Abstract
Acute myeloid leukemia (AML) is the most common acute leukemia that is becoming more prevalent particularly in the older (65 years of age or older) population. For decades, “7 + 3” remission induction therapy with cytarabine and an anthracycline, followed by consolidation therapy, has been the standard of care treatment for AML. This stagnancy in AML treatment has resulted in less than ideal treatment outcomes for AML patients, especially for elderly patients and those with unfavourable profiles. Over the past two years, six new therapeutic agents have received regulatory approval, suggesting that a number of obstacles to treating AML have been addressed and the treatment landscape for AML is finally changing. This review outlines the challenges and obstacles in treating AML and highlights the advances in AML treatment made in recent years, including Vyxeos®, midostaurin, gemtuzumab ozogamicin, and venetoclax, with particular emphasis on combination treatment strategies. We also discuss the potential utility of new combination products such as one that we call “EnFlaM”, which comprises an encapsulated nanoformulation of flavopiridol and mitoxantrone. Finally, we provide a review on the immunotherapeutic landscape of AML, discussing yet another angle through which novel treatments can be designed to further improve treatment outcomes for AML patients.
Key words: acute myeloid leukemia, immunotherapy, liposomes, nanotechnology
Preface
In the last two years, eight new agents have received regulatory approval for the treatment of acute myeloid leukemia (AML), representing a breakthrough given that management of AML has relied primarily upon standard 7 + 3 chemotherapy for over four decades. What have we learned from the past failures and recent successes? How can we combine the lessons learned to create novel therapeutics that will further improve treatment outcomes in AML patients with poor prognosis? Through reviewing the clinical problems of AML along with the unique features of investigational and recently approved treatments, we came to these conclusions: First, the fact that six of the eight new AML treatments have shown superior efficacy and/or have been approved in the combination setting highlights the importance of using multiple agents to target the heterogeneous nature of the disease. Second, optimizing the manner through which broad spectrum agents or any therapeutic agent is administered could have a profound effect on treatment outcomes as demonstrated by the approval of Vyxeos®. Third, improved understandings of the biology of AML have contributed to more effective treatments by enabling resistance mechanisms to be targeted upfront (e.g. the use of MCL-1 inhibitors in combination with potent agents that are known to give rise to MCL-1 overexpressing relapses). Fourth, emerging concepts such as various vaccination approaches, immunogenic cell death, and immunotherapy adjuvants that stimulate the presentation of multiple cancer-derived antigens could be promising for a disease that is normally considered poorly immunogenic. Altogether, we believe that the most effective regimens for AML patients with unfavourable profiles would involve multi-modal treatments comprising combinations of chemotherapy, targeted agents, or immune-based treatments: therapeutic agents that may be further enhanced by leveraging the advantages of nanoscale drug delivery systems.
Background - Acute Myeloid Leukemia
Acute myeloid leukemia (AML) is the most common myeloid leukemia and it is also becoming more prevalent amongst adults aged 65 years or older (1,2). AML can arise as a de novo malignancy in an otherwise healthy patient or as a secondary malignancy resulting from mutagenic events caused by leukemogenic agents such as alkylating chemotherapy drugs or radiation (3–5). AML was initially considered a monolithic disease that can be treated with a “one-for-all” chemotherapy drug (6). However, studies over the years revealed that AML is actually a genetically pleiomorphic disease, characterized by genetic heterogeneity at diagnosis (6,7). A summary of the mutational landscape of AML is presented in Fig. 1. Therefore, in order to capture this diversity to achieve better treatment outcomes, cytogenetic analysis has become a mainstay in the standard diagnosis of AML (6). Despite efforts to improve therapeutic outcomes and to better understand this disease, the standard of care for AML has remained largely unchanged for more than 40 years and it consists of two phases: remission induction therapy followed by consolidation therapy (2,8). Induction therapy consists of a “7 + 3” regimen which entails 7 days of continuous infusion of cytarabine in combination with 3 consecutive days of anthracycline treatment (typically daunorubicin) (8). Upon achieving initial remission from induction therapy, patients then undergo consolidation therapy to eradicate residual leukemia and this regimen typically includes high doses of cytarabine with or without hematopoietic stem cell transplantation (8,9). For most patients who are 60 years of age or younger, a complete remission is achievable with this initial cytotoxic induction therapy (7,10). However, despite the likelihood of achieving initial remission, AML remains challenging to treat, particularly when the patients are older or when the disease relapses or becomes refractory (10). However, the treatment landscape is changing due to (i) a better understanding of what is driving malignancy which points towards targeted therapeutics; (ii) the use of immunotherapy strategies; and (iii) the application of nanoformulation technology to prepare combination products. Here, the clinical challenges of AML, the recently developed therapeutic strategies, and emerging treatment designs are reviewed for the treatment of AML in the newly diagnosed as well as the relapsed or refractory (r/r) setting.
Fig. 1.

Most commonly found mutations in AML.
Challenges of Treating AML
Management of AML in elderly patients is particularly challenging. Patients who are older than 65 years of age often present adverse cytogenetic risk profiles and these patients typically carry more comorbidities. In a retrospective analysis of 968 adult AML patients, the percentage of patients with unfavourable cytogenetics increased from 35% in patients younger than 56 years of age to 51% in those older than 75 (11). Furthermore, there was also an increase from 33% in younger patients to 57% in older patients when considering the percentage of patients that develop multidrug resistance (11). The increased incidence of unfavourable cytogenetics coupled with other comorbidities such as lower white blood cell counts make the older patients less tolerant of intense chemotherapy and hematopoietic stem cell transplantation (HSCT) (9,11,12). As a result, treatment outcome deteriorates markedly with age and the prognosis is particularly grim for older AML patients, with complete remission rates of less than 55% and a 5-year survival rate of less than 10% (9,13).
In addition to age, further challenges in treating AML arise when the disease relapses, and this applies to the majority of patients who achieved initial remission (10,14). The complexities and challenges of treating relapsed and refractory (r/r) AML often stem from the genetic heterogeneity presented at the time of diagnosis (15). Particularly, studies have demonstrated that AML features dynamic clonal evolution at relapse, with the appearance of new mutations that may contribute to the pathogenesis of r/r AML (16). In fact, this clonal evolution has been highlighted as the primary driver of chemotherapy resistance and the cause of treatment failure in r/r AML (16,17). One of the implicated genetic aberrations that has shown to be prominent in chemo-resistance in AML, contributing to relapses, is the elevated expression of proteins belonging to the Bcl-2 protein family, particularly Mcl-1 (18–20). Mcl-1 is a pro-survival, anti-apoptotic protein that primarily functions as an inhibitor of apoptosis effectors Bak and Bax (19,20). As such, elevated levels of Mcl-1 have been shown to delay apoptosis, leading to chemo-resistance and the ensuing relapse of the disease (18,20,21).
Over the past four decades, multiple clinical trials were conducted to examine alternative doses, schedule, and even new cytotoxic agents in an attempt to achieve therapeutic improvements (13,22). Unfortunately, AML patients still face the same therapeutic obstacles and relapse after complete remission (2,8,13). AML clearly presents an unmet medical need and new therapeutic strategies to improve treatment outcomes of AML, in particular r/r AML, are urgently required.
Novel Therapeutic Strategies for AML
Given tumour heterogeneity and the ability of AML cells to activate a plethora of pro-survival signalling pathways, combination treatments that target multiple leukemogenic pathways is viewed as required to achieve improvements in overall survival in this patent population (23,24). Unfortunately, for decades, AML has been a therapeutic graveyard of failed drug programs designed to look for new treatment regimens. The vast majority of the therapeutic studies have typically come from tinkering with existing therapeutics such as increasing doses of daunorubicin and cytarabine for induction and consolidation therapy respectively or alternatively assessing daunorubicin replacements such as mitoxantrone or vosaroxin (25–28). Fortunately, there was a change in the therapeutic landscape for AML in 2017 and 2018 with novel therapeutic agents being approved by the Food and Drug Administration (FDA) for the treatment of AML (Fig. 2).
Fig. 2.
Progression of AML therapeutics over the years.
2017 saw the approval of four new therapeutic options by the FDA: Midostaurin (Novartis Pharmaceuticals), Enasidenib (Celgene and Agios Pharmaceuticals), Vyxeos® (also known as CPX 351; Celator/Jazz Pharmaceuticals), and Gemtuzumab ozogamicin (Pfizer) (29). Further progress was made in 2018 with the approval of Venetoclax (AbbVie and Genentech) and Daurismo™ (Pfizer). Ivosidenib (Agio Pharmaceuticals) and gilteritinib (Astellas Pharma US) were also approved as single-agent treatment for refractory and relapsed AML in 2018. However, as this review is focused on combination treatments, detailed discussions of ivosidenib and gilteritinib will not be pursued. Nevertheless, as we believe that combinations are capable of exhibiting efficacy far greater than single agent activities, new combinations utilizing new and improved single agents should be developed and investigated. The approval of these new therapeutics attempts to address the many challenges of AML treatment and has provided AML patients with alternatives where intensive chemotherapy is not an option. A summary of the therapeutic agents discussed below is presented in Table I.
Table I.
Therapeutic Landscape of AML
Midostaurin
Some of the most common genetic aberrations found in AML are FLT3 mutations (30). Mutations of the tyrosine kinase FLT3 activate downstream pathways such as the PI3K and RAS/ERK pathways (31). FLT3 mutations can occur as internal tandem duplication (ITD) or can be found in the tyrosine kinase domain (TKD) (30,31). FLT3 mutations confer a negative prognosis in AML patients and have emerged as important targets for therapy (31). One of these promising inhibitors that specifically act against FLT3-TKD and FLT3-ITD mutants is midostaurin (30,32). As a single agent, midostaurin has limited utility in AML (30). However, when used in combination with standard of care “7 + 3” chemotherapy, midostaurin significantly prolongs overall survival as demonstrated in a phase 3 clinical trial with 717 patients, where FLT3-positive AML patients survived 49 months longer based on median survival and had a 5.2 month-improvement in event-free survival. Additionally, these patients had a 24.3% lower risk of death compared to just chemotherapy alone (33). In light of the positive data, FDA approved midostaurin on April 28th, 2017 to be used with the traditional standard of care (cycles of cytarabine and daunorubicin) for the treatment of adult AML patients with newly diagnosed FLT3-mutations (30). This marked the first new FDA approval for AML treatment in decades (32). Additionally, midostaurin is currently under investigation for use in combination with the aforementioned Vyxyeos® to treat patients with FLT3 mutations and preliminary findings revealed robust synergy when the two therapeutic agents were used concurrently (34).
Enasidenib
Enasidenib is an isocitrate dehydrogenase 2 (IDH2) inhibitor (35). Isocitrate dehydrogenase mutations are observed in 15–20% of AML patients and cause the reduction of α-ketoglutarate to 2-hydroxyglutarate (36). 2-hydroxyglutarate inhibits the function of histone lysine demethylases, leading to hypermethylation of DNA and histones (36). This, in turn, exerts leukemogenic effects by blocking the differentiation of hematopoietic progenitor cells (36). Enasidenib was approved by the FDA on August 1st, 2017 as a monotherapy drug for the treatment of adult patients with r/r AML with IDH2 mutation. As a single agent, enasidenib is an effective option, with a complete remission (CR) rate of around 20% in AML patients having IDH2 mutations (35,37). However, enasidenib is significantly underutilized if it is only used as a monotherapy agent as enasidenib showed marked improvement in the CR rate when used in combination with standard induction chemotherapy (37). In a phase 1 clinical study with AML patients possessing IDH2 mutations, combining enasidenib with standard induction chemotherapy resulted in an improvement of CR rates to 67% and 58% in de novo and secondary AML respectively (37).
CPX-351 (Vyxeos®)
CPX-351, or Vyxeos®, is a liposomal formulation of cytarabine and daunorubicin that delivers the two drugs at a fixed synergistic cytarabine-to-daunorubicin molar ratio of 5:1 (38,39). Compared to standard of care treatment, Vyxeos® demonstrated superior median overall survival (3.61 months longer), event-free survival (1.22 months longer), and remission rate (14.4% higher) without increasing treatment-related mortality and toxicities (38–40). Liposomes are well-established nanocarriers that are known to prolong circulation time in blood and this remarkable improvement in treatment outcome by Vyxeos® was attributed to the increased circulation lifetime of the cytotoxic agents as well as the administration and maintenance of a fixed synergistic drug combination ratio (40–42). Based on these positive findings, FDA approved Vyxeos® for use in the treatment of adults with newly diagnosed therapy-related AML on August 3, 2017 (43).
Venetoclax
Venetoclax is an orally bioavailable Bcl-2 inhibitor (44). As discussed previously, overexpression of Bcl-2 implicates survival of AML cells and is often associated with treatment resistance (44). Inability to completely eradicate leukemic stem cells (LSC) is often believed to be an important cause of treatment failure and disease relapse in AML and high levels of Bcl-2 have recently been identified as a defining characteristic of LSC (45,46). As a Bcl-2 inhibitor, Venetoclax has shown promising activity when used in combination with hypomethylating agents such as azacitidine or decitabine or with low-dose cytarabine. These venetoclax combinations were granted accelerated approval from the FDA on November 21, 2018 to be used to treat adult AML patients who are aged 75 years or older or patients who have comorbidities that preclude them from being treated with induction chemotherapy (44,47). Complete remission rates were 37%, 54%, and 21% when venetoclax was used in combination with azacitidine, decitabine, and low-dose cytarabine respectively (44,47). Phase 3 studies (NCT02993523 and NCT03069352) are currently underway to serve as confirmatory trials to evaluate overall survival when venetoclax is used in combination with azacitidine or low-dose cytarabine. Additionally, venetoclax has shown synergy when used with MCL-1-inhibitors such as flavopiridol (alvocidib) and this combination is now being tested as a rational combination in phase I clinical trial (NCT03441555) (48).
Glasdegib (Daurismo™)
The Hedgehog signalling pathway is critical to embryonic development by regulating differentiation and proliferation in a time-dependent fashion (49). Dysregulation of the Hedgehog pathway has been detected in a variety of hematopoietic malignancies, predominantly in leukemic stem cells (50,51). Leukemic stem cells are typically quiescent and are thus resistant to conventional chemotherapy treatments (51). Therefore, inhibitors of Hedgehog signalling may be ideal options to target leukemic stem cells. Glasdegib, or Daurismo™, is a small molecule inhibitor of the transmembrane protein Smoothened (SMO) (47). Translocation of SMO into the primary cilium of blood cells is a necessary step in the activation of the Hedgehog pathway (47,52). As a SMO inhibitor, glasdegib prevents this translocation and in turn inhibits the activation of downstream Hedgehog targets (47). Based on a randomized phase 2 trial (NCT01546038), the use of glasdegib with low dose cytarabine extended median overall survival by 3.4 months and improved complete response rates by 12.7% relative to using low-dose cytarabine alone (53). Given its promise, glasdegib was approved by the FDA on November 21st, 2018 to be used in combination with low-dose cytarabine to treat AML patients who are 75 years or older or those who have comorbidities that prohibit them from undergoing intensive induction chemotherapy.
