Abstract
Brown adipose tissue (BAT) is able to rapidly generate heat and metabolise macronutrients, such as glucose and lipids, through activation of mitochondrial uncoupling protein 1 (UCP1). Diet can modulate UCP1 function but the capacity of individual nutrients to promote the abundance and activity of UCP1 is not well established. Caffeine consumption has been associated with loss of body weight and increased energy expenditure, but whether it can activate UCP1 is unknown. This study examined the effect of caffeine on BAT thermogenesis in vitro and in vivo. Stem cell-derived adipocytes exposed to caffeine (1 mM) showed increased UCP1 protein abundance and cell metabolism with enhanced oxygen consumption and proton leak. These functional responses were associated with browning-like structural changes in mitochondrial and lipid droplet content. Caffeine also increased peroxisome proliferator-activated receptor gamma coactivator 1-alpha expression and mitochondrial biogenesis, together with a number of BAT selective and beige gene markers. In vivo, drinking coffee (but not water) stimulated the temperature of the supraclavicular region, which co-locates to the main region of BAT in adult humans, and is indicative of thermogenesis. Taken together, these results demonstrate that caffeine can promote BAT function at thermoneutrality and may have the potential to be used therapeutically in adult humans.
Subject terms: Stem-cell research, Molecular imaging
Introduction
Brown adipose tissue (BAT) is rapidly activated by diet and cold exposure and has the potential to improve metabolic homeostasis in adults1–3. This adaptation is characterised by enhanced function of the BAT specific uncoupling protein 1 (UCP1), located on the inner mitochondrial membrane4. In adult humans, as the amount of BAT decreases with age and is negatively correlated with body mass index (BMI)5–8, changing the rate of BAT loss could have marked benefits for metabolic health. This could be achieved through dietary ingredients but, although it has been shown that diet can stimulate BAT function9, the extent to which individual nutrients can have comparable effects is not well established. Animal studies indicate that brown and beige cells could be activated through nutrients such as capsaicin analogs10,11, and capsinoids exert similar effects in increasing BAT-dependent energy expenditure as cold exposure12. Caffeine (1,3,7-trimethylxantine), a plant alkaloid found in coffee, tea, cola, and chocolate, is widely consumed, has been associated with weight loss and increased energy expenditure in both human and animal models in vivo, and reduces the risk of type 2 diabetes13–18. Although caffeine has been reported to upregulate UCP1 in obese mice19, the extent to which caffeine (or coffee) may directly stimulate BAT is not known. To elucidate this, we utilised a recently established stem cell model of browning20 to determine whether a physiological amount of caffeine could change mitochondrial function and lipid handling through promotion of UCP1 function. Based on the recent literature21–23, a caffeine concentration of 1 mM was used for in vitro cell culture. A human in vivo study was then undertaken to determine whether the amount of caffeine normally present in a standard coffee beverage can elicit a thermogenic effect on supraclavicular BAT in adult humans. This was assessed by an increase in temperature measured using thermal imaging of the supraclavicular region. Increases in temperature in the region is well correlated with the enhanced radiolabelled glucose uptake into BAT measured during cold exposure using positron emission tomography-computed tomography (PET-CT)24.
Methods
Cell culture
Mouse mesenchymal stem cells (mMSCs) were cultured as previously described20 and adipogenesis induced using a growth medium supplemented with 1 μM dexamethasone (Cayman Chemicals, USA), 100 µM isobutylmethylxanthine (IBMX) (Sigma-Aldrich, UK), 1 μM rosiglitazone (Cayman Chemicals, USA), 10 μg/ml insulin (Sigma-Aldrich, UK) and 1 nM triiodothyronine (T3) (Sigma-Aldrich, UK)). To establish the optimal amount of caffeine, mMSCs were differentiated in increasing concentrations ranging from 0.1–10 mM over 7 days. This demonstrated that the optimum concentration for viability and differentiation was 1 mM (Fig. S1) and this was, therefore, used in all subsequent experiments. A similar treatment (with an increased IBMX concentration to 500 μM) was applied to primary human bone marrow-derived stem cells (hMSCs) (Lonza, UK) over 21 days. For both cell types, cell viability assay and Oil Red O (ORO) staining were performed as previously described20. The analysis performed in this study was carried out on 3 batches of cells (each one done with 3 technical replicates).
Mitochondrial staining and Immunocytochemistry
Cells cultured on coverslips were stained with 100 nM MitoTracker Deep Red FM and then examined using a Zeiss Elyra PS.1 microscope to determine the intensity of mitochondrial staining20. Cells were fixed and the abundance of UCP1 and proliferator-activated receptor gamma coactivator (PGC-1α) was determined using validated primary antibodies (ab10983; ab54481, Abcam, UK)20,25–29. Twenty fields of view from three experiments for each condition (three biological replicates) were randomly selected, and the overall fluorescence intensity for UCP1 and MitoTracker signals was measured applying identical instrument settings (scanning laser power 633 nm at 4%, detector gain set at 750 and detector offset was set to zero). Image processing and fluorescence measurements were done using original raw files on the manufacturer’s software ZEN blue 2.1 (https://www.zeiss.com/microscopy/int/products/microscope-software/zen-lite.html).
Transmission electron microscopy analysis
Cells were fixed in pre-warm (37 °C) fixative solution (2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2) and post-fixed in 1% osmium tetroxide in the same buffer following washing. Each sample was then infiltrated with a mixture of epoxy resin and propylene oxide, polymerized with resin and sectioned on a Leica UC6 ultramicrotome (Leica Microsystems, UK). For ultrastructural examination, ultrathin sections were mounted on grids, contrasted with uranyl acetate followed by lead acetate and examined using a FEI Tecnai 12 Biotwin transmission electron microscope (TEM).
