Abstract
The transient receptor potential ion-channel superfamily consists of nonselective cation channels located mostly on the plasma membranes of numerous animal cell types, which are closely related to sensory information transmission (e.g., vision, pain, and temperature perception), as well as regulation of intracellular Ca2+ balance and physiological activities of growth and development. Transient receptor potential ion channel subfamily V (TRPV) is one of the largest and most diverse subfamilies, including TRPV1–TRPV6 involved in the regulation of a variety of cellular functions. TRPV4 can be activated by various physical and chemical stimuli, such as heat, mechanical force, and phorbol ester derivatives participating in the maintenance of normal cellular functions. In recent years, the roles of TRPV4 in cell proliferation, differentiation, apoptosis, and migration have been extensively studied. Its abnormal expression has also been closely related to the onset and progression of multiple tumors, so TRPV4 may be a target for cancer diagnosis and treatment. In this review, we focused on the latest studies concerning the role of TRPV4 in tumorigenesis and the therapeutic potential. As evidenced by the effects on cancerogenesis, TRPV4 is a potential target for anticancer therapy.
Subject terms: Cancer, Biomarkers
Facts
TRPV4 is a broadly expressed, nonselective calcium permeant cation channel that perform an important role in regulating the Ca2+ influx in the cells in which they are expressed.
TRPV4 is a thermosensor, activated by temperatures greater than 24–27 °C, also can be activated by osmotic, mechanical, and chemical cues.
TRPV4 is constitutively expressed and capable of spontaneous activity in the absence of agonist stimulation, which suggests that it serves important physiological functions.
Open questions
What is the underlying cause of TRPV4 expression levels in different types of cancer?
Whether it is possible to lower the toxicity and high-efficiency TRPV4 inhibitors from natural products to treat various diseases?
How to inhibit the development of cancer by targeting TRPV4 of tumor cells without affecting the function of normal cells?
Introduction
Malignant tumors are still the primary health problems that plague people1, which are clinically treated mainly through chemotherapy, biological therapy, radiotherapy, and surgical resection2. However, chemotherapy usually causes severe side effects and proneness to drug resistance2. Therefore, researchers have endeavored to find molecular targeted drugs which are less toxic and more efficient. Targeted therapy using drugs which designed based on antitumor targets interferes with or even blocks the physiological activities of tumors3. Drugs that target cancer cell membrane receptors have the advantages of high affinity and recognition efficiency4. For instance, drugs targeting epidermal growth factor receptors and some other cell membrane receptors have been clinically used5. Several important cellular functions are related to transmembrane potentials and lie under the control of ion channels. The role of ion channels play in connecting the intracellular to the extracellular environment and controlling almost any cellular function makes ion channels ideal potential therapeutic targets. Nowadays, increasing interest has been given to ion channels as potential drug targets in many cancer conditions6,7. Many observations found that some cancer cell lines display unusual ion channel expression, and the altered ion transport may play an important role in human cancer progression. When ion channels open, certain ion species can pass through which affects such basic cellular characteristics, including membrane potential, cell volume, and the state of intracellular signaling pathways. Thus, aberrant expression or function changes of ion channels may produce global or local, spatially restricted changes in these characteristics and driving the transformation of normal cells into malignant derivatives8–11. Some previous research also found that regulating of certain ion channels can inhibit the occurrence and development of tumor12–16. Beyond that, the expression level of transient receptor potential ion channel, subfamily V, member 4 (TRPV4) vary in different tumors and play important role in multiple processes of tumor progression. TRPV4 is a calcium-permeable nonselective cation channel of TRP family, which biologically involved in osmotic sensitivity and mechanosensitivity17,18. TRPV4 participates in many physiological processes, such as liver19, intestinal20, renal21 and bladder22 functions, growth and structural integrity of the skeleton23, together with systemic osmotic pressure induced by the brain24. Until now, TRPV4 has been reported functionally related to cell proliferation25, differentiation26, apoptosis27, migration28, and many other physiological processes. In addition, TRPV4 has also been significantly correlated with tumor angiogenesis29. Targeting TRPV4 may have inhibitory effects on tumor onset and progression, so it is a potential prognostic index and therapeutic target for malignant tumors. We herein reviewed the research focus on the relationship between TRPV4 and cancer, also explored the mechanism of TRPV4-mediated oncogenesis and the strategy that target TRPV4 for tumor metastasis. We hope this finding provide valuable reference for further research and clinical application.
TRP superfamily
Similar to voltage-dependent cation channel, TRP channel also has six-transmembrane (S1–S6) domains, and both the N-terminus and C-terminus are intracellular. The segment between S5 and S6 of the TRP channel is embedded to form an ion-passage channel30. The S4 fragment lacks positively charged amino acid residues of that in normal voltage-dependent cation channel, i.e., the TRP channel is nonvoltage dependent. Besides, there are several ankyrin (ANK) repeat domains at the N-terminus of many TRP subtypes31. According to the amino acid sequence homology, more than 30 mammalian TRP channels have been classified into seven subfamilies: TRPC, TRPV, TRPM, TRPA, TRPN, TRPML, and TRPP32. The TRP channel is a nonselective cation channel on the cell membrane through which calcium and sodium ions mainly pass33. However, the ratios of selectivity of calcium ion to that of sodium ion (PCa/PNa, P stand for permeability) are different, ranging from higher than 100:1 to lower than 0.05:134,35. In addition, many divalent ions, such as magnesium (Mg2+), zinc (Zn2+), manganese (Mn2+), and cobalt (Co2+), can also pass through TRPM6 and TRPM7 channels36–38.
As a crucial sensor of cells, TRP channels can transmit information between intracellular and extracellular, also being regulated by changes in messenger molecules, compounds, temperature, and osmotic pressure39. Different TRP channels have various regulatory mechanisms, many of which are modulated by a variety of stimulations. Although the activation mechanisms of different TRP channels vary, they can be roughly classified into receptor activation, ligand activation, and temperature-change activation. G protein-coupled receptors or tyrosine kinase receptors can activate TRP channels by activating phospholipase C (PLC)40,41. Some exogenous small molecules, compounds, inorganic ions, and endogenous substances can also activate these channels42. Temperature changes directly activate multiple TRP channels, and the activation thresholds of different temperature-sensitive TRP channels vary43. TRPV4 is activated by moderate heat (>24–27 °C), while TRPA1 is activated by noxious cold at 17 °C and below. TRP channel is widely distributed in the peripheral nervous system, skin, cardiovascular system, respiratory system, gastrointestinal system, genitourinary system, and immune systems in addition to the central nervous system44–48. Unlike the classical voltage-dependent cation channel that participates in specific functions, TRP channel has a variety of functions, mainly including temperature and pain perception49, gustation50, feeling of mechanical force51, mediation of PLC-dependent calcium influx52, maintenance of cell ion homeostasis35, as well as regulation of cell growth53, neurotransmitter release, and hormone secretion54,55.
Recently, several members of the TRP superfamily have been confirmed to play certain roles in tumor progression. Accumulating evidence has indicated that TRP members were expressed abundantly in cancer cells and closely related to tumor progression. Kim et al. reported that TRPM7 played a key role in the growth and survival of gastric cancer cells56. Elevated expression of TRPC5 protein is associated with the drug resistance of breast cancer cells57, and promotes colon cancer metastasis via the hypoxia-inducible factor 1-alpha/Twist (HIF-1α/Twist) signaling pathway58. Meanwhile, TRPV4 is aberrantly expressed in many tumors and strongly corelated with the prognosis of cancer. The different members of the TRP family and their main characteristics are summarized in Table 1, including the physiological functions, activation conditions, and their effects on cancer.
Table 1.
Different members of the TRP family and their main characteristics
| Channel subunit | Physiological functions | Activation | Effects on cancer | Refs. |
|---|---|---|---|---|
| TRPC subfamily | ||||
| TRPC1 | Mechanosensation; salivary gland fluid secretion and appetite control; generation of the excitatory postsynaptic potential in brain; promotion of brain development (together with TRPC5) | Ca2+ store depletion stress; mechanical stretch; PLC signaling pathway | Promoted cell migration (H1080 cells) | 127–129 |
| TRPC2 | Gender recognition and aggression behaviors signal transduction (mouse) | Diacylglycerol (DAG) | ND | 127, 128,130 |
| TRPC3 | Regulation of cerebral vasomotor; guide of growth cones; spine formation in brain; motor behavior in the cerebellum | Ca2+ store depletion stress; DAG | Promoted melanoma cell viability and migration | 131–133 |
| TRPC4 | Regulation of endothelium-dependent vasorelaxation; regulation of transcellular permeation of the endothelial layer and endothelium intercellular adhesion; participation in 5-HT signal transduction | GPCR and receptor tyrosine kinases and their downstream components; Ca2+ store depletion stress | Inhibited proliferation (SW982 cells) | 134,135 |
| TRPC5 | Control of anxiety, fear, and reward behaviors; promotion of brain development (together with TRPC1) | GPCR and receptor tyrosine kinases and their downstream components; Ca2+ store depletion stress | Improved colorectal cancer, breast chemoresistance; associated with autophagy, inhibited proliferation | 135–137 |
| TRPC6 | Redox sensor; regulation of artery contractility and angiogenesis; participation in endocannabinoid signal transduction; promotion of dendrite growth and synapse forming in the developing brain | DAG, PtdIns(4,5)P2 (PIP2) | Promoted breast proferation, migration | 127, 128,138 |
| TRPC7 | Control respiratory rhythm activity in pre-Bötzinger complex in the brain; regulation of vasoconstriction | DAG, Ca2+ store depletion stress, GPCR | ND | 128,139 |
| TRPV subfamily | ||||
| TRPV1 | Thermosensation (heat); autonomic thermos regulation; inflammatory hyperalgesia; regulation of osmosensing in the brain by a particular TRPV1 variant; nociception and pain management; endocannabinoid signaling in the brain; food intake signal regulation | Heat (>43 °C), vanilloids (capsaicin) | Promoted cell proliferation (PC3cells); promoted migration; induced cell apoptosis (U373 or HeLa cells) | 140,141 |
| TRPV2 | Thermosensation (noxious heat); nociception; mediated immune response | Heat (>52 °C), osmotic cell swelling and LPLs | Induced apoptosis (T24 cells); promoted migration and invasion (prostate cancer) | 140,142 |
