Summary
Both major subcategories of inflammatory bowel disease (IBD), ulcerative colitis and Crohn’s disease are characterized by infiltration of the gut wall by inflammatory effector cells and elevated biomarkers of inflammation in blood and feces. We investigated the phenotypes of circulating lymphocytes in the two types of IBD in treatment‐naive pediatric patients by analysis of blood samples by flow cytometry. Multivariate analysis was used to compare the phenotypes of the blood lymphocytes of children with ulcerative colitis (n = 17) or Crohn’s disease (n = 8) and non‐IBD control children with gastrointestinal symptoms, but no signs of gut inflammation (n = 23). The two IBD subcategories could be distinguished based on the results from the flow cytometry panel. Ulcerative colitis was characterized by activated T cells, primarily in the CD8+ population, as judged by increased expression of human leukocyte antigen D‐related (HLA‐DR) and the β1‐integrins [very late antigen (VLA)] and a reduced proportion of naive (CD62L+) T cells, compared with the non‐IBD controls. This T cell activation correlated positively with fecal and blood biomarkers of inflammation. In contrast, the patients with Crohn’s disease were characterized by a reduced proportion of B cells of the memory CD27+ phenotype compared to the non‐IBD controls. Both the patients with ulcerative colitis and those with Crohn’s disease showed increased percentages of CD23+ B cells, which we demonstrate here as being naive B cells. The results support the notion that the two major forms of IBD may partially have different pathogenic mechanisms.
Keywords: CD27+ B cells, CD23+ B cells, Crohn’s disease, pediatric, ulcerative colitis
Introduction
Inflammatory bowel disease (IBD) is a chronic, systemic, inflammatory condition that affects the gastrointestinal tract. While the etiology of IBD remains unclear, it is thought to involve a complex interplay between genetic predisposition, gut microbiota composition and dysregulated immune responses. In IBD, the gut mucosa is heavily infiltrated by cytokine‐producing effector T cells 1 and immunoglobulin‐producing plasma cells 2. The invading T cells have an activated phenotype that is associated with the production of T helper type 1 (Th1) and Th17 cytokines 1, 3, 4. Immunoglobulin (Ig)‐producing cells of all isotypes are increased in density, especially the IgG‐producing plasma cells. Among the IgA‐producing cells, those that produce IgA1 and monomeric IgA are selectively increased in density 5. Systemic markers of inflammation may also be present, exemplified by acute‐phase reactants such as C‐reactive protein (CRP), orosomucoid and increased erythrocyte sedimentation rate (ESR) 6. The numbers of blood leukocytes and thrombocytes may be increased in patients with IBD due to cytokine‐driven acceleration of bone marrow synthesis 6, 7. Conversely, the levels of serum albumin and blood hemoglobin may decrease as a function of the acute‐phase reaction 8 and/or due to the loss of blood and proteins across the inflamed gut wall.
IBD encompasses two major subcategories: ulcerative colitis and Crohn’s disease. In ulcerative colitis the intestinal inflammatory infiltrate is restricted to the colonic mucosa, while in Crohn’s disease it may occur at any site along the gastrointestinal tract and often involves the entire gut wall 9. Granulomas, i.e. aggregates of activated macrophages some of which coalesce into multi‐nucleate giant cells, are a hallmark of Crohn’s disease, but they are not found in all patients with the disease 10.
Numerous studies have investigated the potential differences in immune effector mechanisms between the two subcategories of IBD. Although IgG‐producing plasma cells are markedly increased in the inflamed gut mucosa in both diseases, IgG1‐producing cells appear to be particularly increased in ulcerative colitis, whereas IgG2‐producing plasma cells are present in increased proportions in Crohn’s disease 11, 12. Regarding T cell phenotypes, global gene expression studies of inflamed mucosa have shown very similar patterns in ulcerative colitis and Crohn’s disease, characterized by Th1/Th17 differentiation and a low level of Th2 differentiation 4.
Regarding blood lymphocyte subsets, previous research has demonstrated an increase of circulating activated T cells in patients with IBD 13. Blood lymphocytes comprise a mixture of circulating naive and memory T and B lymphocytes, as well as primed effector cells that are en route to the inflamed gut mucosa. Naive T lymphocytes express L‐selectin (CD62L) 14 and CD45RA. Upon encountering the cognate antigen in the secondary lymphoid organs CD62L and CD45RA disappear, while activation markers such as human leukocyte antigen D‐related (HLA‐DR) and the memory marker CD45RO appear, together with members of the very late antigen (VLA) family, or β1‐integrins, which facilitate mobilization of the effector cells from the blood vessels into inflamed tissues 15. B cells are not fully developed when they leave the bone marrow, but undergo a series of transitional phases before they reach the mature naive stage 16. Transitional B cells have the CD24highCD38high phenotype and CD5 and CD23 have also been proposed as markers for the identification of transitional and/or naive B cells 16, 17. B cells that have encountered their cognate antigen and been converted to memory cells express CD27. A larger fraction of transitional B cells 18 and a smaller fraction of IgM‐positive memory B cells have been reported in patients with IBD, compared to healthy controls 19, 20.
Approximately one‐quarter of IBD cases present during childhood and adolescence 21. Compared to adult‐onset IBD, childhood‐onset IBD exhibits more extensive inflammation and pediatric Crohn’s disease more often affects the colon 22. Regarding the etiology of IBD, cases with onset in childhood may be especially interesting to study, as the disease is less likely to have been longstanding and co‐existing disorders and medication are less common in children than in adults.
In the present study, we tested the hypothesis that ulcerative colitis and Crohn’s disease may exhibit a distinct pattern of circulating T and B lymphocytes. Blood samples from children with newly diagnosed active, untreated IBD were analyzed regarding lymphocytic markers of naivety, activation and memory using flow cytometry panels.
Patients and methods
Patients
Children with suspected IBD who were referred to the Pediatric Gastroenterology Unit at the Sahlgrenska University Hospital (Göteborg, Sweden) were eligible for the study. Exclusion criteria were intake of antibiotics, anti‐inflammatory drugs or probiotics or any dietary restrictions during the previous 3 months. All the children included in the study underwent a diagnostic work‐up, which included esophagogastroduodenoscopy, ileocolonoscopy and small bowel imaging, and were diagnosed according to the Porto criteria 22. Blood samples for the study were obtained and analyzed by flow cytometry before diagnosis and treatment. Flow cytometric analysis, including gating and calculation of lymphocyte subset fractions, were performed by researchers blinded to the diagnosis of the patients and the diagnostic code was not broken until completion of these analyses.
The following children, in the age range of 5–16 years, were included; 17 with ulcerative colitis, eight with Crohn’s disease (described in detail in Table 1) and 23 symptomatic non‐IBD controls. The non‐IBD controls comprised children referred to the Pediatric Gastroenterology Unit due to suspected IBD, but in whom the IBD diagnosis was refuted after diagnostic work‐up. These patients showed no macroscopic or microscopic signs of inflammation in the gastrointestinal tract and were found to have functional gastrointestinal disorders. Predominantly, these patients were diagnosed with IBS and a few with functional diarrhea 23.
Table 1.
Clinical and inflammatory characteristics of children with ulcerative colitis and Crohn’s disease
| Clinical characteristics | Disease distribution/behavior | Biomarkers of inflammation | |||||
|---|---|---|---|---|---|---|---|
| Disease subcategory | Sex (male/female) | Age (years) | CRP (mg/l) | ESR (mm) | Orosomucoid (g/l) | F‐calprotectin (mg/kg) | |
| Ulcerative colitis | M | 15 | Pancolitis | 5 | 23 | 0·86 | 9 |
| Ulcerative colitis | F | 15 | Pancolitis | 5 | 29 | 1·3 | 2056 |
| Ulcerative colitis | M | 14 | Pancolitis | 5 | 52 | 1·2 | 1821 |
| Ulcerative colitis | M | 16 | Pancolitis | 5 | 11 | 0·77 | 841 |
| Ulcerative colitis | M | 10 | Pancolitis | 66 | 28 | 1·9 | 420 |
| Ulcerative colitis | M | 15 | Pancolitis | 5 | 5 | 0·77 | 337 |
| Ulcerative colitis | M | 15 | Pancolitis | 33 | 13 | 2·6 | 1737 |
| Ulcerative colitis | F | 10 | Pancolitis | 5 | 11 | 1·2 | 1917 |
| Ulcerative colitis | M | 14 | Pancolitis | 5 | 5 | 0·96 | No data |
| Ulcerative colitis | M | 15 | Pancolitis | 6 | 28 | 1 | 2261 |
| Ulcerative colitis | M | 16 | Pancolitis | 13 | 20 | 1·6 | 1388 |
| Ulcerative colitis | F | 16 | Pancolitis | 5 | 8 | 1·1 | 840 |
| Ulcerative colitis | M | 16 | Pancolitis | 5 | 21 | 0·86 | 2673 |
| Ulcerative colitis | F | 14 | Pancolitis | 6 | 27 | 1·4 | 1541 |
| Ulcerative colitis | M | 9 | Pancolitis | No data | 3 | 0·79 | 912 |
| Ulcerative colitis | F | 15 | Pancolitis | 5 | 21 | 1·0 | 1485 |
| Ulcerative colitis | F | 16 | Proctitis | 5 | 1 | 1·2 | 202 |
| Crohn’s disease | M | 8 | Ileum, colon, perianal, stricturing | 58 | 34 | 2·0 | 1612 |
| Crohn’s disease | F | 12 | Ileum, colon, non‐stricturing | 53 | 20 | 2·2 | 691 |
| Crohn’s disease | F | 11 | Ileum, colon, stricturing | 68 | 34 | 2·8 | 1862 |
| Crohn’s disease | M | 11 | Ileum, perianal, non‐stricturing, OFGa | 19 | 16 | 1·4 | 1394 |
| Crohn’s disease | F | 16 | Ileum, colon, non‐stricturing | 13 | 10 | 1·6 | 738 |
| Crohn’s disease | M | 6 | Ileum, colon, non‐stricturing | 5 | 30 | 1·6 | 1220 |
| Crohn’s disease | M | 12 | Ileum, colon, non‐stricturing | 13 | 30 | 1·3 | 1418 |
| Crohn’s disease | F | 16 | Ileum, colon, non‐stricturing | 63 | 24 | 2·3 | 1078 |
Orofacial granulomatosis (OFG), an oral pathological manifestation associated with Crohn’s disease 49. ESR = erythrocyte sedimentation rate; CRP = C‐reactive protein.