Gene expression analysis
Extraction of total RNA and quantitative PCR were performed as previously described20. Gene expression was determined using the GeNorm normalization algorithm against two selected reference genes (stability value M = 0.28), beta-2-microglobulin (B2M) and acidic ribosomal protein subunit P0 (RPLP0) using GeNorm software (version 3.5; Primer Design Ltd). UCP1, COX8b, P2RX5, DIO2, ARß3, ARα2, TRPV1, TRPV2 and TRPV4 gene expression was determined using TaqMan probe (BioRad TaqMan Gene Expression assays qHsaCEP0050537, qMmuCIP0034367, qRnoCIP0024301, qMmuCEP0052679 qMmuCEP0031789, qMmuCEP0055983, qMmuCIP0031313, qMmuCIP0035343 and qMmuCIP0032629, respectively). Murine-specific oligonucleotide primers (Eurofins) are given in Supplementary Table 1. A principal component analysis (PCA) and heat map were constructed to evaluate the overall changes of caffeine on adipocyte differentiation using ClustVis30. For qPCR analysis, 5 different passages treated and analysed independently were used.
Seahorse assay
Cells were seeded into the XFe96 Microplates (Seahorse Bioscience) to measure the oxygen consumption rate (OCR: an indicator of mitochondrial respiration) and the extracellular acidification rate (ECAR: an indicator of glycolysis) as previously described20. OCR and ECAR readings were measured under basal conditions and after the addition of mitochondrial inhibitors (oligomycin, carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone (FCCP) and rotenone/antimycin A). Coupling efficiency, an indicator of the proportion of respiratory activity used to make ATP, was determined by calculating the percentage of OCR immediately following the oligomycin treatment with the final baseline value. Seahorse datasets were normalised to cell number using Hoechst 33258 fluorescent dye signal measured immediately after Seahorse analysis31.
Human in vivo response to caffeine
Healthy volunteers (4 male; 5 female), aged 27 (SD 6) years of normal BMI (mean 23 (SD 3) kg/m2), were imaged in standard loose fitting clothing to reveal the region of interest, in the morning, without prior vigorous exercise, caffeine, drug or alcohol consumption within 9 hours and at least 2 hours after eating. Thermally-reflective skin markers were placed on precise anatomical landmarks for subsequent data analysis32. Imaging was undertaken within a temperature controlled room (maintained at 22 °C; external ambient temperatures on study days: 16.8 (SD 2.4) °C). Participants acclimatised for 30 minutes prior to imaging before baseline imaging (FLIR T540, FLIR Systems AB, Danderyd, Sweden)) as previously described32. After baseline imaging, participants consumed either a caffeinated beverage (Nescafe© Original 1.8 g sachet ~65 mg caffeine dissolved in 200 ml water at 22 °C; n = 9) or water alone at 22 °C (n = 9), remained seated for 30 minutes (to allow time for caffeine absorption33) and then underwent further thermal imaging. The study was conducted according to the standards of Declaration of Helsinki following approval of the University of Nottingham Medical School Ethics Committee and with informed participant consent. Nine participants in each group gave our study 95% power to detect a biologically significant mean difference of 0.2 °C24.
Thermal images were analysed as described previously with the hottest 10% of the points within the thermal image identified and the medians of those points calculated32. Mean pre-stimulation supraclavicular temperature (TSCV) was calculated 20 minutes prior to ingestion of coffee (n = 9) or water (n = 9), whilst mean post-stimulation TSCV was measured from 30 minutes after ingestion.