| TRPV3 | Thermosensation (moderate heat); nociception; skin integrity, hair growth and sebocyte function, mood regulation | Heat (>33 °C) | Promoted lung cancer proliferation | 143,144 |
| TRPV4 | Thermosensation (moderate heat); mechanosensation; osmo-sensation; nociception; endothelium vasomotor control and shear stress sensor; modulation of cell migration; control adherens junctions in skin | Heat (>24-27°C); mechanical deformation and osmotic stimuli; PH; 5,6-EET | Destabilized cancer vascular integrity (mouse prostate cancer) and promoted migration (breast cancer) | 114,145 |
| TRPV5 | Ca2+ reabsorption in kidney epithelial cells; bone density | Activated via PLC; PH; osmotic stimuli | Promoted proliferation and metastasis (renal cell carcinoma) | 140, 146,147 |
| TRPV6 | Ca2+ reabsorption channel in kidney; bone density; keratinocyte development in the skin; regulation of calcium entry into intestinal enterocyte | Constitutively active; osmotic stimuli; Ca2+ store depletion stress | Promoted proliferation (ovarian cancer) | 148,149 |
| TRPM subfamily | ||||
| TRPM1 | Light-evoked response in ON bipolar retinal ganglia cells; regulation of melanin content in human epidermal melanocyte | Constitutively active | Reduced melanoma cells metastasis | 127, 128,150 |
| TRPM2 | Thermosensation (moderate heat); oxidative and nitrosative stress response; immunity cells infiltration; regulation of pancreas insulin release; apoptosis control | Heat (>35 °C) | Promoted Migration, invasion (AGS cells, breast cancer) and promoted proliferation (prostate cancer) | 15, 127, 128, 150,151 |
| TRPM3 | Regulation of pancreas insulin release and glucose homeostasis; steroid hormone (pregnanolon) sensor | Heat (>35 °C); cell swelling | ND | 127, 128,152 |
| TRPM4 | Regulation of catecholamine release from chromafn cells; involved in mast cell activation and dendritic cell migration; regulation of Ca2+ entry | Heat (~40°C) | Induced EMT and promoted invasion, metastasis (prostate cancer) | 127, 150,153 |
| TRPM5 | A key component of taste (sweet, bitter, umami) transduction; regulator of glucose-induced insulin release | Actived via GPCR | Inhabited metastasis (B16BL6 cells) | 127, 154,155 |
| TRPM6 | A key component of taste (sweet, bitter, umami) transduction; positive regulator of glucose-induced insulin release; trigeminal nasal chemoreception | ND | Promoted proliferation (SHEP-21N cells) | 127,156 |
| TRPM7 | Mg2+ homeostasis and reabsorption in kidney and intestine; development of thymocytes | Membrane stretch activated and Mg-ATP inhibited | Promoted migration and invasion (prostate cancer) | 127,140 |
| TRPM8 | Thermosensation (cold); autonomic thermos regulation (with TRPV1) | Cooling (<28 °C), PIP2 and LPLs | Decreased migration (prostate cancer); increase apoptosis and oxidative stress (Du145 cells); promoted cancer viability (prostate cancer, pancreatic adenocarcinoma) | 140,157–160 |
| TRPA1 subfamily | ||||
| TRPA1 | Thermosensation (noxious cold); mechanosensation; chemosensor; nociception; inflammatory pain | Heat (<=17 °C) | Anti-ROS induced cell death; induced autophagy and promoted invasion (lung cancer) | 161,162 |
| TRPML subfamily | ||||
| TRPML1 | Essential for endocytosis and endosomal/lysosomal function; regulation of autophagy; regulation of neurological function | PIP2, pH | Promoted proliferation and invasion (triple-negative breast cancer) | 163–165 |
| TRPML2 | Endosomal/lysosomal function | PIP2 | Promoted proliferation and viability (glioma) | 166,167 |
| TRPML3 | Endosomal/lysosomal function; autophagy; hair cell maturation; regulation of autophagy | PIP2 | ND | 168,169 |
| TRPP subfamily | ||||
| TRPP2 | Regulator of endogenous mechanosensitive channels; cardiac, skeletal and renal development; integrity of the vessel wall; mechanoreceptor and flow sensor in endothelium; apoptosis | EGF | Promoted EMT and invasion (squamous cell carcinoma) | 170,171 |
| TRPP3 | Renal development; part of putative sour sensor | ND | ND | 172,173 |
| TRPP5 | Spermatogenesis | ND | ND | 174 |
ND not determined
TRPV4
Structure and function of TRPV4
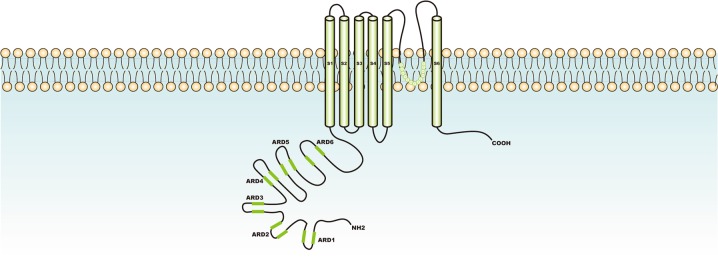
The TRPV family consists of six members (TRPV1–TRPV6), which all function as tetramers. Among them, TRPV1–TRPV4 have moderate permeabilities for calcium ions, with the PCa/PNa ratios of 1–10. With the ratios of over 100, TRPV5–TRPV6 are highly permeable for calcium ions34. The TRPV4 ion channel was described in detail dating back to 2000, and characterized as a volume-regulated channel due to osmotic sensitivity and cell volume regulation59. TRPV4 gene encodes TRPV4 ion channel protein, which was initially referred to as “vanilloid-receptor related osmotically activated channel” and “OSM9-like transient receptor potential channel, member 4”60, as a member of the vanilloid subfamily in the TRP superfamily61. TRPV4 channel protein consisting of 871 amino acids has a homodimeric tetramer structure which is similar to those of other transient potential receptor proteins, with six transmembrane spanning α-helices (S1–S6) per monomer62. The structure of TRPV4 is shown in Fig. 1. In addition to the transmembrane region, the remaining part of this protein is located in the cytoplasm. Similar to other TRPVs, it has six ANK repeats at the N-terminus, which are essential for the regular functioning of ion channels and protein–protein interactions. As a nonselective cation channel (Ca2+ or Mg2+ as the permeating extracellular cation), TRPV4 is characterized with a moderate high Ca2+ permeability ratio (PCa/PNa= 6–10, PMg/PNa = 2–3)63,64. The pore-forming loop that allows the ionic flow is located between S5 and S6 domains of TRPV465. Some molecules, such as phosphatidylinositol 4,5-bisphosphate, can bind firmly to the ANK repeats end of TRPV4, thereby inhibiting the effects of TRPV466. Temperature, mechanical force, hypotonia, phorbol ester derivatives, and other physical and chemical stimuli can activate TRPV4, allowing calcium-based cations to rapidly enter the cytoplasm to maintain osmotic pressure stability and signal transmission66. The representative agonists and antagonists of TRPV4 are organized in Table 2. TRPV4 is widely expressed in the nervous system67, immune system68, eye69, ear70, cardiovascular system71, respiratory system72, urinary system73, and digestive system74. Moreover, TRPV4 maintains osmotic pressure homeostasis by activating, rapidly and efficiently causing the influx of calcium-based cations, and maintaining cell morphology75. When skin tissue is physically and chemically stimulated, opening of the TRPV4 promotes the mechanical responses of subcutaneous fibroblasts and endothelial cells, manifested as vasodilation and skeletal muscle relaxation76. Different physical and chemical stimuli include heat, mechanical force, and endogenous substances, such as arachidonic acid and its cytochrome P450-derived metabolites (epoxyeicosatrienoic acids), endocannabinoids (anandamide and 2-arachidonoylglycerol), as well as synthetic a-phorbol derivatives can activate TRPV4. TRPV4 integrates multiple stimuli, then transmitting calcium signals and inducing a series of stress responses, such as promotion of release of nitric oxide, prostaglandin I2, and endothelial-derived enoic acid in the vascular endothelial system, relaxation of vascular smooth muscles, production of inflammatory factors (e.g., interleukin-6 (IL-6)) in lung tissue, and development of inflammatory responses77,78. At the early stage of vascular and neuronal development, activation of the TRPV4 channel of capillary endothelial cells and neurons activates downstream phosphatidylinositol 3-kinase (PI3K) and induces the activation of α-integrin protein, thereby facilitating the localization and remodeling of neurons and endothelial cells79. In adipocytes, TRPV4 is involved in fatty acid metabolism. Activating TRPV4 not only increases fatty acid synthesis by regulating RAC-alpha serine/threonine-protein kinase (AKT) phosphorylation but also attenuates fatty acid oxidation to reduce heat production80.
Fig. 1. Structure of TRPV4.
Similar to other transient potential receptor proteins, TRPV4 is consisted of 871 amino acids, has a homodimeric tetramer structure with six transmembrane spanning α-helices (S1–S6) per TRPV4 monomer
Table 2.
Data summary for agonists and antagonists of TRPV4
| Compound | Structure | Formula | Speciality | Species | Selectivity | EC50 | Refs. |
|---|---|---|---|---|---|---|---|
| GSK1016790A |
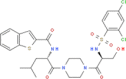
|
C28H32Cl2N4O6S2 | Agonists | Human, mouse | Selecitve | hTRPV4: 2.1 nM mTRPV4: 18 nM | 90, 175,176 |
| 4αPDD |
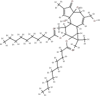
|
C40H64O8 | Agonists | Human, mouse, rat | Nonselective (channel unknown) | hTRPV4: 0.2 µM | 175,177–179 |
| Phorbol 12 myristate 13-acetate |
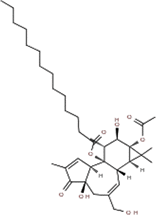
|
C36H56O8 | Agonists | Human, mouse | Nonselective (agonists of TRPV1) | hTRPV4: 11.7 nM | 62, 175,176 |
| N-arachidonoyl taurine |
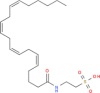
|
C22H37NO4S | Agonists | Mouse | Nonselective (agonists of TRPV1) | mTRPV4: 21 µM | 180 |
| 5,6-EET |
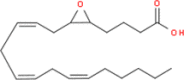
|
C20H32O3 | Agonists | Mouse | Nonselective (inhibitor of T-channel Cav3 currents; agonists of TRPA1) | ND | 181–185 |
| Apigenin |
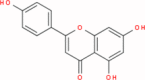
|
C15H10O5 | Agonists | Human, mouse, rat | ND | hTRPV4: 4.32 µM | 186 |
| Bisandrographolide A |
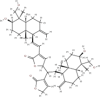
|
C40H56O8 | Agonists | Mouse | Selecitve | hTRPV4: 0.79-0.95 µM | 181,187 |
| Dimethylallyl pyrophosphate |
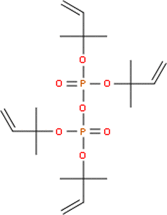
|
C20H36O7P2 | Agonists | Human, mouse | Nonselective (antagonists of TRPV3) | hTRPV4: 2.5 µM | 188 |
| Gd3+ |
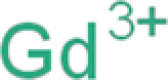
|
Gd | Antagonists | Human, rat | Nonselective (antagonists of non-selective TRPVs channels) | ND | 62, 189,190 |
| La3+ |
|
La | Antagonists | Human, rat | Nonselective (antagonists of non-selective TRPVs channels) | ND | 62, 189,190 |
| Ruthenium red |
|
H42Cl6N14O2Ru3 | Antagonists | Human, rat | Nonselective (inhibitor of SERCA; antagonists of non-selective TRPVs channels) | ND | 175, 189,191 |
| HC-067047 |
|
C26H28F3N3O2 | Antagonists | Mouse, rat | Selecitve | hTRPV4: 0.36 µM | 106, 192,193 |
| RN-1734 |
|
C14H22Cl2N2O2S | Antagonists | Human, mouse, rat | Selecitve | hTRPV4: 0.77 µM | 192 |
| Capsazepine |
|
C19H21ClN2O2S | Antagonists | Human, rat | Nonselective (antagonists of TRPV1) | ND | 192,194–196 |
| GSK2193874 |
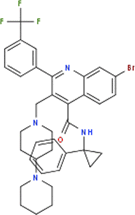
|
C37H38BrF3N4O | Antagonists | Human, mouse | Selective | hTRPV4: 0.65 µM | 192, 197,198 |
ND not determined
TRPV4 is involved in tumor onset and progression
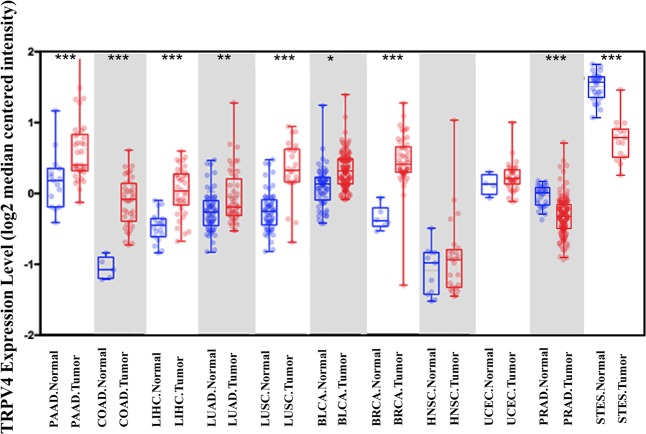
Abnormal expression of TRPV4 is closely related to tumor formation and metastasis, which is higher in gastric cancer, lung cancer, and colorectal cancer cells, but lower in esophageal cancer and prostate cancer cells than in normal tissue cells according to the researches on TRPV4 (Table 3). The expression data of TRPV4 obtained from oncomine (https://www.oncomine.org/resource/main.html) show similar results (Fig. 2). Since TRPV4 can be activated at the condition of body temperature, its high expression in these cancer cells may led the intracellular calcium higher than other cells. Once TRPV4 is activated by some other stimuli, the rapidly increased intracellular calcium can regulate the downstream signaling pathway to affect the different processes of tumorigenesis. However, TRPV4 downregulation in cancers might be related with the differences in tumor microenvironment. Collectively, TRPV4 probably affect cell proliferation, differentiation, apoptosis, and migration by regulating Ca2+ and production of isoforms, thus affecting tumor onset and progression.
