Abstract
To determine the association of consanguinity with the occurrence of genetically transmitted eye diseases in rural and urban populations in Pavagada and Madhugiri taluks, Karnataka state, south India. This study was part of a population based cross-sectional prevalence survey, “The Pavagada pediatric eye disease study 2.” As a part of the demographic data, trained investigators collected information on consanguinity from the parents of children identified for the study. The children underwent visual acuity measurements and were examined by an ophthalmologist. Children with minor eye diseases were treated and those with major eye diseases were seen by a pediatric ophthalmologist. Eight thousand five hundred and fifty-three children were examined. The prevalence of ocular morbidity was 6.54% and blindness was 0.09%. The percentage of consanguineously married couples in the screened population was 34.33%. Among the blind children, 75% were blind with a disease with potential genetic etiology. Out of that, 66.67% were born out of consanguineous marriage (uncle-niece). Among children with diseases with a potential genetic etiology 54.29% of the children were born out of consanguineous union. Most of these children (71.43%) were born out of uncle-niece marriages. Further analysis showed that consanguineous parents were more likely to have children with disease with a potential genetic etiology as compared to nonconsanguineous parents (odds ratio: 2.551, p = 0.012). It is evident that consanguineous marriages, especially uncle-niece unions are common in the study area. Consanguinity is more likely to result in children with eye diseases with potential genetic etiology.
Keywords: Consanguinity, Eye diseases, Pediatric, India, Pavagada Pediatric Eye Disease Study
Introduction
Consanguineous marriages describe unions between couples who share at least one common ancestor (Shawky et al. 2013). Children born out of consanguineous unions may be at increased risk of acquiring genetic disorders because of the expression of autosomal recessive gene mutations inherited from a common ancestor in a homozygous state (Shawky et al. 2013). Consanguinity is prevalent in many Middle Eastern and Arab cultures and societies (Bener and Hussain 2006). Consanguineous marriage is also preferred in the southern Indian states of Tamil Nadu, Karnataka, and Andhra Pradesh (Kumaramanickavel et al. 2002). First-cousin marriages and uncle-niece unions are common. More than 20% of the consanguineous marriages are said to be uncle-niece unions (Bittles et al. 1991). In Belgaum in Karnataka, 36% of the marriages were uncle-niece unions (Nath et al. 2004), whereas in Mysore (Karnataka), there was almost equal distribution of first-cousin (44.68%) and uncle-niece marriages (46.81%) (Ramegowda and Ramachandra 2006). Blindness caused by early-onset retinal dystrophies (Li et al. 2017) and whole-globe anomalies (Ferda et al. 2000; Bardakjian and Schneider 2011) appear to be more common in consanguineous communities.
Objectives
This study is a part of the Pavagada Pediatric Eye Disease Study 2. The main aim of the study was to determine the prevalence of ocular morbidity and childhood blindness in children ≤ 15 years of age. As a part of the demographic data, trained field investigators collected information on consanguinity from the parents of children identified for the study. In this paper, we discuss the association of parental consanguinity in children with eyes diseases with a potential genetic etiology.
Methods
Design
This study was a population based cross-sectional prevalence survey, conducted from August 2012 to December 2013. The study was approved by the Institutional Review Board of the hospital and conducted within the tenets of the Declaration of Helsinki.
Setting
The study area comprised the two taluks (sub-districts) of Pavagada and Madhugiri in Tumkur district of Karnataka state in south India. These are backward areas in terms of education, health, and socioeconomic standpoints (Dr D M Nanjundappa Committee Report. 2002).
Participants
Children ≤ 15 years of age, residing in the study area were eligible to participate in the study. The enumeration and identification of eligible candidates were undertaken by a medical/social/worker and a field investigator, and brought to make shift clinics in village schools. As a part of the demographic profile, data was gathered on the status of parental consanguinity of these children. The children were examined by one ophthalmologist. Children with minor eye diseases like conjunctivitis, refractive errors were treated by the ophthalmologist. In the second phase, children with major eye diseases like strabismus, cataract, retinal pathologies, and amblyopia were seen by the pediatric ophthalmologist when she visited the peripheral hospital in the study area on a periodic basis.