Statistical analyses
All data were analysed by Student’s t test. For data that was not normally distributed the nonparametric Mann Whitney test was used to evaluate the statistical significance between two treatment groups. Statistical significance was accepted at P < 0.05, with *P < 0.05; **P < 0.01; ***P < 0.001. Errors bars plotted on graphs are presented as the mean ± SEM. Data were analysed using Graph Pad Prism Software (https://www.graphpad.com). For principal component analysis, Metaboanalyst (https://www.metaboanalyst.ca) was used.
Results
Caffeine treatment increased UCP1 protein expression, mitochondrial biogenesis and bioenergetics profile in MSCs-derived adipocytes
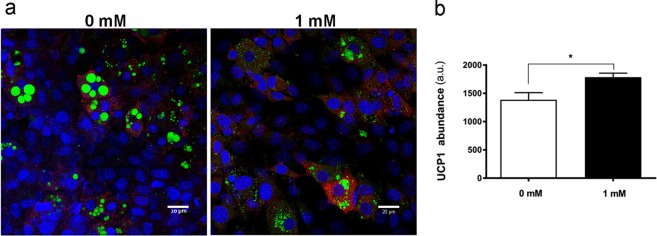
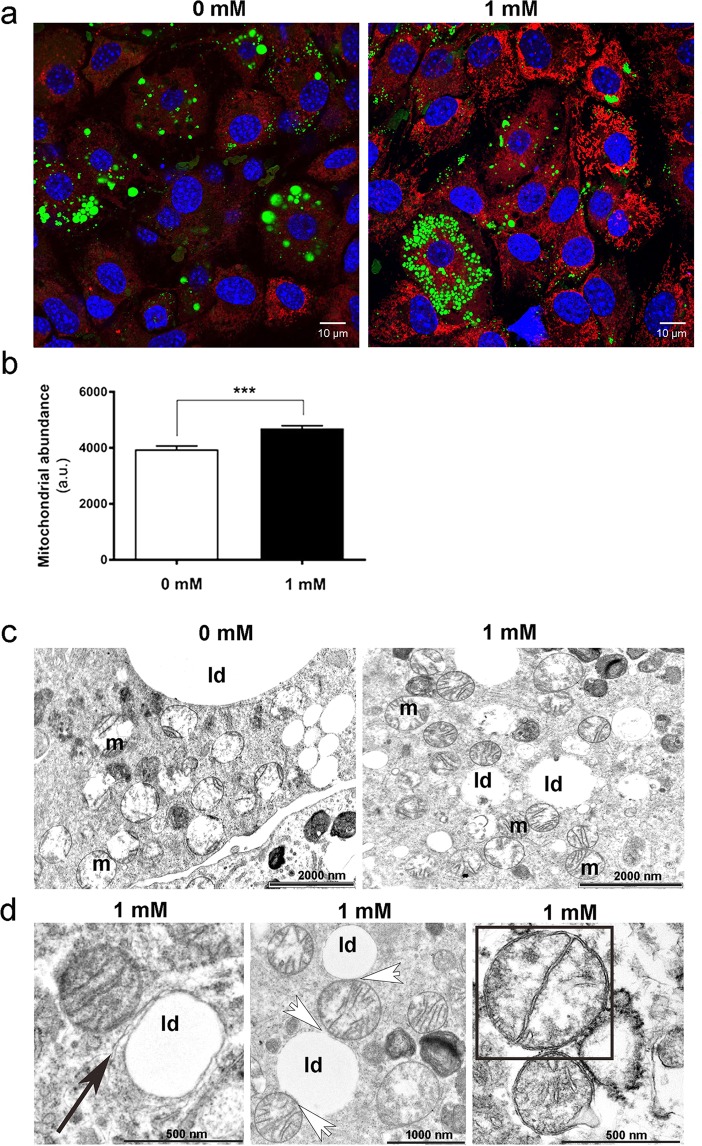
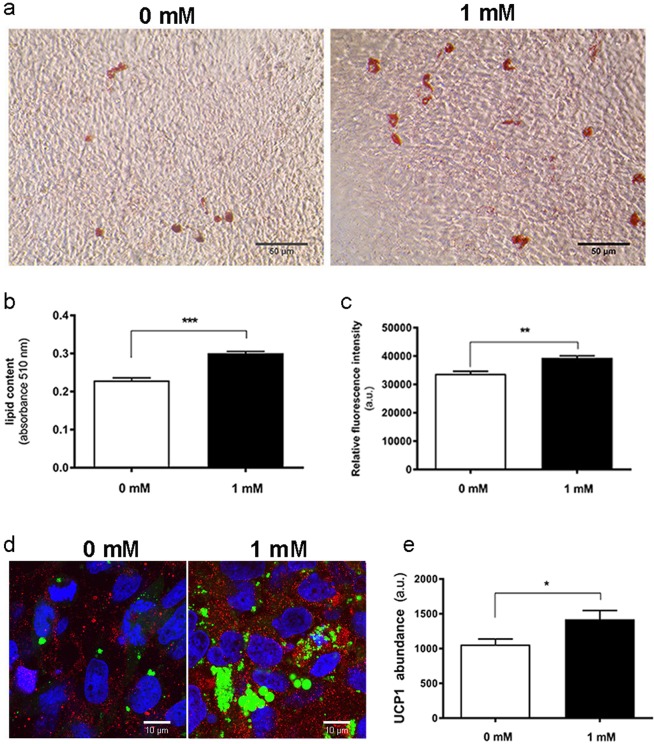
Caffeine promoted the expression of UCP1 in mMSC cultures (Fig. 1a,b), a response accompanied by enhanced abundance of peroxisome proliferator-activated receptor gamma coactivator (PGC)-1α, with the nuclear presence of PGC-1α seen in most adipocytes exposed to caffeine, contrary to controls (Fig. S2). Mitochondrial staining further indicated that caffeine exposure enhanced mitochondriogenesis (Fig. 2a,b). Ultrastructural TEM analysis revealed more numerous and densely packed mitochondria with caffeine treatment, in an interconnected reticular pattern (Fig. 2c). The number of mitochondria undergoing division was enhanced by caffeine which also caused modification in their shape, resulting in a more rounded appearance with abundant lamellar cristae. These adaptations were accompanied with enhanced contact sites between mitochondria and lipid droplets, and between lipid droplets and endoplasmic reticulum (Fig. 2d). Comparable responses were observed in human MSC cultures, with caffeine causing an increase in lipid content, metabolic activity and UCP1 protein abundance (Fig. 3).
Figure 1.
Effect of caffeine on immunofluorescence analysis of UCP1 abundance in adipogenic cultures. (a) Representative images showing upregulation of UCP1 (red) in mMSCs. DAPI was used to identify cell nuclei (blue) and BODIPY was used to identify lipid droplets (green). (b) Mean relative UCP1 abundance (n = 20). Scale bars: 20 μm; *P < 0.05.
Figure 2.
Adaptations in the mitochondrial compartment of adipocytes following 1 mM caffeine treatment. (a) Representative images showing mitochondrial staining (MitoTracker, red). DAPI was used to identify nuclei (blue) and BODIPY was used to identify lipid droplet (green). (b) Relative MitoTracker fluorescence intensity. (c,d) TEM analysis of mitochondria (m) and lipid droplets (ld), with (d) showing higher magnification views of caffeine-treated cells with contact sites between lipid droplets and endoplasmic reticulum (black arrow), contact sites between mitochondria and lipid droplets (white arrows), and mitochondria division (outlined square). Scale bar: 10 μm. ***P < 0.001.
Figure 3.
hMSC response to caffeine exposure during a 21-day period of adipogenic differentiation. (a) Representative images of cells stained for lipid droplets with ORO (Scale bar: 50 μm) and mean (b) staining quantified spectrophotometrically. (c) Cell viability assay. (d) Representative images showing upregulation of UCP1 (red) in hMSCs. DAPI was used to identify cell nuclei (blue) and BODIPY was used to identify lipid droplets (green); scale bar: 10 μm and (e) mean relative UCP1 abundance (n = 20). Data presented as mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001.