Gemtuzumab Ozogamicin
One of the most investigated AML-related target is CD33 (Siglec-3), an antigen that is highly expressed on blasts of 85–90% patients with AML as well as on normal myeloid cells (54). Expression of CD33 is highly variable with about half of AML patients expressing CD33 on >75% of leukemic blast cells (55). Although anti-CD33 monoclonal antibodies (mAbs) such as lintuzumab are tolerated in human clinical trials, they failed to provide meaningful clinical benefits (56,57). The only approved CD33-targeting agent is gemtuzumab ozogamicin (GO; Mylotarg™), a humanized CD33 mAb conjugated to a DNA damaging calicheamicin derivative (57). Gemtuzumab ozogamicin was granted FDA approval in 2000. However, based on a phase 3 trial (Southwest Oncology Group (SWOG); S0106 study; NCT00085709), the addition of GO to standard chemotherapy not only failed to improve treatment outcomes but was associated with statistically significant increase in treatment-related deaths relative to the standard of care (SOC) arm (55,58). This led to the voluntary withdrawal of Mylotarg™ by Pfizer in 2010. In September 2017, Mylotarg™ was re-approved by the FDA to be used in combination with daunorubicin and cytarabine for the treatment of newly diagnosed CD33+ AML patients and for r/r CD33+ adult and pediatric patients over the age of two. The approval was based on results from ALFA-0701 (NCT00927498), a multicenter phase III study of 271 newly diagnosed AML patients. Here, the addition of GO to standard chemotherapy extended median event-free survival by 8.2 months (no significant gain in overall survival) with a hazard ratio of 0.66 (57,59). In light of the success of Mylotarg™ in combination with standard of care chemotherapy, a phase I clinical study was initiated to investigate the potential of using Mylotarg™ with Vyxeos® to treat patients with r/r AML (NCT03672539).
The success of Mylotarg™ highlighted the potential of cancer immunotherapy in hematologic malignancies and to gain a better understanding of the immune aspects of AML, we review below the immunotherapy landscape for AML and discuss the challenges as well as opportunities of the immunotherapeutic strategies for the treatment of AML in the newly diagnosed as well as the relapsed or refractory (r/r) setting.
Application of Immunotherapeutic Concepts for the Treatment of AML
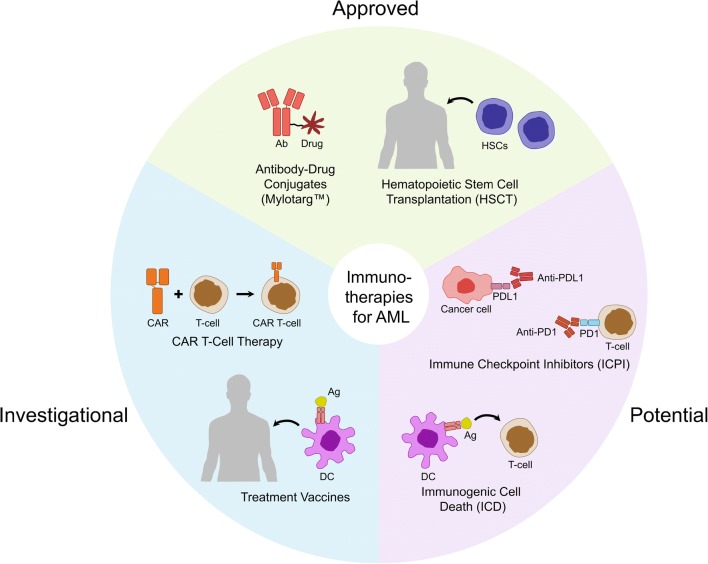
Advances in cancer immunotherapy over the past decade have undoubtedly revolutionized treatment strategies for both solid tumours and hematologic malignancies (60,61). Since 2011, six immune checkpoint inhibitors (ICPIs) have been approved by the FDA to treat various solid tumours including metastatic melanoma and advanced non-small cell lung cancer (NSCLC): illnesses that were considered untreatable at diagnosis until recent years (60). ICPIs have been associated with promising and durable responses extending survival, for the first time, for months or even years in patient populations that would otherwise succumb to their diseases within a year’s time. The use of genetically modified lymphocytes, specifically chimeric antigen receptor (CAR) T cells, on the other hand, has achieved great successes in the treatment of blood cancers, primarily B cell malignancies such as B cell acute lymphoblastic leukemia (ALL), chronic lymphoblastic leukemia (CLL), and non-Hodgkin’s lymphoma (57,61,62). The different immunotherapies that have been considered for treating AML are described in the following sections and are summarized in Fig. 3.
Fig. 3.
Immunotherapy strategies for AML treatment.
Hematopoietic Stem Cell Transplantation (HSCT)
A small population of poor-risk AML patients may be given the option for allogeneic hematopoietic stem cell transplantation (HSCT) based on factors such as comorbidities, age, and availability of donors (63,64). HSCT is one of the oldest and most successful immunotherapeutic approaches in the treatment of AML, which involves ablation of the bone marrow using radiation or chemotherapy followed by re-implantation of stem cells from the host (autologous) or a donor (allogeneic) (57). Unfortunately, the majority of elderly patients are not eligible for HSCT, which is the last curative option for r/r patients (57,65). Even after HSCT, 40–60% patients develop graft-versus-host disease (GVHD) and approximately 20–50% patients invariably relapse and are left with very limited treatment options, underscoring the importance of developing novel immunotherapeutic approaches to improve this dire scenario (66,67). In recent years, efforts are being placed on optimising HSCT procedures, some of which include the use of reduced-intensity conditioning or myeloablative conditioning prior to HSCT (NCT02626715), infusion of donor natural killer cells (NCT03300492), and prophylactic use of IL-2 in combination with HSCT (NCT01517347), all of which are designed to lower the incidence of GVHD, reduce toxicities, extend the application of HSCT to elderly and frail patients, and to prevent relapse (68–70).
Antibody-Drug Conjugates
Novel, non-transplant immunotherapeutic modalities are also being heavily explored for AML. In addition to the CD33-targeting agent, Mylotarg™, which was discussed above, Vadastuximab talirine (SGN-CD33A) is another antibody-drug conjugate (ADC) that has been tested in human clinical trials in recent years. While results from phase I study were promising, a phase III trial evaluating the combination of SGN-CD33A with the hypomethylating agents azacitidine or decitabine in newly diagnosed elderly AML patients was discontinued by Seattle Genetics in 2017 due to increased treatment-related mortality (71,72).
CD123 is another AML-target of interest particularly due to its more restricted expression on normal hematopoietic stem cells, suggesting the potential to reduce “on-target off-leukemia toxicities” (54,57,73,74). To date, talacotuzumab (mAb; J&J; NCT02472145) and SGN-CD123A (ADC; Seattle Genetics; NCT02848248 have been tested in human clinical trials, of which both were halted in development due to unfavourable risks/benefits profiles. Taken together, additional studies are warranted to validate the feasibility and utility of targeting CD123 in the treatment of AML.
AML Vaccines
Vaccination is an attractive strategy for patients who are not eligible for HSCT or who relapse following HSCT. To date, three main types of vaccines are being tested in humans for AML: peptide, granulocyte macrophage colony stimulating factor (GM-CSF), and dendritic cell (DC) vaccines. Peptide vaccines involve the use of AML-associated antigens to stimulate a cytotoxic T cell response. Two common targets are Wilms tumor 1 (WT1) and PR1 (peptide derived from proteinase 3), both of which are overexpressed in AML. Recent results from a phase II clinical trial investigating the multivalent WT1 peptide vaccine galinpepimut-S (NCT01266083) suggest that the peptide vaccine is tolerated and may contribute to increase in overall survival (75). On the other hand, a PR1 peptide vaccine has been shown to induce antigen-specific immune responses in patients based on a Phase I/II trial (NCT00004918) (76,77). However, it is unclear when and how the vaccine should be used to produce optimal treatment efficacy (77). Another vaccination strategy is the use of gene-transduced tumor cell vaccine (GAVX) where cells are modified to express GM-CSF to recruit DCs to the site of intradermal vaccination to elicit an anti-tumour immune response (78). A phase II trial (NCT01773395) is being conducted to evaluate the efficacy of GVAX following allogeneic HSCT. Dendritic cell vaccination, which has the potential of presenting multiple tumour-derived antigens, is of particular interest for the treatment of AML due to the poor immunogenic nature of the disease (57,79). DCP-001 (DCPrime), a vaccine developed through the differentiation of the AML cell line DCOne into mature DCs, is currently recruiting for a phase II clinical trial (ADVANCE-II; NCT03697707) after demonstrating both safety and induction of immune responses in elderly AML patients in its phase I study (NCT01373515) (80). This strategy was designed to replace conventional DC vaccination approaches which involve administration of mature DCs that were isolated from the patients and matured ex vivo: an approach that is known to be costly, cumbersome, and logistically complex (28). Another DC-based treatment being tested in humans involves the vaccination with a hybridoma consisting of patient-derived AML cells fused with autologous DCs (81). In that study, 12/17 patients who had achieved remission experienced no recurrence after 57 months and showed expansion of disease-specific T cells which may help protect against relapse (81). This vaccination approach is being evaluated in combination with PD-1 blockade in a phase II clinical trial (NCT01096602).
Chimeric Antigen Receptor T (CAR-T) Cell Therapy
CAR-T therapy has been greatly successful in the treatment of some hematologic malignancies but this accomplishment has yet to transfer to AML. Briefly, CAR-T cells are patient-derived T cells that have been genetically modified to recognize antigens expressed on the cancer cell’s surface (82). The development of CAR-T therapy for AML, however, has been much more challenging compared to that for B cell malignancies due to poor immunogenicity stemming from low mutation burden, the lack of AML-specific antigens leading to the risk of generating on-target off-leukemia toxicities, and the heterogeneous biology of the disease arising from various myeloid progenitors (57,62,82,83). Numerous targets are being explored in the preclinical and/or clinical development of CAR-T treatments for AML including CD33, CD123, FLT3(CD135), CD7, NKG2D, CD133, FRβ, LeY, and CLL-1 (82,84–91). While CAR-T cells are attractive due to their demonstrated success in other hematologic malignancies, CAR Natural Killer (NK) cells are also being investigated for AML as they are believed to be associated with lower risks of generating severe clinical toxicities such as cytokine release syndrome (92).
Immune Checkpoint Inhibitors (ICPIs)
ICPIs have been associated with remarkable treatment outcomes in various solid tumours including NSCLC and melanoma (57). ICPIs involve the removal of immunosuppressive signals that are often used as a mechanism by cancer cells to evade immune detection. Although no ICPI has been granted approval for the treatment of AML, over 30 clinical trials are underway to evaluate the efficacy of clinically approved ICPIs as single agents or in combinations for newly diagnosed patients as well as post-remission and r/r AML patients. The role of ICPIs in AML will become clearer when results become available from these clinical trials.
Immunomodulators for Immunogenic Cell Death Induction
Immunogenic cell death (ICD) has emerged in recent years as a popular immunotherapeutic concept that is being investigated pre-clinically and in early clinical trials. ICD is a form of cell death wherein cancer cells, in the process of treatment-induced death, emit certain molecular signals in a specific spatiotemporal pattern that result in the recruitment of immune cells, presentation of tumour-specific antigens, and activation of an adaptive immune response that consequently leads to tumour eradication and generation of immunological memory against future re-challenges (93–95). This “therapeutic vaccination” approach has garnered much attention as it has been shown that several existing chemotherapeutics are capable of inducing ICD, characterized by cell surface expression of calreticulin (CRT) and secretion of ATP and HMGB1 (93,96,97). Traditionally, chemotherapeutics are tested in immune-compromised pre-clinical models to ensure that the agents exert cytotoxic and not immunogenic effects. It is now known that certain chemotherapeutics (e.g. doxorubicin, oxaliplatin, mitoxantrone) induce ICD as a secondary mechanism, providing long-term immune protection in immune competent syngeneic models of cancer (95,96,98–100). While ample evidence suggest that ICD induction may provide cures and vaccination in pre-clinical models, evidence of ICD in humans are still being explored. Currently, over 20 clinical trials are investigating the immunological profile of existing chemotherapeutic regimens in various types of cancer (99). There are also multiple phase I/II clinical trials exploring the potential of novel immunomodulators to improve outcomes of standard chemotherapy or radiotherapy through ICD induction (NCT02906800, NCT02721056, NCT02805894). In a recent report, CRT exposure on leukemic blast cells has been correlated with increased AML-specific immune response, superior relapse-free survival and overall survival, suggesting that ICD-associated markers may be clinically relevant in the AML setting (101). While ICD has not been widely considered as an AML treatment strategy, it is important to note that anthracyclines such as doxorubicin and daunorubicin are used as SOC chemotherapy for AML patients while mitoxantrone, also a known bona fide ICD inducer, is an approved chemotherapeutic for r/r AML patients. Currently, there are over 40 trials involving the use of mitoxantrone to treat AML, suggesting that there are great interests in determining how to best use this potent chemotherapeutic. Use of in a combination setting where the anticancer immune response of mitoxantrone, other bona fide ICD inducers or immune-based treatments can be boosted using an adjuvant may prove to be an effective therapeutic/vaccination strategy (96).
FLAM – Opening New Doors in AML
One of the combinatorial therapeutic strategies that has been employed in the treatment of AML is timed sequential therapy (TST). TST was established based on strategic sequential administration of cell cycle dependent anti-leukemic agents to augment the cytotoxic effect on leukemic cells (102,103). Over the years, TST has shown to yield favourable outcomes in AML patients, producing durable disease-free survival and improved remission rates (104–106). A TST that has shown great therapeutic potential is FLAM. FLAM is the sequential administration of flavopiridol followed by cytarabine and mitoxantrone (107). Flavopiridol is an anticancer agent derived from the plant alkaloid rohitukine that has shown to be effective against various types of cancer such as leukemia, prostate carcinoma, breast carcinoma, and bladder cancer (108–112). Flavopiridol works as a multi-cyclin-dependent kinase (CDK) inhibitor (CDK1, 2, 3, 4, and 9), effectively arresting cell cycle in the G1-S and G2-M phases (108,109,113–116). In AML, in vitro studies showed that flavopiridol induces synchronous cell cycling, increasing the proportion of cells in the S phase 48 h after flavopiridol exposure (102). This observation provided the basis for using flavopiridol in the FLAM regimen with S phase-specific agents like cytarabine and mitoxantrone. When administered as a component of FLAM, the combination regimen demonstrated, in a randomized multicentre Phase 2 trial, complete remission rates of nearly 70% in newly diagnosed poor-risk AML patients. This was nearly a 25% improvement in the complete remission rates compared to the 7 + 3 SOC regimen (103).