Table 3.
Expression of TRPV4 in various cancer
| Cancer type | Expression | Refs. |
|---|---|---|
| Gastric cancer | Up | 88 |
| Lung cancer | Up | 199 |
| Colorectal cancer | Up | 200 |
| Esophageal cance | Down | 201–203 |
| Prostate cancer | Down | 204 |
| Pancreatic cancer | Up | 205 |
| Liver cancer | Up | 206 |
The results of lung cancer and colorectal cancer in this table and Fig. 2 are obtained from the same research
Fig. 2. Expression of TRPV4 in human tumor samples obtained from oncomine.
Data obtained from oncomine and the table are made by the data set from PAAD (pancreatic adenocarcinoma): series GSE16515 (title: FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt), with p-value of 8.93E-4, and 52 patients samples were used for this analysis; COAD (colon adenocarcinoma): series GSE5206 (title: Transcriptional recapitulation and subversion of embryonic colon development by mouse colon tumor models and human colon cancer), with p-value of 5.56E-7, and 46 patients samples were used for this analysis; LIHC (liver hepatocellular carcinoma): series GSE14323 (title: Genes involved in viral carcinogenesis and tumor initiation in Hepatitis C virus-induced hepatocellular carcinoma), with p-value of 1.36E-9, and 57 patients samples were used for this analysis; LUAD (lung adenocarcinoma): series GSE19188 (title: Gene expression-based classification of non-small cell lung carcinomas and survival prediction), with p-value of 0.002, and 110 patients samples were used for this analysis; LUSC (lung squamous cell carcinoma): series GSE19188 (title: Gene expression-based classification of non-small cell lung carcinomas and survival prediction), with p-value of 5.18E-9, and 92 patients samples were used for this analysis; BLCA (bladder urothelial carcinoma): series GSE13507 (title: Expression signature of E2F1 and its associated genes predict superficial to invasive progression of bladder tumors), with p-value of 0.035, and 130 patients samples were used for this analysis; BRCA (breast invasive carcinoma): series GSE9014 (Title: Stromal gene expression predicts clinical outcome in breast cancer), with p-value of 3.54E-7, and 59 patients samples were used for this analysis; HNSC (head-and-neck squamous cell carcinoma): series GSE7410 (title: Gene expression in early stage cervical cancer), with p-value of 0.908, and 54 patients samples were used for this analysis; UCEC (uterine corpus endometrial carcinoma): series GSE19188 (title: Fibroblast growth factor 9 has oncogenic activity and is a downstream target of Wnt signaling in ovarian endometrioid adenocarcinomas), with p-value of 0.067, and 45 patients samples were used for this analysis; PRAD (prostate adenocarcinoma): series GSE21034 (title: Whole-transcript and exon-level expression data for human primary and metastatic prostate cancer samples and control normal adjacent benign prostate), with p-value of 3.71E-12, and 160 patients samples were used for this analysis; STES (esophageal carcinoma): series GSE13898 (title: Robust prognostic biomarkers for EAC identified by systems-level characterization of tumor transcriptome), with p-value of 6.58E-9, and 40 patients samples were used for this analysis. We queried oncomine for each of those specific cancers to compare the fold change of TRPV4 in cancer tissue cells with normal tissue cells. All p-values represent a Student’s t test
Role of TRPV4 in cell proliferation and differentiation
Proliferation and differentiation are two basic cell biological events. It is well-accepted that cancer cells require unlimited replicative potential to generate macroscopic tumors81. This capability is different from the behaviors of most normal cell lineages which have limited number of successive growth-and-division cycles82. Most cancer cells have been considered immortal after transformation from normal cells, as the starting point of tumorigenesis. TRPV4 may dominate in the process of proliferation, and it’s upregulation is strongly linked to the proliferation of hepatic stellate cells (HSCs). Song et al. reported that the TRPV4 mRNA and protein expression levels of rat HSC-T6 cell line after treatment with transforming growth factor β1 significantly exceeded those of control group. However, TGF-β1-induced HSC-T6 cell proliferation was inhibited by Ruthenium Red (a nonspecific inhibitor of TRPV4) or synthetic siRNA targeting TRPV483. When TRPV4 activated, HSCs can be transformed into myofibroblasts which in turn secrete a large amount of collagen, leading to liver fibrosis, damage to liver tissue structure, cirrhosis, and eventually liver cancer84,85. Accordingly, increased expression of TRPV4 can indirectly induce liver cancer by stimulating the proliferation of HSCs. Functionally expressed in oligodendrocyte precursor cells (OPCs), TRPV4 can increase their proliferation. Ohashi et al. detected TRPV4 mRNA expressions in OPCs in vivo and primary cultured rat OPCs. Stimulating TRPV4 by GSK1016790A augmented OPC proliferation, which was abolished by co-treatment with HC-06704786. Given that OPCs are the origin of malignant glioma cells, overexpression or overactivation of TRPV4 may exert evident effects on the development of malignancies. Huang et al. showed that the overactivation of TRPV4 promoted the proliferation and/or migration of esophageal squamous cell carcinoma87. In addition, TRPV4 activation may selectively inhibits tumor endothelial cell proliferation via inhibition of ERK1/2 phosphorylation25. Studies have also shown that calcium-sensing receptor (CaSR) and TRPV4 were colocalized in gastric cancer cells, and CaSR activation evoked TRPV4-mediated Ca2+ entry promote gastric cancer cells proliferation88.
If a gene cannot be specifically expressed during maturation, the differentiation process is inhibited, which is primarily responsible for tumorigenesis. The growth and differentiation of keratinocytes are affected by intracellular and extracellular Ca2+ concentrations. When the extracellular Ca2+ concentration is low, primary keratinocytes remain undifferentiated. Conversely, cell proliferation is suppressed and differentiation is thus facilitated in the presence of high-concentration Ca2+89. TRPV4 is highly expressed in healthy or inflamed skin, whereas lowly or even not expressed in precancerous lesions and nonmelanoma skin cancer. In human keratinocytes, activation of TRPV4 stimulates the release of IL-8, which in turn downregulates TRPV4 expression90,91. Hypothetically, low expression of TRPV4 in skin cancer affects the release of ATP and autocrine communication between keratinocytes by regulating Ca2+ homeostasis, then decreasing extracellular Ca2+ concentration, keeping cells intact and ultimately inducing tumor formation91. In summary, TRPV4 plays an important role in the proliferation and differentiation of cells, which further affects the progression of cancer.
Role of TRPV4 in cell apoptosis
In multicellular organisms, the total number of cells is delicately balanced by the cell-generating effects of mitosis and cell death induced through apoptosis, which, when disrupted, results in cancer progression92. As a self-monitoring mechanism of organisms for hyperproliferative cells, apoptosis is also an effective means of tumor treatment. TRPV4 can inhibit the expressions of apoptotic proteins via a variety of (direct and indirect) pathways, and plays an indispensable role in suppressing apoptosis. Knockdown of TRPV4 expression by siRNA or pharmacological inhibition of TRPV4 can attenuate neuronal apoptosis18. Nonetheless, overactivation of TRPV4 can induce apoptosis. Activation of TRPV4 by GSK1016790A induces apoptosis by downregulating PI3K/Akt and upregulating p38 MAPK signaling pathways93. TRPV4 is a nonselective cation channel that works mainly based on channel switches. Zhan et al. treated HSC-T6 cells with 4α-phorbol 12,13-didecanoate (a TRPV4 activator) which then suppressed apoptosis and enhanced autophagy94. There are existing insights suggested substantial cytotoxicity of TRPV4 overactivation. GSK1016790A can cause strong calcium-overload and cellular disarrangement, increased the rate of apoptosis, and strongly inhibited human melanoma cell lines (A375, SK-MEL-28, MKTBR) proliferation/survival, similarly in HaCaT keratinocytes90, also in breast cancer cell line MDA-MB-468 pharmacological activation of TRPV4 produced pronounced cell death through apoptosis and oncosis27. It can be seen that activation of TRPV4 can induce the occurrence of apoptosis from above results. Classical calcium signaling pathway has manifested that calcium influx can regulate apoptosis by regulating caspase signaling pathway which was regulated by calpain. This may also be the main mechanism of TRPV4 in apoptosis.