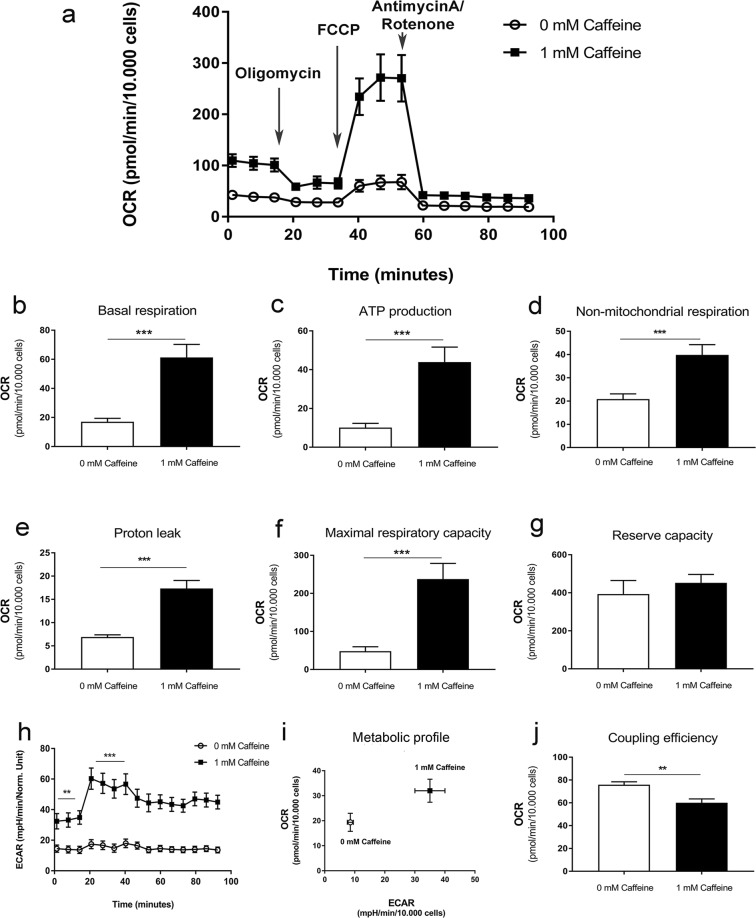
To determine whether the observed caffeine-induced changes in mitochondrial biogenesis were accompanied by functional changes in bioenergetics, cultures were analysed using the Seahorse assay. This demonstrated that caffeine enhanced basal respiration, respiration used for the ATP-production, non-mitochondrial respiration, proton leak and maximal respiratory capacity (Fig. 4a–f). Basal ECAR was also raised by caffeine both before, and after, oligomycin treatment (Fig. 4h), and when plotted against OCR (Fig. 4i). Caffeine increased glycolysis and oxidative phosphorylation, indicative of more metabolically active cells, as well as decreasing coupling efficiency (Fig. 4j).
Figure 4.
Seahorse XF cell mitochondrial stress test assay performed on mouse mesenchymal stem cell-derived adipocytes treated with or without caffeine for 7 days. (a) OCR profile plot. (b) Basal respiration. (c) ATP production. (d) Non-mitochondrial respiration. (e) Proton leak. (f) Maximal respiratory capacity. (g) Reserve capacity. (h) ECAR. (i) Summary metabolic profiles as determined by plotting ECAR against OCR. (j) Coupling efficiency. Histograms represent mean ± SEM. n = 3; **P < 0.01, ***P < 0.001.
Caffeine treatment shows upregulation of beige/brown genes during MSC adipogenesis
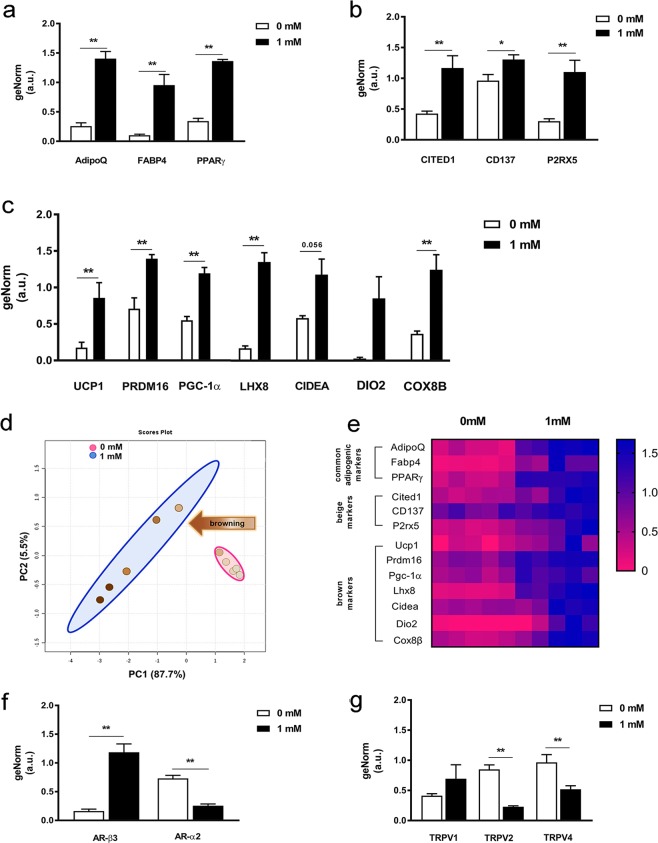
Caffeine increased gene expression of PPARγ, adiponectin and FABP4 (Fig. 5a), as well as beige markers CITED1, CD137 and P2RX5 (Fig. 5b) and brown-selective genes UCP1, PRDM16, PGC-1α, LHX8 and COX8b (Fig. 5c) and AR-ß3 (Fig. 5f). PCA and heat maps further revealed separate gene clusters, visually confirming discrete expression patterns with caffeine (Fig. 5d,e), which also decreased the abundance of AR-α2 (Fig. 5f) TRPV2 and TRPV4 mRNA (Fig. 5g). Raw Ct-values for UCP1 gene expression are presented in Supplementary Table 2.
Figure 5.
Adipogenic gene expression analysis by qPCR during mMSCs differentiation treated with or without caffeine. Relative gene expression of (a) adipogenic markers AdipoQ, FABP4 and PPARγ, (b) beige lineage markers CITED1, CD137 and P2RX5, and (c) brown lineage markers UCP1, PRDM16, PGC-1α, LHX8, CIDEA, DIO2 and COX8b. (d) Scatter plot of the first two principal components, comprising 93.2% of total variance, highlighting two clusters corresponding to cells differentiated in adipogenic medium with 0 mM (pink) and 1 mM (blue) caffeine. (e) Unsupervised hierarchical clustering of expression values for the same genes and samples as those in panel D. Each row represents a gene while each column corresponds to a different sample. Gene expression of (f) AR-ß3 and AR-α2 and (g) TRPV1, TRPV2 and TRPV4. Data represent the mean ± SEM of five replicates; *P < 0.05; ***P < 0.001.