Sample size calculation
A sample size of 8115 was chosen from 30 standardized villages and 25 urban wards of the study area (villages/urban wards with < 200 households were clubbed together, those with household size ranging from 200 to 400 were retained as they were and those with 400+ households were divided into two or more units). The sample size was calculated using the formula 4 pq/l2 where p = prevalence of the factor of interest, q = 1-p or 100-p if it is expressed as a percentage, and I is the acceptable margin of error. The major disorder of interest for sample size calculation was ocular morbidity, 9.39% (95% CI, 8.006–10.93); prevalence of ocular morbidity was taken from personal communication—Babu RB. The Madhugiri Pediatric Eye Disease Study on April 2010 calculated a sample size of 8115 (taking 8% (lower limit of CI) as the prevalence, 10% acceptable margin of error and design effect of 1.5).
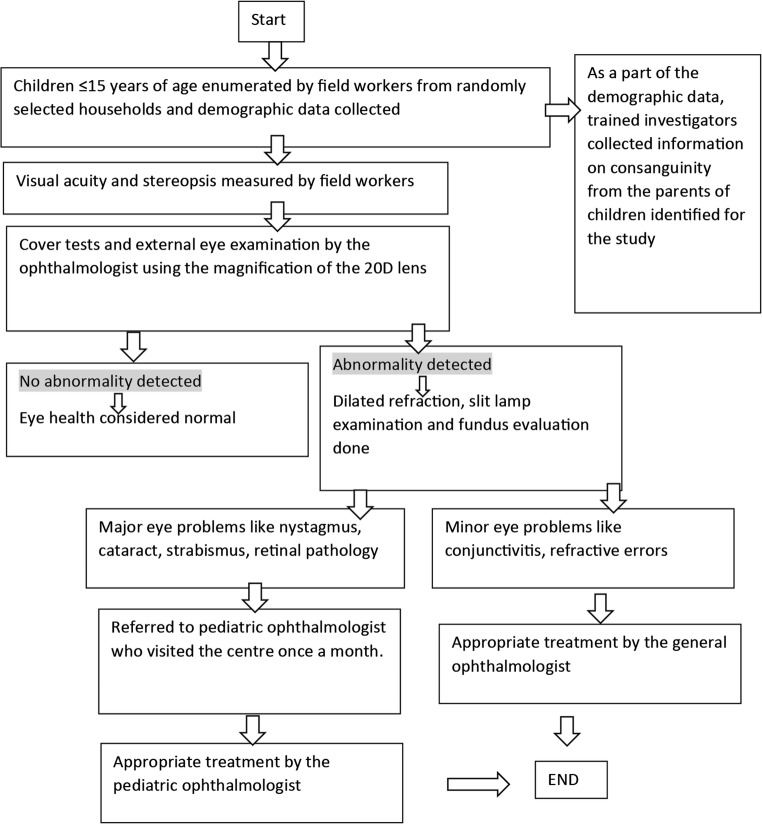
The methodology is illustrated in Fig. 1.
Fig. 1.
Showing the methodology of Pavagada Pediatric Eye Disease Study 2
Statistical analysis
The overall prevalence of blindness, ocular morbidity, and diseases with a potential genetic etiology were calculated in percentages. Ninety-five percent confidence limits were calculated for prevalence rates assuming a binomial distribution, using the formula, p ± 1.96 √(p(1-p))/n, where p is the prevalence of the ocular morbidity and n is the total population screened. The associations between consanguinity and presence of a disease with potential genetic etiology were evaluated using logistic regression models and odds ratio were reported. Statistical analyses were performed with commercially available software (Stata version 13.1; StataCorp, College Station, TX) and a p value of less than or equal to 0.05 was considered statistically significant.
Results
Children in the age group of 0–15 years (mean age, 8 years) were screened. Eight thousand five hundred and fifty-three children were examined. The prevalence of ocular morbidity was 6.54% (CI, 6.01–7.06) and blindness was 0.09% (CI, 0.03–0.16) (Kemmanu et al. 2018). The prevalence of diseases with a potential genetic etiology was 0.41% (CI, 0.29–0.57).
The percentage of consanguineously married couples in the screened population was 34.33%. About 69% of these couples were literate, but when we looked into the number of children with consanguineous parents in whom the mother had > 9 years schooling, only 38.16% had pursued education of > 9 years. Among children who were born to consanguineous parents, 57.20% were products of uncle-niece marriages (Table 1).
Table 1.