In addition to acting as a pan-CDK inhibitor, flavopiridol has also been shown to induce apoptosis through the downregulation of anti-apoptotic proteins such as Bcl-2 and Mcl-1 (117,118). As previously discussed, overexpression of Mcl-1 in AML is often synonymous with disease relapses and the ability of flavopiridol to repress the expression of Mcl-1 is believed to contribute to the synergism found in the FLAM regimen by potentiating the activities of cytarabine and mitoxantrone (119). Despite the remarkable improvement in complete remission rates following FLAM treatment, there were no difference in overall survival or event-free survival when compared to the conventional “7 + 3” regimen (103). Therefore, FLAM clearly has room for improvement.
One approach that our laboratory is considering is to enhance therapeutic effects of sequential flavopiridol, cytarabine and mitoxantrone is through reformulation using nanocarriers, such as liposomes. Nanomedicines are known to alter the pharmacokinetics of drugs, resulting in improved efficacy and reduced toxicities which ultimately lead to better treatment outcomes (120,121). To address the genetic heterogeneity of AML, broad-spectrum chemotherapy drugs have been used in the past and will continue to be used in the future. However, these drugs have associated side effects such as the anthracycline-induced cardiotoxicity that potentially life-threatening to AML patients (122). Additionally, commonly used AML drugs like cytarabine have been shown to have poor retention in blood and show enhanced antitumour activity when the circulation half-life of the drug is extended (123). The use of drug delivery systems, like liposomes, has proven capabilities in altering toxicity and extending circulation lifetime, which are features that are particularly relevant when considering treatments for patients with AML.
Vyxeos® represents one of the most recent success stories for nanomedicines in general and for their use in the treatment of AML in particular. As discussed, Vyxeos® delivers cytarabine and daunorubicin in liposomes at a fixed synergistic ratio and this demonstrates the ability of liposomes to control ratiometric dosing of anticancer drug combinations (124,125). Ratio-dependent dosing was based on in vitro data that showed that some drug combinations, but not all, work optimally at a specified drug-to-drug molar ratio. Prior to the inception of Vyxeos®, Mayer et al. liposomal formulations of several drug combinations based on the in vitro results showing that drug-drug ratio mattered in achieving synergy (126). For example, CPX-1 demonstrated the importance of ratiometric dosing to synergy maintenance between irinotecan and floxuridine (125). At high irinotecan/floxuridine ratios (10:1), irinotecan may antagonize the activity of floxuridine activity by causing cell cycle arrest in S phase (125,127). To build on this concept of ratiometric dosing, Mayer et al. experimented with various molar ratios of cytarabine- to-daunorubicin (1:10, 1:5, 1:1, 5:1, and 10:1) and found that only 1:1, 5:1, and 10:1 were synergistic, with the rest being either additive or antagonistic (125). Due to a lack of mechanistic explanations for the interaction, it can only be postulated that given cytarabine’s effects on newly synthesized DNA (therefore S-phase specific), it is possible that at some ratios, daunorubicin may antagonize cytarabine activity by arresting cells in G1 phase (128).
With Vyxeos® paving the way for nanomedicine and drug delivery systems, studies are underway to evaluate the use of various types of nanocarriers as means to improve the overall efficacy of anti-leukemic agents and to decrease the toxicities presented by these agents of interest. Of the various drug delivery platforms investigated, liposomes remain one of the most widely utilized systems (121,129).
In a study published in 2014, Tan et al. developed a method to co-encapsulate safingol and C2-ceramide, which are both bioactive sphingolipids that have displayed efficacy against AML but are limited clinically due to hemolytic toxicities (130). The resulting formulation significantly reduced the toxicities presented by the sphingolipids when compared to the free drug and the co-encapsulated formulation extended median survival time from 24 to 37 days compared to the singly encapsulated C2-ceramide (130). Other liposomal formulations such as liposomal daunorubicin-emetine (a protein synthesis inhibitor) and liposomal GTI-2040 (ribonucleotide reductase-targeting inhibitor) were also developed and examined preclinically against AML cells (131,132). Co-encapsulation of daunorubicin and emetine resulted in enhanced activity against MOLM-13 cells in vitro, with an increase in the induction of apoptosis by a factor of six (131). Similarly, liposomal GTI-2040 demonstrated significantly improved anti-tumour activity (decreased tumour size and prolonged overall survival of mice) when compared to the free drug (132). In addition to liposomal formulations, other polymer-based nanoparticle delivery systems have been explored for AML indications, including polymeric nanoparticles. In 2012, Simon et al. encapsulated ATRA (all-trans retinoic acid), a chemotherapy drug used to treat acute promyelocytic leukemia (an AML subtype), in polymeric nanoparticles made with poly-D,L-lactadie-co-glycolide (PLGA) (133). This polymer-based formulation allowed ATRA, an otherwise orally administered drug, to be available for intravenous injections, which can be beneficial for patients who are unable to swallow (133). Varshosaz et al. synthesized folate and retinoic acid grafted/dextran (FA-RA/DEX) polymeric micelles for targeted delivery of doxorubicin in AML (134). The doxorubicin-loaded micelles showed enhanced in vitro cytotoxicity against KG-1 cells when compared to the free drug (134). Another example of a nanocarrier demonstrating therapeutic improvements in AML includes dendrimer-based formulations. Dendrimers are nano-scale polymers that are globular in shape with branch-like configurations (135). Szulc et al. used dendrimers to formulate cytarabine triphosphate and the resulting cytarabine-complexed dendrimers significantly enhanced the cytotoxicity of cytarabine against 1301 cells (a T cell leukemia cell line) (135).
Therefore, in light of the recent success of Vyxeos® and the promising potential that liposomes have in improving AML therapeutics, we are developing a novel liposomal combination product derived from FLAM called “EnFlaM”: a product consisting of encapsulated forms of flavopiridol and mitoxantrone that will potentially amplify the synergy that exists between the two cytotoxic agents. In consideration of this effort, the advances in the development of nanoparticulate formulations of mitoxantrone and flavopiridol as single agents is summarized below. These nanoformulations are also summarized in Table II.
Table II.
Nanoparticulate Formulations for Mitoxantrone in Preclinical and Clinical Investigation
| Formulation | Composition | Indication(s) | Development Stage | Ref. |
|---|---|---|---|---|
| Liposome complexed mitoxantrone (LCM) | Soy phosphatidyl choline, cholesterol, phosphatidic acid, D,L-α-tocopherol | Breast cancer | I/II | (136,137) |
| ∆pH Mitoxantrone liposomes | Soy phosphatidyl choline, cholesterol, DPPE-PEG2000 | Leukemia | Preclinical | (138) |
| DSPC/Chol liposomes | DSPC, cholesterol | Leukemia | Preclinical | (139) |
| DMPC/Chol liposomes | DMPC, cholesterol | Leukemia, squamous cell carcinoma, colorectal cancer | Preclinical | (140,141) |
| Liposome-entrapped mitoxantrone Easy-To-Use (LEM-ETU) | DOPC, cholesterol, cardiolipin, alphatocopheryl acid succinate | Various cancers | I | (142,143) |
|
Pegylated liposomal mitoxantrone 60 nm (PLM-60) |
HSPC, cholesterol, DSPE-PEG2000 |
Leukemia, prostate cancer, non-Hodgkin’s lymphoma, various solid tumours, peripheral T cell lymphoma | I/II | (144–146) |
| Mitoxantrone polybutyl cyanacrylate (PBCA) nanoparticles | Butyl cyanacrylate, dextran-70, poloxamer 188 | Leukemia, melanoma | Preclinical | (147,148) |
| Mitoxantrone polybutyl cyanacrylate nanoparticles (DHAQ-PBCA-NP) |
Butyl cyanacrylate, dextran-70, sodium dithionite, sodium chloride |
Hepatocellular carcinoma | II | (149–151) |
| Mitoxantrone solid lipid nanoparticles (MTO-SLN) | Lecithin, Compritol-888, surfactant S-40 | Breast cancer | Preclinical | (152) |
| Mitoxantrone bovine serum albumin nanoparticles (MTO-BSANP) | Bovine serum albumin, glutaraldehyde, folic acid | Ovarian cancer | Preclinical | (153) |
| Mitroxantrone iron oxide magnetic nanoparticles | Iron oxide, dextran | Rhabdomyosarcoma | Preclinical | (154) |
Nanoparticulate Formulations of Mitoxantrone
Mitoxantrone is an active chemotherapeutic drug approved for the treatment of several cancer indications with primary side effects being cardiotoxicity, neutropenia, and bone marrow suppression (155). The controlled release of drugs from nanoparticles can help increase the circulation half-life, reduce renal clearance, and decrease the exposure of normal tissue to high drug concentrations (156). The resulting modified pharmacokinetics and biodistribution could improve the therapeutic index of the drug. Of the various nanoparticle formulations of mitoxantrone that were examined, liposomal mitoxantrone formulations remain the most extensively investigated for use in humans.
The first liposomal formulation of mitoxantrone was produced in the 1990s by Schwendener et al. and was based on the formation of complexes as a result of electrostatic interactions between the cationic drug and phosphatidic acid. The drug was encapsulated in liposomes comprised of soy phosphatidyl choline, D,L-α-tocopherol and cholesterol (Chol). The resulting mitoxantrone formulation was generally more effective and less toxic than the free drug in various tumour models including the murine lymphocytic leukemia model L1210 (136). The liposomal formulation was further evaluated in patients with advanced breast cancer in a Phase I/II study, where it was well tolerated and showed moderate antitumour activity (137). Despite the promising results, mitoxantrone was cleared from the blood circulation relatively rapidly and this triggered follow-up studies to improve the pharmacokinetics of the formulation. In one of the studies, mitoxantrone was encapsulated in novel soy phosphatidyl choline/ cholesterol liposomes modified with 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DPPE-PEG2000) through a pH-gradient-mediated process. This resulting formulation was evaluated only in the preclinical setting and showed superior pharmacokinetic properties with a 40-fold increase of the area under the curve (AUC) (138).
To further optimize the drug release of liposomal mitoxantrone, additional studies were conducted by Madden et al. using the conventional 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC)/ Chol liposomes and sterically stabilized DSPC/Chol/ DPPE-PEG2000 liposomes. Mitoxantrone loading was mediated by a transmembrane pH-gradient and the resulting liposomes extended the survival time of L1210 bearing mice (139). The therapeutic activity of liposomal mitoxantrone was further influenced by the rate of release of the drug from the liposomes. When encapsulated in 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC)/Chol liposomes, mitoxantrone was released at a rate of 1.7 μg/μg lipid/h, which was significantly faster than when encapsulated in DSPC/Chol liposomes (<0.0257 μg/μg lipid/h) (140). The increased release rate resulted in significantly improved long-term survival of the animals treated with DMPC/Chol liposomal mitoxantrone (140). Similar observations were made in mice bearing A431 human squamous cell carcinoma and LS180 human colon cell carcinoma xenografts, where DMPC/ cholesterol liposomes resulted in greater delays in tumour growth compared with animals treated with other liposomal formulations (141).
In the 2000s, research on liposomal mitoxantrone continued with the use of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC)/Chol/cardiolipin liposomes (142). The encapsulation of mitoxantrone was based on the electrostatic interaction between the cationic drug and the negatively charged cardiolipin. The entrapment efficiency of the formulation was 99% and the mean vesicle size was 150 nm (142). The formulation was named liposome-entrapped mitoxantrone Easy-To-Use (LEM-ETU), it was only evaluated in a Phase I clinical trial by the company NeoPharm but no results were disclosed and the formulation has yet to progress further clinically (143).
To explore other liposomal formulations of mitoxantrone, Li et al. encapsulated mitoxantrone in PEGylated (polyethylene glycol-containing) liposomes using a transmembrane copper ion gradient and the formulation significantly enhanced the blood circulation time but did not improve the mean survival time of mice bearing L1210 leukemia cells (157). An additional study with PEGylated liposomes demonstrated that a formulation with hydrogenated soy phosphatidyl choline (HSPC)/ cholesterol/ 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) and high PEG density was the most active against S180 murine sarcoma (158). For this particular formulation, the effect of particle size on the therapeutic efficacy was investigated in a follow-up study (144). The results indicated that the small liposomes (60 nm) had the fastest release rate, exhibited less toxicity, and were the most efficacious (144). Continued research found that the level of antitumour activity of the small-sized formulation, termed PLM-60, directly correlates with increasing level of doses administered (145). The initial in vivo success of PLM-60 led to further investigation of the formulation in a Phase I study in patients with non-Hodgkin’s lymphoma and other malignancies (146). PLM-60 was found to be less toxic, potentially more efficacious, and longer circulating compared to unencapsulated mitoxantrone (146). Recently, in 2018, a randomized Phase I/II clinical trial (NCT03553914) has been scheduled to evaluate the toxicity and overall response rate of PLM-60 in patients with peripheral T cell lymphoma (PTCL).
In addition to liposomes, a polymeric nanoparticle formulation of mitoxantrone based on polybutyl cyanacrylate (PBCA) was tested as an anticancer agent in the clinics. Initial studies demonstrated that PBCA nanoparticles were not able to reduce the toxicity of mitoxantrone but were able to significantly reduce tumour volumes of mice bearing B16 melanoma (147,148). Due to their hepatic seeking tendencies, PBCA formulations serve as suitable drug delivery systems to target liver tumours, reducing toxicity to peripheral organs and enhancing the antitumour effects on hepatic malignancies (149). When a novel PBCA formulation, DHAQ-PBCA-NP, was evaluated in a murine model of orthotopically transplanted human hepatocellular carcinoma, the formulation resulted in inhibition of tumour growth and reduction of acute toxicity compared to free drug (150). This formulation was then evaluated in a Phase II clinical trial in patients with hepatocellular carcinoma. Intravenous administration of DHAQ-PBCA-NP resulted in increased cytotoxicity in hepatic tumours and improved median survival time by 2.23 months compared to patients treated with free mitoxantrone (151).
A myriad of other formulation methods were developed to encapsulate mitoxantrone. These include solid lipid nanoparticles (152) and micelles (159), which are examples of other lipid-based nanoparticles, and cationic surfactant cetyltrimethylammonium bromide (CTAB) micelles (160) and non-ionic surfactant micelles (161,162). Nanoparticles composed of other polymers such as poly(lactic acid-co-lysine) (PLA-PLL) (163), dextran (164), and hyaluronic acid/ chitosan were also used to deliver mitoxantrone (165). Another type of delivery system used were protein-based nanoparticles made with albumin (153) and beta-casein (166). Inorganic nanoparticles were also studied for the delivery of mitoxantrone and some examples include iron oxide magnetic nanoparticles (154,167), silica nanoparticles (168), and zeolite beta nanoparticles (169). The formulations listed here have only been tested in vitro or in preclinical models and none of these formulations have advanced further into clinical trials.