Drinking coffee stimulates the temperature of the supraclavicular region co-locating with BAT in adults
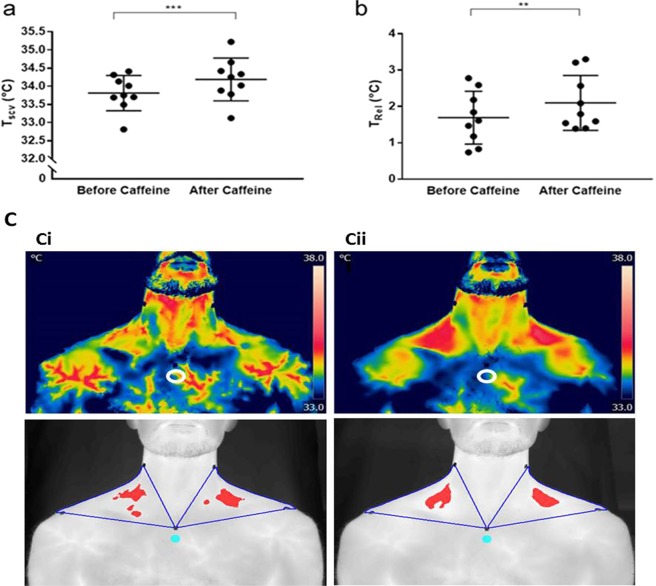
Drinking coffee significantly increased the temperature of the supraclavicular region co-locating with brown adipose tissue in humans23 (Fig. 6), reflecting increasing TSCV relative to reference body surface temperature (Trel;), an effect not seen with consuming water alone (TSCV: before water ingestion: 34.55 ± 0.16 °C; after water ingestion: 34.55 ± 0.17 °C; ΔTREL with water ingestion: 0.13 ± 0.12 °C; n = 9). The temperature of the reference point was unchanged e.g. pre caffeine 31.12 ± 0.27 °C; post caffeine 32.09 ± 0.31 °C (n = 9).
Figure 6.
In vivo effect of drinking caffeine on heat production from brown fat in adult humans. (a) Caffeine resulted in a significant rise in temperature of the supraclavicular region (TSCV) which co-locates with brown fat and this reflects (b) increase in supraclavicular temperature relative to reference body surface temperature (Trel) (n = 9); (c) Representative thermal image (i) pre and (ii) post-caffeine, as either the original FLIR image, or the transformed image high-lighting the hottest 10% of pixels. ***P < 0.001; **P < 0.01. White open and blue closed circles indicate the reference temperature point.
Discussion
This is the first study to determine that the stimulatory effects of caffeine on UCP1 seen in vitro can be translated to adult humans ingesting caffeine in a commonly consumed coffee beverage. The increase in temperature of the region which co-locates with BAT observed with caffeine ingestion is indicative of an increase in BAT activity32 following a relatively low dose of caffeine33 from a single standard cup of coffee. It is supportive of our in vitro observations in human and murine stem-cell derived adipocytes, in which the abundance of UCP1 was increased, together with oxygen consumption, and markers of mitochondrial and lipid metabolism. These findings suggest that the increase in metabolic rate following caffeine ingestion could be mediated by enhanced BAT function, which precedes any change in skin temperature34.
Enhanced BAT activity in adult humans has the potential to improve lipid and carbohydrate homeostasis as well as contributing to weight loss strategies1. The extent to which individual components of the diet can activate BAT in humans is not well established, as the gold standard to assess BAT function is the uptake of radio-labelled glucose (fludeoxyglucose or 18FDG) as measured by PET-CT5,8. Normally, this has to be undertaken in fasted subjects, as feeding is accompanied by such a large increase in glucose uptake by muscle that the capacity to detect BAT is greatly reduced35. We have, therefore, developed thermal imaging as a practical method to detect in vivo changes in BAT activity and which can detect changes in response to ingestion without requiring exposure to radiation and which correlates well with the uptake of 18FDG following cold exposure24. Moreover, there is increasing evidence that dietary ingredients promote brown fat function9, responses which may be mediated by the release of secretin from the gut27.
It has been suggested that thermal imaging may not give an optimal measure of BAT36,37, however, such studies have not considered the changes in temperature of the supraclavicular region with an appropriate reference point that correlate well with BAT function as assessed by PET-CT24. In addition, single point measures of supraclavicular temperature can be misleading as they cannot reliably detect the hottest region of brown fat as identified with thermal imaging approach used here38. Although caffeine has been reported to cause changes in blood flow39–41, this was not expected to cause substantial changes in skin temperature, as this typically does not occur until at least 2 h after caffeine consumption34. Further studies measuring the kinetics of caffeine-induced blood flow changes can help refine its impact on thermal analysis.
Temperature, together with intact innervation42 are primary factors regulating the magnitude of change in brown and/or beige fat function, which is further modulated by the relative amount of UCP1 within a depot. Consequently, transferring mice from 22 to 5 °C results in a modest rise within interscapular BAT, but a much larger increase within inguinal beige fat43. It is thus likely that comparatively small changes in the amount of UCP1 can impact on energy balance, a proposal supported by the present study. We demonstrate that in beige adipocytes20, in which caffeine exposure increased the amount of UCP1 by ~20%, in conjunction with more mitochondria and lipid, this was accompanied by a marked rise in oxygen consumption in vitro. The magnitude of change was, however, lower than that seen when comparing mitochondria sampled from perirenal fat in humans with pheochromocytoma patients living in a warm climate44. It would thus be useful to further examine the extent to which comparable outcomes are found in vitro in brown as opposed to beige adipocytes.