Pattern of consanguinity in the screened population
| Total number of couples in the population screened | 5173 |
| Total number of consanguineous couples in the population screened | 1776 (34.33%) |
| Number of consanguineous couples in whom the mother is literate | 1228 (69.144%) |
| Number of children with consanguineous parents (n = 8553)* | 3068 (35.87%) |
| Number of children who were products of first-cousin marriages** (n = 3068) | 998 (32.53%) |
| Number of children who were products of union between distant relatives** | 316 (10.30%) |
| Number of children who were products of uncle-niece marriages** | 1755 (57.20%) |
| Number of children with consanguineous parents in whom the mother is literate** | 2056 (67.01%) |
| Number of children with consanguineous parents in whom the mother is illiterate to ≤ 8 years schooling** | 1895 (61.84%) |
| Number of children with consanguineous parents in whom the mother > 9 years schooling** | 1169 (38.16%) |
| Number of children with consanguineous parents from rural area** | 1962 (63.95%) |
*8553 is the total number of children screened
**Denomination is 3068, i.e., those children with consanguineous parents
Among children with diseases with a potential genetic etiology, 54.29% of the children were born out of consanguineous union, whereas in the group with normal children along with children with diseases without a genetic etiology, 36.03% were products of consanguineous union (odds ratio = 2.551 and p = 0.012). Among children with diseases with a potential genetic etiology, 71.43% of the children born out of consanguineous union were children of uncle-niece marriages. Children born out of uncle-niece unions were significantly more likely to have diseases with potential genetic etiology (odds ratio, 2.60; p = 0.006) than first-cousin marriages (odds ratio, 1.263; p = 0.629) or marriages between distant relatives (odds ratio, 1.085; p = 0.937) (Table 2). Out of the seven children with cataracts, six were born out of consanguineous unions (85.71%). This was statistically significant with an odds ratio of 10.75 and a p value of 0.028 (Table 3).
Table 2.
Comparing consanguinity in children with diseases with potential genetic etiology versus normal + diseases without potential genetic etiology
| Children with Diseases with a potential genetic etiology (n = 35) | Normal children + children with diseases without genetic etiology (n = 8519) | Odds ratio (CI) and P value | |
|---|---|---|---|
| Children born of union between first cousins | 5 (26.32%)* | 998 (32.52%)# | 1.26 (0.49 to 3.26), 0.63 |
| Children born of union between distant relatives | 1 (5.26%)* | 316 (10.30%)# | 1.09 (0.14 to 8.21), 0.94 |
| Children born of uncle-niece union | 13 (68.42%)* | 1755 (57.18%)# | 2.55 (1.22 to 5.31), 0.01 |
| Total number of children who are product of consanguineous union | 19 (54.29%)** | 3069 (36.03%)† | 2.13 (1.09 to 4.15), 0.03 |
*The denominator is 19, i.e., the total number of children with diseases with genetic etiology, born out of consanguineous union
#The denominator is 3069, i.e., the total number of children who are normal and with diseases of no genetic etiology born out of consanguineous unions
**The denominator is 35, i.e., the total number of children with diseases with a potential genetic etiology
†The denominator is 8519, i.e., the total number of children with diseases without genetic etiology along with the normal children
Table 3.
Pattern of consanguinity in children having specific diseases with a potential genetic etiology
| Disease | Total number of children with the disease/prevalence | Number of children with consanguineous parents | Number of children born out of nonconsanguineous marriage | Odds ratio (CI) and p value | PAR (CI), p value | |||
|---|---|---|---|---|---|---|---|---|
| First cousin* | Distant relative* | Uncle-niece* | Total | |||||
| Whole globe (anophthalmos and microphthalmos) | 13, 0.15% (CI, 0.07–0.23) | 0 | 1 (16.66%) | 5 (83.33%) | 6 (46.15%)† | 7 (53.85%) | 1.53 (0.51 to 4.57), 0.44 | 0.02% (− 0.04 to 0.09), 0.47 |
| Lens (pediatric cataract) | 7, 0.08%, (CI, 0.02–0.14) | 1 (16.66%) | 0 | 5 (83.33%) | 6 (85.71%)† | 1 (44.29%) | 10.75 (1.30 to 89.32), 0.03 | 0.06% (0.01 to 0.12), 0.03 |
| Uvea (coloboma) | 15, 0.18% (CI, 0.09–0.26) | 2 (33.33%) | 0 | 4 (66.66%) | 6 (40%) | 9 (60%) | 1.19 (0.42 to 3.35), 0.74 | 0.01% (− 0.06 to 0.08), 0.74 |
| Retina (retinal dystrophy) | 4, 0.05%, (CI, 0.00–0.04) | 2 (66.66%) | 0 | 1 (33.33%) | 3 (75%) † | 1 (25%) | 5.37 (0.59 to 51.63), 0.15 | 0.03% (−0.01 to0.07), 0.18 |
| Total | 35#, 0.41% (CI, 0.29–0.57) | 5 (23.81%) | 1 (4.76%) | 15 (71.43%) | 19# (54.29%)† | 16# (45.71%) | 2.13 (1.09 to 4.15) 0.03 | 0.12% (0.00 to 0.23), 0.04 |
*The denominator is the total number of children with consanguineous parents in that particular row
†The denominator is the total number of children with the disease
#2 children had whole globe and uveal anomalies and 2 had uveal and lens anomalies. Hence, total is 35 and not 39
Among the eight blind children, six (75%) were blind with a disease with potential genetic etiology (whole globe, − 2; uveal coloboma, 2; cataract, 1; and retinal dystrophy, 1). Out of the six, four (66.67%) were born out of consanguineous marriage. All the marriages were uncle-niece unions.
