Abstract
The risk of osteoporosis in patients with chronic inflammatory neuropathy (CIN) has not been evaluated in detail. We conducted a population-based case-control study nested in a retrospective cohort to analyze osteoporosis risk among patients with CIN using a nationwide database. Patients with CIN based on the Korean Classification of Disease diagnostic code were included and were matched to controls. A Cox proportional hazards regression model was used to evaluate the effect of CIN on osteoporosis. After propensity score matching, 585 CIN patients and 585 controls were selected. Patients with CIN had an increased osteoporosis risk (hazard ratio [HR] = 2.293, 95% confidence interval [CI] 1.460–3.601) compared with controls. The osteoporosis risk was higher among male patients with CIN than among male controls (HR = 5.404, 95% CI 2.252–12.969), while there were no significant differences among women. Among the CIN patients, the average daily dose of corticosteroids was higher in those who developed osteoporosis (19.6 mg [10.8–49.3]) than those who did not (16.2 mg [7.2–29.1], p = 0.001). The osteoporosis risk among CIN patients is higher than among controls. High risk of osteoporosis in male patients may indicate that osteoporosis in CIN patients results from the disease itself or related treatments.
Subject terms: Neuromuscular disease, Peripheral neuropathies
Introduction
Chronic inflammatory neuropathy (CIN), which includes chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) and multifocal motor neuropathy (MMN), is a rare immune-mediated disease of the peripheral nerves that shows a progressive clinical course1–3. Patients with CIDP often develop substantial disability and nearly 20 to 30% of patients experience a functional status of being wheelchair bound or worse4,5. Although not as severe as in CIDP, MMN can also present with lower limb weakness and more than half of patients display abnormal gait before treatment6. Recently, we revealed that the bone mass of patients with CIDP was significantly lower than that of normal controls and low bone density was associated with impaired functional status7. Reduced bone mass, along with lower limb weakness and gait unsteadiness8, can increase the risk of fracture in patients with CIN.
It is important to assess the risk of osteoporosis or fracture in patients with CIN. A previous report revealed that over half of patients with CIDP developed osteoporosis within 3 years9. However, only a few studies with small study populations have assessed the risk of osteoporosis among patients with CIN, and there is a lack of comprehensive analyses of the effect of each risk factor on osteoporosis. This could be due to the low prevalence of CIN, which ranges from 1 to 4.8 per 100,000 for CIDP and 1–2 per 100,000 for MMN10–14. In order to overcome this limitation, a cohort study based on a large study population is required. Thus, we analyzed the occurrence of osteoporosis in patients with CIN using a nationwide database.
Methods
Data resource and study population
The public medical insurance system in South Korea, known as the National Health Insurance Service, covers approximately 98% of the South Korean population as South Korean law requires every resident to be covered by this scheme15,16. Under this national insurance system, the Health Insurance Review and Assessment Service (HIRA) reviews and evaluates medical costs and healthcare service quality. Currently, HIRA provides nationwide claims data, which are collected during this process for researchers. HIRA claims data include age, sex, diagnoses, medical costs claimed, procedures, prescribed drugs, and a unique anonymous number for each patient16. The strength of HIRA data lies in the fact that the National Health Insurance Service covers the entire South Korean population which is ethnically homogeneous. Many population-based studies have been conducted based on HIRA data17–19.
This is a population-based case-control study nested in a retrospective cohort using HIRA claims data collected between 2007 and 2016. Patients and controls were identified based on the Korean Classification of Disease (KCD), which is an adapted version of International Classification of Disease (ICD). In the present study, we used the KCD diagnosis code G61.8 (other inflammatory polyneuropathies) to identify patients with CIN. Currently, the KCD format does not provide a diagnosis code solely for CIDP or MMN and these diagnoses are incorporated in the diagnosis of other inflammatory polyneuropathies (G61.8). For the robustness of the data, an operational definition of CIN was made based on the diagnosis code and the following criteria. After excluding patients with a pre-existing G61.8 diagnosis in 2007, we included patients with the following criteria: (1) age ≥18 years, (2) ≥2 G61.8 claims reported at least 90 days apart, (3) history of inpatient hospitalization or outpatient visit at the department of neurology or rehabilitation medicine, and (4) underwent nerve conduction studies. The inclusion criterion requiring ≥2 claims reported at least 90 days apart was based on a previous epidemiologic study and the diagnostic criteria of CIDP and MMN that require the disease duration to be ≥2 months and ≥1 month, respectively20–22. We excluded the following people: (1) patients with a secondary cause of polyneuropathy that should be differentiated from CIN, including hereditary demyelinating neuropathy (G60.0), monoclonal gammopathy (D47.2), polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, skin change syndrome (G62.9, D47.7), drug or toxin-induced neuropathy (G62), Borrelia burgdorferi infection (A69.2), diphtheria (A36.8), and amyloidosis (E85) and (2) patients with a diagnosis of osteoporosis before the initial diagnosis of CIN. For the control group, we identified patients aged ≥18 years whose claims included the diagnosis code of Z00.0 (general medical examination). Of these patients, those with a previous diagnosis of osteoporosis, a diagnosis code of G61.8, and neurologic disease that could be differentiated from CIN were excluded. The utilization of and access to the HIRA database was approved by HIRA. The Severance Hospital Institutional Review Board approved this research (approval no. 4-2017-0318) and the study was conducted in accordance with the Declaration of Helsinki. Informed consent was waived due to the retrospective nature of the study.