Previous studies reported that caffeine inhibits differentiation, promotes lipolysis and suppresses intracellular lipid accumulation in adipocyte cultures21,45–47, while increasing BAT temperature, guanosine-5′-diphosphate (GDP) binding and oxygen consumption in mitochondria in mice48. The present study found that caffeine used at 1 mM upregulated not only UCP1 but also primary regulatory genes including PPARγ, PRDM16, and PGC-1α49. Caffeine, therefore, activated the main regulators of brown adipocyte thermogenesis50–52, but also gene expression of CD137, LHX8, P2RX5, CITED1 and COX8b, that are indicative of molecular conversion of white/beige cells into brown adipocytes. Consistent with these molecular changes induced by caffeine was the appearance of smaller lipid droplets in UCP1-expressing adipocytes, a major morphological sign of lipolysis49. The response to caffeine exposure was observed to vary in human and mouse cultures, in line with the different level of lipid accumulation noted under adipogenic induction in these models, pointing to intrinsic differences which will require further characterization. The recruitment of thermogenesis results in mitochondriogenesis, a process controlled by PGC-1α50,53,54, and was here accompanied by increased PGC-1α mRNA expression and PGC-1α protein nuclear localization indicating PGC-1α functional activity in caffeine-treated cells. In line with these observations, increased mitochondrial biogenesis and PGC-1α expression after caffeine treatment have been reported in other cell types55–57. PGC-1α not only triggers mitochondrial biogenesis, but also activates several components of the adaptive thermogenic program in BAT, including fatty-acid oxidation, and increases oxygen consumption through co-activation of transcription factors such as PPARγ53. These molecular adaptations with caffeine exposure were accompanied by changes in mitochondrial ultrastructure and the redistribution of adjacent small lipid droplets. The numerous lamellar cristae observed in caffeine-treated adipocytes are characteristic of activated beige/brown adipocytes that expand the surface of their inner mitochondria membrane with enhanced functional UCP14. These contact sites constitute an important way of inter-organelle communication58 in which fatty acids liberated by lipolysis could be used for β-oxidation. Future analysis of sorted lipid-laden cells could provide a more specific measure of the mitochondrial response to caffeine treatment. The TRPV receptor family can also modulate beige/brown adipocyte thermogenesis, and TRPV4 can be a negative regulator of PGC1α59, so caffeine might further induce PGC1α and UCP1 expression by antagonizing TRPV4. The capacity of caffeine-treated adipocytes to undergo mitochondrial uncoupling was confirmed by enhanced bioenergetic capacity accompanied with an increase in ATP-producing metabolic pathways, i.e. oxidative phosphorylation and glycolysis which are indicative of higher energy demands observed during the browning process20, although additional pathways besides UCP1-dependent mechanisms could also be involved. The low Ct values observed here for UCP1 may be consistent with this, and might also underline differences in between the half-life of UCP1 protein (2–20 days) and UCP1 mRNA (3 h)60,61.
Besides acting as an adenosine receptor antagonist, caffeine inhibits phosphodiesterase, increasing intracellular cAMP62, a process also activated by β-adrenergic stimulation4,63. This could be the mechanism by which caffeine enhances UCP1 function. Coupled to G proteins, β3-AR induces cAMP formation, which in turn activates protein kinase A and stimulates fat hydrolysis, with the free fatty acids released activating UCP14,64. Adenylyl cyclase (which synthesizes cAMP from ATP65) is positively coupled to β3-AR but inhibited by ARα266, which was observed here to decrease with caffeine exposure. Taken together, the morphological evidence of lipolysis, plus reduced ARα2 gene suppression induced by caffeine, supports an antilipolytic effect67. Further experiments investigating the effects of PKA inhibition could provide additional information on the role of cAMP levels in the caffeine response.
In conclusion, these results provide new complementary in vitro and in vivo evidence that caffeine (and a coffee beverage) can promote BAT function at doses compatible with human use. Similar approaches could be considered to screen other potential dietary compounds that could target UCP1 and promote BAT function. Future intervention studies can now be undertaken to assess whether caffeine-induced BAT activation in humans is dose-dependent, refine the minimal intake required for a BAT response, and explore whether comparable effects are seen in fully differentiated adipocytes and primary cells, as well as in diabetic and/or obese individuals.
Supplementary information
Acknowledgements
This work was funded by the EU-CASCADE fellowship scheme from the EU’s 7th FP PCOFUND-GA-2012-600181 (K.V.), CONACyT, Mexico (H.A.L.L.) with support from The Cardiometabolic Disease Research Foundation (Los Angeles, USA) (DW). This work was supported by the Biotechnology and Biological Sciences Research Council [grant number BB/L013827/1], the Zeiss Elyra PS.1 microscope, the processing computers and software were funded by BBSRC BB/L013827/1 (Multidisciplinary Super Resolution Microscopy Facility). Technical assistance and expertise from Denise Mclean and Robert Markus (School of Life Sciences, University of Nottingham) are gratefully acknowledged.
Author Contributions
K.V., M.E.S., V.S. and H.B. planned and designed the project. K.V., H.A.L.L. and D.W. assembled the figures and supporting information. K.V. and H.A.L.L. carried out cellular experiments and D.W. carried out the in vivo studies. H.A.L.L. and I.B. carried out the molecular analysis. K.V., M.S. and V.S. finalized the manuscript with input from H.A.L.L., I.B., J.L., D.E.M. and H.S. All authors contributed to scientific review and discussion of the study.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michael E. Symonds, Email: michael.symonds@nottingham.ac.uk
Virginie Sottile, Email: virginie.sottile@nottingham.ac.uk.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-45540-1.