Discussion
Consanguineous marriages are common in populations of North Africa, Middle East, West Asia, and south India and constitute 20–60% of all marriages (Tadmouri et al. 2009). In south India, the rate of consanguineous marriage is said to be about 20–50%; the highest level recorded in urban Pondicherry where the rate was estimated to be about 54.9% (Bittles 2001). Out of the couples in our study population, 34.33% were consanguineously married. A study by Nirmalan PK et al. found 26.7% of the subjects screened to be products of consanguineous union (Nirmalan et al. 2006). Another study in a tertiary care center in Chennai found 28.8% of the families screened to have a history of consanguinity (Kumaramanickavel et al. 2002). In our study, 35.85% of the children screened had consanguineous parents. About 64% of these children were from the rural areas. It is known that consanguineous marriages are more common in the rural areas (Bittles 2001). It is generally believed that education has a decreasing effect on the frequency of consanguineous unions (Fuster and Colantonio 2004; Hussain and Bittles 2000). However, in two studies in Pakistan (Jabeen and Malik 2014; Ahmed et al. 2016), consanguinity was observed to be significantly higher in the literate group compared to nonliterate. Our study found that 67% of the children born out of consanguineous unions had mothers who were literate. When we compared the consanguineous union among the group illiterates and ≤ 8 years of schooling and those with >9 years of schooling, we found that the latter group had 38.16% of children with consanguineous parents as compared to 61.84% in the former group (p = 0.001). This goes to say that mere literacy may not be enough to take pro-health decisions; it is the number of years of schooling, rather than literacy that is important.
In our study, 57% of the children born out of consanguineous unions were of uncle-niece unions, followed by first-cousin unions (32.53%). The preference for uncle-niece unions in south India has already been documented (Hamamy et al. 2011). Our study clearly showed that children born out of uncle-niece unions were more likely to have diseases with potential genetic etiology than first-cousin marriages or marriages between distant relatives. Uncle-niece share 1/4th of their genes and first cousins share 1/8th of their gene pool (Bennett et al. 2002). Hence, there is a greater risk of having an offspring afflicted with a hereditary disease in uncle-niece unions.
A study from south India reported that 44.6% of subjects with nonsyndromic ocular coloboma had consanguineous parents (Hornby et al. 2003). Another study from a genetic center in south India reported retinitis pigmentosa in 63.9% of the patients with consanguineous parents (Kumaramanickavel et al. 2002). In another study (Bener and Mohammad 2017), the odds of finding common eye diseases in people with consanguineous parents was 3.68 times (p value of 0.002) higher than those with nonconsanguineous parents. In our study, we found that the odds of finding cataracts in children born of consanguineous unions were high (10.75 times higher). Consanguinity was not significantly associated with the presence of other diseases (whole globe, uvea, and retina) with potential genetic etiology. There are studies from the West, which have shown that up to one third of the bilateral pediatric cataract cases are genetic, most of which are autosomal dominant (Churchill and Graw 2011; Shiels and Hejtmancik 2007). In areas with a high rate of consanguinity, due to increased expression of potential homozygous recessive genes, autosomal recessive inheritance of pediatric cataract comprises a large percentage of cases (Khan 2012). A study from south India (Nirmalan et al. 2006) did not find consanguinity to be significantly associated with the presence of eye diseases with potential genetic etiology. This could be possibly because, this study comprised of 35.7% of the people with uncle-niece parents, whereas in our study 57.2% of the children were born out of uncle-niece unions.
Our study has shown that consanguineous parents were 2.5 times more likely to have children with diseases with a potential genetic etiology as compared to nonconsanguineous parents (odds ratio = 2.551 and p = 0.012). This could still be an under estimation, considering that children with severe congenital anomalies may not have survived. Parental consanguinity is associated with an increased incidence of death in previous siblings (p < 0.000) (Bromiker et al. 2004).