The clinical characteristics of the IBD patients are shown in Table 1. Nearly all the patients with ulcerative colitis had pancolitis. The Crohn’s disease patients frequently had colon involvement, but none had perianal disease. Six of the children with Crohn’s disease displayed granuloma formation at the time of diagnosis. However, within 2 years of the diagnosis being made, granulomas were found in all the patients. In addition, one Crohn’s patient also presented with orofacial granulomatosis, an inflammatory lesion in the oral region that may be associated with Crohn’s disease 24 (Table 1).
The IBD study was approved by the Ethics Committee at the University of Gothenburg, Sweden. Written informed consent was obtained from the parents of the children.
Inflammatory markers in blood and feces
Blood samples collected at the same time‐point as blood assessed for lymphocyte subsets using flow cytometry (see below) were analyzed for the acute‐phase reactants CRP, orosomucoid and ESR, as well as the negative acute‐phase reactants hemoglobin and albumin, the levels of which decrease with inflammation 6. Furthermore, the numbers of blood leukocytes and thrombocytes were also measured. All analyses were carried out using accredited methodology at the Clinical Chemistry Laboratory, Sahlgrenska University Hospital.
For assessment of mucosal inflammation, fecal samples collected from all the patients before diagnosis and treatment were evaluated for the levels of fecal calprotectin, lactoferrin, myeloperoxidase (MPO) and eosinophil cationic protein (ECP). Analyses were performed at the Clinical Immunology Laboratory, Sahlgrenska University Hospital.
Flow cytometry of blood samples
Phenotype analyses of CD4+ and CD8+ T lymphocytes, as well as of CD19+ B cells, were performed by flow cytometry. Whole blood (50 μl/tube for surface staining or 100 μl/tube for surface and intracellular staining) was incubated for 20 min at 4°C with the anti‐human monoclonal antibodies (mAb) listed in Supporting information, Table S1. Red blood cells were then lysed [fluorescence activated cell sorter (FACS) lysing solution; BD Biosciences, San Jose, CA, USA], and the remaining cells were washed twice with FACS buffer. After completion of the cell‐surface staining, intracellular staining for cytotoxic T lymphocyte antigen (CTLA)‐4 was performed using the Cytofix/Cytoperm Kit (BD Biosciences), according to the manufacturer’s instructions.
To determine the absolute counts of CD4+, CD8+ and CD19+ lymphocytes, the TruCOUNT method was used. Undiluted blood was stained with anti‐human CD45 antibody (Supporting information, Table S1) in TruCOUNT tubes (BD Biosciences) and incubated for 15 min at room temperature. The red blood cells were then lysed with FACS lysing solution for 15 min at room temperature. All the samples were analyzed within 2 h of staining. Lymphocytes were defined as having low side‐scatter (SSC) and high expression of CD45, whereas TruCOUNT beads were identified as FL1 and FL2high. The absolute cell counts for lymphocytes were calculated using the following formula: events of lymphocytes/events of beads × number of beads per TruCOUNT tube/blood volume.
Samples were analyzed in a FACSCalibur (BD Biosciences) equipped with the CellQuestPro software and the data were analyzed using FlowJo software (TreeStar Inc., Ashland, OR, USA). All flow cytometric analyses were made blinded, i.e. all the samples were coded so that there was no previous knowledge of which samples were from patients or belonged to the control group when staining and setting the gates. Each sample had internal isotype controls, which were used to set the gates. Gating strategy can be found in Supporting information, Fig. S1. Representative FACS plots are depicted in Figs 1k and 3g.
Figure 1.
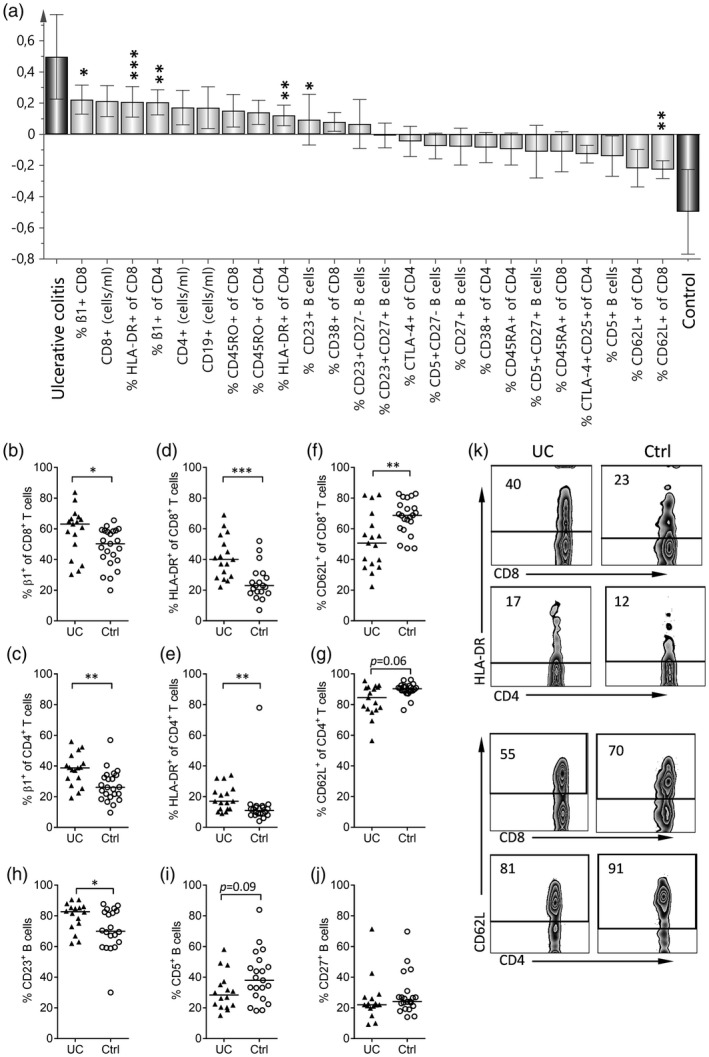
Comparison of the populations of circulating lymphocytes in children with newly diagnosed ulcerative colitis and non‐inflammatory bowel disease (IBD) controls. (a) Orthogonal projections to latent structures discriminant analysis (OPLS‐DA) was used to identify markers on circulating lymphocytes (X‐variables, light gray) that distinguish between children with newly diagnosed ulcerative colitis and non‐IBD controls (Y‐variables, dark gray). The X‐variables whose bars point in the same direction as the ‘ulcerative colitis’ Y‐variable have higher values in the children with the disease, whereas the X‐variables whose bars point in the same direction to the ‘control’ Y‐variable have higher values in the non‐IBD controls. The taller the bar and shorter the error bar, the larger and more certain is the contribution to the model of the X‐variable. Individual variables were tested for statistically significant differences by the Mann–Whitney U‐test and the levels of significance are denoted by asterisks above the variable in question in (a), as well as being shown in individual diagrams, i.e. the proportions of CD8+ T cells (b) and CD4+ T cells (c) that express the β1‐integrin in children with ulcerative colitis (UC) and non‐IBD controls (Ctrl), the proportions of CD8+ T cells (d) and CD4+ T cells (e) that express human leukocyte antigen D‐related (HLA‐DR) in the respective groups, the proportions of CD8+ T cells (f) and CD4+ T cells (g) that express CD62L, and the proportion of B cells that express CD23 (h), CD5 (i) or CD27 (j) in children with ulcerative colitis and non‐IBD controls. Each dot represents an individual, and horizontal bars indicate the median values. (k) Representative Zebra‐plots showing the expression of HLA‐DR, as well as the levels of CD62L on CD8+ and CD4+ T cells in a child with newly diagnosed ulcerative colitis (UC) and a non‐IBD control (Ctrl) patient are shown. *P < 0·05, **P < 0·01 or ***P < 0·001 (Mann–Whitney U‐test).
Analysis of the nature of CD23+ B cells
To analyze the origins of the CD23+ B cells that were found in increased numbers in the patients with IBD, we used cord blood (n = 6) and peripheral blood from healthy adults (control subjects in a study described previously 25; median age = 61 years, range = 29–72; n = 16). The CD23‐positive and ‐negative populations were identified within the CD19+ B cell population (Fig. 5a,b). Thereafter, the proportions of mature naive, class‐switched and IgM+ memory B cells within the CD23+ or CD23neg B cell populations were analyzed using the surface markers IgD and IgM 26, and transitional, naive and memory B cells were identified using CD24 and CD38 expression 16, 17. Mononuclear cells from cord blood and peripheral blood samples were isolated by density gradient centrifugation (900 g, 20 min, at room temperature) over Ficoll (GE Healthcare, Little Chalfont, UK), and stained for 20 min at 4°C with the anti‐human monoclonal antibody (mAb) listed in Supporting information, Table S1. The analysis was carried out as specified for the IBD blood samples, except that the samples were analyzed in a FACSCanto 2 (BD Biosciences) equipped with the FacsDiva software. Representative FACS plots are shown in Fig. 5a–c,e.
Figure 5.