Role of TRPV4 in tumor metastasis
It is well-documented that the expression level of TRPV4 in tumor cells was positively correlated with their metastatic ability. Lee et al. performed high-throughput sequencing for isogenic breast cancer cell lines, and found that the expression levels of TRPV4 in 4T07 and 4T1 cells which were prone to exudation and spread during metastasis were abnormally elevated95. Fusi et al. detected the expression of TRPV4 by immunohistochemical assay and found that the TRPV4 in weakly metastatic squamous cell carcinoma and basal cell epithelial cancer were significantly lower than that of normal skin tissue, whereas the expression in strongly metastatic malignant melanoma was higher than that of normal tissue89. Mrkonjić et al. transfected HEK293 cells with TRPV4. As a result, the cell migration ability was significantly enhanced compared with that of the blank control group. After transfecting with TRPV4 lacking a phosphoinositide-binding site, TRPV4 of HEK293 cell opens continuously which allowing further augmentation of cell motility and directionality96. Tumor-related death has mainly been attributed to metastasis97. Taken together, TRPV4 is abnormally highly expressed in many tumors which significantly correlated with their metastasis potential. Hence, it may be a therapeutic target for inhibiting tumor metastasis.
Influence of TRPV4 on tumor metastasis and possible mechanism
Regardless of significant advances achieved in research, diagnosis, and treatment of cancers, the vast majority of advanced metastatic cases, with rare exception, cannot be cured by current regimens. Being closely related to the prognosis of tumor patients, TRPV4 is probably one of the main participants in tumor metastasis. Therefore, it is of great significance to clarify the mechanism of TRPV4 in tumor metastasis.
TRPV4 promotes epithelial–mesenchymal transition (EMT)
EMT confers on tumor cells properties that are critical to invasion and metastatic dissemination, notably increased motility, invasiveness, and degradation of components of the extracellular matrix. The cells are transformed into spindle-shaped fibroblast-like ones, accompanied by enhanced motility98. TRPV4 can mediate the occurrence of EMT. E-cadherin is a key inhibitor of neonatal epithelial tumor cell movement and invasion, thus the decreased expression of E-cadherin is considered to be a significant marker of EMT99. TRPV4 is highly expressed in bladder cancer cells, which, when suppressed, can significantly downregulate the expression of E-cadherin. Meanwhile, inhibiting TRPV4 can induce the activation of AKT and FAK to further alter the expression level of E-cadherin100. Cell motility enhancement is typified by cytoskeletal protein remodeling, also as a crucial feature of EMT101,102. In mammal cells, TRPV4 and cytoskeletal protein actin have extensive colocalized expression. Breast cancer cells in which TRPV4 is activated undergo cytoskeletal remodeling. As an upstream “signal emitter”, TRPV4 regulates not only microtubule–microfilament polymerization but also the dynamic changes of microvilli, filopodia, and slab pseudopods, thereby affecting cell motility103. The release of many factors in the tumor microenvironment, such as epidermal growth factor (EGF), tumor necrosis factor-α (TNF-α), and signal transducer and activator of transcription 3 (STAT3), can induce the occurrence of tumor EMT, and these factors are regulated by intracellular calcium signaling104. Calcium ion chelators can inhibit the activation of EGF transduction signals and STAT3 in breast cancer cells105. Furthermore, the levels of calcium ions in tumor epithelioid cells and mesenchymal cells are different62. Raising calcium concentration can induce EMT of breast cancer MDA-MB-468 cells, probably by mediating their morphological changes105. Using the specific antagonist HC-067047 to inhibit TRPV4 in hepatocellular carcinoma cells suppressed cell proliferation, induced apoptosis, and decreased the migration capability by attenuating the EMT process in vitro and intraperitoneal injection of HC-067047 could obviously suppress tumor growth in NOD-SCID mouse xenograft models106. Since TRPV4 is an essential ion channelregulating cell morphology, it may predominantly regulate EMT. TRPV4-mediated calcium signaling participates in the EMT process via multiple signaling pathways, including the Wnt/β-catenin and PI3K/AKT pathway. As to gastric cancer metastasis, TRPV4 can enhance PI3K/AKT activity upon activation, induce β-catenin to enter the nucleus, and activate EMT by interacting with nuclear transcription factor T lymphocyte factor/lymphoid enhancer88.
TRPV4 promotes expressions of tumor metastasis-associated proteins
The promotive effects of TRPV4 on the EMT process have been elucidated above. When TRPV4 is continuously activated, the expressions of genes that facilitate tumor metastasis increase, but those of genes that inhibit metastasis reduce. Lee et al. found that the expressions of cell adhesion-associated tumor suppressor genes in mouse breast cancer cell line 4T07, such as Fn1, Clu, Tubb2c, and Spp1, decreased after administration with TRPV4 agonist 4α-PDD. At the same time, the expression of tumor metastasis-promoting gene Talin secreted by exosomes increased. The Kaplan–Meier survival analysis of 4142 patients with breast cancer revealed a significant positive correlation between Talin gene expression and lymphatic metastasis95. In the meantime, tumor cells released metastasis-promoting factors through exosomes to mediate tumor metastasis28. As the main histological barrier of tumor metastasis, ECM comprises collagen, glycoprotein, proteoglycan, and other components107. TRPV4 seems to be colocalized with F-actin in highly dynamic membrane structures, such as filopodia, microvilli, and lamellipodia edges. The interaction between TRPV4 and F-actin may be necessary for the activation of TRPV4 by hypotonic cell swelling, and disrupting the F-actin structure abolishes TRPV4-actin co-localization and leads to the loss of hypotonicity-induced Ca2+ influx and RVD108. Moreover, matrix metalloproteinases (MMPs) are important enzymes for the degradation of ECM109. Villalta et al. evaluated the effects of TRPV4 on mouse brain edema, and found that the expression of MMP2/MMP9 in the hippocampus was significantly inhibited by using HC-067046, a TRPV4 antagonist110. Furthermore, MMP2/MMP9 is associated with the metastasis of many types of cancers such as lung cancer111. Microtubule-associated protein 7 (MAP7) interacts with residues 798–809 at the C-terminus of TRPV4, possibly by increasing the expression of TRPV4 in the plasma membrane and linking the channel to cytoskeletal microtubules, forming a mechanosensitive molecular complex112. VPAC1, a member of the G protein-coupled receptor (GPCR) superfamily, is mainly activated by vasoactive intestinal peptide (VIP). Previous studies reveal that VPAC1 activation promotes migration and invasion of GC cells through TRPV4 channel-dependent Ca2+ entry, which in turn augments VIP expression. VIP significantly increased the lung metastasis of gastric cancer cells in vivo through the VPAC1/TRPV4/Ca2+ signaling axis113. In vivo angiogenesis assay demonstrated that TRPV4 regulates tumor vessel integrity by maintaining VE-cadherin expression at cell–cell contacts114.
Regulatory effects of TRPV4 on tumor angiogenesis
Access to the host vascular system and generation of tumor blood supply are important processes in tumor progression. A large number of new blood vessels not only provide sufficient nutrients for promoting the continuous growth of tumors but also drive tumor spread and metastasis115. TRPV4 is critical for tumor angiogenesis, but it may affect various tumors differently. Under normal conditions, activation of TRPV4-mediated Ca2+ entry is translated into a pro-angiogenic signal by several decoders, such as the Ca2+-dependent nuclear factor of activated T cells, cytoplasmic 1 (NFATc1), myocyte enhancer factor 2C (MEF2C), and Kv channel interacting protein 3, calsenilin (KCNIP3/CSEN/DREAM), which drive endothelial cell proliferation, β-integrin, and PI3K, which promote endothelial cell motility116. When TRPV4 expression is subjected to interference with shRNA or an inhibitor is used, retinal vascular cells fail to form tubules, indicating that TRPV4 significantly participates in retinal vascular endothelial cell migration and tubule formation117. The functions of TRPV4 protein from breast cancer-derived endothelial cells are similar to those of normal endothelial cells. Activating TRPV4 by using arachidonic acid can significantly promote the migration of endothelial cells derived from human breast cancer, but not that of normal human microvascular endothelial cells, which may be ascribed to the significantly higher expression of TRPV4 in breast cancer. As a result, intracellular calcium increased to promote endothelial cell migration. In contrast, loss of TRPV4 expression inhibits arachidonic acid-induced breast cancer cell migration118. For lung cancer and prostate cancer, the expression levels of TRPV4 in tumor-derived endothelial cells are lower than those of normal endothelial cells, thus elevating the sensitivity of endothelial cells to extracellular matrix stiffness and being conducive to the formation of abnormal blood vessels29. In TRPV4 knockout mice, the density and diameter of new tumor blood vessels enlarge, and the coverage of surrounding tumor capillary endothelial cells shrinks. Therefore, the formation of blood vessels is “abnormalized” to further promote the progression of lung cancer. On the contrary, activating TRPV4 with GSK1016790A can “normalize” the vascular endothelium, enhance the permeability of chemotherapeutic drugs, and ultimately reduce the exudation of cancer cells and block tumor growth119. With the study mentioned before, these results demonstrate the tumor angiogenesis regulatory role of TRPV4. In short, inhibition of tumor angiogenesis by targeting TRPV4 should be based on a thorough understanding of the organ specificity of the tumor microenvironment. Nevertheless, the underlying mechanisms require further in-depth studies.
TRPV4 interacts with tumor microenvironment to promote tumor metastasis
The tumor microenvironment is characterized by hypoxia, low pH, and high tissue pressure, often accompanied by inflammatory reactions, which preferentially selects more invasive and aggressive tumor cells and also impedes the tumor-killing action of immune cells120. Proinflammatory cytokines in the inflammatory microenvironment recruit and hijack immune cells to aid the immune escape of tumor cells121. These inflammatory factors also stimulate blood vessels and lymphatic vessels to change their permeability to increase protein and cell leakage, ultimately contributing to tumor metastasis122. TRPV4 is crucial throughout inflammation. At the initial stage of inflammation, cathepsin produced by the microenvironment of tumor inflammation can simultaneously activate TRPV4 and proteinase 2 which then synergize to continue the development of inflammatory response123. Meanwhile, TRPV4 is an essential effector protein that mediates inflammation and signal transduction. Inflammatory factors released in the tumor microenvironment, such as IL-1, IL-8, arachidonic acid, and phosphatase A2, as well as interstitial plasticity changes can promote opening of the tumor TRPV4, at which point the inflammatory factor release is reinforced by cascade amplification. Walter et al. reported that the expressions of inflammatory factors IL-1β, IL-6, and IL-8 decreased when intervertebral disc cells were inhibited by TRPV4124. Besides, Kim et al. found that inflammatory factors, such as TNF-α and IL-6, promoted the metastasis of Lewis lung cancer by increasing toll-like receptor 2 (TLR2) and its community toll-like receptor 6 (TLR6)125. When inflammatory response occurs, inflammatory factors recruit immune cells, and activation of the TRPV4 channel therein enables tumor cells to escape from the immune system. Scheraga et al. verified that activating the TRPV4 in bone marrow-derived macrophages was beneficial to the release of inflammatory factors, such as IL-1β and IL-10. Furthermore, activation of the TRPV4 of T cells at the site of inflammation facilitates the release of interferon-ɣ which is also an important mediator of tumor immune escape126. Collectively, targeting TRPV4 can simultaneously act on tumor cells and their “hijacked” immune cells, reduce the production of inflammatory factors, and finally ameliorate the inflammatory microenvironment for tumor metastasis.