Strengths and limitations of this study: strengths
There are only a few population-based studies that actually look into the relationship between childhood eye diseases and consanguinity. This study demonstrates the increased risk in children of consanguineous unions, and the increase of this risk with the degree of consanguinity (coefficient of inbreeding).
Limitations
We have not collected data on the number of abortions and child mortality and the pedigree was not recorded due to lack of trained investigators on the field. We have only taken the data of parental relationship of the children examined. This may result in an underestimation of the real amount of inbreeding especially in populations with a long standing history of inbreeding. We must also understand that prevalence estimates from cross-sectional studies will underestimate risk to a certain extent as young children who are still unaffected may turn out to be affected later on; for example, an infant examined may not manifest diseases like retinitis pigmentosa, but may manifest in the first decade of life.
To conclude, consanguinity may play a significant role in the occurrence of congenital eye diseases. Genetic counseling could be offered to consanguineous couples to prevent the possibility of having children with congenital malformations. Also, increasing awareness about the consequences of consanguineous marriages through education and awareness programs may help in decreasing the prevalence of congenital anomalies and their comorbidities, caused as a result of consanguineous unions.
Acknowledgements
We would like to sincerely thank Swami Japanandaji, Chairman and all the staff of Shree Sharada Devi Eye Hospital and Research Centre, Pavagada, Karnataka, India.
Funding Organization
This project was supported by the Indian Council of Medical Research (ICMR) vide Ref: 5/4/6/2/09 – NCD-11 dated 22.03.2011.Pavagada Pediatric Eye Disease Study/Beyond Blindness.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
For studies with human subjects
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was a population based cross-sectional study and was approved by the Institutional Review Board of the hospital and conducted within the tenets of the Declaration of Helsinki.
Informed consent
Informed consent for participating in the study has been taken from the parent/caregiver of the child.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed J, Rehman UA, Malik S. Determinants of consanguinity and inbreeding coefficient F in Dir lower district, north-west Pakistan: a multivariate approach. Iran J Public Health. 2016;45:537–539. [PMC free article] [PubMed] [Google Scholar]
- Bardakjian TM, Schneider A. The genetics of anophthalmia and microphthalmia. Curr Opin Ophthalmol. 2011;22:309–313. doi: 10.1097/ICU.0b013e328349b004. [DOI] [PubMed] [Google Scholar]
- Bener A, Hussain R. Consanguineous unions and child health in the State of Qatar. Paediatr Perinat Epidemiol. 2006;20:372–378. doi: 10.1111/j.1365-3016.2006.00750.x. [DOI] [PubMed] [Google Scholar]
- Bener A, Mohammad RR. Global distribution of consanguinity and their impact on complex diseases: genetic disorders from an endogamous population. Egypt J Med Hum Genet. 2017;18:315–320. doi: 10.1016/j.ejmhg.2017.01.002. [DOI] [Google Scholar]
- Bennett RL, Motulsky AG, Bittles A, Hudgins L, Uhrich S, Doyle DL, Silvey K, Scott CR, Cheng E, McGillivray B, Steiner RD, Olson D. Genetic counselling and screening of consanguineous couples and their offspring: recommendations of the National Society of Genetic Counsellors. J Genet Couns. 2002;11:97–120. doi: 10.1023/A:1014593404915. [DOI] [PubMed] [Google Scholar]
- Bittles AH. Consanguinity and its relevance to clinical genetics. Clin Genet. 2001;60:89–98. doi: 10.1034/j.1399-0004.2001.600201.x. [DOI] [PubMed] [Google Scholar]
- Bittles AH, Mason WM, Greene J, Rao AN. Reproductive behaviour and health in consanguineous marriages. Science. 1991;252:789–794. doi: 10.1126/science.2028254. [DOI] [PubMed] [Google Scholar]
- Bromiker R, Glam-Baruch M, Gofin R, Hammerman C, Amitai Y. Association of parental consanguinity with congenital malformations among Arab newborns in Jerusalem. Clin Genet. 2004;66:63–66. doi: 10.1111/j.0009-9163.2004.00264.x. [DOI] [PubMed] [Google Scholar]