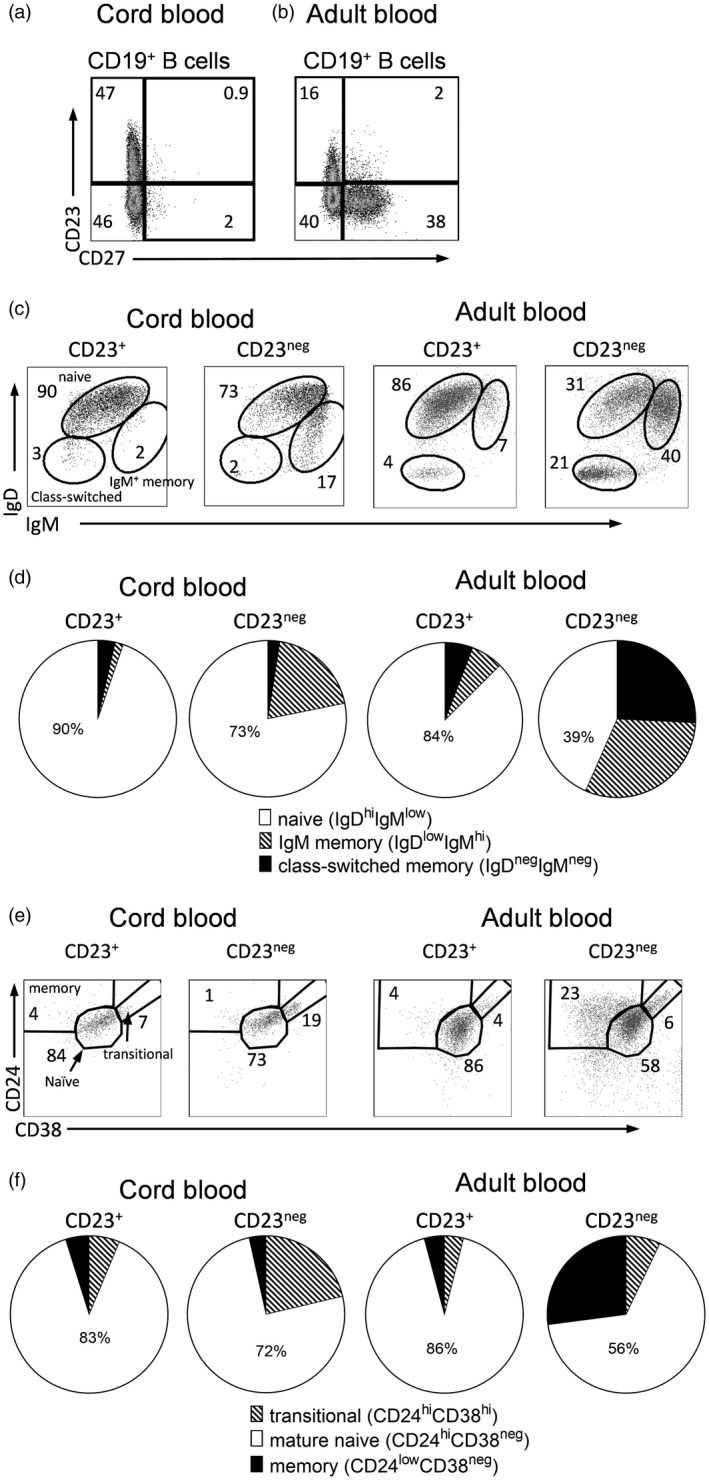
Characterization of blood CD23+ B cells as mature naive B cells. Cord blood (n = 6) and peripheral blood samples from healthy adults (n = 16) were analyzed by flow cytometry; CD19+ B cells were stained with anti‐CD23 and anti‐CD27, anti‐immunoglobulin (Ig)D, anti‐IgM, anti‐CD24 and anti‐CD38 antibodies. Representative gating strategies for cord blood (a) and adult blood (b) are shown for (c) CD23+ and CD23neg B cells that were gated for naive, IgM+ memory and class‐switched memory B cells based on CD23 and CD27 gating. The majority of the CD23+ B cells are naive IgMlowIgD+ B cells. (d) Summary of the results obtained for the six cord and the 16 adult blood samples; (e) gating of CD23+ and CD23neg B cells from cord and adult blood; CD23+ B cells are mainly mature naive B cells. (f) Summary of the results obtained for the six cord and the 16 adult blood samples.
Statistical analysis
The multivariate method orthogonal projection to latent structures by means of partial least‐squares – discriminant analysis (OPLS‐DA) (SIMCA‐P+ software version 13; Umetrics, Umeå, Sweden) was performed to ascertain whether the subcategories of IBD (children with ulcerative colitis or Crohn’s disease; discriminants) could be distinguished based on the childrens’ percentage of circulating naive, activated and memory cells within the CD4+, CD8+ T cell and CD19+ B cell populations (X‐variables). OPLS was performed to elucidate the associations between a certain lymphocyte population (Y‐variable) and the levels of inflammatory biomarkers in blood and feces (X‐variables). In both OPLS and OPLS‐DA, the contribution of each X‐variable to the model, i.e. in predicting Y, is represented by a column bar. X‐variables that are projected in the same direction as the Y‐variable are positively associated, whereas X‐variables that are projected in the opposite direction are inversely associated. The quality levels of the models were assessed based on the parameters R2 and Q2, i.e. the percentages of the variation of the data set that were explained (R2) and predicted (Q2) by the model, respectively 27.
Univariate analyses were performed to corroborate the multivariate analysis using the Mann–Whitney U‐test or Spearman’s rank correlation test (GraphPad Prism; GraphPad, San Diego, CA, USA), as specified in the figure legends. P < 0·05 was considered to be significant (*P < 0·05, ** P < 0·01, *** P < 0·001 and ****P < 0·0001).
Results
Children with newly diagnosed IBD, either ulcerative colitis or Crohn’s disease, were analyzed regarding the circulating lymphocyte subsets and associated markers of activation, homing and memory. The non‐IBD control group consisted of children who underwent the same diagnostic procedures (as they experienced symptoms suggestive of IBD), but for whom the diagnosis of IBD was excluded after a thorough diagnostic work‐up. None of the included children had received medication or had any diet restrictions.
All the patients were investigated while they were in the active phase of the disease. Mucosal inflammation, as indicated by increased levels of the four investigated fecal inflammatory biomarkers (calprotectin, lactoferrin, myeloperoxidase and eosinophil cationic protein), was pronounced in the patients with IBD (Table 2).
Table 2.
The concentration of different inflammation markers in children with ulcerative colitis, Crohn’s disease and non‐IBD controls
| Blood inflammation biomarkers | Fecal inflammation biomarkers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (range) | Median (range) | ||||||||||
| CRP (mg/l) | ESR (mm) | Orosomucoid (g/l) | Albumin (g/l) | Hemoglobin (g/l) | Leukocytes (×109/l) | Thrombocytes (×109/l) | Calprotectin (mg/kg) | Lactoferrin (μg/l) | MPO (μg/g) | ECP (μg/l) | |
| Ulcerative colitis | 5 | 20**, a | 1.1** | 40 | 120 | 9.3** | 370** | 1400**** | 150*** | 30*** | 220**** |
| (5–66) | (1–52) | (0·8–2·6) | (31–44) | (82–150) | (3·6–22) | (240–630) | (9–2700) | (5–41 000) | (0–580) | (2–1240) | |
| Crohn’s disease | 19****, a | 30**** | 1·6*** + | 34**** | 114** | 7·7 | 380** | 1400*** | 182* | 110**** | 160** |
| (5–68) | (10–38) | (1·2–2·8) | (22–39) | (93–120) | (5·6–9·1) | (300–560) | (690–2600) | (35–480) | (17–450) | (27–430) | |
| Non‐ IBD controls | 5 | 3 | 0·7 | 41 | 130 | 5·6 | 268 | 15 | 2 | 1 | 10 |
| (5–11) | (1–27) | (0·4–1·2) | (31–52) | (110–150) | (3·1–11) | (140–510) | (4–940) | (1–690) | (0–320) | (2–180) | |
| P ulcerative colitis versus Crohn’s disease | 0·003 | 0·4 | 0·1 | 0·03 | 0·2 | 0·5 | >0·99 | >0·99 | >0·99 | 0·9 | >0·99 |
Blood and fecal inflammation markers were measured in blood and stool samples obtained from children with ulcerative colitis (n = 17) or Crohn’s disease (n = 8) or from non‐inflammatory bowel disease (IBD) control children (n = 23).
CRP = C‐reactive protein; ESR = erythrocyte sedimentation rate; MPO = myeloperoxidase; ECP = eosinophil cationic protein.
P‐value for comparison with non‐IBD control children, Kruskal–Wallis test followed by Dunn’s multiple comparison test:
P < 0·05,
P < 0·01,
P < 0·001 and
P < 0·0001.
As shown in Table 2, blood biomarkers of inflammation were also detected in the children with IBD, with wide variations between individuals. These included increased levels of the positive acute‐phase reactants CRP, ESR and orosomucoid, as well as decreased concentrations of serum albumin and hemoglobin, observed particularly in the patients with Crohn’s disease, which is in agreement with previous findings 28. Serum albumin and hemoglobin are negative acute‐phase markers, the levels of which decrease with systemic inflammation 6. Increased counts of thrombocytes were noted in both types of IBD, while increased blood leukocyte counts mainly characterized patients with ulcerative colitis (Table 2).
Children with newly diagnosed ulcerative colitis have high percentages of circulating CD8+ and CD4+ T cells with an activated phenotype
First, we investigated which blood lymphocyte markers characterized active ulcerative colitis by comparing blood lymphocyte phenotypes (e.g. naive and activated lymphocytes) between children with newly diagnosed ulcerative colitis (n = 17) and non‐IBD controls (n = 23). For this, we used the multivariate pattern recognition method OPLS‐DA. Figure 1a shows the results; markers for which the bars are projected in the same direction as the ulcerative colitis variable have higher values in those patients, whereas X‐variables projected in the same direction as the control variable are higher in the control subjects. The larger the bar and the smaller the error bar, the stronger and more certain is the contribution to the model. The variables that showed the strongest contributions to the model were tested by univariate analysis; statistically significant differences are denoted by asterisks above the bar in question.
As shown in Fig. 1a, the variables most strongly associated with ulcerative colitis (positioned furthest to the left) were: the proportion of circulating CD8+ T cells that expressed β1‐integrin; the concentration of CD8+ T cells in the blood; the proportion of circulating CD8+ T cells that expressed HLA‐DR; the concentration of CD4+ T cells in the blood; and the proportion of circulating CD4+ T cells that expressed β1‐integrin (Fig. 1a). The higher proportions of β1‐integrin‐expressing CD8+ and CD4+ T cells were corroborated in the univariate analysis (Fig. 1b,c); β1 is the common β‐chain of the VLA integrins. Furthermore, the univariate analysis confirmed that CD8+ T cells from patients with ulcerative colitis expressed the activation marker HLA‐DR more often than the corresponding cells from control patients (Fig. 1d), with the same holding true for CD4+ T cells (Fig. 1e). However, other characteristics of the ulcerative colitis group, according to the OPLS model (Fig. 1a), did not reach significance in the univariate analysis, including the slightly higher concentrations of CD8+ (median = 0·53 × 106/ml versus 0·44 × 106/ml; P = 0·2) and CD4+ T cells (median = 0·53 × 106/ml versus 0·44 × 106/ml; P = 0·2) and the increased expression level of the memory marker CD45RO on CD8+ (40 versus 33%, P = 0·3) and CD4+ (44 versus 41%, P = 0·3) T cells, compared to the control group.