Data collection
The primary outcome of this study was a new diagnosis of osteoporosis. Such diagnoses are made and entered by physicians at each site based on their clinical impression. HIRA provides guidelines regarding the clinical indications for conducting dual energy x-ray absorptiometry and prescribing medication for osteoporosis. It also reviews whether payment claims correctly follow these guidelines. Previous studies demonstrated a concordance between the HIRA database and actual diagnoses made using clinical information23,24. Data regarding the basic demographics (age and sex), past medical history, and Charlson comorbidity index (CCI) score were collected. CCI is the most widely used comorbidity index; it categorizes comorbidities of patients based on the ICD codes and calculates a comorbidity score by summing the score of each category weighted according to the adjusted risk of mortality. A higher CCI score indicates greater comorbidities25. In the current study, CCI score was measured according to the algorithms suggested by Jang et al., which is based on the algorithm of Quan et al.24,26. The use of corticosteroids, intravenous immunoglobulin, immunosuppressants, or immunomodulatory drugs (alemtuzumab, azathioprine, cyclophosphamide, cyclosporine, etanercept, interferon, mycophenolate mofetil, methotrexate, and rituximab) were recorded. In South Korea, intravenous immunoglobulin obtained reimbursement approval for use in patients with CIDP in June 2015 and in the patients with MMN in April 2018. As medical service providers have processed claims for insurance benefits since this time point, the intravenous immunoglobulin prescription data before this point or unclaimed prescriptions after this point were not recorded in the HIRA database and could not be analyzed in the present study. Corticosteroid doses were converted to the prednisone equivalent dose, and the average daily dose of corticosteroid was calculated by dividing the cumulative prednisone dose by the duration of treatment. Pulsed dexamethasone treatment was defined as the prescription of ≥40 mg dexamethasone for ≥4 consecutive days and pulsed methylprednisolone treatment was defined as the prescription of ≥500 mg methylprednisolone for ≥4 consecutive days27,28. The annual incidence and prevalence was calculated by using patients of all ages. The annual incidence and prevalence of CIN was calculated by dividing the number of newly diagnosed patients with CIN and the number of the patients who visited healthcare facilities with a diagnosis of CIN by the population of each year, respectively. The total population of South Korea was obtained from the Korean Statistical Information Service (KOSIS; http://kosis.kr/), which provides official statistics for South Korea.
Propensity score matching
Osteoporosis is associated with age and sex29. Therefore, a propensity score matching technique was adopted to attenuate the compounding effects of age, sex, index year, and CCI score between patients with CIN and controls. The propensity score was calculated using a logit model for matching variables (age, sex, index year, and CCI score) by considering patients with CIN as the treatment group and predicting probabilities. A 1:1 propensity score matching was fulfilled to match patients with CIN with controls within a caliper of 0.05. Propensity score matching revealed that there were no significant differences in age, sex, and CCI score between the groups. A total of 1170 participants (585 patients with CIN and 585 controls) were used in the final analysis.
Statistical analysis
The results are presented as numbers (percentages) for categorical variables and median [Q1–Q3] for continuous variables as these did not meet the normality assumption. For data before propensity score matching, the Chi-square test and Mann–Whitney U test were used for the comparison of categorical variables and continuous variables between the 2 groups, respectively. For data after propensity score matching, the McNemar test and the Wilcoxon signed rank test were used for the comparison of categorical variables and continuous variables between the 2 groups, respectively. We used a stratified Cox proportional hazards regression model to estimate the effect of CIN on the development of osteoporosis when taking the matching into account. Kaplan–Meier analysis was performed to analyze the cumulative incidence curve of osteoporosis between patients with CIN and controls. The log-rank test was performed to assess the difference in the incidence curves between the 2 groups. All p-values were two-sided and a p value < 0.05 was considered statistically significant. SAS 9.4 software (SAS Institute, Cary, NC) was used for the statistical analysis. R software (version 3.4.0, R Foundation for Statistical Computing, Vienna, Austria) was used to draw the cumulative incidence curves.
Results
Baseline demographics and clinical features of CIN and control groups
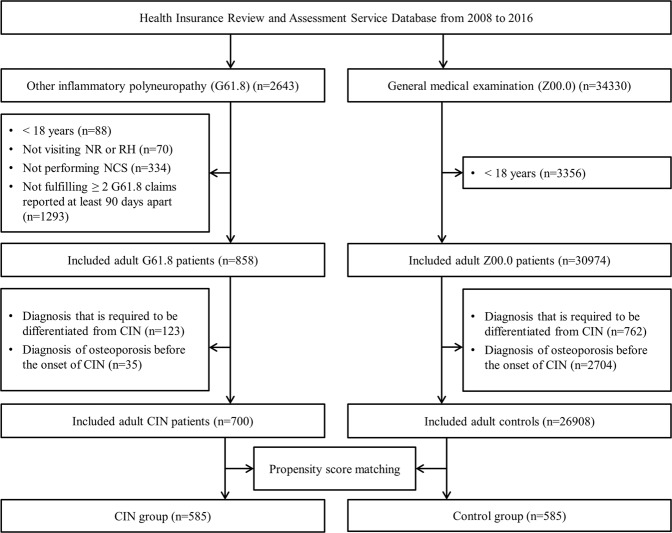
After excluding patients with pre-existing CIN in 2007, a total of 2643 patients with a diagnosis code of other inflammatory polyneuropathy (G61.8) were identified between 2008 and 2016. Of these, 858 fulfilled the inclusion criteria. The exclusion criteria applied to a further 158 patients. Finally, 700 patients who satisfied the operational definition of CIN were included in the study (Fig. 1). The annual incidence of CIN was 0.2 per 100,000 per year and prevalence rate was 0.6 per 100,000 population. Baseline demographic and clinical characteristics of patients with CIN and controls are presented in Table 1. There were 427 (61.0%) male and 273 (39.0%) female participants with CIN with a male to female ratio of 1.6:1. There was significant difference in terms of sex (p < 0.001) and CCI score (p < 0.001) between the CIN and control groups before propensity score matching. Using propensity score matching, 585 patients with CIN and 585 controls were finally used in the analysis and it was confirmed that there were no significant differences in age, sex, and CCI scores between the 2 groups.