Nanoparticulate Formulations for Flavopiridol
As discussed previously, flavopiridol is an active drug under investigation for many modalities of cancer including acute myeloid leukemia. However, clinical use of flavopiridol is largely hindered by its relatively low aqueous solubility (170) and high binding affinity to plasma proteins (171). In order to solubilize flavopiridol, it is dissolved in 30% hydroxypropyl beta-cyclodextrin/0.1 M citrate buffer (pH 4.52) to form a complex that can be clinically administered to patients (172). However, this formulation was reported to induce several side effects such as nausea/vomiting, diarrhea, fatigue, and neutropenia (173). Nanoparticles such as liposomes are well-documented to improve solubility and to provide steric barriers for encapsulated compounds (174–176). Therefore, the use of well-designed nanoparticle formulations can help increase the solubility of the drug, prevent the interaction with plasma proteins, and reduce toxicities associated with flavopiridol formulations.
Two different types of nanoparticles were used to encapsulate flavopiridol. In one study, flavopiridol was loaded by a transmembrane pH-gradient into liposomes composed of different lipids: HSPC/Chol, HSPC/Chol/ Tween-80, and HSPC/Chol/ DSPE-PEG2000 (177). HSPC/Chol/ DSPE-PEG2000, with a mean diameter of 120.7 nm and entrapment efficiency of 70.4%, was selected to conduct further pharmacokinetic studies in mice. Liposomal flavopiridol increased the elimination phase half-life from 57.0 min for free drug to 339.7 min for liposomal drug, decreased clearance from 0.036 L/min to 0.012 L/min, and augmented AUC from 3.4 min μmol/L to 10.8 min μmol/L (177). These results highlighted the capacity of liposomes to modulate the pharmacokinetic profile of flavopiridol.
In another study, flavopiridol was encapsulated into poly(lactic-co-glycolic acid) (PLGA) nanoparticles to produce a formulation for local delivery and sustained release of the drug (178). Drug release assays in vitro showed that the nanoparticles had a sustained release of flavopiridol for up to 3 days. More specifically, the formulation was prepared to inhibit astrocyte growth and inflammatory factor synthesis during the reparation of spinal cord injuries (178). In this context, flavopiridol nanoparticles decreased cell-cycle activation, reduced glial scarring, and facilitated survival and regeneration of neurons in vivo. Presumably, the use of PLGA nanoparticles of flavopiridol could be extrapolated to other diseases such as cancer, where modified pharmacokinetics resulting in extended sustained release of flavopiridol would be of advantage.
To our knowledge, the development of flavopiridol nanoparticles is limited to the two formulations described above. However, data from other flavonoids with similar structure, such as quercetin, suggest that flavopiridol could be loaded into a variety of different nanomaterials. There are some examples of quercetin nanoparticles designed for the treatment of cancer. Quercetin was entrapped in HSPC/Chol/ PE-PEG2000 liposomes (179), soy lecithin/ cholesterol/ PEG4000 liposomes (180), egg sphingomyelin/ cholesterol/ ceramide-PEG2000 liposomes (181), and lipoid S75/ oleic acid liposomes (182). Additionally, polymers like poly(lactic acid) nanoparticles (183), superparamagnetic iron oxide nanoparticles (184), and mesoporous silica nanoparticles (185) have all been shown to encapsulate quercetin. Additionally, a quercetin-encapsulated liposomal product was successfully developed in our lab using a metal complexation technology that enhanced quercetin’s circulation longevity and improved its apparent solubility (186). Thus, it would be of particular interest to see if these formulations can be used to encapsulate flavopiridol to increase the therapeutic potential of the drug.
EnFlaM - a Simpler Method to Achieve Time Sequenced Treatments like FLAM?
The intriguing improvements in complete remission rates achieved by the FLAM regimen has shed new light on timed sequential therapy (TST) in the treatment of AML. However, FLAM is a treatment with limitations that could be possibly overcome by altering the pharmacokinetics and biodistribution of the drugs. As proposed, EnFlaM will be a liposomal combination product of mitoxantrone and flavopiridol that could potentially be a new treatment regimen implemented to address the challenges of AML treatment. We believe that EnFlaM will be a better alternative to FLAM with the potential of eliminating the need for TST. EnFlaM could be designed to achieve optimized drug delivery and enhanced therapeutic activity.
The principle of TSTs is to expose cancer cells to cell cycle-dependent drugs in sequence in an effort to achieve synergistic effects. As discussed previously, nanoparticles like liposomes are drug delivery systems whose lipid composition and structure can be varied to achieve desired release kinetics ranging from rapid release to intermediate release to slow release, providing long-term sustained drug delivery – an effect that is separate and distinct from the ability of nanoformulations to deliver contents to selected cell populations through nanoparticle cell interactions (187). The variety of lipid compositions and loading methods provide great versatility in the design of suitable formulations that can be selected for development (187). Liposomes have previously been shown to increase the plasma concentration of drugs, mediating an increase in the drug’s anticancer activity (188). Due to its high tendency to bind serum protein and its rapid elimination from the plasma, flavopiridol is particularly well-suited to be therapeutically enhanced through liposomal delivery (189). Therefore, when encapsulated, flavopiridol in an appropriate liposomal formulation should be therapeutically more active. Similarly, the therapeutic potential of mitoxantrone benefits from liposomal encapsulation. Mitoxantrone, although developed as a less cardiotoxic agent compared to anthracyclines, also causes dose-limiting cardiotoxicity and the use of liposomes can curb this cardiotoxicity (190,191). Therefore, an encapsulated formulation of mitoxantrone should be less toxic and better tolerated. Preliminary in vitro studies have shown that flavopiridol and mitoxantrone interact synergistically in a ratio-independent way (unpublished data) and it is reasonable to consider these data in the development of more efficacious and less toxic formulations. We aim to capture the synergistic potential of flavopiridol and mitoxantrone through liposomal technology, but recognize that there is also a potential to capture synergistic toxicity when using the combination product.
Additionally, one of the causes of AML relapses is attributed to leukemic stem cells and these stem cells home to and engraft within bone marrow, where they remain quiescent and protected from the effects of cell cycle-dependent chemotherapeutic agents (192–194). With the use of liposomes or other nanotechnology-based formulations, it will be possible to improve delivery of cytotoxic agents to the bone marrow to target leukemic stem cells. For example, liposomes have previously been shown to be preferentially taken up by bone marrow macrophages and this process facilitates the transport of drugs along with the liposomes to the bone marrow (195,196).
Conclusions and Future Perspectives on AML
xThe standard of care for AML has remained almost unchanged for more than 40 years. Only recently in 2017 and 2018, a series of new treatments that provide therapeutic benefits especially to patients of older age or those who have recurrent disease have been approved for clinical use. The emergence of these treatments, namely midostaurin, enasidenib, Vyxeos®, gemtuzumab ozogamicin, venetoclax, and glasdegib, provide new hopes for patients in the r/r setting. With the development of novel immunotherapeutics, a plethora of clinical trials are being conducted to improve the prognosis of AML patients and to overcome the inherent challenge of activating an anticancer immune response against a heterogeneous disease that is poorly immunogenic.
However, AML still presents a number of clinical challenges and novel therapeutic strategies are necessary to provide additional treatment options for patients with high-risk disease. The FLAM regimen based on timed sequential therapy with flavopiridol, mitoxantrone and cytarabine has shown favourable outcomes with remarkable improvements in complete remission rates in AML. However, the FLAM regimen has some limitations and failed to improve overall survival. Thus, we proposed the use of nanomedicine to circumvent these limitations by introducing EnFlaM. “EnFlaM” will consist of a liposomal combination product of mitoxantrone and flavopiridol that will potentially improve the pharmacokinetics and biodistribution and increase the therapeutic efficacy of the drugs.
Through the lessons we learned from reviewing the clinical challenges of AML and the breakthroughs with recently approved treatments, we expect that the most effective treatments, as in the current standard of care of AML and other cancers, would rely on multi-modal therapeutics comprising of combinations of chemotherapy, targeted therapy, or immunotherapeutic modalities. It is therefore anticipated that many more clinical trials will be designed to focus on combining two or more drugs with immunotherapeutic agents. For instance, the use of a PD-1-targeting checkpoint inhibitor in conjunction of the DC/AML fusion vaccine is being investigated in a phase II clinical trial for AML patients who have achieved remission after chemotherapy (NCT01096602). There are also clinical trials combining various immunotherapeutic strategies with standard treatments (e.g. NCT03286114, NCT00006045) and studies where combinations of different ICPIs are being explored (e.g. NCT01822509, NCT02397720). With novel nanoformulations such as Vyxeos® becoming standard treatment for subpopulations of AML patients and the potential of nanoparticles to enhance immunotherapeutic effects, it will be of great interests to evaluate treatment combinations of EnFlaM with immune checkpoint inhibitors approved for other cancer indications to determine whether or not improved immunotherapeutic effects and extended survival can be achieved.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368(9550):1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 2.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341(14):1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 3.Szotkowski T., Rohon P., Zapletalova L., Sicova K., Hubacek J., Indrak K. Secondary acute myeloid leukemia – a single center experience. Neoplasma. 2010;57(2):170–178. doi: 10.4149/neo_2010_02_170. [DOI] [PubMed] [Google Scholar]
- 4.Larson RA. Therapy-related myeloid neoplasms. Haematologica. 2009;94(4):454–459. doi: 10.3324/haematol.2008.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016 Jul 1 6(7):e441. Pubmed Central PMCID: 5030376. [DOI] [PMC free article] [PubMed]
- 6.Lowenberg B. Acute Myeloid Leukemia: The Challenge of Capturing Disease Variety. Hematology. 2008;2008(1):1–11. doi: 10.1182/asheducation-2008.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 8.Kumar CC. Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes & cancer 2011 Feb;2(2):95–107. Pubmed Central PMCID: 3111245. [DOI] [PMC free article] [PubMed]
- 9.Klepin HD, Balducci L. Acute myelogenous leukemia in older adults. Oncologist. 2009;14(3):222–232. doi: 10.1634/theoncologist.2008-0224. [DOI] [PubMed] [Google Scholar]
- 10.Ramos Nestor, Mo Clifton, Karp Judith, Hourigan Christopher. Current Approaches in the Treatment of Relapsed and Refractory Acute Myeloid Leukemia. Journal of Clinical Medicine. 2015;4(4):665–695. doi: 10.3390/jcm4040665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appelbaum F. R. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finn L, Dalovisio A, Foran J. Older patients with acute myeloid leukemia: treatment challenges and future directions. Ochsner J 2017 Winter;17(4):398–404. Pubmed Central PMCID: 5718453. [PMC free article] [PubMed]
- 13.Ferrara Felicetto, Schiffer Charles A. Acute myeloid leukaemia in adults. The Lancet. 2013;381(9865):484–495. doi: 10.1016/S0140-6736(12)61727-9. [DOI] [PubMed] [Google Scholar]
- 14.Verma Dushyant, Kantarjian Hagop, Faderl Stefan, O'Brien Susan, Pierce Sherry, Vu Khanh, Freireich Emil, Keating Michael, Cortes Jorge, Ravandi Farhad. Late relapses in acute myeloid leukemia: analysis of characteristics and outcome. Leukemia & Lymphoma. 2010;51(5):778–782. doi: 10.3109/10428191003661852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Research N, Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013 May 30;368(22):2059–2074. Pubmed Central PMCID: 3767041. [DOI] [PMC free article] [PubMed]
- 16.Ding Li, Ley Timothy J., Larson David E., Miller Christopher A., Koboldt Daniel C., Welch John S., Ritchey Julie K., Young Margaret A., Lamprecht Tamara, McLellan Michael D., McMichael Joshua F., Wallis John W., Lu Charles, Shen Dong, Harris Christopher C., Dooling David J., Fulton Robert S., Fulton Lucinda L., Chen Ken, Schmidt Heather, Kalicki-Veizer Joelle, Magrini Vincent J., Cook Lisa, McGrath Sean D., Vickery Tammi L., Wendl Michael C., Heath Sharon, Watson Mark A., Link Daniel C., Tomasson Michael H., Shannon William D., Payton Jacqueline E., Kulkarni Shashikant, Westervelt Peter, Walter Matthew J., Graubert Timothy A., Mardis Elaine R., Wilson Richard K., DiPersio John F. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506–510. doi: 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paguirigan Amy L., Smith Jordan, Meshinchi Soheil, Carroll Martin, Maley Carlo, Radich Jerald P. Single-cell genotyping demonstrates complex clonal diversity in acute myeloid leukemia. Science Translational Medicine. 2015;7(281):281re2–281re2. doi: 10.1126/scitranslmed.aaa0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes & Development. 2012 January 15, 2012;26(2):120–5. [DOI] [PMC free article] [PubMed]
- 19.Bose P, Grant S. Mcl-1 as a therapeutic target in acute myelogenous leukemia (AML) Leukemia research reports. 2013;2(1):12–14. doi: 10.1016/j.lrr.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyle L, Daver N. Current and emerging therapies for patients with acute myeloid leukemia: a focus on MCL-1 and the CDK9 pathway. Am J Manag Care. 2018 Aug;24(16 Suppl):S356–S65 Epub 2018/08/23. eng. [PubMed]
- 21.Kaufmann SH, Karp JE, Svingen PA, Krajewski S, Burke PJ, Gore SD, et al. Elevated expression of the apoptotic regulator mcl-1 at the time of leukemic relapse. Blood. 1998 Feb 1;91(3):991–1000 Epub 1998/02/03. eng. [PubMed]
- 22.Feldman Eric J., Lancet Jeffrey E., Kolitz Jonathan E., Ritchie Ellen K., Roboz Gail J., List Alan F., Allen Steven L., Asatiani Ekatherine, Mayer Lawrence D., Swenson Christine, Louie Arthur C. First-In-Man Study of CPX-351: A Liposomal Carrier Containing Cytarabine and Daunorubicin in a Fixed 5:1 Molar Ratio for the Treatment of Relapsed and Refractory Acute Myeloid Leukemia. Journal of Clinical Oncology. 2011;29(8):979–985. doi: 10.1200/JCO.2010.30.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scholl Claudia, Gilliland D. Gary, Fröhling Stefan. Deregulation of Signaling Pathways in Acute Myeloid Leukemia. Seminars in Oncology. 2008;35(4):336–345. doi: 10.1053/j.seminoncol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Lam SS, He AB, Leung AY. Treatment of acute myeloid leukemia in the next decade - towards real-time functional testing and personalized medicine. Blood Rev. 2017;31(6):418–425. doi: 10.1016/j.blre.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Sekeres MA, Steensma DP. Boulevard of broken dreams: drug approval for older adults with acute myeloid leukemia. J Clinical Oncology : Official J American Society of Clinical Oncology. 2012;30(33):4061–4063. doi: 10.1200/JCO.2012.44.2962. [DOI] [PubMed] [Google Scholar]
- 26.Lowenberg B, Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C, et al. Cytarabine dose for acute myeloid leukemia. N Engl J Med. 2011;364(11):1027–1036. doi: 10.1056/NEJMoa1010222. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez Hugo F., Sun Zhuoxin, Yao Xiaopan, Litzow Mark R., Luger Selina M., Paietta Elisabeth M., Racevskis Janis, Dewald Gordon W., Ketterling Rhett P., Bennett John M., Rowe Jacob M., Lazarus Hillard M., Tallman Martin S. Anthracycline Dose Intensification in Acute Myeloid Leukemia. New England Journal of Medicine. 2009;361(13):1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravandi Farhad, Ritchie Ellen K, Sayar Hamid, Lancet Jeffrey E, Craig Michael D, Vey Norbert, Strickland Stephen A, Schiller Gary J, Jabbour Elias, Erba Harry P, Pigneux Arnaud, Horst Heinz-August, Recher Christian, Klimek Virginia M, Cortes Jorge, Roboz Gail J, Odenike Olatoyosi, Thomas Xavier, Havelange Violaine, Maertens Johan, Derigs Hans-Günter, Heuser Michael, Damon Lloyd, Powell Bayard L, Gaidano Gianluca, Carella Angelo-Michele, Wei Andrew, Hogge Donna, Craig Adam R, Fox Judith A, Ward Renee, Smith Jennifer A, Acton Gary, Mehta Cyrus, Stuart Robert K, Kantarjian Hagop M. Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): a randomised, controlled, double-blind, multinational, phase 3 study. The Lancet Oncology. 2015;16(9):1025–1036. doi: 10.1016/S1470-2045(15)00201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei AH, Tiong IS. Midostaurin, enasidenib, CPX-351, gemtuzumab ozogamicin, and venetoclax bring new hope to AML. Blood. 2017;130(23):2469–2474. doi: 10.1182/blood-2017-08-784066. [DOI] [PubMed] [Google Scholar]
- 30.Levis M. Midostaurin approved for FLT3-mutated AML. Blood. 2017;129(26):3403–3406. doi: 10.1182/blood-2017-05-782292. [DOI] [PubMed] [Google Scholar]
- 31.Grafone Tiziana, Palmisano Michela, Nicci Chiara, Storti Sergio. An overview on the role of FLT3-tyrosine kinase receptor in acute myeloid leukemia: biology and treatment. Oncology Reviews. 2012;6(1):8. doi: 10.4081/oncol.2012.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullard Asher. FDA approves first targeted drug for acute myelogenous leukaemia. Nature Reviews Drug Discovery. 2017;16(6):375–375. [Google Scholar]
- 33.Stone Richard M., Mandrekar Sumithra J., Sanford Ben L., Laumann Kristina, Geyer Susan, Bloomfield Clara D., Thiede Christian, Prior Thomas W., Döhner Konstanze, Marcucci Guido, Lo-Coco Francesco, Klisovic Rebecca B., Wei Andrew, Sierra Jorge, Sanz Miguel A., Brandwein Joseph M., de Witte Theo, Niederwieser Dietger, Appelbaum Frederick R., Medeiros Bruno C., Tallman Martin S., Krauter Jürgen, Schlenk Richard F., Ganser Arnold, Serve Hubert, Ehninger Gerhard, Amadori Sergio, Larson Richard A., Döhner Hartmut. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. New England Journal of Medicine. 2017;377(5):454–464. doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards DK, Javidi-Sharifi N, Rofelty A, Rosenfeld C, Roth-Carter R, Tardi P, et al. Effective Combination of CPX-351 with FLT3 Inhibitors in AML Blasts Harboring the FLT3-ITD Mutation. Blood. 2016;128(22):5124-.