Those markers that were more often expressed on the circulating lymphocytes of the non‐IBD controls than on the lymphocytes obtained from children with ulcerative colitis appear on the right‐hand side of the OPLS plot (Fig. 1a). Most striking was the higher proportion of CD8+ T cells that expressed the marker CD62L (Fig. 1f); a similar tendency was observed for CD4+ T cells (Fig. 1g). This marker is expressed by cells that circulate through the lymph nodes, i.e. naive cells and non‐activated memory cells, but is not expressed on effector T cells that are activated by their cognate antigen 14.
Regarding the B cell numbers and their markers, the children with ulcerative colitis had significantly higher proportions of B cells that expressed the marker CD23 (Fig. 1h) and they showed a tendency towards lower proportions of CD5+ B cells (Fig. 1i), compared to the non‐IBD controls. The proportions of memory CD27+ B cells were in the same range for the patients with ulcerative colitis and the control subjects (Fig. 1j).
Frequency of α‐chain usage on β1‐intergrin‐positive cells in children with ulcerative colitis
High‐level expression of β1‐integrin was found to be characteristic of both the CD4+ and CD8+ T cells in the children with ulcerative colitis (Fig. 1a–c). The β1‐chain can be combined with any of six different α‐chains (α1–α6) to produce the six different VLA integrin isoforms (VLA 1–6). VLA is up‐regulated upon encountering naive T cells with their cognate antigen in secondary lymphoid organs 29. We examined which α‐chain was expressed on the β1‐integrin‐positive CD4+ and CD8+ T cells in the children with ulcerative colitis. A large majority (>80%) of the β1‐integrin‐positive CD4+ cells expressed the α‐3, α‐4, α‐5 and α‐6 chains, suggesting that VLA‐3–VLA 6 were expressed by these cells. The β1‐integrin‐positive CD8+ T cells in the children with ulcerative colitis mainly expressed the α5 and α6 chains (Supporting information, Table S2).
The proportion of activated T cells in children with ulcerative colitis correlates positively with the levels of biomarkers of systemic and mucosal inflammation
We examined whether the activated phenotype of the circulating T cells that characterized the children with newly diagnosed ulcerative colitis correlated with measures of systemic and mucosal inflammation in these patients (see Table 2). The analysis was performed using OPLS and was confirmed with Spearman’s rank correlation test. As shown in Fig. 2a, a high proportion of the circulating CD8+ T cells that expressed HLA‐DR correlated positively with systemic biomarkers of inflammation, most notably the blood thrombocyte and leukocyte concentrations (Fig. 2a,b). Furthermore, the levels of biomarkers of mucosal inflammation tended to be increased in parallel with the levels of HLA‐DR expression on circulating CD8+ T cells, e.g. fecal eosinophil cationic protein, ECP (r = 0·48, P = 0·06, Spearman’s rank correlation test; Fig.·2a) and fecal calprotectin (r = 0·36, P = 0·2). Regarding the expression of HLA‐DR on circulating CD4+ T cells, similar relationships with systemic and mucosal biomarkers of inflammation were noted, although there were no significant associations in the univariate analysis (Supporting information, Fig. S2a).
Figure 2.
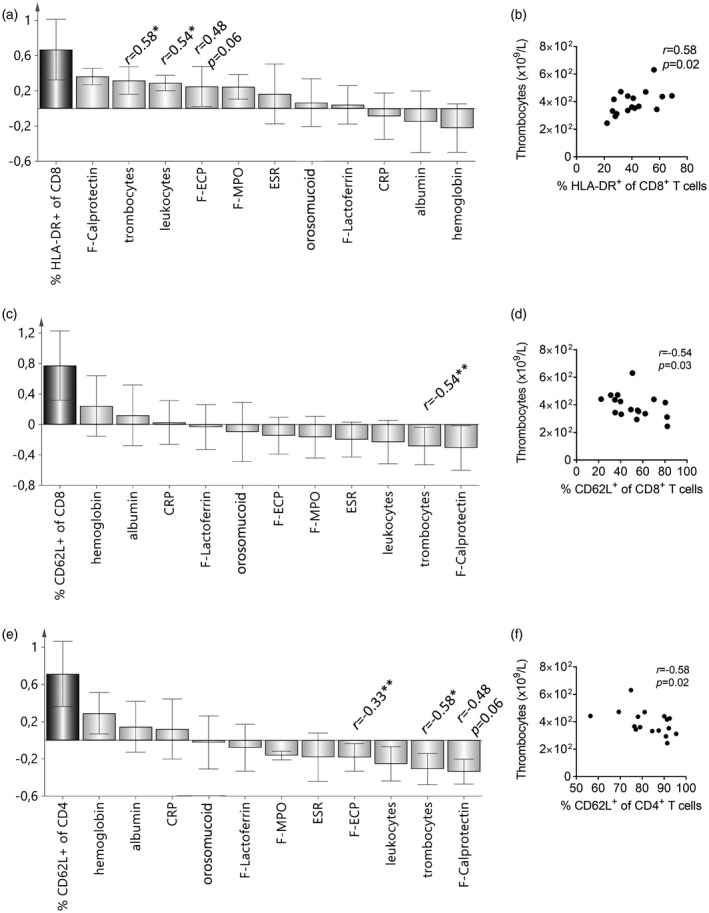
The numbers of circulating activated T cells correlate with the levels of biomarkers of systemic and mucosal inflammation in newly diagnosed ulcerative colitis. Orthogonal projections to latent structures (OPLS) was used to analyze the associations between different T cell populations and the concentrations of systemic and mucosal inflammatory biomarkers (X‐variables). CRP = C‐reactive protein: ESR = erythrocyte sedimentation rate; F‐ECP = fecal eosinophil cationic protein; F‐MPO = fecal myeloperoxidase; F‐calprotectin = fecal calprotectin. X‐variables whose bars project in the same direction as the Y‐variable are positively correlated with the Y‐variable, whereas X‐variables that project in the opposite direction are negatively correlated. The larger the bar and smaller the error bar, the stronger and more certain is the contribution to the model. Statistically significant differences between the Y‐variables and the different inflammation markers in the univariate analysis (Spearman’s rank correlation test) are denoted with the r‐value and asterisks; *P < 0·05, **P < 0·01. (a) OPLS analysis of the association between the proportions of human leukocyte antigen D‐related (HLA‐DR)+ CD8+ T cells and levels of inflammatory biomarkers. (b) Univariate analysis showing a positive correlation between the percentages of circulating CD8+ T cells expressing HLA‐DR and the numbers of blood thrombocytes. (c) OPLS analysis showing a negative association between the percentages of CD8+ T cells having a non‐activated CD62L+ phenotype and levels of inflammatory biomarkers. (d) Negative correlation between the proportion of CD8+ T cells expressing CD62L and blood thrombocyte concentration. (e) OPLS analysis of the association between the percentages of non‐activated CD62L+ CD4+ cells and levels of inflammatory biomarkers. (f) Negative correlation between the percentages of CD62L+CD4+ cells and blood thrombocyte concentration.
The proportion of naive CD8+ T cells (CD62L+) was negatively associated with exactly the same parameters as were positively associated with HLA‐DR expression on CD8+ T cells (Fig. 2c; compare with Fig. 2a). Fig. 2d shows the significant negative correlation between thrombocyte counts in the blood and the proportion of CD62L+CD8+ T cells. The proportion of naive CD4+ T cells (CD62L+) was also significantly negatively associated with the thrombocyte counts (Fig. 2e,f), and tended to correlate negatively with the levels of fecal ECP and calprotectin (Fig. 2e).
The degree of expression of β1‐integrin on CD8+ T cells was positively associated with systemic and mucosal biomarkers of inflammation, although none of these associations was significant by Spearman’s rank correlation test (Supporting information, Fig. S2b). Regarding the proportion of β1+CD4+ T cells in relation to inflammatory markers, the associations were weak and not statistically significant (Supporting information, Fig. S2c).
In summary, children with newly diagnosed ulcerative colitis had a high proportion of circulating T lymphocytes that displayed an activated phenotype, and the presence of such cells correlated positively with the systemic and mucosal inflammatory responses. Naive T cells were less numerous in these patients, and their proportions were negatively associated with the aforementioned inflammatory markers.
Children with newly diagnosed Crohn’s disease have a low percentage of circulating memory CD27+ B cells
Next, we analyzed what characterized circulating lymphocytes in children with newly diagnosed Crohn’s disease by comparing their proportions of different T and B cell subsets to those found in non‐IBD controls. Analyses were performed using OPLS‐DA and associations found in the multivariate model were confirmed by univariate analysis. Figure 3a shows that the children with Crohn’s disease had a higher percentage of B cells that expressed CD23 but not the B cell memory marker CD27 (CD23+CD27neg), compared with controls. The same was true for all B cells that expressed CD23 (Fig. 3a; univariate analyses are shown in Fig. 3b,c). Conversely, the control children had significantly higher percentages of circulating B cells that expressed the memory marker CD27, as evidenced by the bar pointing in the same direction as the ‘control’ variable (Fig. 3a). The difference was found to be highly significant in the univariate analysis (Fig. 3d). Expression of CD27 in the absence of CD23 or CD5 was a more common phenotype of the B cells from the controls than of those from the patients with Crohn’s disease (Fig. 3e,f). Fig. 3g shows representative FACS plots that depict how the gates were set for CD23 and CD27 on CD19+ B cells. We examined the correlation between the proportions of B cells that expressed CD23 and CD27, respectively, in individual patients (patients with Crohn’s disease represented by filled squares, non‐IBD controls represented by open circles). There was a strong inverse correlation between CD23 and CD27 expression on CD19+ B cells (Fig. 3h).