Figure 1.
Flowchart of case inclusion process. NR, department of neurology; RH, department of rehabilitation medicine; NCS, nerve conduction study; CIN, chronic inflammatory neuropathy.
Table 1.
Baseline demographic and clinical characteristics of patients with chronic inflammatory neuropathy and controls before and after propensity score matching.
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| CIN | Control | P-value | CIN | Control | P-value | |
| (n = 700) | (n = 26908) | (n = 585) | (n = 585) | |||
| Age, years | 55.0 [44.0–66.0] | 56.0 [45.0–68.0] | 0.060 | 55.0 [43.0–66.0] | 56.0 [43.0–68.0] | 0.139 |
| Sex | <0.001 | 0.882 | ||||
| Women | 273 (39.0) | 13321 (49.5) | 235 (40.2) | 233 (39.8) | ||
| Men | 427 (61.0) | 13587 (50.5) | 350 (59.8) | 352 (60.2) | ||
| CCI score | <0.001 | 0. 780 | ||||
| 0 | 132 (18.9) | 8347 (31.0) | 132 (22.6) | 132 (22.6) | ||
| 1 | 137 (19.6) | 6218 (23.1) | 120 (20.5) | 130 (22.2) | ||
| ≥2 | 431 (61.6) | 12343 (45.9) | 333 (56.9) | 323 (55.2) | ||
| Median CCI score | 2.0 [1.0–4.0] | 1.0 [0–3.0] | <0.001 | 2.0 [1.0–4.0] | 2.0 [1.0–4.0] | 0.895 |
| Treatment | ||||||
| IVIG* | 31 (4.4) | 29 (5.0) | ||||
| Corticosteroid | 600 (85.7) | 501 (85.6) | ||||
| Immunosuppressant | 304 (43.4) | 253 (43.3) | ||||
*Intravenous immunoglobulins that have been prescribed and claimed since June 2015.
Data are expressed as count (percentage) or median [Q1–Q3] values.
CCI, Charlson comorbidity index; CIN, chronic inflammatory neuropathy; IVIG, intravenous immunoglobulin.
Incidence of osteoporosis in CIN and control groups
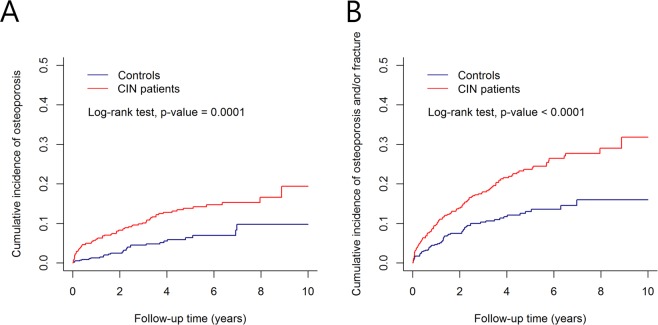
Cumulative incidences of 1) osteoporosis and 2) osteoporosis and/or any fracture for the CIN group and control group are shown in Fig. 2. The cumulative incidences of osteoporosis and osteoporosis/fracture were significantly higher among patients with CIN than among controls. Patients with CIN had a 2.293-fold (hazard ratio [HR] = 2.293, 95% confidence interval [CI] 1.460–3.601) increased risk of developing osteoporosis compared with controls (Table 2). Patients with CIN also had an increased risk of developing any fracture (HR = 1.753, 95% CI 1.178–2.608) and osteoporosis and/or fracture (HR = 1.943, 95% CI 1.411–2.678). We further evaluated the risk of osteoporosis in subgroups of patients stratified by age and sex. In the sex-specific analysis, osteoporosis occurred in 10% of male patients with CIN and 15.7% of female patients with CIN. The risk of osteoporosis was significantly higher among male patients with CIN than among male controls (HR = 5.404, 95% CI 2.252–12.969). Conversely, there were no significant differences in the risk of osteoporosis between female patients with CIN and female controls.
Figure 2.
Cumulative incidence curves: (A) osteoporosis and (B) osteoporosis and/or fracture for chronic inflammatory neuropathy (CIN) and control groups.
Table 2.
Hazard ratios for osteoporosis and/or fracture in patients with chronic inflammatory neuropathy and the risk of osteoporosis stratified by age and sex.
| Characteristics | CIN | Control | CIN vs. control | ||||
|---|---|---|---|---|---|---|---|
| Total | Case (%) | Total | Case (%) | HR | 95% CI | P-value | |
| Outcomes | |||||||
| Osteoporosis | 585 | 72 (12.3) | 585 | 28 (4.8) | 2.293 | 1.460–3.601 | <0.001 |
| Fracture | 585 | 74 (12.6) | 585 | 37 (6.3) | 1.753 | 1.178–2.608 | 0.006 |
| Osteoporosis or fracture | 585 | 124 (21.2) | 585 | 58 (9.9) | 1.943 | 1.411–2.678 | <0.001 |
| Occurrence of osteoporosis | |||||||
| Sex | |||||||
| Men | 350 | 35 (10.0) | 352 | 6 (1.7) | 5.404 | 2.252–12.969 | <0.001 |
| Women | 235 | 37 (15.7) | 233 | 22 (9.4) | 1.41 | 0.809–2.459 | 0.225 |
| Age, years | |||||||
| 18–49 | 228 | 22 (9.7) | 213 | 5 (2.4) | 3.684 | 1.375–9.873 | 0.010 |
| ≥50 | 357 | 50 (14.0) | 372 | 23 (6.2) | 2.027 | 1.211–3.391 | 0.007 |
Data are expressed as count (percentage) values.
CIN, chronic inflammatory neuropathy; HR, hazard ratio; CI, confidence interval.