- 35.Dugan James, Pollyea Daniel. Enasidenib for the treatment of acute myeloid leukemia. Expert Review of Clinical Pharmacology. 2018;11(8):755–760. doi: 10.1080/17512433.2018.1477585. [DOI] [PubMed] [Google Scholar]
- 36.Medeiros BC, Fathi AT, DiNardo CD, Pollyea DA, Chan SM, Swords R. Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia. 2016;31:272. doi: 10.1038/leu.2016.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein EM, DiNardo CD, Mims AS, Savona MR, Pratz K, Stein AS, et al. Ivosidenib or Enasidenib Combined with Standard Induction Chemotherapy Is Well Tolerated and Active in Patients with Newly Diagnosed AML with an IDH1 or IDH2 Mutation: Initial Results from a Phase 1 Trial. Blood. 2017;130(Suppl 1):726-.
- 38.Lancet Jeffrey E., Uy Geoffrey L., Cortes Jorge E., Newell Laura F., Lin Tara L., Ritchie Ellen K., Stuart Robert K., Strickland Stephen Anthony, Hogge Donna, Solomon Scott R., Stone Richard M., Bixby Dale L., Kolitz Jonathan E., Schiller Gary J., Wieduwilt Matthew Joseph, Ryan Daniel H., Hoering Antje, Chiarella Michael, Louie Arthur Chin, Medeiros Bruno C. Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML. Journal of Clinical Oncology. 2016;34(15_suppl):7000–7000. [Google Scholar]
- 39.Lancet Jeffrey E., Uy Geoffrey L., Cortes Jorge E., Newell Laura F., Lin Tara L., Ritchie Ellen K., Stuart Robert K., Strickland Stephen A., Hogge Donna, Solomon Scott R., Stone Richard M., Bixby Dale L., Kolitz Jonathan E., Schiller Gary J., Wieduwilt Matthew J., Ryan Daniel H., Hoering Antje, Banerjee Kamalika, Chiarella Michael, Louie Arthur C., Medeiros Bruno C. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. Journal of Clinical Oncology. 2018;36(26):2684–2692. doi: 10.1200/JCO.2017.77.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeidner JF, Karp JE. Reason for CPXcitement in AML. Blood. 2014;123(21):3211–3212. doi: 10.1182/blood-2014-04-568725. [DOI] [PubMed] [Google Scholar]
- 41.Tolcher AW, Mayer LD. Improving combination cancer therapy: the CombiPlex((R)) development platform. Future Oncol. 2018 Jun;14(13):1317–32. PubMed PMID: WOS:000437055500010. English. [DOI] [PubMed]
- 42.Gabizon A, Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci. 1988;85(18):6949–6953. doi: 10.1073/pnas.85.18.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krauss AC, Gao X, Li L, Manning ML, Patel P, Fu W, et al. FDA approval summary: (daunorubicin and cytarabine) liposome for injection for the treatment of adults with high-risk acute myeloid leukemia. Clin Cancer Res. 2018:clincanres.2990.018. [DOI] [PubMed]
- 44.DiNardo C, Pollyea D, Pratz K, Thirman MJ, Letai A, Frattini M, et al. A Phase 1b Study of Venetoclax (ABT-199/GDC-0199) in Combination with Decitabine or Azacitidine in Treatment-Naive Patients with Acute Myelogenous Leukemia Who Are ≥ to 65 Years and Not Eligible for Standard Induction Therapy. Blood. 2015;126(23):327-.
- 45.Lagadinou Eleni D., Sach Alexander, Callahan Kevin, Rossi Randall M., Neering Sarah J., Minhajuddin Mohammad, Ashton John M., Pei Shanshan, Grose Valerie, O’Dwyer Kristen M., Liesveld Jane L., Brookes Paul S., Becker Michael W., Jordan Craig T. BCL-2 Inhibition Targets Oxidative Phosphorylation and Selectively Eradicates Quiescent Human Leukemia Stem Cells. Cell Stem Cell. 2013;12(3):329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pullarkat VA, Newman EM. BCL2 inhibition by Venetoclax: targeting the Achilles' heel of the acute myeloid leukemia stem cell? Cancer discovery. 2016;6(10):1082–1083. doi: 10.1158/2159-8290.CD-16-0921. [DOI] [PubMed] [Google Scholar]
- 47.Cortes Jorge E., Douglas Smith B., Wang Eunice S., Merchant Akil, Oehler Vivian G., Arellano Martha, DeAngelo Daniel J., Pollyea Daniel A., Sekeres Mikkael A., Robak Tadeusz, Ma Weidong Wendy, Zeremski Mirjana, Naveed Shaik M., Douglas Laird A., O'Connell Ashleigh, Chan Geoffrey, Schroeder Mark A. Glasdegib in combination with cytarabine and daunorubicin in patients with AML or high-risk MDS: Phase 2 study results. American Journal of Hematology. 2018;93(11):1301–1310. doi: 10.1002/ajh.25238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wahlin A, Hornsten P, Hedenus M, Malm C. Mitoxantrone and cytarabine versus daunorubicin and cytarabine in previously untreated patients with acute myeloid leukemia. Cancer Chemother Pharmacol. 1991;28(6):480–483. doi: 10.1007/BF00685827. [DOI] [PubMed] [Google Scholar]
- 49.Rubin LL, de Sauvage FJ. Targeting the hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5(12):1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 50.Aberger F, Hutterer E, Sternberg C, Del Burgo PJ, Hartmann TN. Acute myeloid leukemia - strategies and challenges for targeting oncogenic hedgehog/GLI signaling. Cell communication and signaling : CCS 2017 Jan 25;15(1):8. Pubmed Central PMCID: 5267446. [DOI] [PMC free article] [PubMed]
- 51.Irvine DA, Copland M. Targeting hedgehog in hematologic malignancy. Blood. 2012;119(10):2196–2204. doi: 10.1182/blood-2011-10-383752. [DOI] [PubMed] [Google Scholar]
- 52.Singh Mohan, Chaudhry Parvesh, Merchant Akil A. Primary cilia are present on human blood and bone marrow cells and mediate Hedgehog signaling. Experimental Hematology. 2016;44(12):1181-1187.e2. doi: 10.1016/j.exphem.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortes JE, Heidel FH, Heuser M, Fiedler W, Smith BD, Robak T, et al. A Phase 2 Randomized Study of Low Dose Ara-C with or without Glasdegib (PF-04449913) in Untreated Patients with Acute Myeloid Leukemia or High-Risk Myelodysplastic Syndrome. Blood. 2016;128(22):99-.
- 54.Ehninger A, Kramer M, Röllig C, Thiede C, Bornhäuser M, von Bonin M, Wermke M, Feldmann A, Bachmann M, Ehninger G, Oelschlägel U. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer Journal. 2014;4(6):e218–e218. doi: 10.1038/bcj.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Godwin CD, Gale RP, Walter RB. Gemtuzumab ozogamicin in acute myeloid leukemia. Leukemia. 2017;31(9):1855–1868. doi: 10.1038/leu.2017.187. [DOI] [PubMed] [Google Scholar]
- 56.Feldman Eric J., Brandwein Joseph, Stone Richard, Kalaycio Matt, Moore Joseph, O'Connor Julie, Wedel Nancy, Roboz Gail J., Miller Carole, Chopra Raj, Jurcic Joseph C., Brown Randy, Ehmann W. Christopher, Schulman Philip, Frankel Stanley R., De Angelo Daniel, Scheinberg David. Phase III Randomized Multicenter Study of a Humanized Anti-CD33 Monoclonal Antibody, Lintuzumab, in Combination With Chemotherapy, Versus Chemotherapy Alone in Patients With Refractory or First-Relapsed Acute Myeloid Leukemia. Journal of Clinical Oncology. 2005;23(18):4110–4116. doi: 10.1200/JCO.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 57.Lichtenegger FS, Krupka C, Haubner S, Kohnke T, Subklewe M. Recent developments in immunotherapy of acute myeloid leukemia. J Hematol Oncol 2017 Jul 25;10(1):142. Pubmed Central PMCID: 5526264. [DOI] [PMC free article] [PubMed]
- 58.Petersdorf S. H., Kopecky K. J., Slovak M., Willman C., Nevill T., Brandwein J., Larson R. A., Erba H. P., Stiff P. J., Stuart R. K., Walter R. B., Tallman M. S., Stenke L., Appelbaum F. R. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121(24):4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lambert J, Pautas C, Terré C, Raffoux E, Turlure P, Caillot D, et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: final efficacy and safety updates from the open-label, phase 3 ALFA-0701 trial. Haematologica. 2018. [DOI] [PMC free article] [PubMed]
- 60.Hargadon Kristian M., Johnson Coleman E., Williams Corey J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. International Immunopharmacology. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016 03/15/online;13:273. [DOI] [PMC free article] [PubMed]
- 62.Grosso DA, Hess RC, Weiss MA. Immunotherapy in acute myeloid leukemia. Cancer. 2015;121(16):2689–2704. doi: 10.1002/cncr.29378. [DOI] [PubMed] [Google Scholar]
- 63.Bose P, Vachhani P, Cortes JE. Treatment of relapsed/refractory acute myeloid leukemia. Curr Treat Options in Oncol. 2017;18(3):17. doi: 10.1007/s11864-017-0456-2. [DOI] [PubMed] [Google Scholar]
- 64.Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62–70. doi: 10.1182/blood-2015-07-604546. [DOI] [PubMed] [Google Scholar]
- 65.Armistead PM, de Lima M, Pierce S, Qiao W, Wang X, Thall PF, Giralt S, Ravandi F, Kantarjian H, Champlin R, Estey E Quantifying the survival benefit for allogeneic hematopoietic stem cell transplantation in relapsed acute myelogenous leukemia. Biology of Blood and Marrow Transplantation : J American Society for Blood and Marrow Transplantation 2009 Nov;15(11):1431–1438. Pubmed Central PMCID: 4067765. [DOI] [PMC free article] [PubMed]
- 66.Choi Y, Lee JH, Lee KH, Park HS, Choi E, Ko SH, et al. Treatment outcomes and prognostic factors of patients with relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. Blood. 2016 Dec 2;128(22). PubMed PMID: WOS:000394452304159. English.