References
- 1.Symonds Michael E., Aldiss Peter, Pope Mark, Budge Helen. Recent advances in our understanding of brown and beige adipose tissue: the good fat that keeps you healthy. F1000Research. 2018;7:1129. doi: 10.12688/f1000research.14585.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U Din Mueez, Saari Teemu, Raiko Juho, Kudomi Nobu, Maurer Stefanie F., Lahesmaa Minna, Fromme Tobias, Amri Ez-Zoubir, Klingenspor Martin, Solin Olof, Nuutila Pirjo, Virtanen Kirsi A. Postprandial Oxidative Metabolism of Human Brown Fat Indicates Thermogenesis. Cell Metabolism. 2018;28(2):207-216.e3. doi: 10.1016/j.cmet.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Ouellet V, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 5.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 6.Cypess AM, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virtanen KA, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 8.van Marken Lichtenbelt WD, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 9.Scotney H, et al. Glucocorticoids modulate human brown adipose tissue thermogenesis in vivo. Metabolism. 2017;70:125–132. doi: 10.1016/j.metabol.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darre L, Domene C. Binding of Capsaicin to the TRPV1 Ion Channel. Mol Pharm. 2015;12:4454–4465. doi: 10.1021/acs.molpharmaceut.5b00641. [DOI] [PubMed] [Google Scholar]
- 11.Derbenev AV, Zsombok A. Potential therapeutic value of TRPV1 and TRPA1 in diabetes mellitus and obesity. Semin Immunopathol. 2016;38:397–406. doi: 10.1007/s00281-015-0529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Z, et al. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1alpha upregulation in mice. Cell Res. 2012;22:551–564. doi: 10.1038/cr.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Astrup A, et al. Caffeine: a double-blind, placebo-controlled study of its thermogenic, metabolic, and cardiovascular effects in healthy volunteers. Am J Clin Nutr. 1990;51:759–767. doi: 10.1093/ajcn/51.5.759. [DOI] [PubMed] [Google Scholar]
- 14.Bhupathiraju SN, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97:155–166. doi: 10.3945/ajcn.112.048603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bracco D, Ferrarra JM, Arnaud MJ, Jequier E, Schutz Y. Effects of caffeine on energy metabolism, heart rate, and methylxanthine metabolism in lean and obese women. Am J Physiol. 1995;269:E671–678. doi: 10.1152/ajpendo.1995.269.4.E671. [DOI] [PubMed] [Google Scholar]
- 16.Bukowiecki LJ, Lupien J, Follea N, Jahjah L. Effects of sucrose, caffeine, and cola beverages on obesity, cold resistance, and adipose tissue cellularity. Am J Physiol. 1983;244:R500–507. doi: 10.1152/ajpregu.1983.244.4.R500. [DOI] [PubMed] [Google Scholar]
- 17.Dulloo AG, Geissler CA, Horton T, Collins A, Miller DS. Normal caffeine consumption: influence on thermogenesis and daily energy expenditure in lean and postobese human volunteers. Am J Clin Nutr. 1989;49:44–50. doi: 10.1093/ajcn/49.1.44. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi-Hattori K, Mogi A, Matsumoto Y, Takita T. Effect of caffeine on the body fat and lipid metabolism of rats fed on a high-fat diet. Biosci Biotechnol Biochem. 2005;69:2219–2223. doi: 10.1271/bbb.69.2219. [DOI] [PubMed] [Google Scholar]
- 19.Kogure A, et al. Effects of caffeine on the uncoupling protein family in obese yellow KK mice. Clin Exp Pharmacol Physiol. 2002;29:391–394. doi: 10.1046/j.1440-1681.2002.03675.x. [DOI] [PubMed] [Google Scholar]
- 20.Velickovic K, et al. Low temperature exposure induces browning of bone marrow stem cell derived adipocytes in vitro. Scientific reports. 2018;8:4974. doi: 10.1038/s41598-018-23267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim AR, et al. Caffeine inhibits adipogenesis through modulation of mitotic clonal expansion and the AKT/GSK3 pathway in 3T3-L1 adipocytes. BMB Rep. 2016;49:111–115. doi: 10.5483/BMBRep.2016.49.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto T, et al. Caffeine inhibits cell proliferation by G0/G1 phase arrest in JB6 cells. Cancer Res. 2004;64:3344–3349. doi: 10.1158/0008-5472.CAN-03-3453. [DOI] [PubMed] [Google Scholar]
- 23.Okano J, Nagahara T, Matsumoto K, Murawaki Y. Caffeine inhibits the proliferation of liver cancer cells and activates the MEK/ERK/EGFR signalling pathway. Basic Clin Pharmacol Toxicol. 2008;102:543–551. doi: 10.1111/j.1742-7843.2008.00231.x. [DOI] [PubMed] [Google Scholar]
- 24.Law J, et al. Thermal Imaging is a Noninvasive Alternative to PET/CT for Measurement of Brown Adipose Tissue Activity in Humans. J Nucl Med. 2018;59:516–522. doi: 10.2967/jnumed.117.190546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bond LM, Burhans MS, Ntambi JM. Uncoupling protein-1 deficiency promotes brown adipose tissue inflammation and ER stress. PLoS One. 2018;13:e0205726. doi: 10.1371/journal.pone.0205726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazak L, et al. UCP1 deficiency causes brown fat respiratory chain depletion and sensitizes mitochondria to calcium overload-induced dysfunction. Proc Natl Acad Sci USA. 2017;114:7981–7986. doi: 10.1073/pnas.1705406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veniant MM, et al. Pharmacologic Effects of FGF21 Are Independent of the “Browning” of White Adipose Tissue. Cell Metab. 2015;21:731–738. doi: 10.1016/j.cmet.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Q, et al. Reconstitution of UCP1 using CRISPR/Cas9 in the white adipose tissue of pigs decreases fat deposition and improves thermogenic capacity. Proc Natl Acad Sci USA. 2017;114:E9474–E9482. doi: 10.1073/pnas.1707853114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu B, et al. PGC-1alpha Controls Skeletal Stem Cell Fate and Bone-Fat Balance in Osteoporosis and Skeletal Aging by Inducing TAZ. Cell stem cell. 2018;23:615–623. doi: 10.1016/j.stem.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43:W566–570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papadimitriou E, Lelkes PI. Measurement of cell numbers in microtiter culture plates using the fluorescent dye Hoechst 33258. J Immunol Methods. 1993;162:41–45. doi: 10.1016/0022-1759(93)90405-V. [DOI] [PubMed] [Google Scholar]
- 32.Law J, et al. Thermal imaging is a noninvasive alternative to PET-CT for measurement of brown adipose tissue activity in humans. Journal of Nuclear Medicine. 2018;59:516–522. doi: 10.2967/jnumed.117.190546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White JR, Jr., et al. Pharmacokinetic analysis and comparison of caffeine administered rapidly or slowly in coffee chilled or hot versus chilled energy drink in healthy young adults. Clin Toxicol (Phila) 2016;54:308–312. doi: 10.3109/15563650.2016.1146740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koot P, Deurenberg P. Comparison of changes in energy expenditure and body temperatures after caffeine consumption. Annals of nutrition & metabolism. 1995;39:135–142. doi: 10.1159/000177854. [DOI] [PubMed] [Google Scholar]