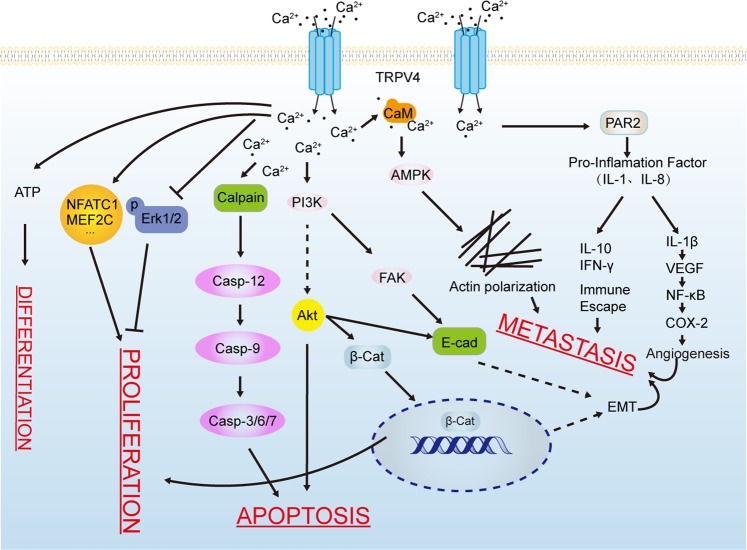
Conclusion
In summary, this review clarified the roles of TRPV4 in tumor onset, progression, and metastasis together with the mechanisms, verifying its potential antitumor effects. The specific process of TRPV4 acting on tumor occurrence and development and its possible mechanism is shown in Fig. 3. It is explicitly that the expression and function of TRPV4 are closely related to the occurrence and development of tumors. Hence, TRPV4 is worthy of extensive research for the diagnosis, treatment, and prognosis of tumors. In recent years, the remarkable effects of calcium signal and relative channel protein on the progression of tumors have gradually been recognized. Cancer therapy can be improved by evaluating channel protein expression feature of different tumors then rationally selecting specific antagonists or agonists. Although TRPV4 inhibitors have been employed to treat various diseases such as pulmonary edema and heart failure, there are no commercially available low-toxic and efficient drugs hitherto. Furthermore, the functions of TRPV4 in tumors varied depending on the original tissue type. TRPV4 regulates cellular function by modulating calcium signaling, and finally participates in tumor onset and progression. As summarized above, TRPV4 is closely related with proliferation, differentiation, apoptosis, and migration of tumor cell by regulation of Ca2+ and its downstream, then finally participates in tumor onset and progression. Consequently, TRPV4 could be a potential therapeutic target of cancer treatment, thereby providing a new direction that develop drugs for cancer to reach the clinic. Regardless of burgeoning studies on the roles of TRPV4 in tumors, its differential expressions in different tumor tissues and the underlying mechanisms are still largely unknown. Therefore, developing antitumor drug targeting TRPV4 remains rather challenging. In addition, targeting TRPV4 channels may also affect other stromal cells, so it is imperative to assess the overall biological effects before possible clinical use.
Fig. 3. The specific process of TRPV4 acting on tumor occurrence and development and its possible mechanism.
TRPV4 is closely related with the proliferation, differentiation, apoptosis, and migration of tumor cell by regulation of Ca2+ and production of isoforms, and finally participates in tumor onset and progression
Acknowledgements
The project was partially supported by the National Natural Science Foundation of China (81573859, 81673725, 81673648, 81673795).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by M. Piacentini
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Suyun Yu, Shuai Huang, Yushi Ding
References
- 1.Lai Y, et al. Current status and perspectives of patient-derived xenograft models in cancer research. J. Hematol. Oncol. 2017;10:106. doi: 10.1186/s13045-017-0470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ethun CG, et al. Frailty and cancer: implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J. Clin. 2017;67:362–377. doi: 10.3322/caac.21406. [DOI] [PubMed] [Google Scholar]
- 3.Schrank Zachary, Chhabra Gagan, Lin Leo, Iderzorig Tsatsral, Osude Chike, Khan Nabiha, Kuckovic Adijan, Singh Sanjana, Miller Rachel, Puri Neelu. Current Molecular-Targeted Therapies in NSCLC and Their Mechanism of Resistance. Cancers. 2018;10(7):224. doi: 10.3390/cancers10070224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pang Xuehui, Cui Cheng, Wan Shuo, Jiang Ying, Zhang Liangliang, Xia Lian, Li Long, Li Xiaowei, Tan Weihong. Bioapplications of Cell-SELEX-Generated Aptamers in Cancer Diagnostics, Therapeutics, Theranostics and Biomarker Discovery: A Comprehensive Review. Cancers. 2018;10(2):47. doi: 10.3390/cancers10020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yewale C, Baradia D, Vhora I, Patil S, Misra A. Epidermal growth factor receptor targeting in cancer: a review of trends and strategies. Biomaterials. 2013;34:8690–8707. doi: 10.1016/j.biomaterials.2013.07.100. [DOI] [PubMed] [Google Scholar]
- 6.Leanza L, Manago A, Zoratti M, Gulbins E, Szabo I. Pharmacological targeting of ion channels for cancer therapy: In vivo evidences. Biochim. Biophys. Acta. 2016;1863:1385–1397. doi: 10.1016/j.bbamcr.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 7.Kale VP, Amin SG, Pandey MK. Targeting ion channels for cancer therapy by repurposing the approved drugs. Biochim. Biophys. Acta. 2015;1848:2747–2755. doi: 10.1016/j.bbamem.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Biasiotta A, D’Arcangelo D, Passarelli F, Nicodemi EM, Facchiano A. Ion channels expression and function are strongly modified in solid tumors and vascular malformations. J. Transl. Med. 2016;14:285. doi: 10.1186/s12967-016-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Arcangelo Daniela, Scatozza Francesca, Giampietri Claudia, Marchetti Paolo, Facchiano Francesco, Facchiano Antonio. Ion Channel Expression in Human Melanoma Samples: In Silico Identification and Experimental Validation of Molecular Targets. Cancers. 2019;11(4):446. doi: 10.3390/cancers11040446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuszynski J, Tilli TM, Levin M. Ion channel and neurotransmitter modulators as electroceutical approaches to the control of cancer. Curr. Pharm. Des. 2017;23:4827–4841. doi: 10.2174/1381612823666170530105837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardo LA, Stuhmer W. The roles of K(+) channels in cancer. Nat. Rev. Cancer. 2014;14:39–48. doi: 10.1038/nrc3635. [DOI] [PubMed] [Google Scholar]
- 12.Chantome A, et al. Pivotal role of the lipid Raft SK3-Orai1 complex in human cancer cell migration and bone metastases. Cancer Res. 2013;73:4852–4861. doi: 10.1158/0008-5472.CAN-12-4572. [DOI] [PubMed] [Google Scholar]
- 13.Davis GC, et al. Asymmetric synthesis and evaluation of a hydroxyphenylamide voltage-gated sodium channel blocker in human prostate cancer xenografts. Bioorg. Med. Chem. 2012;20:2180–2188. doi: 10.1016/j.bmc.2011.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin C, et al. Inhibition of metastatic tumor growth and metastasis via targeting metastatic breast cancer by chlorotoxin-modified liposomes. Mol. Pharm. 2014;11:3233–3241. doi: 10.1021/mp400691z. [DOI] [PubMed] [Google Scholar]
- 15.Almasi S, et al. TRPM2 ion channel promotes gastric cancer migration, invasion and tumor growth through the AKT signaling pathway. Sci. Rep. 2019;9:4182. doi: 10.1038/s41598-019-40330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raphael M, et al. TRPV6 calcium channel translocates to the plasma membrane via Orai1-mediated mechanism and controls cancer cell survival. Proc. Natl Acad. Sci. USA. 2014;111:E3870–E3879. doi: 10.1073/pnas.1413409111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phan MN, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60:3028–3037. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi M, et al. Glial cell-expressed mechanosensitive channel TRPV4 mediates infrasound-induced neuronal impairment. Acta Neuropathol. 2013;126:725–739. doi: 10.1007/s00401-013-1166-x. [DOI] [PubMed] [Google Scholar]
- 19.Seth RK, et al. TRPV4 activation of endothelial nitric oxide synthase resists nonalcoholic fatty liver disease by blocking CYP2E1-mediated redox toxicity. Free Radic. Biol. Med. 2017;102:260–273. doi: 10.1016/j.freeradbiomed.2016.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vergnolle N. TRPV4: new therapeutic target for inflammatory bowel diseases. Biochem. Pharmacol. 2014;89:157–161. doi: 10.1016/j.bcp.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Mamenko MV, et al. The renal TRPV4 channel is essential for adaptation to increased dietary potassium. Kidney Int. 2017;91:1398–1409. doi: 10.1016/j.kint.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deruyver Y, et al. Intravesical activation of the cation channel TRPV4 improves bladder function in a rat model for detrusor underactivity. Eur. Urol. 2018;74:336–345. doi: 10.1016/j.eururo.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Lyons James S., Joca Humberto C., Law Robert A., Williams Katrina M., Kerr Jaclyn P., Shi Guoli, Khairallah Ramzi J., Martin Stuart S., Konstantopoulos Konstantinos, Ward Christopher W., Stains Joseph P. Microtubules tune mechanotransduction through NOX2 and TRPV4 to decrease sclerostin abundance in osteocytes. Science Signaling. 2017;10(506):eaan5748. doi: 10.1126/scisignal.aan5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benfenati V, et al. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc. Natl Acad. Sci. USA. 2011;108:2563–2568. doi: 10.1073/pnas.1012867108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thoppil RJ, et al. TRPV4 channel activation selectively inhibits tumor endothelial cell proliferation. Sci. Rep. 2015;5:14257. doi: 10.1038/srep14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H, Caterina MJ. TRPV channels as thermosensory receptors in epithelial cells. Pflugers Arch. 2005;451:160–167. doi: 10.1007/s00424-005-1438-y. [DOI] [PubMed] [Google Scholar]
- 27.Peters AA, et al. Oncosis and apoptosis induction by activation of an overexpressed ion channel in breast cancer cells. Oncogene. 2017;36:6490–6500. doi: 10.1038/onc.2017.234. [DOI] [PubMed] [Google Scholar]
- 28.Lee WH, et al. TRPV4 plays a role in breast cancer cell migration via Ca(2+) -dependent activation of AKT and downregulation of E-cadherin cell cortex protein. Oncogenesis. 2017;6:e338. doi: 10.1038/oncsis.2017.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thoppil RJ, et al. TRPV4 channels regulate tumor angiogenesis via modulation of Rho/Rho kinase pathway. Oncotarget. 2016;7:25849–25861. doi: 10.18632/oncotarget.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol. Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 31.Harteneck C, Plant TD, Schultz G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–166. doi: 10.1016/S0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- 32.Venkatachalam K, Montell C. TRP channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harper AG, Sage SO. TRP-Na(+)/Ca(2+) exchanger coupling. Adv. Exp. Med. Biol. 2016;898:67–85. doi: 10.1007/978-3-319-26974-0_4. [DOI] [PubMed] [Google Scholar]
- 34.Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacological Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- 35.Gees M, Owsianik G, Nilius B, Voets T. TRP channels. Compr. Physiol. 2012;2:563–608. doi: 10.1002/cphy.c110026. [DOI] [PubMed] [Google Scholar]
- 36.Chubanov V, Mittermeier L, Gudermann T. Role of kinase-coupled TRP channels in mineral homeostasis. Pharmacol. Ther. 2018;184:159–176. doi: 10.1016/j.pharmthera.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, et al. TRPM7 is required for normal synapse density, learning, and memory at different developmental stages. Cell Rep. 2018;23:3480–3491. doi: 10.1016/j.celrep.2018.05.069. [DOI] [PubMed] [Google Scholar]