Figure 3.

Low percentages of circulating memory B cells in children with newly diagnosed Crohn’s disease. (a) Orthogonal projections to latent structures discriminant analysis (OPLS‐DA) showing differences in the blood lymphocyte populations and their activation and memory markers (X‐variables, light gray) between children with Crohn’s disease and non‐IBD controls (Y‐variables). X‐variables with bars that project in the same direction as the bar representing Crohn’s disease are higher in these patients than in the controls, whereas X‐variables that project in the same direction as the control group are higher in the controls. The larger the bar and the smaller the error bar, the stronger and more certain is the contribution of the variable to the model. Statistically significant differences between patients with Crohn’s disease and controls by univariate analysis (Mann–Whitney U‐test) are denoted with asterisks above the respective bars. (b–f) The proportions of circulating CD19+ B cells that express different surface markers in the patients with Crohn’s disease (CD) and non‐IBD controls (Ctrl); CD23+CD27neg (b), CD23+ (c), CD27+ (d), CD23negCD27+ (e), and CD5negCD27+ (f). Each dot represents an individual and the horizontal bars indicate the median values; **P < 0·01, ***P < 0·001 and **** P < 0·0001 (Mann–Whitney U‐test). (g) Representative Zebra plots of a patient with Crohn’s disease and a non‐IBD control patient, showing the expression of CD23 and CD27 on CD19+ B cells. (h) Inverse correlation between the proportions of B cells that express CD23 and CD27, with each symbol representing one child, the filled squares representing children with Crohn’s disease, and the open circles representing controls (Spearman’s correlation test).
Children with Crohn’s disease do not display the T cell‐activation pattern typical of patients with ulcerative colitis
The children with newly diagnosed Crohn’s disease did not show the same pattern of activated T cells observed in patients with ulcerative colitis. In fact, the β1‐integrin and HLA‐DR expression on CD8+ T cells both appeared in the center of the diagram (Fig. 3a), indicating equally high levels of β1‐integrin and HLA‐DR expression on CD8+ T cells in Crohn’s disease patients and controls. Furthermore, high counts of CD8+ T cells in the blood tended to be associated with being a control subject (Fig. 3a), as opposed to the situation in the ulcerative colitis group (Fig. 1a).
Children with newly diagnosed ulcerative colitis and children with Crohn’s disease differ regarding the phenotype of circulating T and B lymphocytes
As the two IBD subcategories seemed to be associated with distinct lymphocyte phenotypes, we next investigated whether patients with ulcerative colitis or Crohn’s disease could be distinguished based on the numbers of circulating CD4+ and CD8+ T cells and CD19+ B cells, as well as their expression of activation and memory markers at the time of diagnosis. As illustrated in the OPLS‐DA in Fig. 4a, ulcerative colitis and Crohn’s disease could be separated from one another based on the overall patterns of lymphocyte subset counts and their surface markers. Being diagnosed with ulcerative colitis was associated with high proportions of memory CD27+ B cells, as well as activated HLA‐DR+ CD4+ and CD8+ T cells, whereas a diagnosis of Crohn’s disease was associated with a high percentage of B cells that expressed CD23 (Fig. 4b).
Figure 4.

Children with newly diagnosed ulcerative colitis or Crohn’s disease differ in the pattern of circulating naive, activated and memory T and B lymphocytes. Orthogonal projections to latent structures discriminant analysis (OPLS‐DA) was applied to separate children with newly diagnosed ulcerative colitis (filled triangles) and children with Crohn’s disease (open squares). (a) Score scatter‐plot; separation is based on the proportions of circulating naive, activated and memory CD4+ and CD8+ T cells, as well as B cells expressing the markers CD5, CD23 and CD27, as shown in the column plot. (b) OPLS‐DA column plot showing the differences in the blood lymphocyte populations and their activation and memory markers (X‐variables, light gray) between children with ulcerative colitis and Crohn’s disease (Y‐variables, dark gray). X‐variables with bars that project in the same direction as the bar representing ulcerative colitis are higher in these patients than in children with Crohn’s disease, whereas X‐variables that project in the same direction as Crohn’s disease are higher in the children with Crohn’s disease. The larger the bar and the smaller the error bar, the stronger and more certain is the contribution of the variable to the model. Statistically significant differences between patients with ulcerative colitis and Crohn’s disease by univariate analysis (Mann–Whitney U‐test) are denoted with asterisks above the respective bars. (c,d) The proportions of circulating CD19+ B cells that express CD27 (c) and CD8+ T cells that express human leukocyte antigen D‐related (HLA‐DR) (d) in patients with ulcerative colitis (UC) and Crohn’s disease (CD). Each dot represents an individual and the horizontal bars indicate the median values. (e,f) ROC analysis of the proportion of CD27+ B cells (e) and HLA‐DR+ of CD8+ T cells (f) in children with ulcerative colitis and Crohn’s disease. **P < 0·01, ***P < 0·001 and ****P < 0·0001 (Mann–Whitney U‐test).
Accordingly, univariate analysis confirmed that children who suffered from Crohn’s disease had a significantly lower proportion of CD27+ B cells than those with ulcerative colitis (median 11 versus 22%, P = 0·0007, Fig. 4c), while the two IBD subcategories had similar proportions of CD23+ B cells (median 87% of the circulating B cells in Crohn’s disease versus 83% in ulcerative colitis, P = 0·2). We evaluated the diagnostic value of the proportion of CD27+ B cells in children with Crohn’s disease compared to those with ulcerative colitis (Fig. 4e) and found that with a cut‐off of 17, the area under the curve was 0·91 (0·78–1) and the accuracy was 0·88 (0·68–0·97). We also performed a receiver operating characteristic (ROC) analysis on activated CD8+ T cells that expressed HLA‐DR; as shown in Fig. 4f, the analysis had an area under the curve of 0·79 (0·55–1) and an accuracy of 0·83 (0·63–1).
CD23 is chiefly expressed on mature naive B cells
The inverse relationship between the proportion of B cells that had the CD27+ memory phenotype and the proportion that expressed CD23 (Fig. 3h) suggested that CD23, the low‐affinity IgE receptor 30 might be a marker of naive B cells. To the best of our knowledge, the nature of the human CD23+ B cell subset has not been elucidated. To study this, we gated on the CD23+ and CD23neg populations from cord blood of healthy newborn infants (n = 6, Fig. 5a) or from blood of healthy middle‐aged control individuals (n = 16, Fig. 5b). We then analyzed the expression of established markers of naivety, memory and the transitional state on these two populations.
Naive B cells express both surface IgM and IgD while cells that only express IgM represent IgM+ memory cells, and B cells that express neither IgM nor IgD represent cells that have undergone class‐switch. As seen in Fig. 5c, the CD23+ B cells in cord blood contained almost exclusively naive non‐switched B cells, this was also true for CD23+ B cells from adults. On average, 90% of the CD23+ cord blood cells and 84% of the CD23+ adult blood cells were such non‐switched IgM+IgD+ cells (Fig. 5d).
Further, CD23+ and CD23neg B cells were subdivided, based on staining for CD24 and CD38, into transitional (CD24highCD38high), naive (CD24+CD38+) and memory (CD24+CD38neg) B cells. Both in the cord blood samples and the adult blood samples (Fig. 5e), the great majority of the CD23+ cells appeared in the naive gate (84 and 86%, respectively). Transitional B cells were not frequent among the CD23+ B cells, either in the cord blood (7%, Fig. 5e) or the peripheral blood from adults (4% Fig. 5e). On average, transitional B cells accounted for only 5% within the cord blood CD23+ population, compared to 21% of the CD23neg B cell population (Fig. 5f). In adults, transitional B cells accounted for 4% of the CD23+ B cells and 6% of the CD23neg B cells (Fig. 5f). To conclude, B cells expressing high surface levels of CD23 chiefly represented mature naive B cells.
The percentage of CD27+ B cells correlate positively with systemic biomarkers of inflammation in children with Crohn’s disease
We next investigated whether the increased percentage of putative naive CD23+ B cells and the decreased percentage of CD27+ memory B cells that characterized the children with Crohn’s disease were related to the ongoing systemic and mucosal inflammation observed in these patients (Table 2). To this end, the proportion of CD27+ memory B cells was related to the levels of systemic and mucosal biomarkers of inflammation in patients with Crohn’s disease using OPLS (Fig. 6). Remarkably, the proportion of B cells that expressed the CD27+ memory marker correlated strongly and positively with the ESR, which is a classical biomarker of systemic inflammation (r = 0·89, P = 0·005; Fig. 6a,b). In general, most of the blood and fecal inflammation biomarkers were positively associated with the proportion of CD27+ B cells, whereas albumin and hemoglobin were negatively associated with this variable in the multivariate OPLS‐analysis (Fig. 6a).
Figure 6.
