rusinaru D, Tribouilloy C, Berry C, Richards AM, Whalley GA, Earle N, et al. Relationship of serum sodium concentration to mortality in a wide spectrum of heart failure patients with preserved and with reduced ejection fraction: an individual patient data meta-analysis(†): Meta-Analysis Global Group in Chronic heart failure (MAGGIC) Eur J Heart Fail. 2012;14:1139–1146. doi: 10.1093/eurjhf/hfs099. [DOI] [PubMed] [Google Scholar]
- [3].Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O'Connor CM, et al. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007;28:980–988. doi: 10.1093/eurheartj/ehl542. [DOI] [PubMed] [Google Scholar]
- [4].Corona G, Giuliani C, Parenti G, Norello D, Verbalis JG, Forti G, et al. Moderate hyponatremia is associated with increased risk of mortality: evidence from a meta-analysis. PLoS One. 2013;8:e80451. doi: 10.1371/journal.pone.0080451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, et al. The Seattle heart failure model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- [6].Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–2667. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- [7].Krüger S, Ewig S, Giersdorf S, Hartmann O, Frechen D, Rohde G, et al. Dysnatremia, vasopressin, atrial natriuretic peptide and mortality in patients with community-acquired pneumonia. Respir Med. 2014;108:1696–1705. doi: 10.1016/j.rmed.2014.09.014. [DOI] [PubMed] [Google Scholar]
- [8].Degoricija V. Uloga sekundarnog hiperaldosteronizma i atrijskog natriuretskog peptida u održavanju ravnoteže soli i ishodu bolesti u cirozi jetre(PhD thesis) University of Zagreb; Croatia: 2004. [Google Scholar]
- [9].Degoricija V, Zjacic-Rotkvic V, Marout J, Sefer S, Troskot B. Clinical and neurohumoral response to posture, physical exercise, and ascites treatment in Child-Pugh C liver cirrhosis: randomized prospective trial. Croat Med J. 2003;44:178–186. [PubMed] [Google Scholar]
- [10].Dong KK, Kwon WJ. Hyponatremia in patients with neurologic disorders. Electrolyte Blood Press. 2009;7:51–57. doi: 10.5049/EBP.2009.7.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rossi J, Bayram M, Udelson JE, Lloyd-Jones D, Adams KF, Oconnor CM, et al. Improvement in hyponatremia during hospitalization for worsening heart failure is associated with improved outcomes: insights from the Acute and Chronic Therapeutic Impact of a Vasopressin antagonist in Chronic Heart Failure (ACTIV in CHF) trial. Acute Card Care. 2007;9:82–86. doi: 10.1080/17482940701210179. [DOI] [PubMed] [Google Scholar]
- [12].Deubner N, Berliner D, Frey A, Güder G, Brenner S, Fenske W, et al. Dysnatraemia in heart failure. Eur J Heart Fail. 2012;14:1147–1154. doi: 10.1093/eurjhf/hfs115. [DOI] [PubMed] [Google Scholar]
- [13].Lee CR, Watkins ML, Patterson JH, Gattis W, O'Connor CM, Gheorghiade M, et al. Vasopressin: a new target for the treatment of heart failure. Am Heart J. 2003;146:9–18. doi: 10.1016/S0002-8703(02)94708-3. [DOI] [PubMed] [Google Scholar]
- [14].Lee SE, Choi D-J, Yoon C-H, Oh I-Y, Jeon E-S, Kim J-J, et al. Improvement of hyponatraemia during hospitalisation for acute heart failure is not associated with improvement of prognosis: an analysis from the Korean Heart Failure (KorHF) registry. Heart. 2012;98:1798–1804. doi: 10.1136/heartjnl-2012-302334. [DOI] [PubMed] [Google Scholar]
- [15].Yamamura Y, Ogawa H, Yamashita H, Chihara T, Miyamoto H, Nakamura S, et al. Characterization of a novel aquaretic agent, OPC-31260, as an orally effective, nonpeptide vasopressin V2 receptor antagonist. Br J Pharmacol. 1992;105:787–791. doi: 10.1111/j.1476-5381.1992.tb09058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yamamura Y, Nakamura S, Itoh S, Hirano T, Onogawa T, Yamashita T, et al. OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther. 1998;287:860–867. [PubMed] [Google Scholar]
- [17].Konstam MA, Gheorghiade M, Burnett JC, Grinfeld L, Maggioni AP, Swedberg K, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- [18].World Medical Association. World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. JAMA. 2013:E1–E4. doi: 10.1001/jama.2013.281053. (JAMA Publi(jama.com)) [DOI] [PubMed] [Google Scholar]
- [19].Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- [20].Fencl V, Leith DE. Stewart's quantitative acid–base chemistry: applications in biology and medicine. Respir Physiol. 1993;91:1–16. doi: 10.1016/0034-5687(93)90085-o. [DOI] [PubMed] [Google Scholar]
- [21].Stewart PA. Modern quantitative acid–base chemistry. Can J Physiol Pharmacol. 1983;61:1444–1461. doi: 10.1139/y83-207. [DOI] [PubMed] [Google Scholar]
- [22].Yunos NM, Bellomo R, Story D, Kellum J. Bench-to-bedside review: chloride in critical illness. Crit Care. 2010;14:226. doi: 10.1186/cc9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tani M, Morimatsu H, Takatsu F, Morita K. The incidence and prognostic value of hypochloremia in critically ill patients. Scientific World Journal. 2012;2012 doi: 10.1100/2012/474185. 474185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Berend K, Van Hulsteijn LH, Gans ROB. Chloride: the queen of electrolytes? Eur J Intern Med. 2012 Apr;23(3):203–211. doi: 10.1016/j.ejim.2011.11.013. [DOI] [PubMed] [Google Scholar]
- [25].Yoo B, Park JJ, Choi D, Kang S, Hwang J, Lin S. Prognostic Value of Hyponatremia in Heart Failure Patients : An Analysis of the Clinical Characteristics and Outcomes in the Relation with Serum Sodium Level in Asian Patients Hospitalized for Heart Failure (COAST) study. 2015:460–470. doi: 10.3904/kjim.2015.30.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]