- 38.Monteilh-Zoller MK, et al. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banner KH, Igney F, Poll C. TRP channels: emerging targets for respiratory disease. Pharmacol. Ther. 2011;130:371–384. doi: 10.1016/j.pharmthera.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- 41.Chuang HH, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 42.Clapham DE. SnapShot: mammalian TRP channels. Cell. 2007;129:220. doi: 10.1016/j.cell.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 43.Inoue M, Fujita T, Goto M, Kumamoto E. Presynaptic enhancement by eugenol of spontaneous excitatory transmission in rat spinal substantia gelatinosa neurons is mediated by transient receptor potential A1 channels. Neuroscience. 2012;210:403–415. doi: 10.1016/j.neuroscience.2012.02.040. [DOI] [PubMed] [Google Scholar]
- 44.Moran MM, McAlexander MA, Biro T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 2011;10:601–620. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- 45.Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu. Rev. Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- 46.Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol. Ther. 2011;131:142–170. doi: 10.1016/j.pharmthera.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bertin S, Raz E. Transient receptor potential (TRP) channels in T cells. Semin. Immunopathol. 2016;38:309–319. doi: 10.1007/s00281-015-0535-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nilius B. TRP channels in disease. Biochim. Biophys. Acta. 2007;1772:805–812. doi: 10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Laing RJ, Dhaka A. ThermoTRPs and Pain. Neuroscientist. 2016;22:171–187. doi: 10.1177/1073858414567884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/S0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 51.Inoue R, Jian Z, Kawarabayashi Y. Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol. Ther. 2009;123:371–385. doi: 10.1016/j.pharmthera.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Pires PW, Earley S. No static at all: tuning into the complexities of Ca2+ signaling in the endothelium. Circ. Res. 2016;118:1042–1044. doi: 10.1161/CIRCRESAHA.116.308519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aarts M, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/S0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 54.Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron. 2006;52:485–496. doi: 10.1016/j.neuron.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 55.Togashi K, et al. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. 2006;25:1804–1815. doi: 10.1038/sj.emboj.7601083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim BJ, Hong C. Role of transient receptor potential melastatin type 7 channel in gastric cancer. Integr. Med. Res. 2016;5:124–130. doi: 10.1016/j.imr.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sousa D, Lima RT, Vasconcelos MH. Intercellular transfer of cancer drug resistance traits by extracellular vesicles. Trends Mol. Med. 2015;21:595–608. doi: 10.1016/j.molmed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Chen Z, et al. Overexpression of TrpC5 promotes tumor metastasis via the HIF-1alpha-Twist signaling pathway in colon cancer. Clin. Sci. (Lond) 2017;131:2439–2450. doi: 10.1042/CS20171069. [DOI] [PubMed] [Google Scholar]
- 59.Liedtke W, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/S0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 61.Kang SS, Shin SH, Auh CK, Chun J. Human skeletal dysplasia caused by a constitutive activated transient receptor potential vanilloid 4 (TRPV4) cation channel mutation. Exp. Mol. Med. 2012;44:707–722. doi: 10.3858/emm.2012.44.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White JP, et al. TRPV4: molecular conductor of a diverse orchestra. Physiol. Rev. 2016;96:911–973. doi: 10.1152/physrev.00016.2015. [DOI] [PubMed] [Google Scholar]
- 63.Voets T, et al. Molecular determinants of permeation through the cation channel TRPV4. J. Biol. Chem. 2002;277:33704–33710. doi: 10.1074/jbc.M204828200. [DOI] [PubMed] [Google Scholar]
- 64.Watanabe H, et al. Modulation of TRPV4 gating by intra- and extracellular Ca2. Cell Calcium. 2003;33:489–495. doi: 10.1016/S0143-4160(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 65.Xu H, Fu Y, Tian W, Cohen DM. Glycosylation of the osmoresponsive transient receptor potential channel TRPV4 on Asn-651 influences membrane trafficking. Am. J. Physiol. Renal. Physiol. 2006;290:F1103–F1109. doi: 10.1152/ajprenal.00245.2005. [DOI] [PubMed] [Google Scholar]
- 66.Vincent F, Duncton MA. TRPV4 agonists and antagonists. Curr. Top Med. Chem. 2011;11:2216–2226. doi: 10.2174/156802611796904861. [DOI] [PubMed] [Google Scholar]
- 67.Jie P, et al. Blockage of transient receptor potential vanilloid 4 inhibits brain edema in middle cerebral artery occlusion mice. Front. Cell Neurosci. 2015;9:141. doi: 10.3389/fncel.2015.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balakrishna S, et al. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;307:L158–L172. doi: 10.1152/ajplung.00065.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ryskamp DA, et al. TRPV4 regulates calcium homeostasis, cytoskeletal remodeling, conventional outflow and intraocular pressure in the mammalian eye. Sci. Rep. 2016;6:30583. doi: 10.1038/srep30583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karasawa T, Wang Q, Fu Y, Cohen DM, Steyger PS. TRPV4 enhances the cellular uptake of aminoglycoside antibiotics. J. Cell Sci. 2008;121:2871–2879. doi: 10.1242/jcs.023705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Randhawa PK, Jaggi AS. TRPV4 channels: physiological and pathological role in cardiovascular system. Basic Res. Cardiol. 2015;110:54. doi: 10.1007/s00395-015-0512-7. [DOI] [PubMed] [Google Scholar]
- 72.Goldenberg NM, Ravindran K, Kuebler WM. TRPV4: physiological role and therapeutic potential in respiratory diseases. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:421–436. doi: 10.1007/s00210-014-1058-1. [DOI] [PubMed] [Google Scholar]
- 73.Thorneloe KS, et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl) sulfonyl] amino} -3-hydroxypropanoyl)-1-piperazinyl] carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J. Pharmacol. Exp. Ther. 2008;326:432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- 74.Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol. Therapeut. 2011;131:142–170. doi: 10.1016/j.pharmthera.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Phelps CB, Wang RR, Choo SS, Gaudet R. Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J. Biol. Chem. 2010;285:731–740. doi: 10.1074/jbc.M109.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sokabe T, Fukumi-Tominaga T, Yonemura S, Mizuno A, Tominaga M. The TRPV4 channel contributes to intercellular junction formation in keratinocytes. J. Biol. Chem. 2010;285:18749–18758. doi: 10.1074/jbc.M110.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen G, Suzuki H, Weston AH. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br. J. Pharmacol. 1988;95:1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- 79.Thodeti CK, et al. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ. Res. 2009;104:1123–1130. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ye L, et al. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell. 2012;151:96–110. doi: 10.1016/j.cell.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 82.Buys CH. Telomeres, telomerase, and cancer. N. Engl. J. Med. 2000;342:1282–1283. doi: 10.1056/NEJM200004273421710. [DOI] [PubMed] [Google Scholar]
- 83.Song Y, et al. TRPV4 channel inhibits TGF-beta1-induced proliferation of hepatic stellate cells. PLoS ONE. 2014;9:e101179. doi: 10.1371/journal.pone.0101179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dou C, et al. P300 acetyltransferase mediates stiffness-induced activation of hepatic stellate cells into tumor-promoting myofibroblasts. Gastroenterology. 2018;154:2209–2221. doi: 10.1053/j.gastro.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sung YC, et al. Combined delivery of sorafenib and a MEK inhibitor using CXCR4-targeted nanoparticles reduces hepatic fibrosis and prevents tumor development. Theranostics. 2018;8:894–905. doi: 10.7150/thno.21168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ohashi K, et al. TRPV4 is functionally expressed in oligodendrocyte precursor cells and increases their proliferation. Pflugers Arch. 2018;470:705–716. doi: 10.1007/s00424-018-2130-3. [DOI] [PubMed] [Google Scholar]
- 87.Huang R, et al. Recurrent activations of transient receptor potential vanilloid-1 and vanilloid-4 promote cellular proliferation and migration in esophageal squamous cell carcinoma cells. FEBS Open Bio. 2019;9:206–225. doi: 10.1002/2211-5463.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xie R, et al. Calcium promotes human gastric cancer via a novel coupling of calcium-sensing receptor and TRPV4 channel. Cancer Res. 2017;77:6499–6512. doi: 10.1158/0008-5472.CAN-17-0360. [DOI] [PubMed] [Google Scholar]
- 89.Fusi C, et al. Transient receptor potential vanilloid 4 (TRPV4) is downregulated in keratinocytes in human non-melanoma skin cancer. J. Invest. Dermatol. 2014;134:2408–2417. doi: 10.1038/jid.2014.145. [DOI] [PubMed] [Google Scholar]
- 90.Olivan-Viguera A, et al. Pharmacological activation of TRPV4 produces immediate cell damage and induction of apoptosis in human melanoma cells and HaCaT keratinocytes. PLoS ONE. 2018;13:e0190307. doi: 10.1371/journal.pone.0190307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ohsaki, A., Tanuma, S. I. & Tsukimoto, M. TRPV4 channel-regulated ATP release contributes to γ-irradiation-induced production of IL-6 and IL-8 in epidermal keratinocytes. Biol. Pharm. Bull.41, 2018. [DOI] [PubMed]
- 92.Cotter TG. Apoptosis and cancer: the genesis of a research field. Nature Rev. Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 93.Jie P, et al. Activation of transient receptor potential vanilloid 4 induces apoptosis in hippocampus through downregulating PI3K/Akt and upregulating p38 MAPK signaling pathways. Cell Death Dis. 2015;6:e1775. doi: 10.1038/cddis.2015.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhan L, et al. Transient receptor potential vanilloid 4 inhibits rat HSC-T6 apoptosis through induction of autophagy. Mol. Cell Biochem. 2015;402:9–22. doi: 10.1007/s11010-014-2298-6. [DOI] [PubMed] [Google Scholar]
- 95.Lee WH, et al. TRPV4 regulates breast cancer cell extravasation, stiffness and actin cortex. Sci. Rep. 2016;6:27903. doi: 10.1038/srep27903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mrkonjic S, et al. TRPV4 participates in the establishment of trailing adhesions and directional persistence of migrating cells. Pflugers Arch. 2015;467:2107–2119. doi: 10.1007/s00424-014-1679-8. [DOI] [PubMed] [Google Scholar]
- 97.Massague J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nature Rev. Cancer. 2018;18:128–134. doi: 10.1038/nrc.2017.118. [DOI] [PubMed] [Google Scholar]