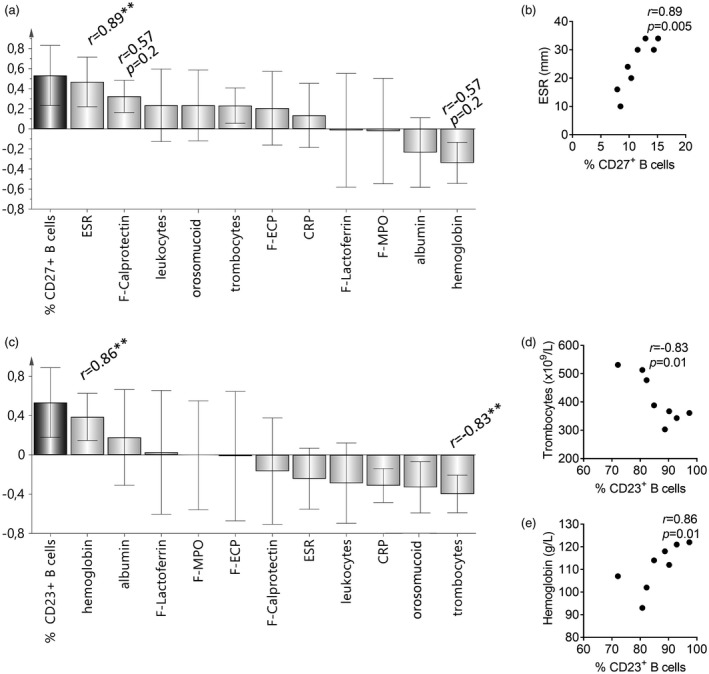
Associations between the percentages of naive (CD23+) and memory (CD27+) B cells and inflammatory biomarkers levels in children with newly diagnosed Crohn’s disease. Orthogonal projections to latent structures (OPLS) and univariate analysis (Spearman’s rank correlation) were used to uncover the associations between the percentages of B cells that express the proposed marker of naivety CD23, or the memory marker CD27, as well as markers of systemic and local inflammation in children with newly diagnosed Crohn’s disease; CRP = C‐reactive protein; ESR = erythrocyte sedimentation rate; F‐ECP = fecal eosinophil cationic protein; F‐MPO = fecal myeloperoxidase; and F‐calprotectin = fecal calprotectin. In OPLS, X‐variables with bars that project in the same direction as the Y‐variables are positively associated, whereas X‐variables that project in the opposite direction are inversely related to the Y‐variable. The larger the bar and smaller the error bar, the stronger and more certain is the contribution to the model. Statistically significant correlations (positive or negative) obtained from the univariate analysis are denoted by asterisks above the respective variable bars; **P < 0·01. (a) Positive correlation between the proportion of memory CD27+ B cells and the levels of systemic and mucosal inflammatory markers. (b) Strong positive correlation between the proportions of memory CD27+ B cells and the ESR. (c) OPLS analysis showing inverse association between the proportions of putative naive CD23+ B cells and the levels of several fecal and blood markers of inflammation. (d) Strong negative correlation between the proportions of putative naive CD23+ B cells and thrombocyte concentrations. (e) Strong positive correlation between the proportions of putative naive CD23+ B cells and the hemoglobin concentrations.
Conversely, the proportion of B cells that expressed the putative naive marker CD23 correlated negatively with the levels of systemic and mucosal biomarkers of inflammation (Fig. 6c). For example, the proportion of CD23+ B cells correlated strongly and negatively with the numbers of thrombocytes (r = –0·83, P < 0·01; Fig. 6d). Similarly, CD23 expression was positively associated with the blood hemoglobin levels (r = 0·86, P < 0·01; Fig. 6e).
To summarize, an inflammatory environment correlated positively with the conversion of B cells from a naive (CD23+) to a memory (CD27+) phenotype. In children with Crohn’s disease, memory B cell conversion was hampered despite the strong inflammatory drive in these patients.
Discussion
In the present study, we investigated the expression profiles of selected markers of immunological naivety, activation and memory in the circulating lymphocytes of children with newly diagnosed IBD. Care was taken to exclude children who had consumed any type of medication that could, in a primary or secondary fashion, affect the immune system, including anti‐inflammatory drugs, antibiotics and probiotics. Thus, our results reflect the immune response at an early, active stage of the disease process that is unaffected by medications.
We identified differences in the pattern of circulating lymphocyte phenotypes between children with ulcerative colitis and children with Crohn’s disease. In the patients with ulcerative colitis, both circulating CD4+ and CD8+ T lymphocytes displayed signs of activation, including increased levels of HLA‐DR, expression of the common β1‐integrin chain and down‐regulated expression of CD62L. The CD8+ subset showed the clearest evidence of activation. The common β1‐chain can be combined with six different α‐chains to produce the six members of the VLA family, VLA1–6. They are involved in cell migration and interactions with tissue matrix components. For example, VLA‐4 is expressed by T cells that are activated by their cognate antigens in secondary lymphoid organs, and VLA‐4, ‐5 and ‐6 permit effector T cells to exit the blood stream and enter into inflamed tissues 29, 31, 32. Our results show that in newly diagnosed ulcerative colitis, CD8+ T cells chiefly express VLA‐5 and VLA‐6, while the CD4+ T cells express a broader range of integrins, from VLA‐3 to VLA‐6.
The activated phenotype of the circulating CD8+ T cells that typify patients with newly diagnosed ulcerative colitis correlated positively with the increased levels of biomarkers of systemic and mucosal inflammation seen in these patients. For example, the proportion of circulating CD8+ T cells that expressed HLA‐DR correlated significantly with the numbers of thrombocytes and leukocytes, both of which are produced in the bone‐marrow in response to inflammatory cytokines 6, 7. Conversely, the proportion of CD62L‐expressing, non‐activated CD8+ and CD4+ T cells correlated negatively with the thrombocyte counts, and the proportion of CD62L‐expressing, non‐activated CD4+ T cells correlated negatively with the fecal levels of eosinophil cationic protein and calprotectin, which are two biomarkers of mucosal inflammation. In accordance with these findings, a recent study has shown increased percentages of HLA‐DR‐ and CD38‐positive circulating CD8+ T cells in patients with ulcerative colitis, and has reported that such markers of T cell activation correlate with blood biomarkers of inflammation 13. Thus, severe inflammation is linked to the activation and maturation of T cells towards an effector phenotype that is ready to egress into the inflamed tissues. Which is the cause and which is the consequence may be discussed. It may be that activated CD8+ T cells attack targets in the gut mucosa of patients with ulcerative colitis, causing tissue breakdown that triggers inflammation. However, it is also known that local inflammation in the gut mucosa could promote T cell activation, because it would increase the traffic of dendritic cells that are carrying antigens from the mucosa to the draining lymph nodes 33 and would also augment their capacity to activate naive T cells to which they present antigens, compared to dendritic cells that arrive from a mucosa that is in homeostasis 34. The mucosal inflammation may be caused by microbial dysbiosis that starves the mucosal epithelium 35, 36, 37, leading to failure to uphold mucosal integrity and increased leakage of microbial products across the mucosa. The two scenarios are obviously not mutually exclusive; it may well be that there is a vicious cycle wherein local inflammation promotes T cell activation, which leads to more local inflammation.
In contrast to the ulcerative colitis group, the patients with Crohn’s disease did not show increased levels of activation markers on their circulating CD4+ or CD8+ T cells, compared to the corresponding cells from non‐IBD controls. This was especially surprising, considering that the children with Crohn’s disease displayed at least equally severe inflammation, as reflected by blood and fecal biomarkers, compared with patients with ulcerative colitis. Funderburg et al. have shown similar results in which the entire IBD patient group had a greater population of activated CD8+ T cells compared to the controls, but when dividing the IBD subjects into those with ulcerative colitis and Crohn’s disease, only patients with ulcerative colitis had elevated activated T cells compared to the control group 13. This may indicate that the two IBD subcategories have, at least partly, differing immunological backgrounds and mechanisms.
Notably, the children with Crohn’s disease displayed a markedly reduced proportion of CD27+ memory B cells among the circulating B cells, compared to both the children with ulcerative colitis and the non‐IBD controls. A reduced proportion of circulating IgM+CD27+ memory B cells in adults with IBD has been reported previously, albeit with no difference between patients with Crohn’s disease and ulcerative colitis 19, 20. Furthermore, children with Crohn’s disease exhibit an impaired CD27+IgDneg memory B cell response to pneumococcal vaccination 38. In addition, a paucity of IgM memory B cells and reduced evidence of somatic hypermutation have recently been reported in patients with Crohn’s disease 18. Although none of the other studies examined treatment‐naive subjects, the findings are in accordance with ours. Another study has reported that adult patients with Crohn’s disease have increased numbers of circulating transitional B cells and CD21low B cells 18. We could not corroborate this finding in this pediatric patient group, as CD5+ B cells, which represent early transitional cells 16, 17, were not increased in number in the children with Crohn’s disease in our study.
The proportions of B cells that expressed the surface marker CD23 were increased in children with Crohn’s disease, as well as in the patients with ulcerative colitis. The antibody panel that we used to analyze blood lymphocytes from children with IBD and control subjects did not include surface IgM and IgD or other markers that may be used to distinguish transitional and naive B cells. However, control experiments performed using cord blood and adult blood from healthy individuals identified the CD23+ B cells as mainly being mature naive B cells that expressed moderate to high levels of surface IgM, in combination with high levels of surface IgD. Notably, CD23+ and CD23neg B cells were not two obvious distinct populations, but B cells showed a continuous distribution of CD23 on their surface. CD23 is the low‐affinity receptor for IgE 30. It has been suggested that both naive and/or transitional B cells express CD23, although no data were shown to support these claims 16, 39. In the present study, we found no evidence to suggest that CD23 is a marker of transitional B cells. Instead, the transitional B cells in the cord blood were localized mainly to the CD23neg subset using gating based on the expression of CD24 and CD38.
In the children with Crohn’s disease, we observed a significant reduction in the proportion of memory B cells, combined with a significantly increased proportion of putative CD23+ B cells, and the respective proportion of these two cell subsets were highly inversely correlated. This gives rise to our main hypothesis: that Crohn’s disease may be associated with reduced conversion of naive B cells into memory cells. An alternative explanation would be that memory B cells disappear from the blood stream in an increased amount than normal in patients with Crohn’s disease, e.g. because the environment favors recruitment into the inflamed gut mucosa. However, ulcerative colitis patients also have extensive gut inflammation with prominent plasma cell infiltration 12, but we noted no reduction in circulating memory B cells in these patients compared with non‐IBD controls. Further, inflammatory markers in patients with Crohn’s disease correlated positively, not negatively, with the proportion of CD27+ B cells in blood. Indeed, interleukin (IL‐6), which is the major inducer of the acute‐phase reaction 40, is a stimulator of thrombocyte production in the bone marrow 41 and also a B cell‐maturation factor 42. Thus, the cytokines that circulate in the patients with Crohn’s disease with ongoing inflammatory state tend to drive B cell maturation. To conclude, our patients with Crohn’s disease did not have such high percentages of naive B cells and so few memory B cells because of inflammation but, rather, despite inflammation. The third possibility would be that Crohn’s disease patients have an increased production of naive B cells in the bone marrow that would tend to ‘dilute’ the memory CD27+ B cells. This is, however, disputed by the negative correlation between the proportion of putative naive CD23+ B cells and inflammatory biomarkers in the patients with Crohn’s disease. Furthermore, children with Crohn’s disease had no higher levels of CD19+ B cells in the circulation compared to non‐IBD controls. Lastly, children with ulcerative colitis had a significantly elevated proportion of putative naive CD23+ B cells, without any signs of low proportion of memory CD27+ B cells.