Comparison between male and female patients with CIN
We compared the clinical factors between male and female patients with CIN to evaluate the possible factors that may have affected the high risk of osteoporosis among male patients with CIN (Table 3). Male patients with CIN were older than female patients with CIN (p = 0.022) and had a higher CCI score (p = 0.006). With regard to comorbidities, the prevalence of diabetes mellitus, liver disease, and cerebrovascular disease was higher among male patients with CIN than among female patients (p = 0.020, p = 0.003 and p = 0.003, respectively). There were no significant differences in the use of corticosteroids and immunosuppressive or immunomodulating agents between male and female patients.
Table 3.
Comparison of clinical and treatment-related features between male and female patients with chronic inflammatory neuropathy.
| Male (n = 350) | Female (n = 235) | P-value | |
|---|---|---|---|
| Age, years | 56.0 [45.0–66.0] | 53.0 [39.0–64.0] | 0.022 |
| CCI score | 0.013 | ||
| 0 | 73 (20.9) | 59 (25.1) | |
| 1 | 61 (17.4) | 59 (25.1) | |
| ≥2 | 216 (61.7) | 117 (49.8) | |
| Median CCI score | 2.0 [1.0–4.0] | 1.0 [0–3.0] | 0.006 |
| Past medical history | |||
| Connective tissue disease | 15 (4.3) | 18 (7.7) | 0.083 |
| Diabetes mellitus | 148 (42.3) | 77 (32.8) | 0.020 |
| Renal disease | 32 (9.1) | 12 (5.1) | 0.070 |
| Liver disease | 84 (24.0) | 33 (14.0) | 0.003 |
| Cerebrovascular disease | 84 (24.0) | 33 (14.0) | 0.003 |
| Cancer | 47 (13.4) | 21 (8.9) | 0.097 |
| Metastatic cancer | 9 (2.6) | 6 (2.6) | 0.989 |
| Number of patients using corticosteroids | 306 (87.4) | 195 (83.0) | 0.132 |
| Average daily dose ≤ 12 m from onset | 17.5 [7.5–31.3] | 15.8 [7.6–31.5] | 0.478 |
| Number of patients using IVIG* | 13 (3.7) | 16 (6.8) | 0.091 |
| Number of patients using immunosuppressant | 151 (43.1) | 102 (43.4) | 0.950 |
*Intravenous immunoglobulins that have been prescribed and claimed since June 2015.
Data are expressed as count (percentage) or median [Q1–Q3] values.
CCI, Charlson comorbidity index; IVIG, intravenous immunoglobulin.
Comparison between patients with CIN who developed osteoporosis and those who did not
We compared clinical factors between 72 patients with CIN who developed osteoporosis (osteoporosis group) and 513 patients with CIN who did not (non-osteoporosis group). The results are shown in Table 4. The proportion of female patients was significantly higher in the osteoporosis group than in the non-osteoporosis group (p = 0.038). Regarding treatment, the average daily dose of corticosteroids within 12 months from CIN diagnosis was significantly higher in the osteoporosis group than in the non-osteoporosis group (p = 0.001). The difference was found to be significant in terms of the average daily dose of oral prednisolone (p < 0.001), whereas no difference was observed in the dose of pulsed methylprednisolone (p = 0.842). Conversely, the proportion of patients who received intravenous immunoglobulin or immunosuppressive agents was significantly lower in the osteoporosis group than in the non-osteoporosis group (p = 0.038 and p = 0.020, respectively). In terms of preventive therapy, 38.9% of patients with osteoporosis and 23.4% of patients without osteoporosis were treated with vitamin D and/or calcium.
Table 4.
Comparison of clinical and treatment-related feature between patients with chronic inflammatory neuropathy who developed osteoporosis and those who did not.
| Osteoporosis | Non-osteoporosis | P-value | |
|---|---|---|---|
| (n = 72) | (n = 513) | ||
| Age, years | 57.0 [44.0–64.0] | 54.0 [43.0–66.0] | 0.378 |
| Sex | 0.038 | ||
| Female | 37 (51.4) | 198 (38.6) | |
| Male | 35 (48.6) | 315 (61.4) | |
| Median CCI score | 2.0 [0–3.0] | 2.0 [1.0–4.0] | 0.479 |
| Past medical history | |||
| Connective tissue disease | 5 (6.9) | 28 (5.5) | 0.585 |
| Diabetes mellitus | 26 (36.1) | 199 (38.8) | 0.662 |
| Renal disease | 2 (2.8) | 42 (8.2) | 0.103 |
| Liver disease | 11 (15.3) | 106 (20.7) | 0.285 |
| Cerebrovascular disease | 16 (22.2) | 101 (19.7) | 0.615 |
| Cancer | 10 (13.9) | 58 (11.3) | 0.522 |
| Metastatic cancer | 3 (4.2) | 12 (2.3) | 0.413 |
| Number of patients using corticosteroids | 64 (88.9) | 437 (85.2) | 0.401 |
| Average daily dose ≤12 m from onset | 19.6 [10.8–49.3] | 16.2 [7.2–29.1] | 0.001 |
| Pulsed methylprednisolone during follow-up | 22 (30.6) | 178 (34.7) | 0.488 |
| Total dose, mg | 6250.0 [3750.0–6250.0] | 6250.0 [3750.0–6250.0] | 0.842 |
| Oral prednisolone during follow-up | 61 (84.7) | 404 (78.8) | 0.240 |
| Average daily dose, mg | 14.6 [8.7–31.8] | 4.9 [1.8–10.0] | <0.001 |
| Number of patients using IVIG* | 0 (0) | 29 (5.7) | 0.038 |
| Number of patients using immunosuppressant | 22 (30.6) | 231 (45.0) | 0.020 |
| Azathioprine | 18 (25.0) | 191 (37.2) | 0.043 |
| Cyclosporine | 0 (0) | 24 (4.7) | 0.060 |
| Cyclophosphamide | 2 (2.8) | 25 (4.9) | 0.561 |
| Mycophenolate mofetil | 3 (4.2) | 43 (8.4) | 0.213 |
| Methotrexate | 1 (1.4) | 9 (1.8) | >0.999 |
*Intravenous immunoglobulins that have been prescribed and claimed since June 2015.