- 67.Fletcher Alexander G., Cooper Fergus, Baker Ruth E. Mechanocellular models of epithelial morphogenesis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1720):20150519. doi: 10.1098/rstb.2015.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Henig Israel, Zuckerman Tsila. Hematopoietic Stem Cell Transplantation—50 Years of Evolution and Future Perspectives. Rambam Maimonides Medical Journal. 2014;5(4):e0028. doi: 10.5041/RMMJ.10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao Xiang-Yu, Zhao Xiao-Su, Wang Yu-Tong, Chen Yu-Hong, Xu Lan-Ping, Zhang Xiao-Hui, Han Wei, Chen Huan, Wang Yu, Yan Chen-Hua, Wang Feng-Rong, Wang Jing-Zhi, Liu Kai-Yan, Chang Ying-Jun, Huang Xiao-Jun. Prophylactic use of low-dose interleukin-2 and the clinical outcomes of hematopoietic stem cell transplantation: A randomized study. OncoImmunology. 2016;5(12):e1250992. doi: 10.1080/2162402X.2016.1250992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Locatelli F, Pende D, Falco M, Della Chiesa M, Moretta A, Moretta L. NK cells mediate a crucial graft-versus-leukemia effect in Haploidentical-HSCT to cure high-risk acute leukemia. Trends Immunol. 2018;39(7):577–590. doi: 10.1016/j.it.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 71.Assi R, Kantarjian H, Ravandi F, Daver N. Immune therapies in acute myeloid leukemia: a focus on monoclonal antibodies and immune checkpoint inhibitors. Curr Opin Hematol. 2018;25(2):136–145. doi: 10.1097/MOH.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 72.Fathi Amir T., Erba Harry P., Lancet Jeffrey E., Stein Eytan M., Ravandi Farhad, Faderl Stefan, Walter Roland B., Advani Anjali S., DeAngelo Daniel J., Kovacsovics Tibor J., Jillella Anand, Bixby Dale, Levy Moshe Y., O’Meara Megan M., Ho Phoenix A., Voellinger Jenna, Stein Anthony S. A phase 1 trial of vadastuximab talirine combined with hypomethylating agents in patients with CD33-positive AML. Blood. 2018;132(11):1125–1133. doi: 10.1182/blood-2018-03-841171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munoz L, Nomdedeu JF, Lopez O, Carnicer MJ, Bellido M, Aventin A, et al. Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica. 2001 Dec 86(12):1261–9. PubMed PMID: WOS:000172637500006. English. [PubMed]
- 74.Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, Meyerrose T, Rossi R, Grimes B, Rizzieri DA, Luger SM, Phillips GL. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14(10):1777–1784. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 75.Maslak Peter G., Dao Tao, Bernal Yvette, Chanel Suzanne M., Zhang Rong, Frattini Mark, Rosenblat Todd, Jurcic Joseph G., Brentjens Renier J., Arcila Maria E., Rampal Raajit, Park Jae H., Douer Dan, Katz Laura, Sarlis Nicholas, Tallman Martin S., Scheinberg David A. Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia. Blood Advances. 2018;2(3):224–234. doi: 10.1182/bloodadvances.2017014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qazilbash M H, Wieder E, Thall P F, Wang X, Rios R, Lu S, Kanodia S, Ruisaard K E, Giralt S A, Estey E H, Cortes J, Komanduri K V, Clise-Dwyer K, Alatrash G, Ma Q, Champlin R E, Molldrem J J. PR1 peptide vaccine induces specific immunity with clinical responses in myeloid malignancies. Leukemia. 2016;31(3):697–704. doi: 10.1038/leu.2016.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alatrash G, Molldrem JJ, Qazilbash MH. Targeting PR1 in myeloid leukemia. Oncotarget. 2018 Jan 12 9(4):4280–4281. Pubmed Central PMCID: 5796973. [DOI] [PMC free article] [PubMed]
- 78.Avigan D, Rosenblatt J. Vaccine therapy in hematologic malignancies. Blood. 2018;131(24):2640–2650. doi: 10.1182/blood-2017-11-785873. [DOI] [PubMed] [Google Scholar]
- 79.Austin Rebecca, Smyth Mark J., Lane Steven W. Harnessing the immune system in acute myeloid leukaemia. Critical Reviews in Oncology/Hematology. 2016;103:62–77. doi: 10.1016/j.critrevonc.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 80.van de Loosdrecht AA, van Wetering S, Santegoets S, Singh SK, Eeltink CM, den Hartog Y, et al. A novel allogeneic off-the-shelf dendritic cell vaccine for post-remission treatment of elderly patients with acute myeloid leukemia. Cancer immunology, immunotherapy : CII 2018 Oct;67(10):1505–1518. Pubmed Central PMCID: 6182404. [DOI] [PMC free article] [PubMed]
- 81.Rosenblatt J., Stone R. M., Uhl L., Neuberg D., Joyce R., Levine J. D., Arnason J., McMasters M., Luptakova K., Jain S., Zwicker J. I., Hamdan A., Boussiotis V., Steensma D. P., DeAngelo D. J., Galinsky I., Dutt P. S., Logan E., Bryant M. P., Stroopinsky D., Werner L., Palmer K., Coll M., Washington A., Cole L., Kufe D., Avigan D. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Science Translational Medicine. 2016;8(368):368ra171–368ra171. doi: 10.1126/scitranslmed.aag1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Przespolewski A, Szeles A, Wang ES. Advances in immunotherapy for acute myeloid leukemia. Future Oncol. 2018;14(10):963–978. doi: 10.2217/fon-2017-0459. [DOI] [PubMed] [Google Scholar]
- 83.Cummins KD, Gill S. Will CAR T cell therapy have a role in AML? Promises and pitfalls. Semin Hematol. 2018 2018/08/29/. [DOI] [PubMed]
- 84.Bakker A, Lebrec H, Lindl B, Adams G, Mardiros A, Koppikar P, et al. Abstract 2559: Generation and evaluation of an FLT3 CAR-T cell therapy for the treatment of acute myeloid leukemia. Cancer Res. 2018;78(13 Supplement):2559-.
- 85.Gomes-Silva D, Tashiro H, Srinivasan M, Lulla P, Brenner MK, Mamonkin M. Chimeric Antigen Receptor (CAR) T Cell Therapy for CD7-Positive Acute Myeloid Leukemia. Blood. 2017;130(Suppl 1):2642-.
- 86.Sallman DA, Brayer JB, Poire X, Kerre T, Lewalle P, Wang ES, et al. Abstract CT129: The THINK clinical trial: Preliminary evidence of clinical activity of NKG2D chimeric antigen receptor T cell therapy (CYAD-01) in acute myeloid leukemia. Cancer Res. 2018;78(13 Supplement):CT129-CT.
- 87.Sallman David A., Brayer Jason, Sagatys Elizabeth M., Lonez Caroline, Breman Eytan, Agaugué Sophie, Verma Bikash, Gilham David E., Lehmann Frédéric F., Davila Marco L. NKG2D-based chimeric antigen receptor therapy induced remission in a relapsed/refractory acute myeloid leukemia patient. Haematologica. 2018;103(9):e424–e426. doi: 10.3324/haematol.2017.186742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Xin, Xiao Qing, Wang Zhe, Feng Wen-Li. CAR-T therapy for leukemia: progress and challenges. Translational Research. 2017;182:135–144. doi: 10.1016/j.trsl.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 89.Gomes-Silva D, Atilla E, Atilla PA, Mo F, Tashiro H, Srinivasan M, et al. CD7 CAR T Cells for the Therapy of Acute Myeloid Leukemia. Molecular Therapy. 2018 2018/10/04/. [DOI] [PMC free article] [PubMed]
- 90.Wang J, Chen S, Xiao W, Li W, Wang L, Yang S, et al. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. J Hematol Oncol. 2018 January 10;11(1):7. [DOI] [PMC free article] [PubMed]
- 91.Cummins KD, Frey N, Nelson AM, Schmidt A, Luger S, Isaacs RE, et al. Treating Relapsed / Refractory (RR) AML with Biodegradable Anti-CD123 CAR Modified T Cells. Blood. 2017;130(Suppl 1):1359-.
- 92.Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T, et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res. 2018;8(6):1083–9. [PMC free article] [PubMed]
- 93.Garg AD, Dudek-Peric AM, Romano E, Agostinis P. Immunogenic cell death. The International J Develop Bio. 2015;59(1–3):131–140. doi: 10.1387/ijdb.150061pa. [DOI] [PubMed] [Google Scholar]
- 94.Gubin MM, Schreiber RD. CANCER. The odds of immunotherapy success. Science. 2015;350(6257):158–159. doi: 10.1126/science.aad4140. [DOI] [PubMed] [Google Scholar]
- 95.Li X. The inducers of immunogenic cell death for tumor immunotherapy. Tumori. 2018;104(1):1–8. doi: 10.5301/tj.5000675. [DOI] [PubMed] [Google Scholar]
- 96.Bezu L, Gomes-de-Silva LC, Dewitte H, Breckpot K, Fucikova J, Spisek R, et al. Combinatorial strategies for the induction of immunogenic cell death. Front Immunol 2015;6:187. Pubmed Central PMCID: 4408862. [DOI] [PMC free article] [PubMed]
- 97.Obeid Michel, Tesniere Antoine, Ghiringhelli François, Fimia Gian Maria, Apetoh Lionel, Perfettini Jean-Luc, Castedo Maria, Mignot Grégoire, Panaretakis Theoharis, Casares Noelia, Métivier Didier, Larochette Nathanael, van Endert Peter, Ciccosanti Fabiola, Piacentini Mauro, Zitvogel Laurence, Kroemer Guido. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature Medicine. 2006;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 98.Pol Jonathan, Vacchelli Erika, Aranda Fernando, Castoldi Francesca, Eggermont Alexander, Cremer Isabelle, Sautès-Fridman Catherine, Fucikova Jitka, Galon Jérôme, Spisek Radek, Tartour Eric, Zitvogel Laurence, Kroemer Guido, Galluzzi Lorenzo. Trial Watch: Immunogenic cell death inducers for anticancer chemotherapy. OncoImmunology. 2015;4(4):e1008866. doi: 10.1080/2162402X.2015.1008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garg Abhishek D., More Sanket, Rufo Nicole, Mece Odeta, Sassano Maria Livia, Agostinis Patrizia, Zitvogel Laurence, Kroemer Guido, Galluzzi Lorenzo. Trial watch: Immunogenic cell death induction by anticancer chemotherapeutics. OncoImmunology. 2017;6(12):e1386829. doi: 10.1080/2162402X.2017.1386829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kepp Oliver, Senovilla Laura, Vitale Ilio, Vacchelli Erika, Adjemian Sandy, Agostinis Patrizia, Apetoh Lionel, Aranda Fernando, Barnaba Vincenzo, Bloy Norma, Bracci Laura, Breckpot Karine, Brough David, Buqué Aitziber, Castro Maria G., Cirone Mara, Colombo Maria I., Cremer Isabelle, Demaria Sandra, Dini Luciana, Eliopoulos Aristides G., Faggioni Alberto, Formenti Silvia C., Fučíková Jitka, Gabriele Lucia, Gaipl Udo S., Galon Jérôme, Garg Abhishek, Ghiringhelli François, Giese Nathalia A., Guo Zong Sheng, Hemminki Akseli, Herrmann Martin, Hodge James W., Holdenrieder Stefan, Honeychurch Jamie, Hu Hong-Min, Huang Xing, Illidge Tim M., Kono Koji, Korbelik Mladen, Krysko Dmitri V., Loi Sherene, Lowenstein Pedro R., Lugli Enrico, Ma Yuting, Madeo Frank, Manfredi Angelo A., Martins Isabelle, Mavilio Domenico, Menger Laurie, Merendino Nicolò, Michaud Michael, Mignot Gregoire, Mossman Karen L., Multhoff Gabriele, Oehler Rudolf, Palombo Fabio, Panaretakis Theocharis, Pol Jonathan, Proietti Enrico, Ricci Jean-Ehrland, Riganti Chiara, Rovere-Querini Patrizia, Rubartelli Anna, Sistigu Antonella, Smyth Mark J., Sonnemann Juergen, Spisek Radek, Stagg John, Sukkurwala Abdul Qader, Tartour Eric, Thorburn Andrew, Thorne Stephen H., Vandenabeele Peter, Velotti Francesca, Workenhe Samuel T., Yang Haining, Zong Wei-Xing, Zitvogel Laurence, Kroemer Guido, Galluzzi Lorenzo. Consensus guidelines for the detection of immunogenic cell death. OncoImmunology. 2014;3(9):e955691. doi: 10.4161/21624011.2014.955691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fucikova J, Truxova I, Hensler M, Becht E, Kasikova L, Moserova I, et al. Calreticulin exposure by malignant blasts correlates with robust anticancer immunity and improved clinical outcome in AML patients. Blood. 2016 Dec 29 128(26):3113–24. Pubmed Central PMCID: 5201098. [DOI] [PMC free article] [PubMed]
- 102.Karp JE, Ross DD, Yang WD, Tidwell ML, Wei YT, Greer J, et al. Timed sequential therapy of acute leukemia with flavopiridol: In vitro model for a phase I clinical trial. Clin Cancer Res. 2003 Jan, 9(1):307–15. PubMed PMID: WOS:000180430600039. English. [PubMed]
- 103.Zeidner J. F., Foster M. C., Blackford A. L., Litzow M. R., Morris L. E., Strickland S. A., Lancet J. E., Bose P., Levy M. Y., Tibes R., Gojo I., Gocke C. D., Rosner G. L., Little R. F., Wright J. J., Doyle L. A., Smith B. D., Karp J. E. Randomized multicenter phase II study of flavopiridol (alvocidib), cytarabine, and mitoxantrone (FLAM) versus cytarabine/daunorubicin (7+3) in newly diagnosed acute myeloid leukemia. Haematologica. 2015;100(9):1172–1179. doi: 10.3324/haematol.2015.125849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Burke PJ, Karp JE, Braine HG, Vaughan WP. Timed Sequential Therapy of Human Leukemia Based upon the Response of Leukemic Cells to Humoral Growth Factors. Cancer Res. 1977;37(7):2138–2146. [PubMed] [Google Scholar]
- 105.Rytting Michael, Ravandi Farhad, Estey Elihu, Cortes Jorge, Faderl Stefan, Garcia-Manero Guillermo, Jeha Sima, Ouzounian Souzanne, Pierce Sherry, Kantarjian Hagop. Intensively timed combination chemotherapy for the induction of adult patients with acute myeloid leukemia. Cancer. 2010;116(22):5272–5278. doi: 10.1002/cncr.25516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Geller R, Burke P, Karp J, Humphrey R, Braine H, Tucker R, et al. A two-step timed sequential treatment for acute myelocytic leukemia. Blood. 1989;74(5):1499–1506. [PubMed] [Google Scholar]
- 107.Knapper S. The FLAM regimen: revisiting time sequential induction therapy for patients with poor-risk acute myeloid leukemia. Haematologica. 2015;100(9):1105–1107. doi: 10.3324/haematol.2015.134023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wirger A, Perabo FGE, Burgemeister S, Haase L, Schmidt DH, Doehn C, et al. Flavopiridol, an inhibitor of cyclin-dependent kinases, induces growth inhibition and apoptosis in bladder cancer cells in vitro and in vivo. Anticancer Res 2005 Nov-Dec;25(6B):4341–4347. PubMed PMID: WOS:000233278300081. English. [PubMed]
- 109.Garcia-Cuellar M-P, Füller E, Mäthner E, Breitinger C, Hetzner K, Zeitlmann L, Borkhardt A, Slany R K. Efficacy of cyclin-dependent-kinase 9 inhibitors in a murine model of mixed-lineage leukemia. Leukemia. 2014;28(7):1427–1435. doi: 10.1038/leu.2014.40. [DOI] [PubMed] [Google Scholar]