Thus, we believe that the findings of our study fit best with a hampered conversion of B cells to a memory phenotype in patients with Crohn’s disease. Interestingly, a number of disorders that, similarly to Crohn’s disease, manifest with granuloma formation, are also characterized by lower than normal percentages of CD27+ memory B cells. These include chronic granulomatous disease (CGD) 43, Blau syndrome, early‐onset sarcoidosis 44 and a subtype of common variable immunodeficiency (CVID) 45. Blau syndrome and early‐onset sarcoidosis are linked to mutations in the NOD2 gene, a feature shared with Crohn’s disease 44, 46. One may speculate that a low proportion of CD27+ memory B cells reflects reduced capacity to activate B cells and, hence, to elicit efficient antibody responses that may be required to eliminate microbial antigens that enter via the gut mucosa. Instead, such antigens may persist in the gut wall, leading to chronic inflammation and subsequent granuloma formation. Another explanation for the relative shortage of memory B cells in children with Crohn’s disease might be that they have experienced fewer infections and/or been less exposed to commensal microbes compared with children in general. This is compatible with the fact that Crohn’s disease is associated with a highly hygienic lifestyle 47, 48, 49, 50, 51. These hypotheses are not mutually exclusive; a genetic shortcoming in mounting efficient B cell responses, in combination with a hygienic life‐style that gives too little immune stimulation, may well synergize to precipitate the disease.
A weakness of the present study is the lack of healthy controls and the low number of participating children, especially those with Crohn’s disease. Therefore, the results should be interpreted with caution and need to be verified in a larger study. The strengths of the study include well‐characterized treatment‐naive subjects with recent onset of disease which minimizes the risk of confounders due to prolonged disease duration and concomitant anti‐inflammatory therapy. Analysis of the blood samples within some hours of collection allowed us to evaluate immune markers, such as CD62L, that could be down‐regulated by freezing before analysis. Flow cytometryc gating was performed by a researcher blinded to the diagnosis of the patient from whom the sample derived which reduced the risk of bias. A further strength of this study is the use of multivariate factor analysis to find patterns and trends in the large data set, before proceeding with univariate analyses.
Crohn’s disease and ulcerative colitis are sometimes difficult to differentiate in clinical practice, especially in patients diagnosed in childhood; for instance, pediatric Crohn’s disease often presents with colitis involving a large proportion of the colon, mimicking the inflammatory distribution of ulcerative colitis 22. Whether measurements of CD27 expression on B cells may be used as complement in the diagnostic work‐up to distinguish between the major subcategories of IBD remains to be investigated in a larger cohort of pediatric patients, as well as in adult patients with IBD.
The results of the present study suggest that Crohn’s disease and ulcerative colitis exhibit distinct patterns of lymphocytic activation and memory marker expression, indicating that the diseases have at least partly different etiologies. In ulcerative colitis, a vicious circle of gut inflammation and activation of T cells, mainly of the CD8+ phenotype, appears to be a hallmark. In Crohn’s disease, arrested B cell activation and hampered maturation beyond the naive stage seems to be a characteristic sign.
Disclosures
Based on the described findings, a patent application regarding a diagnostic tool to distinguish ulcerative colitis and Crohn’s disease with the use of the marker CD27 has been filed. The intellectual property rights have been assigned to Flora Innovation AB, in which R. S. and A. W. are shareholders. The remaining authors have no conflicts of interest to declare. This does not alter our adherence to sharing data and materials.
Author contributions
R. S. and A. W. designed the study. H. R., M. M., C. B. and S. Ö. analyzed the raw data and H. R. made the multivariate statistics. I. G. overviewed the experiments and analyses regarding the expression of CD23 on different B cell subsets, with which H. R. set the FACS gaits and performed the statistics. H. R., M. M., R. S. and A. W. wrote the manuscript. R. S. and A. W. supervised all the analyses and writing of the manuscript. All of the authors have given their consent to submit the manuscript to Clinical & Experimental Immunology.
Supporting information
Fig. S1. Gating strategy for B‐ and T‐lymphocytes. (a) Gating strategy for CD5, CD23 and CD27 of CD19+ B‐cells, in peripheral blood from a patient with Crohn’s disease. First, lymphocytes were identified with the use of forward (FSC) and side scatter (SSC). Thereafter, B‐cells were recognized as CD19+ cells, that were studied for their expression of CD23, CD27 and CD5. (b) Gating strategy for CD62L, CD45RA, CD45RO, CD38, HLA‐DR on CD4+ or CD8+ T cells, in peripheral blood from a patient with ulcerative colitis. With the use of forward and side scatter the lymphocytes were identified. Subsequently, the CD4+ and CD8+ T cells were gated, and the expression of CD62L. CD45RA, CD45RO, CD38 or HLA‐DR was examined with the two T‐cell populations. (c) The gating strategy for the expression of β1 on CD4+ and CD8+ T‐cell populations in a patient with ulcerative colitis. First, the lymphocyte population was identified with the use of forward and side scatter. Thereafter, the CD4+ T‐cell population was identified as CD3+CD4+ lymphocytes, whereas the CD8+ T‐cell population was recognized as CD3+CD4neg lymphocytes. Finally, the expression of β1 was studied on these two subsets of T cells.
Fig. S2. Markers of T cell activation in relation to systemic and mucosal inflammation markers in children with ulcerative colitis. Orthogonal projection to latent structures by means of partial least squares (OPLS) that depicts the association between the proportion of (a) CD4+ cells expressing HLA‐DR, (b) CD8+ T cells that express β1 and (c) CD4+ T cells that express β1 (Y variables), and the concentration of different inflammation markers (X‐variables). X‐variables with bars projected in the same direction as the Y‐variables are positively associated, whereas X‐variables projected in the opposite direction are inversely related to Y. The larger the bar and smaller the error bar the stronger andmore certain is the contribution to the model. Statistically significant differences between the Y‐variables and the different inflammation markers are denoted with the r‐valueand asterisks. *P < 0·05, **P < 0·01 (Spearman’s rank correlation test).
Table S1. Monoclonal antibodies used for the characterization of CD4+, CD8+ T cells and CD19+ B cells.
Table S2. Alpha‐chain expression of β1+ T cells (CD4+ and CD8+) in children with newly diagnosed ulcerative colitis and in non‐IBD controls.
Acknowledgments
We thank study nurse Anette Ekberg at the Gastroenterology Unit at The Queen Silvia Children’s Hospital for excellent help with sample collection. We greatly appreciate the skillful technical assistance of Eva Ågren, Ingela Kinell and Jolanta Bonislavska, along with the staff of the Clinical Immunology Laboratory of Sahlgrenska University Hospital. Finally, we thank all the families who took part in the study. This work was funded by the Swedish Medical Research Council, The Medical Faculty of Gothenburg under the LUA agreement, The Samaritan Foundation, Mayflower Foundation, The Nine Meter Life Foundation, The Wilhelm and Martina Lundgren Research Foundation, The Gothenburg Orphanage Foundation, and a non‐conditional research grant from Astra Zeneca Sweden.