Data are expressed as count (percentage) or median [Q1–Q3] values.
CCI, Charlson comorbidity index; IVIG, intravenous immunoglobulin.
Discussion
The present study revealed a high risk of osteoporosis among patients with CIN based on nationwide cohort. The results showed that patients with CIN had a 2.293-fold increased risk of osteoporosis compared with matched controls. Although osteoporosis is frequent among women in general, the risk of osteoporosis was significantly higher among male patients with CIN than among male controls. These results highlight the importance of early screening to detect osteoporosis in both male and female patients with CIN. In addition, patients with CIN who developed osteoporosis received higher doses of corticosteroids than controls, which may suggest that high dose corticosteroid use is one of the risk factors for osteoporosis among patients with CIN.
Only a few studies have evaluated the occurrence of osteoporosis among patients with CIN, with varying results7,9,30. One study evaluated bone mineral density in 9 patients with CIDP during pulsed steroid treatment and 5 (55.6%) patients developed osteoporosis over 3 years9. Our recent retrospective study also demonstrated that 39.0% of CIDP patients had a bone mineral density in the osteoporotic range7. In contrast, another study that measured bone mineral density during pulsed steroid treatment in 40 patients with CIDP reported that none of the patients developed osteoporosis after 12 months30. Previous results are based on small study populations with a short follow-up duration and it is difficult to determine whether patients with CIN have an increased risk of osteoporosis compared with those without CIN. Therefore, a population-based study with an adequate follow-up period is necessary to assess the risk of osteoporosis for this relatively uncommon disease.
The high incidence of osteoporosis among patients with CIN may result from prolonged use of corticosteroids, which causes bone loss by inhibiting osteoblast function and increasing osteoclast formation31,32. In the present analysis, patients with CIN who developed osteoporosis received higher doses of steroids than those who did not. In addition, the difference was attributed to the difference in the dose of oral prednisolone but not to the dose of intravenous pulsed steroids. This is consistent with previous reports showing that the cumulative steroid dose is inversely correlated with bone mineral density and that prolonged oral steroid treatment is more deleterious to bone density than pulsed intravenous administration31,33,34. In contrast, patients who did not develop osteoporosis were more likely to have undergone treatment with immunosuppressive agents or intravenous immunoglobulin. The steroid-sparing effect of these alternative treatments may have reduced the risk of osteoporosis, although further studies are required to investigate this.
There are some other possible explanations for the high incidence of osteoporosis in patients with CIN. First, functional disability resulting from CIN may have an adverse effect on bone density. Previous studies showed that 39.5% of patients with CIDP had a functional status of chair-bound or worse at the time of initial treatment5, and more than half of the patients with MMN displayed abnormality in gait before treatment6. This disability can result in decreased muscle mass, lack of physical activity, and low sunlight exposure, all of which are known risk factors for bone loss35–37. Concordantly, our recent study revealed that osteoporosis among patients with CIDP was associated with impaired functional status7. Second, CIN itself can cause bone loss. Previous studies have revealed an increased risk of osteoporosis among patients with autoimmune neurological diseases38–40. Chronic inflammation in these diseases is thought to promote bone loss through pro-inflammatory cytokines by inhibiting osteoblast activity and promoting osteoclast activity41,42. Interleukin-17 is one of the proinflammatory cytokines that contributes to bone loss43, and interleukin-17 levels were found to be high in both active CIDP patients and in an animal model of CIDP44,45. In addition, tumor necrosis factor alpha (TNF-α), which is known to induce osteoclastogenesis, plays a main role in animal models of autoimmune neuropathy, and autoimmune neuritis was attenuated in TNF-α knockout mice, probably by altering the balance between proinflammatory and anti-inflammatory macrophages46–48.
It is interesting to note that the risk of osteoporosis was especially high among male patients with CIN. In general, women have a significantly higher risk of osteoporosis than men, and the rate of osteoporosis among women aged ≥50 years is nearly 4 times higher than that among men29. Thus, although the absolute proportion of patients with CIN with newly developed osteoporosis was higher among women than among men, the relative risk was not significantly different from that among controls because of the high baseline risk among the general female population. In contrast, the risk of osteoporosis among male patients with CIN was significantly higher than that among controls. This may reflect both a low incidence of osteoporosis among the general male population and an increased risk of osteoporosis among male patients with CIN. Overall, the male to female ratio of osteoporosis occurrence in patients with CIN was not as markedly different as in controls. This finding may imply that the development of osteoporosis in both male and female patients with CIN is attributable to a common factor: this could be CIN itself, disability from the disease, or related treatments.
The high risk of osteoporosis or fracture in male patients with CIN may be partially due to an older age or a higher proportion of diabetes mellitus among male patients with CIN compared with female patients. Previous studies revealed that male patients with CIDP were older than female patients with CIDP. One study reported that the prevalence of CIDP was higher among male than female patients, particularly those over the age of 55 years12. Other studies showed that the prevalence of CIDP continuously increased after the age of 60–70 years among male patients with CIDP, while the prevalence started to decrease from this point in female patients11,13. The risk of osteoporosis increases with age29 and older age in male patients with CIN may further increase the risk of osteoporosis. In addition, diabetes mellitus was observed more frequently among male patients with CIN than among female patients, which is consistent with the previous studies49,50. Patients with type 1 diabetes mellitus have decreased bone mineral density51,52, and the risk of fracture is increased among patients with type 2 diabetes mellitus53. Thus, the higher incidence of fracture may result from the higher proportion of diabetes mellitus among male patients with CIN.