- 99.Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell. 2003;4:499–515. doi: 10.1016/S1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- 100.Janssen DA, et al. The mechanoreceptor TRPV4 is localized in adherence junctions of the human bladder urothelium: a morphological study. J. Urol. 2011;186:1121–1127. doi: 10.1016/j.juro.2011.04.107. [DOI] [PubMed] [Google Scholar]
- 101.Mouneimne G, et al. Differential remodeling of actin cytoskeleton architecture by profilin isoforms leads to distinct effects on cell migration and invasion. Cancer Cell. 2012;22:615–630. doi: 10.1016/j.ccr.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morita T, Mayanagi T, Sobue K. Dual roles of myocardin-related transcription factors in epithelial mesenchymal transition via slug induction and actin remodeling. J. Cell Biol. 2007;179:1027–1042. doi: 10.1083/jcb.200708174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fiorio Pla A, et al. TRPV4 mediates tumor-derived endothelial cell migration via arachidonic acid-activated actin remodeling. Oncogene. 2012;31:200–212. doi: 10.1038/onc.2011.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 105.Davis FM, et al. Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene. 2014;33:2307–2316. doi: 10.1038/onc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fang Y, et al. Pharmacological inhibition of TRPV4 channel suppresses malignant biological behavior of hepatocellular carcinoma via modulation of ERK signaling pathway. Biomed. Pharmacother. 2018;101:910–919. doi: 10.1016/j.biopha.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 107.Afratis NA, Klepfish M, Karamanos NK, Sagi I. The apparent competitive action of ECM proteases and cross-linking enzymes during fibrosis: applications to drug discovery. Adv. Drug Deliv. Rev. 2018;129:4–15. doi: 10.1016/j.addr.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 108.Becker D, Bereiter-Hahn J, Jendrach M. Functional interaction of the cation channel transient receptor potential vanilloid 4 (TRPV4) and actin in volume regulation. Eur. J. Cell Biol. 2009;88:141–152. doi: 10.1016/j.ejcb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 109.Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10:415–433. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- 110.Villalta PC, Rocic P, Townsley MI. Role of MMP2 and MMP9 in TRPV4-induced lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;307:L652–L659. doi: 10.1152/ajplung.00113.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iizasa T, et al. Elevated levels of circulating plasma matrix metalloproteinase 9 in non-small cell lung cancer patients. Clin. Cancer Res. 1999;5:149–153. [PubMed] [Google Scholar]
- 112.Suzuki M, Hirao A, Mizuno A. Microtubule-associated [corrected] protein 7 increases the membrane expression of transient receptor potential vanilloid 4 (TRPV4) J. Biol. Chem. 2003;278:51448–51453. doi: 10.1074/jbc.M308212200. [DOI] [PubMed] [Google Scholar]
- 113.Tang Bo, Wu Jilin, Zhu Michael X., Sun Xuemei, Liu Jingjing, Xie Rui, Dong Tobias Xiao, Xiao Yufeng, Carethers John M., Yang Shiming, Dong Hui. VPAC1 couples with TRPV4 channel to promote calcium-dependent gastric cancer progression via a novel autocrine mechanism. Oncogene. 2019;38(20):3946–3961. doi: 10.1038/s41388-019-0709-6. [DOI] [PubMed] [Google Scholar]
- 114.Cappelli HC, et al. Mechanosensitive TRPV4 channels stabilize VE-cadherin junctions to regulate tumor vascular integrity and metastasis. Cancer Lett. 2019;442:15–20. doi: 10.1016/j.canlet.2018.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Folkman J. The role of angiogenesis in tumor growth. Semin. Cancer Biol. 1992;3:65–71. [PubMed] [Google Scholar]
- 116.Troidl C, et al. Calcium-dependent signalling is essential during collateral growth in the pig hind limb-ischemia model. J. Mol. Cell Cardiol. 2010;49:142–151. doi: 10.1016/j.yjmcc.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 117.Wen L, et al. TRPV4 regulates migration and tube formation of human retinal capillary endothelial cells. BMC Ophthalmol. 2018;18:38. doi: 10.1186/s12886-018-0697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fiorio Pla A, et al. Arachidonic acid-induced Ca2+ entry is involved in early steps of tumor angiogenesis. Mol. Cancer Res. 2008;6:535–545. doi: 10.1158/1541-7786.MCR-07-0271. [DOI] [PubMed] [Google Scholar]
- 119.Adapala RK, et al. Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene. 2016;35:314–322. doi: 10.1038/onc.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5:915–919. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Borsig L, Wolf MJ, Roblek M, Lorentzen A, Heikenwalder M. Inflammatory chemokines and metastasis–tracing the accessory. Oncogene. 2014;33:3217–3224. doi: 10.1038/onc.2013.272. [DOI] [PubMed] [Google Scholar]
- 123.Zhao P, et al. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J. Biol. Chem. 2014;289:27215–27234. doi: 10.1074/jbc.M114.599712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Walter BA, et al. Reduced tissue osmolarity increases TRPV4 expression and pro-inflammatory cytokines in intervertebral disc cells. Eur. Cell Mater. 2016;32:123–136. doi: 10.22203/eCM.v032a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim S, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Scheraga RG, et al. TRPV4 mechanosensitive ion channel regulates lipopolysaccharide-stimulated macrophage phagocytosis. J. Immunol. 2016;196:428–436. doi: 10.4049/jimmunol.1501688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang JB, Kindzelskii AL, Clark AJ, Petty HR. Identification of channels promoting calcium spikes and waves in HT1080 tumor cells: their apparent roles in cell motility and invasion. Cancer Res. 2004;64:2482–2489. doi: 10.1158/0008-5472.CAN-03-3501. [DOI] [PubMed] [Google Scholar]
- 130.Miller BA. TRPC2. Handb. Exp. Pharmacol. 2014;222:53–65. doi: 10.1007/978-3-642-54215-2_3. [DOI] [PubMed] [Google Scholar]
- 131.Tiapko, O. & Groschner, K. TRPC3 as a target of novel therapeutic interventions. Cells7, 83 (2018). [DOI] [PMC free article] [PubMed]
- 132.Gao J, Zeng K, Liu Y, Gao L, Liu L. LncRNA SNHG5 promotes growth and invasion in melanoma by regulating the miR-26a-5p/TRPC3 pathway. Onco. Targets Ther. 2019;12:169–179. doi: 10.2147/OTT.S184078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grayson TH, Murphy TV, Sandow SL. Transient receptor potential canonical type 3 channels: Interactions, role and relevance - A vascular focus. Pharmacol. Ther. 2017;174:79–96. doi: 10.1016/j.pharmthera.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 134.Cheung SY, et al. TRPC4/TRPC5 channels mediate adverse reaction to the cancer cell cytotoxic agent (-)-Englerin A. Oncotarget. 2018;9:29634–29643. doi: 10.18632/oncotarget.25659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rubaiy HN. Treasure troves of pharmacological tools to study transient receptor potential canonical 1/4/5 channels. Br. J. Pharmacol. 2019;176:832–846. doi: 10.1111/bph.14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ma X, et al. Essential role for TrpC5-containing extracellular vesicles in breast cancer with chemotherapeutic resistance. Proc. Natl Acad. Sci. USA. 2014;111:6389–6394. doi: 10.1073/pnas.1400272111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ma X, et al. Transient receptor potential channel TRPC5 is essential for P-glycoprotein induction in drug-resistant cancer cells. Proc. Natl Acad. Sci. USA. 2012;109:16282–16287. doi: 10.1073/pnas.1202989109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Diez-Bello R, et al. (−)Oleocanthal inhibits proliferation and migration by modulating Ca(2+) entry through TRPC6 in breast cancer cells. Biochim. Biophys. Acta. Mol. Cell Res. 2019;1866:474–485. doi: 10.1016/j.bbamcr.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 139.Zhang X, Spinelli AM, Masiello T, Trebak M. Transient receptor potential canonical 7 (TRPC7), a calcium (Ca(2+)) permeable non-selective cation channel. Adv. Exp. Med. Biol. 2016;898:251–264. doi: 10.1007/978-3-319-26974-0_11. [DOI] [PubMed] [Google Scholar]
- 140.Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat. Rev. Cancer. 2011;11:609–618. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 141.Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- 142.Monet M, et al. Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Res. 2010;70:1225–1235. doi: 10.1158/0008-5472.CAN-09-2205. [DOI] [PubMed] [Google Scholar]
- 143.Xu H, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 144.Li X, et al. Overexpression of TRPV3 correlates with tumor progression in non-small cell lung cancer. Int. J. Mol. Sci. 2016;17:437. doi: 10.3390/ijms17040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hope JM, Greenlee JD, King MR. Mechanosensitive ion channels: TRPV4 and P2X7 in disseminating cancer cells. Cancer J. 2018;24:84–92. doi: 10.1097/PPO.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen Y, et al. Vitamin D receptor suppresses proliferation and metastasis in renal cell carcinoma cell lines via regulating the expression of the epithelial Ca2+ channel TRPV5. PLoS ONE. 2018;13:e0195844. doi: 10.1371/journal.pone.0195844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.de Groot T, Bindels RJ, Hoenderop JG. TRPV5: an ingeniously controlled calcium channel. Kidney Int. 2008;74:1241–1246. doi: 10.1038/ki.2008.320. [DOI] [PubMed] [Google Scholar]
- 148.Wissenbach, U. & Niemeyer, B. A. TRPV6. Handb. Exp. Pharm.179, 221–234 (2007). [DOI] [PubMed]
- 149.Xue H, et al. Inhibition of transient receptor potential vanilloid 6 channel, elevated in human ovarian cancers, reduces tumour growth in a xenograft model. J. Cancer. 2018;9:3196–3207. doi: 10.7150/jca.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hantute-Ghesquier, A., Haustrate, A., Prevarskaya, N. & Lehen'kyi, V. TRPM family channels in Cancer. Pharmaceuticals (Basel, Switzerland)11, 58 (2018). [DOI] [PMC free article] [PubMed]
- 151.Tan CH, McNaughton PA. The TRPM2 ion channel is required for sensitivity to warmth. Nature. 2016;536:460–463. doi: 10.1038/nature19074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Held K, Voets T, Vriens J. TRPM3 in temperature sensing and beyond. Temperature (Austin) 2015;2:201–213. doi: 10.4161/23328940.2014.988524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hong X, Yu JJ. MicroRNA-150 suppresses epithelial-mesenchymal transition, invasion, and metastasis in prostate cancer through the TRPM4-mediated beta-catenin signaling pathway. Am. J. Physiol. Cell Physiol. 2019;316:C463–C480. doi: 10.1152/ajpcell.00142.2018. [DOI] [PubMed] [Google Scholar]