- Churchill A, Graw J. Clinical and experimental advances in congenital and paediatric cataracts. Philos Trans R Soc Lond B: BiolSci. 2011;366:1234–1249. doi: 10.1098/rstb.2010.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dr D M Nanjundappa Committee Report (2002) High-Power Committee for Redressal of Regional Imbalances (HPCRRI) headed by the late D.M. Nanjundappa was submitted to the State Government
- Ferda PE, Ploder LA, Yu JJ, Arici K, Horsford DJ, Rutherford A, et al. Human microphthalmia associated with mutations in the retinal homeobox gene CHX10. Nat Genet. 2000;25:397–401. doi: 10.1038/78071. [DOI] [PubMed] [Google Scholar]
- Fuster V, Colantonio SE. Socioeconomic, demographic, and geographic variables affecting the diverse degrees of consanguineous marriages in Spain. Hum Biol. 2004;76:1–14. doi: 10.1353/hub.2004.0021. [DOI] [PubMed] [Google Scholar]
- Hamamy H, Antonarakis SE, Cavalli-Sforza LL, Temtamy S, Romeo G, Kate T, et al. Consanguineous marriages, pearls and perils: Geneva international consanguinity workshop report. Genet Med. 2011;13:841–847. doi: 10.1097/GIM.0b013e318217477f. [DOI] [PubMed] [Google Scholar]
- Hornby SJ, Dandona L, Jones RB, Stewart H, Gilbert CE. The familial contribution to non-syndromic ocular coloboma in south India. Br J Ophthalmol. 2003;87:336–340. doi: 10.1136/bjo.87.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain R, Bittles AH. Sociodemographic correlates of consanguineous marriage in the Muslim population of India. J Biosoc Sci. 2000;32:433–442. doi: 10.1017/S0021932000004338. [DOI] [PubMed] [Google Scholar]
- Jabeen N, Malik S. Consanguinity and its sociodemographic differentials in Bhimber District, Azad Jammu and Kashmir, Pakistan. J Health Popul Nutr. 2014;32:301–313. [PMC free article] [PubMed] [Google Scholar]
- Kemmanu V, Giliyar SK, Shetty BK, Singh AK, Kumaramanickavel G, McCarty CA. Emerging trends in childhood blindness and ocular morbidity in India: the Pavagada Pediatric Eye Disease Study 2. Eye (Lond) 2018;32(10):1590–1598. doi: 10.1038/s41433-018-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AO. Hereditary pediatric cataract on the Arabian Peninsula. Saudi J Ophthalmol. 2012;26:67–71. doi: 10.1016/j.sjopt.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaramanickavel G, Joseph B, Vidhya A, Arokiasamy T, Shetty SN. Consanguinity and ocular genetic diseases in South India: analysis of a five-year study. Community Genet. 2002;5:182–185. doi: 10.1159/000066334. [DOI] [PubMed] [Google Scholar]
- Li L, Chen Y, Jiao X, Jin C, Jiang D, Tanwar M, et al. Homozygosity mapping and genetic analysis of autosomal recessive retinal dystrophies in 144 consanguineous Pakistani families. Invest Ophthalmol Vis Sci. 2017;58:2218–2238. doi: 10.1167/iovs.17-21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Patil C, Naik VA. Prevalence of consanguineous marriages in a rural community and its effect on pregnancy outcome. Indian J Community Med. 2004;29:41–43. [Google Scholar]
- Nirmalan PK, Krishnaiah S, Nutheti R, Shamanna BR, Rao GN, Thomas R. Consanguinity and eye diseases with a potential genetic etiology. Data from a prevalence study in Andhra Pradesh, India. Ophthalmic Epidemiol. 2006;13:7–13. doi: 10.1080/09286580500473795. [DOI] [PubMed] [Google Scholar]
- Ramegowda S, Ramachandra NB. Parental consanguinity increases congenital heart diseases in South India. Ann Hum Biol. 2006;33:519–528. doi: 10.1080/03014460600909349. [DOI] [PubMed] [Google Scholar]
- Shawky RM, Elsayed SM, Zaki ME, El-Din SMN, Kamal FM. Consanguinity and its relevance to clinical genetics. Egypt J Med Hum Genet. 2013;14:157–164. doi: 10.1016/j.ejmhg.2013.01.002. [DOI] [Google Scholar]
- Shiels A, Hejtmancik JF. Genetic origins of cataract. Arch Ophthalmol. 2007;125:165–173. doi: 10.1001/archopht.125.2.165. [DOI] [PubMed] [Google Scholar]
- Tadmouri GO, Nair P, Obeid T, Al Ali MT, Al Khaja N, Hamamy HA. Consanguinity and reproductive health among Arabs. Reprod Health. 2009;6:17. doi: 10.1186/1742-4755-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]