Despite a high incidence of osteoporosis among patients with CIN, only 38.9% of patients were treated with Vitamin D and/or calcium. Recent guidelines recommend that all adults taking ≥2.5 mg/day of prednisone for more than 3 months should take optimal doses of calcium and vitamin D54. However, not enough attention is paid to the risk of osteoporosis in patients taking corticosteroids. Hougardy et al. showed that only 31% of those taking oral corticosteroids over a prolonged period were given medication to prevent osteoporosis55. A previous survey conducted by the Korea Centers for Disease Control & Prevention similarly showed that less than half of women over 50 diagnosed with osteoporosis were being treated56. Lack of awareness has been similarly observed among neurologists. In previous studies regarding bone health in patients with myasthenia gravis, only half of patients undergoing corticosteroid treatment were treated with bisphosphonates57,58. In addition, previous studies show that the physician diagnosis rate and treatment rate of osteoporosis is lower for male patients than female patients55,59. Thus, the current study may contribute to raising awareness among neurologists regarding bone loss in patients with CIN, especially in male patients.
Some limitations to this study should be acknowledged. First, the results are based on the HIRA data, which are obtained for the purposes of administrative billing. We were unable to assess risk factors for osteoporosis that are not collected in the HIRA database. Second, data regarding the clinical features, laboratory results, disease course and severity, and results of nerve conduction studies, as well as bone mineral density and biochemical parameters, are not provided in the HIRA data. Therefore, the diagnosis of CIN and osteoporosis had to rely upon the KCD diagnosis code and operational definition. However, the demographic characteristics of patients with CIN in the present study, including age, gender ratio, and annual incidence, correlates well with those reported in previous studies10–13,60, which may imply that the diagnosis is reliable. In addition, previous reports demonstrated a considerable correlation between prevalence calculated based on the HIRA database and actual diagnoses made using clinical data23,24. Third, the use of prophylactic therapy with vitamin D or calcium could not be adequately analyzed. In contrast to the other medications, including steroids and immunosuppressive agents, calcium and vitamin D supplements are available to the patients without prescription. As the current study is based on the data obtained for the purposes of administrative billing, the use of medications without prescription could not be analyzed.
In conclusion, this nationwide, population-based study showed that the risk of osteoporosis among patients with CIN is significantly higher than among matched controls. The risk was particularly high among male patients with CIN, who are usually perceived to have a lower risk of osteoporosis than female patients. Moreover, it was found that not enough patients were taking preventive treatment for osteoporosis. The result highlights the importance of early screening for osteoporosis and initiating (or switching) pharmacologic treatment for osteoporosis in patients with CIN.
Acknowledgements
This study was supported by Green Cross Corp.
Author Contributions
Seung Woo Kim drafted the manuscript and contributed to study concept and design and interpretation of data. Eun Hwa Kim conducted statistical analysis and contributed to study concept and design and interpretation of data. Jinae Lee conducted statistical analysis and contributed to study concept and design, interpretation of data, and critical revision of the manuscript for important intellectual content. Young-Chul Choi and Seung Min Kim contributed to study concept and design and critical revision of the manuscript for important intellectual content. Ha Young Shin was responsible for study supervision and contributed to study concept and design, interpretation of data, and critical revision of the manuscript for important intellectual content.
Data Availability
Data are available at Healthcare Bigdata Hub (http://opendata.hira.or.kr) after the qualification process and approval from HIRA for the utilization of and access to the HIRA database.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van Asseldonk J-TH, Franssen H, Van den Berg-Vos RM, Wokke JHJ, Van den Berg LH. Multifocal motor neuropathy. Lancet Neurol. 2005;4:309–319. doi: 10.1016/s1474-4422(05)70074-0. [DOI] [PubMed] [Google Scholar]
- 2.Koller H, Kieseier BC, Jander S, Hartung HP. Chronic inflammatory demyelinating polyneuropathy. N Engl J Med. 2005;352:1343–1356. doi: 10.1056/NEJMra041347. [DOI] [PubMed] [Google Scholar]
- 3.Mahdi-Rogers M, Hughes RA. Epidemiology of chronic inflammatory neuropathies in southeast England. Eur J Neurol. 2014;21:28–33. doi: 10.1111/ene.12190. [DOI] [PubMed] [Google Scholar]
- 4.Dimachkie MM, Barohn RJ. Chronic Inflammatory Demyelinating Polyneuropathy. Curr Treat Options Neurol. 2013;15:350–366. doi: 10.1007/s11940-013-0229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuwabara S, et al. Long term prognosis of chronic inflammatory demyelinating polyneuropathy: a five year follow up of 38 cases. J Neurol Neurosurg Psychiatry. 2006;77:66–70. doi: 10.1136/jnnp.2005.065441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nobile-Orazio E, et al. Multifocal motor neuropathy: clinical and immunological features and response to IVIg in relation to the presence and degree of motor conduction block. J Neurol Neurosurg Psychiatry. 2002;72:761–766. doi: 10.1136/jnnp.72.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SW, Choi YC, Kim SM, Shin HY. Risk factors for osteoporosis in chronic inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve. 2018;58:407–412. doi: 10.1002/mus.26175. [DOI] [PubMed] [Google Scholar]