References
- 1. Rovedatti L, Kudo T, Biancheri P et al Differential regulation of interleukin 17 and interferon gamma production in inflammatory bowel disease. Gut 2009; 58:1629–36. [DOI] [PubMed] [Google Scholar]
- 2. Keren DF, Appelman HD, Dobbins WO III et al Correlation of histopathologic evidence of disease activity with the presence of immunoglobulin‐containing cells in the colons of patients with inflammatory bowel disease. Hum Pathol 1984; 15:757–63. [DOI] [PubMed] [Google Scholar]
- 3. Fujino S, Andoh A, Bamba S et al Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003; 52:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Granlund AV, Flatberg A, Ostvik AE et al Genome gene expression meta‐analysis of inflammatory bowel disease colon mucosa demonstrates lack of major differences between Crohn’s disease and ulcerative colitis. PLOS ONE 2013; 8:e5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacDermott RP, Nash GS, Bertovich MJ et al Altered patterns of secretion of monomeric IgA and IgA subclass 1 by intestinal mononuclear cells in inflammatory bowel disease. Gastroenterology 1986; 91:379–85. [DOI] [PubMed] [Google Scholar]
- 6. Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut 2006; 55:426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muller KE, Lakatos PL, Kovacs JB et al Baseline characteristics and disease phenotype in inflammatory bowel disease results of a paediatric IBD cohort. J Pediatr Gastroenterol Nutr 2015; 62:50–55. [DOI] [PubMed] [Google Scholar]
- 8. Ganz T. Hepcidin and iron regulation, 10 years later. Blood 2011; 117:4425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Limbergen J, Russell RK, Drummond HE et al Definition of phenotypic characteristics of childhood‐onset inflammatory bowel disease. Gastroenterology 2008; 135:1114–22. [DOI] [PubMed] [Google Scholar]
- 10. Rubio CA. My approach to reporting a gastric biopsy. J Clin Pathol 2007; 60:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Philipsen EK, Bondesen S, Andersen J, Larsen S. Serum immunoglobulin G subclasses in patients with ulcerative colitis and Crohn’s disease of different disease activities. Scand J Gastroenterol 1995; 30:50–3. [DOI] [PubMed] [Google Scholar]
- 12. Ruthlein J, Ibe M, Burghardt W, Mossner J, Auer IO. Immunoglobulin G (IgG), IgG1, and IgG2 determinations from endoscopic biopsy specimens in control, Crohn's disease, and ulcerative colitis subjects. Gut 1992; 33:507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Funderburg NT, Stubblefield Park SR, Sung HC et al Circulating CD4(+) and CD8(+) T cells are activated in inflammatory bowel disease and are associated with plasma markers of inflammation. Immunology 2013; 140:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401:708–12. [DOI] [PubMed] [Google Scholar]
- 15. Elices MJ, Osborn L, Takada Y et al VCAM‐1 on activated endothelium interacts with the leukocyte integrin VLA‐4 at a site distinct from the VLA‐4/fibronectin binding site. Cell 1990; 60:577–84. [DOI] [PubMed] [Google Scholar]
- 16. Bemark M, Holmqvist J, Abrahamsson J, Mellgren K. Translational mini‐review series on B cell subsets in disease. Reconstitution after haematopoietic stem cell transplantation – revelation of B cell developmental pathways and lineage phenotypes. Clin Exp Immunol 2012; 167:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lundell AC, Johansen S, Adlerberth I, Wold AE, Hesselmar B, Rudin A. High proportion of CD5+ B cells in infants predicts development of allergic disease. J Immunol (Balt) 2014; 193:510–8. [DOI] [PubMed] [Google Scholar]
- 18. Timmermans WM, van Laar JA, van der Houwen TB et al B‐cell dysregulation in Crohn’s disease is partially restored with infliximab therapy. PLOS ONE 2016; 11:e0160103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di Sabatino A, Carsetti R, Rosado MM et al Immunoglobulin M memory B cell decrease in inflammatory bowel disease. Eur Rev Med Pharmacol Sci 2004; 8:199–203. [PubMed] [Google Scholar]
- 20. Di Sabatino A, Rosado MM, Ciccocioppo R et al Depletion of immunoglobulin M memory B cells is associated with splenic hypofunction in inflammatory bowel disease. Am J Gastroenterol 2005; 100:1788–95. [DOI] [PubMed] [Google Scholar]
- 21. Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis 2011; 17:423–39. [DOI] [PubMed] [Google Scholar]
- 22. Levine A, Koletzko S, Turner D et al ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr 2014; 58:795–806. [DOI] [PubMed] [Google Scholar]
- 23. Simren M, Palsson OS, Whitehead WE. Update on Rome IV criteria for colorectal disorders: implications for clinical practice. Curr Gastroenterol Rep 2017; 19:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saalman R, Mattsson U, Jontell M. Orofacial granulomatosis in childhood – a clinical entity that may indicate Crohn’s disease as well as food allergy. Acta Paediatr 2009; 98:1162–7. [DOI] [PubMed] [Google Scholar]
- 25. Pandya JM, Lundell AC, Hallstrom M, Andersson K, Nordstrom I, Rudin A. Circulating T helper and T regulatory subsets in untreated early rheumatoid arthritis and healthy control subjects. J Leukoc Biol 2016; 100:823–33. [DOI] [PubMed] [Google Scholar]
- 26. Kruetzmann S, Rosado MM, Weber H et al Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med 2003; 197:939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bylesjo M, Rantalainen M, Cloarec O, Nicholson JK, Holmes E, Trygg J. OPLS discriminant analysis: combining the strengths of PLS‐DA and SIMCA classification. J Chemometr 2006; 20:341–51. [Google Scholar]
- 28. Fagan EA, Dyck RF, Maton PN et al Serum levels of C‐reactive protein in Crohn’s disease and ulcerative colitis. Eur J Clin Invest 1982; 12:351–9. [DOI] [PubMed] [Google Scholar]
- 29. Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA Jr. Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med 1993; 177:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yokota A, Kikutani H, Tanaka T et al Two species of human Fc epsilon receptor II (Fc epsilon RII/CD23): tissue‐specific and IL‐4‐specific regulation of gene expression. Cell 1988; 55:611–8. [DOI] [PubMed] [Google Scholar]
- 31. Haworth O, Hardie DL, Burman A et al A role for the integrin alpha6beta1 in the differential distribution of CD4 and CD8 T‐cell subsets within the rheumatoid synovium. Rheumatology (Oxf) 2008; 47:1329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pinto‐Mariz F, Carvalho LR, de Mello W et al Differential integrin expression by T lymphocytes: potential role in DMD muscle damage. J Neuroimmunol 2010; 223:128–30. [DOI] [PubMed] [Google Scholar]
- 33. Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol 2003; 21:685–711. [DOI] [PubMed] [Google Scholar]
- 34. Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC‐205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med 2002; 196:1627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roediger WE. The colonic epithelium in ulcerative colitis: an energy‐deficiency disease? Lancet 1980; 2:712–5. [DOI] [PubMed] [Google Scholar]
- 36. Thibault R, Blachier F, Darcy‐Vrillon B, de Coppet P, Bourreille A, Segain JP. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis 2010; 16:684–95. [DOI] [PubMed] [Google Scholar]
- 37. Sjoberg F, Barkman C, Nookaew I et al Low‐complexity microbiota in the duodenum of children with newly diagnosed ulcerative colitis. PLOS ONE 2017; 12:e0186178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fallahi G, Aghamohammadi A, Khodadad A et al Evaluation of antibody response to polysaccharide vaccine and switched memory B cells in pediatric patients with inflammatory bowel disease. Gut Liver 2014; 8:24–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perez‐Andres M, Paiva B, Nieto WG et al Human peripheral blood B‐cell compartments: a crossroad in B‐cell traffic. Cytom B Clin Cytom 2010; 78(Suppl 1):S47–60. [DOI] [PubMed] [Google Scholar]
- 40. Castell JV, Gomez‐Lechon MJ, David M, Hirano T, Kishimoto T, Heinrich PC. Recombinant human interleukin‐6 (IL‐6/BSF‐2/HSF) regulates the synthesis of acute phase proteins in human hepatocytes. FEBS Lett 1988; 232:347–50. [DOI] [PubMed] [Google Scholar]
- 41. Gangarossa S. Interleukin‐6 and thrombocytopoiesis: probable role of platelets during acute phase responses. Pediatr Hematol Oncol 1991; 8:281–2. [DOI] [PubMed] [Google Scholar]
- 42. Hirano T, Taga T, Yamasaki K et al A multifunctional cytokine (IL‐6/BSF‐2) and its receptor. Int Arch Allergy Appl Immunol 1989; 88:29–33. [DOI] [PubMed] [Google Scholar]
- 43. Cotugno N, Finocchi A, Cagigi A et al Defective B‐cell proliferation and maintenance of long‐term memory in patients with chronic granulomatous disease. J Allergy Clin Immunol 2015; 135:753–61.e2. [DOI] [PubMed] [Google Scholar]
- 44. Caso F, Galozzi P, Costa L, Sfriso P, Cantarini L, Punzi L. Autoinflammatory granulomatous diseases: from Blau syndrome and early‐onset sarcoidosis to NOD2‐mediated disease and Crohn’s disease. RMD Open 2015; 1:e000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Montfrans JM, Hoepelman AI, Otto S et al CD27 deficiency is associated with combined immunodeficiency and persistent symptomatic EBV viremia. J Allergy Clin Immunol 2012; 129:787–93.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Strober W, Asano N, Fuss I, Kitani A, Watanabe T. Cellular and molecular mechanisms underlying NOD2 risk‐associated polymorphisms in Crohn’s disease. Immunol Rev 2014; 260:249–60. [DOI] [PubMed] [Google Scholar]
- 47. Farrokhyar F, Swarbrick ET, Irvine EJ. A critical review of epidemiological studies in inflammatory bowel disease. Scand J Gastroenterol 2001; 36:2–15. [DOI] [PubMed] [Google Scholar]
- 48. Gent AE, Hellier MD, Grace RH, Swarbrick ET, Coggon D. Inflammatory bowel disease and domestic hygiene in infancy. Lancet 1994; 343:766–7. [DOI] [PubMed] [Google Scholar]
- 49. Kim SK, Lee ES. Orofacial granulomatosis associated with Crohn’s disease. Ann Dermatol 2010; 22:203–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Radon K, Windstetter D, Poluda AL, Mueller B, von Mutius E, Koletzko S. Contact with farm animals in early life and juvenile inflammatory bowel disease: a case–control study. Pediatrics 2007; 120:354–61. [DOI] [PubMed] [Google Scholar]
- 51. Springmann V, Brassard P, Krupoves A, Amre D. Timing, frequency and type of physician‐diagnosed infections in childhood and risk for Crohn’s disease in children and young adults. Inflammatory Bowel Diseases 2014; 20:1346–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Gating strategy for B‐ and T‐lymphocytes. (a) Gating strategy for CD5, CD23 and CD27 of CD19+ B‐cells, in peripheral blood from a patient with Crohn’s disease. First, lymphocytes were identified with the use of forward (FSC) and side scatter (SSC). Thereafter, B‐cells were recognized as CD19+ cells, that were studied for their expression of CD23, CD27 and CD5. (b) Gating strategy for CD62L, CD45RA, CD45RO, CD38, HLA‐DR on CD4+ or CD8+ T cells, in peripheral blood from a patient with ulcerative colitis. With the use of forward and side scatter the lymphocytes were identified. Subsequently, the CD4+ and CD8+ T cells were gated, and the expression of CD62L. CD45RA, CD45RO, CD38 or HLA‐DR was examined with the two T‐cell populations. (c) The gating strategy for the expression of β1 on CD4+ and CD8+ T‐cell populations in a patient with ulcerative colitis. First, the lymphocyte population was identified with the use of forward and side scatter. Thereafter, the CD4+ T‐cell population was identified as CD3+CD4+ lymphocytes, whereas the CD8+ T‐cell population was recognized as CD3+CD4neg lymphocytes. Finally, the expression of β1 was studied on these two subsets of T cells.
Fig. S2. Markers of T cell activation in relation to systemic and mucosal inflammation markers in children with ulcerative colitis. Orthogonal projection to latent structures by means of partial least squares (OPLS) that depicts the association between the proportion of (a) CD4+ cells expressing HLA‐DR, (b) CD8+ T cells that express β1 and (c) CD4+ T cells that express β1 (Y variables), and the concentration of different inflammation markers (X‐variables). X‐variables with bars projected in the same direction as the Y‐variables are positively associated, whereas X‐variables projected in the opposite direction are inversely related to Y. The larger the bar and smaller the error bar the stronger andmore certain is the contribution to the model. Statistically significant differences between the Y‐variables and the different inflammation markers are denoted with the r‐valueand asterisks. *P < 0·05, **P < 0·01 (Spearman’s rank correlation test).
Table S1. Monoclonal antibodies used for the characterization of CD4+, CD8+ T cells and CD19+ B cells.
Table S2. Alpha‐chain expression of β1+ T cells (CD4+ and CD8+) in children with newly diagnosed ulcerative colitis and in non‐IBD controls.