- 154.Schutz B, et al. Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front. Physiol. 2015;6:87. doi: 10.3389/fphys.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Maeda T, et al. TRPM5 mediates acidic extracellular pH signaling and TRPM5 inhibition reduces spontaneous metastasis in mouse B16-BL6 melanoma cells. Oncotarget. 2017;8:78312–78326. doi: 10.18632/oncotarget.20826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang Z, et al. N-Myc-induced up-regulation of TRPM6/TRPM7 channels promotes neuroblastoma cell proliferation. Oncotarget. 2014;5:7625–7634. doi: 10.18632/oncotarget.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bas E, Naziroglu M, Pecze L. ADP-Ribose and oxidative stress activate TRPM8 channel in prostate cancer and kidney cells. Sci. Rep. 2019;9:4100. doi: 10.1038/s41598-018-37552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang L, Barritt GJ. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004;64:8365–8373. doi: 10.1158/0008-5472.CAN-04-2146. [DOI] [PubMed] [Google Scholar]
- 159.Gkika D, Flourakis M, Lemonnier L, Prevarskaya N. PSA reduces prostate cancer cell motility by stimulating TRPM8 activity and plasma membrane expression. Oncogene. 2010;29:4611–4616. doi: 10.1038/onc.2010.210. [DOI] [PubMed] [Google Scholar]
- 160.Yee NS, Zhou W, Lee M. Transient receptor potential channel TRPM8 is over-expressed and required for cellular proliferation in pancreatic adenocarcinoma. Cancer Lett. 2010;297:49–55. doi: 10.1016/j.canlet.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Reczek CR, Chandel NS. ROS promotes cancer cell survival through calcium signaling. Cancer Cell. 2018;33:949–951. doi: 10.1016/j.ccell.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 162.Du GJ, et al. The combination of TRPM8 and TRPA1 expression causes an invasive phenotype in lung cancer. Tumour Biol. 2014;35:1251–1261. doi: 10.1007/s13277-013-1167-3. [DOI] [PubMed] [Google Scholar]
- 163.Xu M, et al. The lysosomal TRPML1 channel regulates triple negative breast cancer development by promoting mTORC1 and purinergic signaling pathways. Cell Calcium. 2019;79:80–88. doi: 10.1016/j.ceca.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fine M, Schmiege P, Li X. Structural basis for PtdInsP2-mediated human TRPML1 regulation. Nat. Commun. 2018;9:4192. doi: 10.1038/s41467-018-06493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li X, et al. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat. Cell Biol. 2016;18:404–417. doi: 10.1038/ncb3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Morelli, M. B. et al. Overexpression of transient receptor potential mucolipin-2 ion channels in gliomas: role in tumor growth and progression. Oncotarget. 7, 43654–43668 (2016). [DOI] [PMC free article] [PubMed]
- 167.Di Paola S, Scotto-Rosato A, Medina DL. TRPML1: the Ca((2+))retaker of the lysosome. Cell Calcium. 2017;69:112. doi: 10.1016/j.ceca.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 168.Kim HJ, Soyombo AA, Tjon-Kon-Sang S, So I, Muallem S. The Ca(2+) channel TRPML3 regulates membrane trafficking and autophagy. Traffic. 2009;10:1157–1167. doi: 10.1111/j.1600-0854.2009.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang X, et al. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wu K, et al. TRPP2 enhances metastasis by regulating epithelial-mesenchymal transition in laryngeal squamous cell carcinoma. Cell. Physiol. Biochem. 2016;39:2203–2215. doi: 10.1159/000447914. [DOI] [PubMed] [Google Scholar]
- 171.Bai CX, et al. Activation of TRPP2 through mDia1-dependent voltage gating. EMBO J. 2008;27:1345–1356. doi: 10.1038/emboj.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Park EYJ, Kwak M, Ha K, So I. Identification of clustered phosphorylation sites in PKD2L1: how PKD2L1 channel activation is regulated by cyclic adenosine monophosphate signaling pathway. Pflugers Arch. 2017;470:505–516. doi: 10.1007/s00424-017-2095-7. [DOI] [PubMed] [Google Scholar]
- 173.Lu Z, et al. Deficiency of PKD2L1 (TRPP3) exacerbates pathological cardiac hypertrophy by augmenting NCX1-mediated mitochondrial calcium overload. Cell Rep. 2018;24:1639–1652. doi: 10.1016/j.celrep.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 174.Semmo M, Köttgen M, Hofherr A. The TRPP subfamily and polycystin-1 proteins. Handb. Exp. Pharmacol. 2014;222:675–711. doi: 10.1007/978-3-642-54215-2_27. [DOI] [PubMed] [Google Scholar]
- 175.Watanabe H, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J. Biol. Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 176.Xu F, Satoh E, Iijima T. Protein kinase C-mediated Ca2+ entry in HEK 293 cells transiently expressing human TRPV4. Br. J. Pharmacol. 2003;140:413–421. doi: 10.1038/sj.bjp.0705443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vriens J, et al. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl Acad. Sci. USA. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Klausen TK, et al. Single point mutations of aromatic residues in transmembrane helices 5 and -6 differentially affect TRPV4 activation by 4alpha-PDD and hypotonicity: implications for the role of the pore region in regulating TRPV4 activity. Cell Calcium. 2014;55:38–47. doi: 10.1016/j.ceca.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 179.Alexander R, et al. 4α-phorbol 12,13-didecanoate activates cultured mouse dorsal root ganglia neurons independently of TRPV4. Br. J. Pharmacol. 2013;168:761–772. doi: 10.1111/j.1476-5381.2012.02186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Saghatelian A, McKinney MK, Bandell M, Patapoutian A, Cravatt BF. A FAAH-regulated class of N-acyl taurines that activates TRP ion channels. Biochemistry. 2006;45:9007–9015. doi: 10.1021/bi0608008. [DOI] [PubMed] [Google Scholar]
- 181.Vriens J, Owsianik G, Janssens A, Voets T, Nilius B. Determinants of 4 alpha-phorbol sensitivity in transmembrane domains 3 and 4 of the cation channel TRPV4. J. Biol. Chem. 2007;282:12796–12803. doi: 10.1074/jbc.M610485200. [DOI] [PubMed] [Google Scholar]
- 182.Berna-Erro A, et al. Structural determinants of 5',6'-epoxyeicosatrienoic acid binding to and activation of TRPV4 channel. Sci. Rep. 2017;7:10522. doi: 10.1038/s41598-017-11274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996;78:415–423. doi: 10.1161/01.RES.78.3.415. [DOI] [PubMed] [Google Scholar]
- 184.Randall MD, Kendall DA. Anandamide and endothelium-derived hyperpolarizing factor act via a common vasorelaxant mechanism in rat mesentery. Eur. J. Pharmacol. 1998;346:51–53. doi: 10.1016/S0014-2999(98)00003-X. [DOI] [PubMed] [Google Scholar]
- 185.Sisignano M, et al. 5,6-EET is released upon neuronal activity and induces mechanical pain hypersensitivity via TRPA1 on central afferent terminals. J. Neurosci. 2012;32:6364–6372. doi: 10.1523/JNEUROSCI.5793-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ma X, et al. Apigenin, a plant-derived flavone, activates transient receptor potential vanilloid 4 cation channel. Br. J. Pharmacol. 2012;166:349–358. doi: 10.1111/j.1476-5381.2011.01767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Smith PL, Maloney KN, Pothen RG, Clardy J, Clapham DE. Bisandrographolide from Andrographis paniculata activates TRPV4 channels. J. Biol. Chem. 2006;281:29897–29904. doi: 10.1074/jbc.M605394200. [DOI] [PubMed] [Google Scholar]
- 188.Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. Nociceptive and pro-inflammatory effects of dimethylallyl pyrophosphate via TRPV4 activation. Br. J. Pharmacol. 2012;166:1433–1443. doi: 10.1111/j.1476-5381.2012.01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am. J. Physiol. Cell Physiol. 2004;286:C195–C205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- 190.Zhu X, Jiang M, Birnbaumer L. Receptor-activated Ca2+ influx via human Trp3 stably expressed in human embryonic kidney (HEK)293 cells. Evidence for a non-capacitative Ca2+ entry. J. Biol. Chem. 1998;273:133–142. doi: 10.1074/jbc.273.1.133. [DOI] [PubMed] [Google Scholar]
- 191.Yamada A, et al. Ca2+ sensitization of smooth muscle contractility induced by ruthenium red. Am. J. Physiol. 1999;276:C566–C575. doi: 10.1152/ajpcell.1999.276.3.C566. [DOI] [PubMed] [Google Scholar]
- 192.Vincent F, et al. Identification and characterization of novel TRPV4 modulators. Biochem. Biophys. Res. Commun. 2009;389:490–494. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 193.Everaerts W, et al. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc. Natl Acad. Sci. USA. 2010;107:19084–19089. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Skogvall S, et al. Effects of capsazepine on human small airway responsiveness unravel a novel class of bronchorelaxants. Pulm. Pharmacol. Ther. 2007;20:273–280. doi: 10.1016/j.pupt.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 195.Zhao LM, et al. Effect of TRPV1 channel on proliferation and apoptosis of airway smooth muscle cells of rats. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2014;34:504–509. doi: 10.1007/s11596-014-1306-0. [DOI] [PubMed] [Google Scholar]
- 196.Bratz IN, et al. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H2489–H2496. doi: 10.1152/ajpheart.01191.2007. [DOI] [PubMed] [Google Scholar]
- 197.Pairet N, et al. TRPV4 inhibition attenuates stretch-induced inflammatory cellular responses and lung barrier dysfunction during mechanical ventilation. PLoS ONE. 2018;13:e0196055. doi: 10.1371/journal.pone.0196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Thorneloe KS, et al. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci. Transl. Med. 2012;4:159ra148. doi: 10.1126/scitranslmed.3004276. [DOI] [PubMed] [Google Scholar]
- 199.Hou J, et al. Gene expression-based classification of non-small cell lung carcinomas and survival prediction. PLoS One. 2010;5:e10312. doi: 10.1371/journal.pone.0010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kaiser S, et al. Transcriptional recapitulation and subversion of embryonic colon development by mouse colon tumor models and human colon cancer. Genome Biol. 2007;8:R131. doi: 10.1186/gb-2007-8-7-r131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kim SM, et al. Prognostic biomarkers for esophageal adenocarcinoma identified by analysis of tumor transcriptome. PLoS ONE. 2010;5:e15074. doi: 10.1371/journal.pone.0015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hao Y, et al. Gene expression profiling reveals stromal genes expressed in common between Barrett’s esophagus and adenocarcinoma. Gastroenterology. 2006;131:925–933. doi: 10.1053/j.gastro.2006.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kimchi ET, et al. Progression of Barrett’s metaplasia to adenocarcinoma is associated with the suppression of the transcriptional programs of epidermal differentiation. Cancer Res. 2005;65:3146–3154. doi: 10.1158/0008-5472.CAN-04-2490. [DOI] [PubMed] [Google Scholar]
- 204.Grasso CS, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Buchholz M, et al. Transcriptome analysis of microdissected pancreatic intraepithelial neoplastic lesions. Oncogene. 2005;24:6626–6636. doi: 10.1038/sj.onc.1208804. [DOI] [PubMed] [Google Scholar]
- 206.Wurmbach E, et al. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology. 2007;45:938–947. doi: 10.1002/hep.21622. [DOI] [PubMed] [Google Scholar]