- 8.Vo ML, Chin RL, Miranda C, Latov N. Changes in spatiotemporal gait parameters following intravenous immunoglobulin treatment for chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2017;56:732–736. doi: 10.1002/mus.25553. [DOI] [PubMed] [Google Scholar]
- 9.Muley SA, Kelkar P, Parry GJ. Treatment of chronic inflammatory demyelinating polyneuropathy with pulsed oral steroids. Arch Neurol. 2008;65:1460–1464. doi: 10.1001/archneur.65.11.1460. [DOI] [PubMed] [Google Scholar]
- 10.Rajabally YA, Simpson BS, Beri S, Bankart J, Gosalakkal JA. Epidemiologic variability of chronic inflammatory demyelinating polyneuropathy with different diagnostic criteria: Study of a UK population. Muscle Nerve. 2009;39:432–438. doi: 10.1002/mus.21206. [DOI] [PubMed] [Google Scholar]
- 11.Chio A, et al. Idiopathic chronic inflammatory demyelinating polyneuropathy: an epidemiological study in Italy. J Neurol Neurosurg Psychiatry. 2007;78:1349–1353. doi: 10.1136/jnnp.2007.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iijima M, et al. Prevalence and incidence rates of chronic inflammatory demyelinating polyneuropathy in the Japanese population. J Neurol Neurosurg Psychiatry. 2008;79:1040–1043. doi: 10.1136/jnnp.2007.128132. [DOI] [PubMed] [Google Scholar]
- 13.McLeod JG, et al. Prevalence of chronic inflammatory demyelinating polyneuropathy in New South Wales, Australia. Ann Neurol. 1999;46:910–913. doi: 10.1002/1531-8249(199912)46:6<910::AID-ANA14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhry V, Swash M. Multifocal motor neuropathy. Is conduction block essential? Neurology. 2006;67:558–559. doi: 10.1212/01.wnl.0000233832.13749.ac. [DOI] [PubMed] [Google Scholar]
- 15.Song YJ. The South Korean health care system. JMAJ. 2009;52:206–209. [Google Scholar]
- 16.Kim, L., Kim, J. A. & Kim, S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol Health36, 10.4178/epih/e2014008 (2014). [DOI] [PMC free article] [PubMed]
- 17.Lee HS, Lee HS, Shin HY, Choi YC, Kim SM. The Epidemiology of Myasthenia Gravis in Korea. Yonsei Med J. 2016;57:419–425. doi: 10.3349/ymj.2016.57.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahn IM, et al. Incidence, prevalence, and survival of moyamoya disease in Korea: a nationwide, population-based study. Stroke. 2014;45:1090–1095. doi: 10.1161/strokeaha.113.004273. [DOI] [PubMed] [Google Scholar]
- 19.Jang MJ, Bang SM, Oh D. Incidence of venous thromboembolism in Korea: from the Health Insurance Review and Assessment Service database. J Thromb Haemost. 2011;9:85–91. doi: 10.1111/j.1538-7836.2010.04108.x. [DOI] [PubMed] [Google Scholar]
- 20.Bril V, et al. The dilemma of diabetes in chronic inflammatory demyelinating polyneuropathy. J Diabetes Complications. 2016;30:1401–1407. doi: 10.1016/j.jdiacomp.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van den Bergh PY, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society - first revision. Eur J Neurol. 2010;17:356–363. doi: 10.1111/j.1468-1331.2009.02930.x. [DOI] [PubMed] [Google Scholar]
- 22.Joint Task Force of the EFNS and the PNS European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society–first revision. J Peripher Nerv Syst15, 295–301, 10.1111/j.1529-8027.2010.00290.x (2010). [DOI] [PubMed]
- 23.Park IB, et al. Diabetes Epidemics in Korea: Reappraise Nationwide Survey of Diabetes “Diabetes in Korea 2007”. Diabetes Metab J. 2013;37:233–239. doi: 10.4093/dmj.2013.37.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jang S, et al. Medical service utilization with osteoporosis. Endocrinology and Metabolism. 2010;25:326–339. doi: 10.3803/EnM.2010.25.4.326. [DOI] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Quan, H. et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care, 1130–1139 (2005). [DOI] [PubMed]
- 27.van Lieverloo GGA, et al. Corticosteroids in chronic inflammatory demyelinating polyneuropathy: A retrospective, multicentre study, comparing efficacy and safety of daily prednisolone, pulsed dexamethasone, and pulsed intravenous methylprednisolone. J Neurol. 2018;265:2052–2059. doi: 10.1007/s00415-018-8948-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eftimov F, Vermeulen M, van Doorn PA, Brusse E, van Schaik IN. Long-term remission of CIDP after pulsed dexamethasone or short-term prednisolone treatment. Neurology. 2012;78:1079–1084. doi: 10.1212/WNL.0b013e31824e8f84. [DOI] [PubMed] [Google Scholar]
- 29.Alswat KA. Gender Disparities in Osteoporosis. J Clin Med Res. 2017;9:382–387. doi: 10.14740/jocmr2970w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Schaik IN, et al. Pulsed high-dose dexamethasone versus standard prednisolone treatment for chronic inflammatory demyelinating polyradiculoneuropathy (PREDICT study): a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:245–253. doi: 10.1016/s1474-4422(10)70021-1. [DOI] [PubMed] [Google Scholar]
- 31.van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–787. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 32.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 33.van Staa TP, Leufkens HGM, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology. 2000;39:1383–1389. doi: 10.1093/rheumatology/39.12.1383. [DOI] [PubMed] [Google Scholar]