- 35.Roman S, et al. Brown adipose tissue and novel therapeutic approaches to treat metabolic disorders. Transl Res. 2015;165:464–479. doi: 10.1016/j.trsl.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Sarasniemi JT, et al. Skin temperature may not yield human brown adipose tissue activity in diverse populations. Acta Physiol (Oxf) 2018;224:e13095. doi: 10.1111/apha.13095. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Tellez B, et al. Concurrent validity of supraclavicular skin temperature measured with iButtons and infrared thermography as a surrogate marker of brown adipose tissue. Journal of Thermal Biology. 2019;82:186–196. doi: 10.1016/j.jtherbio.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Law J, Morris DE, Budge H, Symonds ME. Infrared Thermography. Handb Exp Pharmacol. 2019;251:259–282. doi: 10.1007/164_2018_137. [DOI] [PubMed] [Google Scholar]
- 39.Kim BJ, Seo Y, Kim JH, Lee DT. Effect of caffeine intake on finger cold-induced vasodilation. Wilderness Environ Med. 2013;24:328–336. doi: 10.1016/j.wem.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Noguchi K, et al. Effect of caffeine contained in a cup of coffee on microvascular function in healthy subjects. J Pharmacol Sci. 2015;127:217–222. doi: 10.1016/j.jphs.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Addicott MA, et al. The effect of daily caffeine use on cerebral blood flow: How much caffeine can we tolerate? Hum Brain Mapp. 2009;30:3102–3114. doi: 10.1002/hbm.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer AW, Schlein C, Cannon B, Heeren J, Nedergaard J. Intact innervation is essential for diet-induced recruitment of brown adipose tissue. Am J Physiol Endocrinol Metab. 2019;316:E487–E503. doi: 10.1152/ajpendo.00443.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalinovich AV, de Jong JM, Cannon B, Nedergaard J. UCP1 in adipose tissues: two steps to full browning. Biochimie. 2017;134:127–137. doi: 10.1016/j.biochi.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Vergnes L, et al. Adipocyte Browning and Higher Mitochondrial Function in Periadrenal But Not SC Fat in Pheochromocytoma. J Clin Endocrinol Metab. 2016;101:4440–4448. doi: 10.1210/jc.2016-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su SH, et al. Caffeine inhibits adipogenic differentiation of primary adipose-derived stem cells and bone marrow stromal cells. Toxicol In Vitro. 2013;27:1830–1837. doi: 10.1016/j.tiv.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Nakabayashi H, Hashimoto T, Ashida H, Nishiumi S, Kanazawa K. Inhibitory effects of caffeine and its metabolites on intracellular lipid accumulation in murine 3T3-L1 adipocytes. Biofactors. 2008;34:293–302. doi: 10.3233/BIO-2009-1083. [DOI] [PubMed] [Google Scholar]
- 47.Akiba T, et al. Inhibitory mechanism of caffeine on insulin-stimulated glucose uptake in adipose cells. Biochem Pharmacol. 2004;68:1929–1937. doi: 10.1016/j.bcp.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 48.Yoshioka K, Yoshida T, Kamanaru K, Hiraoka N, Kondo M. Caffeine activates brown adipose tissue thermogenesis and metabolic rate in mice. J Nutr Sci Vitaminol (Tokyo) 1990;36:173–178. doi: 10.3177/jnsv.36.173. [DOI] [PubMed] [Google Scholar]
- 49.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 50.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Seale P, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walczak R, Tontonoz P. PPARadigms and PPARadoxes: expanding roles for PPARgamma in the control of lipid metabolism. J Lipid Res. 2002;43:177–186. [PubMed] [Google Scholar]
- 53.Uldry M, et al. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006;3:333–341. doi: 10.1016/j.cmet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Petrovic N, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Thermogenically competent nonadrenergic recruitment in brown preadipocytes by a PPARgamma agonist. Am J Physiol Endocrinol Metab. 2008;295:E287–296. doi: 10.1152/ajpendo.00035.2008. [DOI] [PubMed] [Google Scholar]
- 55.Vaughan RA, Garcia-Smith R, Bisoffi M, Trujillo KA, Conn CA. Effects of caffeine on metabolism and mitochondria biogenesis in rhabdomyosarcoma cells compared with 2,4-dinitrophenol. Nutr Metab Insights. 2012;5:59–70. doi: 10.4137/NMI.S10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McConell GK, et al. Central role of nitric oxide synthase in AICAR and caffeine-induced mitochondrial biogenesis in L6 myocytes. J Appl Physiol (1985) 2010;108:589–595. doi: 10.1152/japplphysiol.00377.2009. [DOI] [PubMed] [Google Scholar]
- 57.Wnuk M, et al. Evaluation of the cyto- and genotoxic activity of yerba mate (Ilex paraguariensis) in human lymphocytes in vitro. Mutat Res. 2009;679:18–23. doi: 10.1016/j.mrgentox.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 58.Schuldiner M, Bohnert M. A different kind of love - lipid droplet contact sites. Biochim Biophys Acta. 2017;1862:1188–1196. doi: 10.1016/j.bbalip.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Ye L, et al. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell. 2012;151:96–110. doi: 10.1016/j.cell.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacobsson A, Cannon B, Nedergaard J. Physiological activation of brown adipose tissue destabilizes thermogenin mRNA. FEBS Lett. 1987;224:353–356. doi: 10.1016/0014-5793(87)80483-0. [DOI] [PubMed] [Google Scholar]
- 61.Patel HV, Freeman KB, Desautels M. Selective loss of uncoupling protein mRNA in brown adipose tissue on deacclimation of cold-acclimated mice. Biochem Cell Biol. 1987;65:955–959. doi: 10.1139/o87-124. [DOI] [PubMed] [Google Scholar]
- 62.Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol. 2006;147(Suppl 1):S252–257. doi: 10.1038/sj.bjp.0706495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Puigserver P, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/S0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 64.Himms-Hagen J. Brown adipose tissue thermogenesis and obesity. Prog Lipid Res. 1989;28:67–115. doi: 10.1016/0163-7827(89)90009-X. [DOI] [PubMed] [Google Scholar]
- 65.Rivera-Oliver M, Diaz-Rios M. Using caffeine and other adenosine receptor antagonists and agonists as therapeutic tools against neurodegenerative diseases: a review. Life Sci. 2014;101:1–9. doi: 10.1016/j.lfs.2014.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lafontan M, et al. Adrenergic regulation of adipocyte metabolism. Hum Reprod. 1997;12(Suppl 1):6–20. doi: 10.1093/humrep/12.suppl_1.6. [DOI] [PubMed] [Google Scholar]
- 67.Fontana E, Morin N, Prevot D, Carpene C. Effects of octopamine on lipolysis, glucose transport and amine oxidation in mammalian fat cells. Comp Biochem Physiol C Toxicol Pharmacol. 2000;125:33–44. doi: 10.1016/s0742-8413(99)00086-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.