- 110.Carlson BA, Dubay MM, Sausville EA, Brizuela L, Worland PJ. Flavopiridol induces G(1) arrest with inhibition of cyclin-dependent kinase (CDK)2 and CDK4 in human breast carcinoma cells. Cancer Res 1996 Jul 1;56(13):2973–2978. PubMed PMID: WOS:A1996UT39800021. English. [PubMed]
- 111.Jackman Kelly M., Frye Carole B., Hunger Stephen P. Flavopiridol displays preclinical activity in acute lymphoblastic leukemia. Pediatric Blood & Cancer. 2008;50(4):772–778. doi: 10.1002/pbc.21386. [DOI] [PubMed] [Google Scholar]
- 112.Drees M, Dengler WA, Roth T, Labonte H, Mayo J, Malspeis L, et al. Flavopiridol (L86–8275): Selective antitumor activity in vitro and activity in vivo for prostate carcinoma cells. Clin Cancer Res 1997 Feb;3(2):273–279. PubMed PMID: WOS:A1997WY36600019. English. [PubMed]
- 113.Kelland Lloyd R. Flavopiridol, the first cyclin-dependent kinase inhibitor to enter the clinic: current status. Expert Opinion on Investigational Drugs. 2000;9(12):2903–2911. doi: 10.1517/13543784.9.12.2903. [DOI] [PubMed] [Google Scholar]
- 114.Losiewicz M.D., Carlson B.A., Kaur G., Sausville E.A., Worland P.J. Potent Inhibition of Cdc2 Kinase Activity by the Flavonoid L86-8275. Biochemical and Biophysical Research Communications. 1994;201(2):589–595. doi: 10.1006/bbrc.1994.1742. [DOI] [PubMed] [Google Scholar]
- 115.deAzevedo WF, MuellerDieckmann HJ, SchulzeGahmen U, Worland PJ, Sausville E, Kim SH. Structural basis for specificity and potency of a flavonoid inhibitor of human CDK2, a cell cycle kinase. P Natl Acad Sci USA 1996 Apr 2;93(7):2735–2740. PubMed PMID: WOS:A1996UD37500021. English. [DOI] [PMC free article] [PubMed]
- 116.Kaiser Astrid, Nishi Kayoko, Gorin Fredric A., Walsh Donal A., Bradbury E.Morton, Schnier Joachim B. The Cyclin-Dependent Kinase (CDK) Inhibitor Flavopiridol Inhibits Glycogen Phosphorylase. Archives of Biochemistry and Biophysics. 2001;386(2):179–187. doi: 10.1006/abbi.2000.2220. [DOI] [PubMed] [Google Scholar]
- 117.Gojo I, Zhang B, Fenton RG. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clinical Cancer Research : An Official J American Association Cancer Research. 2002;8(11):3527–3538. [PubMed] [Google Scholar]
- 118.König A, Schwartz GK, Mohammad RM, Al-Katib A, Gabrilove JL. The Novel Cyclin-Dependent Kinase Inhibitor Flavopiridol Downregulates Bcl-2 and Induces Growth Arrest and Apoptosis in Chronic B-Cell Leukemia Lines. Blood. 1997;90(11):4307–4312. [PubMed] [Google Scholar]
- 119.Kim W, Bahr BL, Soh KK, Bearss JJ, Sol Lee Y, Peterson P, et al. The MCL-1 targeting effect of alvocidib potentiates the activity of cytarabine and mitoxantrone in a time-sequential regimen in AML. Clinical Lymphoma, Myeloma and Leukemia. 2015;15:S18. [Google Scholar]
- 120.Wicki Andreas, Witzigmann Dominik, Balasubramanian Vimalkumar, Huwyler Jörg. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. Journal of Controlled Release. 2015;200:138–157. doi: 10.1016/j.jconrel.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 121.Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Briot T, Roger E, Thepot S, Lagarce F. Advances in treatment formulations for acute myeloid leukemia. Drug Discov Today. 2018;23(12):1936–1949. doi: 10.1016/j.drudis.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 123.Hamada A, Kawaguchi T, Nakano M. Clinical pharmacokinetics of cytarabine formulations. Clin Pharmacokinet. 2002;41(10):705–718. doi: 10.2165/00003088-200241100-00002. [DOI] [PubMed] [Google Scholar]
- 124.Tardi P, Johnstone S, Harasym N, Xie S, Harasym T, Zisman N, et al. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33(1):129–139. doi: 10.1016/j.leukres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 125.Mayer LD, Harasym TO, Tardi PG, Harasym NL, Shew CR, Johnstone SA, et al. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5(7):1854–1863. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- 126.Batist G, Gelmon KA, Chi KN, Miller WH, Jr, Chia SK, Mayer LD, et al. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clinical Cancer Research : an Official J American Association Cancer Research. 2009;15(2):692–700. doi: 10.1158/1078-0432.CCR-08-0515. [DOI] [PubMed] [Google Scholar]
- 127.Goldwasser F, Shimizu T, Jackman J, Hoki Y, O'Connor PM, Kohn KW, et al. Correlations between S and G2 Arrest and the Cytotoxicity of Camptothecin in Human Colon Carcinoma Cells. Cancer Res. 1996;56(19):4430–4437. [PubMed] [Google Scholar]
- 128.Qin T, Youssef EM, Jelinek J, Chen R, Yang AS, Garcia-Manero G, et al. Effect of cytarabine and decitabine in combination in human leukemic cell lines. Clinical Cancer Research : An Official J American Association Cancer Research. 2007;13(14):4225–4232. doi: 10.1158/1078-0432.CCR-06-2762. [DOI] [PubMed] [Google Scholar]
- 129.Bulbake Upendra, Doppalapudi Sindhu, Kommineni Nagavendra, Khan Wahid. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics. 2017;9(4):12. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tan KB, Ling LU, Bunte RM, Chng WJ, Chiu GN. Liposomal codelivery of a synergistic combination of bioactive lipids in the treatment of acute myeloid leukemia. Nanomedicine. 2014;9(11):1665–1679. doi: 10.2217/nnm.13.123. [DOI] [PubMed] [Google Scholar]
- 131.Myhren L, Nilssen IM, Nicolas V, Doskeland SO, Barratt G, Herfindal L. Efficacy of multi-functional liposomes containing daunorubicin and emetine for treatment of acute myeloid leukaemia. Eur J Pharm Biopharm. 2014;88(1):186–193. doi: 10.1016/j.ejpb.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 132.Li Hong, Xu Songlin, Quan Jishan, Yung Bryant C., Pang Jiuxia, Zhou Chenguang, Cho Young-Ah, Zhang Mengzi, Liu Shujun, Muthusamy Natarajan, Chan Kenneth K., Byrd John C., Lee L. James, Marcucci Guido, Lee Robert J. CD33-Targeted Lipid Nanoparticles (aCD33LNs) for Therapeutic Delivery of GTI-2040 to Acute Myelogenous Leukemia. Molecular Pharmaceutics. 2015;12(6):2010–2018. doi: 10.1021/mp5008212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Simon AM, Jagadeeshan S, Abraham E, Akhilandeshwaran A, Pillai JJ, Kumar NA, et al. Poly (D,L-lactic-co-glycolide) nanoparticles for the improved therapeutic efficacy of all-trans-retinoic acid: a study of acute myeloid leukemia (AML) cell differentiation in vitro. Med Chem. 2012;8(5):805–810. doi: 10.2174/157340612802084333. [DOI] [PubMed] [Google Scholar]
- 134.Varshosaz J, Hassanzadeh F, Sadeghi Aliabadi H, Nayebsadrian M, Banitalebi M, Rostami M. Synthesis and characterization of folate-targeted dextran/retinoic acid micelles for doxorubicin delivery in acute leukemia. Biomed Res Int. 2014;2014:525684. doi: 10.1155/2014/525684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Szulc Aleksandra, Pulaski Lukasz, Appelhans Dietmar, Voit Brigitte, Klajnert-Maculewicz Barbara. Sugar-modified poly(propylene imine) dendrimers as drug delivery agents for cytarabine to overcome drug resistance. International Journal of Pharmaceutics. 2016;513(1-2):572–583. doi: 10.1016/j.ijpharm.2016.09.063. [DOI] [PubMed] [Google Scholar]
- 136.Schwendener Reto A., Fiebig Heinz H., Berger Martin R., Berger Dietmar P. Evaluation of incorporation characteristics of mitoxantrone into unilamellar liposomes and analysis of their pharmacokinetic properties, acute toxicity, and antitumor efficacy. Cancer Chemotherapy and Pharmacology. 1991;27(6):429–439. doi: 10.1007/BF00685156. [DOI] [PubMed] [Google Scholar]
- 137.Pestalozzi B, Schwendener R, Sauter C. Phase-I/Ii Study of Liposome-Complexed Mitoxantrone in Patients with Advanced Breast-Cancer. Ann Oncol 1992 Jun;3(6):445–449. PubMed PMID: WOS:A1992JC55000014. English. [DOI] [PubMed]
- 138.Schwendener R.A., Horber D.H., Rentsch K., Hänseler E., Pestalozzi B., Sauter Chr. Preclinical and Clinical Experience with Liposome-Encapsulated Mitoxantrone. Journal of Liposome Research. 1994;4(1):605–639. [Google Scholar]
- 139.Chang CW, Barber L, Ouyang C, Masin D, Bally MB, Madden TD. Plasma clearance, biodistribution and therapeutic properties of mitoxantrone encapsulated in conventional and sterically stabilized liposomes after intravenous administration in BDF1 mice. British Journal of Cancer. 1997;75(2):169–177. doi: 10.1038/bjc.1997.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lim HJ, Masin D, Madden TD, Bally MB. Influence of drug release characteristics on the therapeutic activity of liposomal mitoxantrone. J Pharmacol Exp Ther 1997 Apr;281(1):566–573. PubMed PMID: WOS:A1997WU52200073. English. [PubMed]
- 141.Lim HJ, Masin D, McIntosh NL, Madden TD, Bally MB. Role of drug release and liposome-mediated drug delivery in governing the therapeutic activity of liposomal mitoxantrone used to treat human A431 and LS180 solid tumors. J Pharmacol Exp Ther 2000 Jan;292(1):337–345. PubMed PMID: WOS:000084331700045. English. [PubMed]
- 142.Ugwu Sydney, Zhang Allen, Parmar Manjeet, Miller Bruce, Sardone Tommaso, Peikov Viktor, Ahmad Imran. Preparation, Characterization, and Stability of Liposome-Based Formulations of Mitoxantrone. Drug Development and Industrial Pharmacy. 2005;31(2):223–229. doi: 10.1081/ddc-200047850. [DOI] [PubMed] [Google Scholar]
- 143.Ahmad A, Wang YF, Ahmad I. Separation of liposome-entrapped mitoxantrone from nonliposomal mitoxantrone in plasma: Pharmacokinetics in mice. Methods Enzymol 2005;391:176–185. PubMed PMID: WOS:000228160000011. English. [DOI] [PubMed]
- 144.Li ChunLei, Cui JingXia, Wang CaiXia, Li YinGui, Zhang HongWu, Wang JinXu, Li YanHui, Zhang Lan, Zhang Li, Guo WenMin, Wang YongLi. Encapsulation of mitoxantrone into pegylated SUVs enhances its antineoplastic efficacy. European Journal of Pharmaceutics and Biopharmaceutics. 2008;70(2):657–665. doi: 10.1016/j.ejpb.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 145.Li ChunLei, Zhao Xi, Deng ChenXin, Wang CaiXia, Wei Na, Cui JingXia. Pegylated liposomal mitoxantrone is more therapeutically active than mitoxantrone in L1210 ascitic tumor and exhibits dose-dependent activity saturation effect. International Journal of Pharmaceutics. 2014;460(1-2):165–172. doi: 10.1016/j.ijpharm.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 146.Yang Jianliang, Shi Yuankai, Li Chunlei, Gui Lin, Zhao Xi, Liu Peng, Han Xiaohong, Song Yuanyuan, Li Ning, Du Ping, Zhang Shuxiang. Phase I clinical trial of pegylated liposomal mitoxantrone plm60-s: pharmacokinetics, toxicity and preliminary efficacy. Cancer Chemotherapy and Pharmacology. 2014;74(3):637–646. doi: 10.1007/s00280-014-2523-8. [DOI] [PubMed] [Google Scholar]
- 147.Beck P., Kreuter J., Reszka R., Fichtner I. Influence of polybutylcyanoacrylate nanoparticles and liposomes on the efficacy and toxicity of the anticancer drug mitoxantrone in murine tumour models. Journal of Microencapsulation. 1993;10(1):101–114. doi: 10.3109/02652049309015316. [DOI] [PubMed] [Google Scholar]
- 148.Reszka R, Beck P, Fichtner I, Hentschel M, Richter J, Kreuter J. Body distribution of free, liposomal and nanoparticle-associated mitoxantrone in B16-melanoma-bearing mice. J Pharmacol Exp Ther 1997 Jan;280(1):232–237. PubMed PMID: WOS:A1997WC04100028. English. [PubMed]
- 149.Zhang Zhirong, Liao Gongtie, Nagai Tsuneji, Hou Shixiang. Mitoxantrone polybutyl cyanoacrylate nanoparticles as an anti-neoplastic targeting drug delivery system. International Journal of Pharmaceutics. 1996;139(1-2):1–8. [Google Scholar]
- 150.Zhang Zhi-Rong. Study on the anticarcinogenic effect and acute toxicity of liver-targeting mitoxantrone nanoparticles. World Journal of Gastroenterology. 1999;5(6):511. doi: 10.3748/wjg.v5.i6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou Qinghua, Sun Xun, Zeng Lingyuan, Liu Jie, Zhang Zhirong. A randomized multicenter phase II clinical trial of mitoxantrone-loaded nanoparticles in the treatment of 108 patients with unresected hepatocellular carcinoma. Nanomedicine: Nanotechnology, Biology and Medicine. 2009;5(4):419–423. doi: 10.1016/j.nano.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 152.Lu Bin, Xiong Su-Bin, Yang Hong, Yin Xiao-Dong, Chao Ruo-Bing. Solid lipid nanoparticles of mitoxantrone for local injection against breast cancer and its lymph node metastases. European Journal of Pharmaceutical Sciences. 2006;28(1-2):86–95. doi: 10.1016/j.ejps.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 153.Zhang Liang-ke, Hou Shi-xiang, Zhang Jing-qin, Hu Wen-jing, Wang Cheng-yuan. Preparation, characterization, and in vivo evaluation of mitoxantrone-loaded, folate-conjugated albumin nanoparticles. Archives of Pharmacal Research. 2010;33(8):1193–1198. doi: 10.1007/s12272-010-0809-x. [DOI] [PubMed] [Google Scholar]
- 154.Krukemeyer Manfred G., Krenn Veit, Jakobs Martin, Wagner Wolfgang. Mitoxantrone-Iron Oxide Biodistribution in Blood, Tumor, Spleen, and Liver—Magnetic Nanoparticles in Cancer Treatment. Journal of Surgical Research. 2012;175(1):35–43. doi: 10.1016/j.jss.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 155.Seiter Karen. Toxicity of the topoisomerase II inhibitors. Expert Opinion on Drug Safety. 2005;4(2):219–234. doi: 10.1517/14740338.4.2.219. [DOI] [PubMed] [Google Scholar]
- 156.Gilabert-Oriol R, Chernov L, Deyell RJ, Bally MB. Chapter 9 - Developing liposomal nanomedicines for treatment of patients with neuroblastoma. In: Grumezescu AM, editor. Lipid Nanocarriers for Drug Targeting: William Andrew Publishing; 2018. p. 361–411.