- 34.Farkas K, et al. Bolus administration of steroid therapy is more favorable than the conventional use in preventing decrease of bone density and the increase of body fat percentage in patients with inflammatory bowel disease. J Crohns Colitis. 2014;8:992–997. doi: 10.1016/j.crohns.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 35.Proctor DN, et al. Relative Influence of Physical Activity, Muscle Mass and Strength on Bone Density. Osteoporos Int. 2000;11:944–952. doi: 10.1007/s001980070033. [DOI] [PubMed] [Google Scholar]
- 36.Elf K, Askmark H, Nygren I, Punga AR. Vitamin D deficiency in patients with primary immune-mediated peripheral neuropathies. J Neurol Sci. 2014;345:184–188. doi: 10.1016/j.jns.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 37.Zhu K, et al. Impaired Bone Homeostasis in Amyotrophic Lateral Sclerosis Mice with Muscle Atrophy. J Biol Chem. 2015;290:8081–8094. doi: 10.1074/jbc.M114.603985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dionyssiotis Y. Bone loss and fractures in multiple sclerosis: focus on epidemiologic and physiopathological features. Int J Gen Med. 2011;4:505–509. doi: 10.2147/ijgm.s22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeh JH, Chen HJ, Chen YK, Chiu HC, Kao CH. Increased risk of osteoporosis in patients with myasthenia gravis: a population-based cohort study. Neurology. 2014;83:1075–1079. doi: 10.1212/wnl.0000000000000804. [DOI] [PubMed] [Google Scholar]
- 40.Lloyd ME, Spector TD, Howard R. Osteoporosis in neurological disorders. J Neurol Neurosurg Psychiatry. 2000;68:543–547. doi: 10.1136/jnnp.68.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amarasekara DS, Yu J, Rho J. Bone Loss Triggered by the Cytokine Network in Inflammatory Autoimmune Diseases. J Immunol Res. 2015;2015:832127. doi: 10.1155/2015/832127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11:234–250. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- 43.Miossec P. IL-17 and Th17 cells in human inflammatory diseases. Microbes Infect. 2009;11:625–630. doi: 10.1016/j.micinf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Chi LJ, et al. Distribution of Th17 cells and Th1 cells in peripheral blood and cerebrospinal fluid in chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2010;15:345–356. doi: 10.1111/j.1529-8027.2010.00294.x. [DOI] [PubMed] [Google Scholar]
- 45.Iijima M. Animal models of chronic inflammatory demyelinating polyneuropathy. Clin Exp Neuroimmunol. 2018;9:101–109. doi: 10.1111/cen3.12462. [DOI] [Google Scholar]
- 46.Soliven B. Animal models of autoimmune neuropathy. ILAR J. 2014;54:282–290. doi: 10.1093/ilar/ilt054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H-L, et al. Attenuated EAN in TNF-α Deficient Mice Is Associated with an Altered Balance of M1/M2 Macrophages. PLoS One. 2012;7:e38157. doi: 10.1371/journal.pone.0038157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardy R, Cooper MS. Bone loss in inflammatory disorders. J Endocrinol. 2009;201:309–320. doi: 10.1677/joe-08-0568. [DOI] [PubMed] [Google Scholar]
- 49.Sharma KR, Cross J, Ayyar D, Martinez-Arizala A, Bradley WG. Diabetic demyelinating polyneuropathy responsive to intravenous immunoglobulin therapy. Arch Neurol. 2002;59:751–757. doi: 10.1001/archneur.59.5.751. [DOI] [PubMed] [Google Scholar]
- 50.Gorson KC, Ropper AH, Adelman LS, Weinberg DH. Influence of diabetes mellitus on chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2000;23:37–43. doi: 10.1002/(SICI)1097-4598(200001)23:1<37::AID-MUS5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 51.Lopez-Ibarra PJ, et al. Bone mineral density at time of clinical diagnosis of adult-onset type 1 diabetes mellitus. Endocr Pract. 2001;7:346–351. doi: 10.4158/ep.7.5.346. [DOI] [PubMed] [Google Scholar]
- 52.Kemink SA, Hermus AR, Swinkels LM, Lutterman JA, Smals AG. Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. J Endocrinol Invest. 2000;23:295–303. doi: 10.1007/bf03343726. [DOI] [PubMed] [Google Scholar]
- 53.de L, II, et al. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int. 2005;16:1713–1720. doi: 10.1007/s00198-005-1909-1. [DOI] [PubMed] [Google Scholar]
- 54.Buckley L, et al. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol. 2017;69:1521–1537. doi: 10.1002/art.40137. [DOI] [PubMed] [Google Scholar]
- 55.Hougardy P. Bleasel & Randall Is enough attention being given to the adverse effects of corticosteroid therapy? J Clin Pharm Ther. 2000;25:227–234. doi: 10.1046/j.1365-2710.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 56.Oh SM, et al. Development and Validation of Osteoporosis Risk-Assessment Model for Korean Men. Yonsei Med J. 2016;57:187–196. doi: 10.3349/ymj.2016.57.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konno S, et al. Association between Glucocorticoid-Induced Osteoporosis and Myasthenia Gravis: A Cross-Sectional Study. PloS One. 2015;10:e0126579. doi: 10.1371/journal.pone.0126579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lewis SJ, Smith PE. Osteoporosis prevention in myasthenia gravis: a reminder. Acta Neurol Scand. 2001;103:320–322. doi: 10.1034/j.1600-0404.2001.103005320.x. [DOI] [PubMed] [Google Scholar]
- 59.Choi YJ, Oh HJ, Kim DJ, Lee Y, Chung YS. The prevalence of osteoporosis in Korean adults aged 50 years or older and the higher diagnosis rates in women who were beneficiaries of a national screening program: the Korea National Health and Nutrition Examination Survey 2008–2009. J Bone Miner Res. 2012;27:1879–1886. doi: 10.1002/jbmr.1635. [DOI] [PubMed] [Google Scholar]
- 60.Lunn MP, Manji H, Choudhary PP, Hughes RA, Thomas PK. Chronic inflammatory demyelinating polyradiculoneuropathy: a prevalence study in south east England. J Neurol Neurosurg Psychiatry. 1999;66:677–680. doi: 10.1136/jnnp.66.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at Healthcare Bigdata Hub (http://opendata.hira.or.kr) after the qualification process and approval from HIRA for the utilization of and access to the HIRA database.