- 157.Li ChunLei, Cui JingXia, Li YinGui, Wang CaiXia, Li YanHui, Zhang Lan, Zhang Li, Guo WenMin, Wang JinXu, Zhang HongWu, Hao YanLi, Wang YongLi. Copper ion-mediated liposomal encapsulation of mitoxantrone: The role of anions in drug loading, retention and release. European Journal of Pharmaceutical Sciences. 2008;34(4-5):333–344. doi: 10.1016/j.ejps.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 158.Li ChunLei, Cui JingXia, Wang CaiXia, Wang JinXu, Li YanHui, Zhang Lan, Zhang Li, Guo WenMin, Wang YongLi. Lipid composition and grafted PEG affect in vivo activity of liposomal mitoxantrone. International Journal of Pharmaceutics. 2008;362(1-2):60–66. doi: 10.1016/j.ijpharm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 159.Chen Han, Zhang Tong, Zhou Zhimin, Guan Man, Wang Jingjie, Liu Lingrong, Zhang Qiqing. Enhanced uptake and cytotoxity of folate-conjugated mitoxantrone-loaded micelles via receptor up-regulation by dexamethasone. International Journal of Pharmaceutics. 2013;448(1):142–149. doi: 10.1016/j.ijpharm.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 160.Enache Mirela, Volanschi Elena. Spectral Studies on the Molecular Interaction of Anticancer Drug Mitoxantrone with CTAB Micelles. Journal of Pharmaceutical Sciences. 2011;100(2):558–565. doi: 10.1002/jps.22289. [DOI] [PubMed] [Google Scholar]
- 161.Enache Mirela, Volanschi Elena. Spectroscopic investigations of the molecular interaction of anticancer drug mitoxantrone with non-ionic surfactant micelles. Journal of Pharmacy and Pharmacology. 2012;64(5):688–696. doi: 10.1111/j.2042-7158.2012.01445.x. [DOI] [PubMed] [Google Scholar]
- 162.Li Hui, Xu Yingqi, Wu Minghui, Fan Lijiao, He Chengwei, Wan Jianbo, Li Peng, Chen Meiwan, Liu Yuling. Vitamin E succinate-conjugated F68 micelles for mitoxantrone delivery in enhancing anticancer activity. International Journal of Nanomedicine. 2016;Volume 11:3167–3178. doi: 10.2147/IJN.S103556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Qi Xuelian, Chen Xiaoyan, Sun Ying, Ma Zhichao, Guo Xiaojuan, Lu Wei, Duan Yourong. Cytotoxicity and cellular uptake evaluation of mitoxantrone-loaded poly(lactic acid-co-lysine) arginine-glycine-aspartic acid nanoparticles. Journal of Applied Polymer Science. 2010;119(2):1011–1015. [Google Scholar]
- 164.Wang Huan, Han Siyuan, Sun Jihong, Fan Tengfei, Tian Cixia, Wu Yan. Amphiphilic dextran derivatives nanoparticles for the delivery of mitoxantrone. Journal of Applied Polymer Science. 2012;126(S1):E35–E43. [Google Scholar]
- 165.Xu Yurui, Asghar Sajid, Gao Shiya, Chen Zhipeng, Huang Lin, Yin Lining, Ping Qineng, Xiao Yanyu. Polysaccharide-based nanoparticles for co-loading mitoxantrone and verapamil to overcome multidrug resistance in breast tumor. International Journal of Nanomedicine. 2017;Volume 12:7337–7350. doi: 10.2147/IJN.S145620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shapira Alina, Markman Gilad, Assaraf Yehuda G., Livney Yoav D. β-casein–based nanovehicles for oral delivery of chemotherapeutic drugs: drug-protein interactions and mitoxantrone loading capacity. Nanomedicine: Nanotechnology, Biology and Medicine. 2010;6(4):547–555. doi: 10.1016/j.nano.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 167.Hornung A, Poettler M, Friedrich RP, Weigel B, Duerr S, Zaloga J, et al. Toxicity of Mitoxantrone-loaded Superparamagnetic Iron Oxide Nanoparticles in a HT-29 Tumour Spheroid Model. Anticancer Res 2016 Jun;36(6):3093–3101. PubMed PMID: WOS:000377464200063. English. [PubMed]
- 168.Wani Amit, Muthuswamy Elayaraja, Savithra Galbokka H. Layan, Mao Guangzhao, Brock Stephanie, Oupický David. Surface Functionalization of Mesoporous Silica Nanoparticles Controls Loading and Release Behavior of Mitoxantrone. Pharmaceutical Research. 2012;29(9):2407–2418. doi: 10.1007/s11095-012-0766-9. [DOI] [PubMed] [Google Scholar]
- 169.Grund Stefan, Doussineau Tristan, Fischer Dagmar, Mohr Gerhard J. Mitoxantrone-loaded zeolite beta nanoparticles: Preparation, physico-chemical characterization and biological evaluation. Journal of Colloid and Interface Science. 2012;365(1):33–40. doi: 10.1016/j.jcis.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 170.Li Ping, Tabibi S.Esmail, Yalkowsky Samuel H. Solubilization of flavopiridol by pH control combined with cosolvents, surfactants, or complexants. Journal of Pharmaceutical Sciences. 1999;88(9):945–947. doi: 10.1021/js990097r. [DOI] [PubMed] [Google Scholar]
- 171.Myatt Daniel, Johnson Louise, Baumli Sonja, Siligardi Giuliano. The binding of flavopiridol to blood serum albumin. Chirality. 2010;22(1E):E40–E43. doi: 10.1002/chir.20925. [DOI] [PubMed] [Google Scholar]
- 172.Dannenfelser RM, Surakitbanharn Y, Tabibi SE, Yalkowsky SH. Parenteral formulation of flavopiridol (NSC-649890). PDA J Pharm Sci Technol 1996 Nov-Dec;50(6):356–359. PubMed PMID: WOS:A1996WG45300004. English. [PubMed]
- 173.Senderowicz AM. Development of cyclin-dependent kinase modulators as novel therapeutic approaches for hematological malignancies. Leukemia. 2001;15(1):1–9. doi: 10.1038/sj.leu.2401994. [DOI] [PubMed] [Google Scholar]
- 174.Semple Sean C, Chonn Arcadio, Cullis Pieter R. Interactions of liposomes and lipid-based carrier systems with blood proteins: Relation to clearance behaviour in vivo. Advanced Drug Delivery Reviews. 1998;32(1-2):3–17. doi: 10.1016/s0169-409x(97)00128-2. [DOI] [PubMed] [Google Scholar]
- 175.Kalra J, Bally MB. Liposomes. In: Uchegbu IF, Schätzlein AG, Cheng WP, Lalatsa A, editors. Fundamentals of Pharmaceutical Nanoscience. New York, NY: Springer New York; 2013. pp. 27–63. [Google Scholar]
- 176.Coimbra Maria, Isacchi Benedetta, van Bloois Louis, Torano Javier Sastre, Ket Aldo, Wu Xiaojie, Broere Femke, Metselaar Josbert M., Rijcken Cristianne J.F., Storm Gert, Bilia Rita, Schiffelers Raymond M. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. International Journal of Pharmaceutics. 2011;416(2):433–442. doi: 10.1016/j.ijpharm.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 177.YANG X, ZHAO X, PHELPS M, PIAO L, ROZEWSKI D, LIU Q, LEE L, MARCUCCI G, GREVER M, BYRD J. A novel liposomal formulation of flavopiridol. International Journal of Pharmaceutics. 2009;365(1-2):170–174. doi: 10.1016/j.ijpharm.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ren Hao, Han Min, Zhou Jing, Zheng Ze-Feng, Lu Ping, Wang Jun-Juan, Wang Jia-Qiu, Mao Qi-Jiang, Gao Jian-Qing, Ouyang Hong Wei. Repair of spinal cord injury by inhibition of astrocyte growth and inflammatory factor synthesis through local delivery of flavopiridol in PLGA nanoparticles. Biomaterials. 2014;35(24):6585–6594. doi: 10.1016/j.biomaterials.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 179.Yang Wei, Ahmed Muneeb, Tasawwar Beenish, Levchenko Tatyana, Sawant Rupa R., Collins Michael, Signoretti Sabina, Torchilin Vladimir, Goldberg S. Nahum. Radiofrequency ablation combined with liposomal quercetin to increase tumour destruction by modulation of heat shock protein production in a small animal model. International Journal of Hyperthermia. 2011;27(6):527–538. doi: 10.3109/02656736.2011.582474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yuan Z.-p. Liposomal Quercetin Efficiently Suppresses Growth of Solid Tumors in Murine Models. Clinical Cancer Research. 2006;12(10):3193–3199. doi: 10.1158/1078-0432.CCR-05-2365. [DOI] [PubMed] [Google Scholar]
- 181.Wong Man-Yi, Chiu Gigi N.C. Simultaneous liposomal delivery of quercetin and vincristine for enhanced estrogen-receptor-negative breast cancer treatment. Anti-Cancer Drugs. 2010;21(4):401–410. doi: 10.1097/CAD.0b013e328336e940. [DOI] [PubMed] [Google Scholar]
- 182.Caddeo Carla, Nacher Amparo, Vassallo Antonio, Armentano Maria Francesca, Pons Ramon, Fernàndez-Busquets Xavier, Carbone Claudia, Valenti Donatella, Fadda Anna Maria, Manconi Maria. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. International Journal of Pharmaceutics. 2016;513(1-2):153–163. doi: 10.1016/j.ijpharm.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 183.Pandey Sanjeev K., Patel Dinesh K., Thakur Ravi, Mishra Durga P., Maiti Pralay, Haldar Chandana. Anti-cancer evaluation of quercetin embedded PLA nanoparticles synthesized by emulsified nanoprecipitation. International Journal of Biological Macromolecules. 2015;75:521–529. doi: 10.1016/j.ijbiomac.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 184.Kumar SR, Priyatharshni S, Babu VN, Mangalaraj D, Viswanathan C, Kannan S, et al. Quercetin conjugated superparamagnetic magnetite nanoparticles for in-vitro analysis of breast cancer cell lines for chemotherapy applications. J Colloid Interface Sci. 2014 Dec 15, 436:234–42. PubMed PMID: WOS:000344441700031. English. [DOI] [PubMed]
- 185.Sarkar Abhijit, Ghosh Shatadal, Chowdhury Sayantani, Pandey Bhawna, Sil Parames C. Targeted delivery of quercetin loaded mesoporous silica nanoparticles to the breast cancer cells. Biochimica et Biophysica Acta (BBA) - General Subjects. 2016;1860(10):2065–2075. doi: 10.1016/j.bbagen.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 186.Wehbe Mohamed, Chernov Lina, Chen Kent, Bally Marcel B. PRCosomes: pretty reactive complexes formed in liposomes. Journal of Drug Targeting. 2016;24(9):787–796. doi: 10.1080/1061186X.2016.1186169. [DOI] [PubMed] [Google Scholar]
- 187.Drummond Daryl C., Noble Charles O., Hayes Mark E., Park John W., Kirpotin Dmitri B. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. Journal of Pharmaceutical Sciences. 2008;97(11):4696–4740. doi: 10.1002/jps.21358. [DOI] [PubMed] [Google Scholar]
- 188.Dos Santos Nancy, Waterhouse Dawn, Masin Dana, Tardi Paul G., Karlsson Göran, Edwards Katarina, Bally Marcel B. Substantial increases in idarubicin plasma concentration by liposome encapsulation mediates improved antitumor activity. Journal of Controlled Release. 2005;105(1-2):89–105. doi: 10.1016/j.jconrel.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 189.Blum W., Phelps M. A., Klisovic R. B., Rozewski D. M., Ni W., Albanese K. A., Rovin B., Kefauver C., Devine S. M., Lucas D. M., Johnson A., Schaaf L. J., Byrd J. C., Marcucci G., Grever M. R. Phase I clinical and pharmacokinetic study of a novel schedule of flavopiridol in relapsed or refractory acute leukemias. Haematologica. 2010;95(7):1098–1105. doi: 10.3324/haematol.2009.017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010 Jun 29, 10:337. Pubmed Central PMCID: 2907344. [DOI] [PMC free article] [PubMed]
- 191.Creutzig U, Zimmermann M, Kaspers G, Reinhardt D. Liposomal Daunorubicin Causes Only Low Cardiotoxicity in Pediatric AML Patients. Blood. 2008;112(11):1940. [Google Scholar]
- 192.Shlush LI, Mitchell A, Heisler L, Abelson S, Ng SWK, Trotman-Grant A, et al. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature. 2017;547(7661):104–108. doi: 10.1038/nature22993. [DOI] [PubMed] [Google Scholar]
- 193.Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007 10/21/online;25:1315. [DOI] [PubMed]
- 194.Jordan Craig T. The leukemic stem cell. Best Practice & Research Clinical Haematology. 2007;20(1):13–18. doi: 10.1016/j.beha.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tardi Paul, Wan Chung Ping Leon, Mayer Lawrence. Passive and semi-active targeting of bone marrow and leukemia cells using anionic low cholesterol liposomes. Journal of Drug Targeting. 2016;24(9):797–804. doi: 10.1080/1061186X.2016.1184669. [DOI] [PubMed] [Google Scholar]
- 196.Sou Keitaro, Goins Beth, Oyajobi Babatunde O, Travi Bruno L, Phillips William T. Bone marrow-targeted liposomal carriers. Expert Opinion on Drug Delivery. 2011;8(3):317–328. doi: 10.1517/17425247.2011.553218. [DOI] [PMC free article] [PubMed] [Google Scholar]