Abstract
Different desert truffles, collected from Algerian Saharan soils, were identified and their capacity to produce bioactive substances with antimicrobial activity was analyzed. Based on morphological characterization using Melzer’s reagent staining, the collected strains were identified as Terfezia arenaria. The bioactive substances from T. arenaria were extracted using the following techniques: maceration with methanol and Soxhlet with dichloromethane. The former led to a yield much higher than that of the latter (i.e., 15% and 0.48%, respectively). Both extracts presented antifungal activities against all the tested strains (i.e., A. niger, Penicillium sp., and C. albicans). However, the dichloromethane extracts showed much higher antibacterial activities against all the tested bacteria (i.e., S. aureus, E. faecalis, E. coli, and P. aeruginosa) than the methanol extracts. The thin layer chromatography of both extracts confirmed the presence of polyphenols and flavonoids.
Keywords: Terfezia arenaria, Soxhlet extraction, Antimicrobial activity, Chromatography, Flavonoids, Polyphenols
Introduction
Desert truffles, commonly called Terfez, are the fruiting bodies of some symbiotic edible hypogenous fungi belonging to the phylum Ascomycota. Regardless of the Terfez’s species, it is not only highly appreciated within the local population but also worldwide and considered as a luxury foodstuff (Bradai 2006). Truffles live in mycorrhizal association with Cistaceae plants, especially of the genus Helianthemum and Cistus (Khanaqa 2006). Apart from some studies done on the mycorrhizal association of various species of Terfez with plants (Tadja 1996; Bassah 1999; Bradai 2006; Khanaqa 2006), research on Terfez in Southern Algeria is still limited. However, antibacterial and antifungal potential of some Terfez species such as Terfezia boudieri have been reported (Dib-Bellahouel and Fortas 2014; Hamza et al. 2016).
Many superior fungi are of interest in nutrition and human health. Thus, more than 2000 species are edible and nearly 700 species have interesting pharmaceutical properties (Wasser 2010). The medicinal properties of the superior fungi, particularly in Asian medicinal sectors, have been known for millennia (Chatin 1892). As for mycotherapy, it has emerged during the 1970s and 1980s and since then hundreds of studies conducted by Asian researchers have confirmed its interest. Currently, mycotherapy has been recognized universally due to its application on the treatment of degenerative diseases, cancer, and other pathologies (Attia et al. 2018). Due to the antioxidant properties of truffles’ extracts, several studies on prevention of chronic diseases, such as cardiovascular disorders, cancer, diabetes, high blood pressure, and Alzheimer’s and Parkinson’s diseases, were carried out (Riboli and Norat 2003; Cole et al. 2005).
In the present study, desert truffle species collected from Algerian Saharan soils were identified and the antimicrobial activity of their extracts was assessed. In addition, the composition of the extracts was examined by thin layer chromatography (TLC) and quantified by analytical techniques.
Materials and methods
Desert truffle collection, identification, and characterization
One type of desert truffles (100 g) was collected from the border region between Libya and Algeria (Djanet, Illizi city province (24°33′18″ North, 9°29′06″ East). The fresh truffles were cleaned and dried in the shade. Then they were ground into fine powder and stored at room temperature under dark and dry conditions until used. The genus identification of the harvested specimens was based on the staining by Melzer’s reagent (Josserand 1983). For this, a fragment of the fungus was taken and its wall was withered and crushed. Then a few drops of the Melzer’s reagent were added and, after 10 min, observed under an optical microscope (Optika) at 400× and 1000× magnification, and photomicrographs were taken with a camera (Bio microscopique Motic Image plus 2.0). Several successive observations after 4, 6, 24, and 48 h were required to confirm the final coloration of the asci. The development of yellow or orange coloration on the walls of the asci indicates that the specimen belongs to the genus Terfezia whereas the appearance of a gray–blue color indicates that it belongs to the genus Tirmania (Trappe 1979).
The identification of the Terfez species was based on the morphological characteristics of the fruiting bodies and the color of the glebe as well as the number of spores, asci, and their morphology. The characterization was done by comparing the macroscopic and microscopic observations of the identified species using the classification descriptions according to Trappe (1979), Bassah (1999), and Bouchet and Siebert (1999). Macroscopic characteristics were examined for each of the following parts of the fungus: the ascocarp, the peridium, and the glebe, in terms of shape, color, and size (Fig. 1).
Fig. 1.

Photograph of the ascocarp of different Terfez
The microscopic characterization was made by taking a fresh fragment of the species with a sterile needle, putting it on a blade, adding a drop of distilled water, and covering it with a coverslip. Then it was observed under an optic microscopic (Optika) at different magnifications (i.e., 10×, 40× and 100×), which allowed observing the morphology of the asci and the ascospores and measure their size. The measurements were made using the Bio-microscopic software Motic Image Plus 2.0 which gave the real size of the asci and the ascospores.
Antimicrobial activity of Terfezia arenaria
Pathogenic strains
Five bacterial strains were used for the study of the antibacterial activity of T. arenaria crude extract. Three of them were Gram-negative (i.e., Escherichia coli ATCC25922, Pseudomonas aeruginosa ATCC23853, and Salmonella typhimurium ATCC14028) and the other two were Gram-positive (i.e., Staphylococcus aureus ATCC 6538 and Enterococcus faecalis ATCC29212). For the antifungal activity of T. arenaria crude extracts, two filamentous fungi (i.e., Aspergillus niger ATCC16404 and Penicillium sp.) and the yeast Candida albicans ATCC10231 were used. All tested strains were obtained from the culture collection of the Pasteur Institute of Algeria (IPA). They were maintained on nutritive agar and stored at 4 °C.
Extraction of T. arenaria bioactive substances
To optimize the extraction yields, two extraction methods (i.e., maceration with methanol and Soxhlet with dichloromethane) were assessed. The extraction by maceration with methanol consisted of mixing 10 g of T. arenaria dry powder with 100 mL of methanol for 48 h in the dark at room temperature (Table 1). The methanol extracts obtained were filtered (cotton filter), concentrated using a rotary evaporator, and stored at 4 °C (Dib-Bellahouel and Fortas 2014; Neggaz et al. 2015). The Soxhlet extraction was carried out by mixing 50 g of dry powder of T. arenaria with 750 mL of dichloromethane (Table 1). The mixture was transferred into a filter paper extraction thimble and inserted into a Soxhlet assembly fitted with a 250-mL flask for 24 h. The heating mantle was set at a temperature higher than the boiling temperature of the solvent used. The ball contained some pieces of pumice to regulate the boiling. When the solvent came into contact with the T. arenaria dry powder (Soxhlet filter), it turned from brown to orange yellowish. The extraction was stopped when the liquid surrounding the extraction thimble became clear. Then the dichloromethane extract was concentrated under vacuum using a rotary evaporator and stored in an opaque bottle at 4 °C (Gouzi et al. 2011; Neggaz et al. 2015).
Table 1.
Yields and characteristics of methanol and dicholoromethane extracts of truffles from the species Terfezia arenaria
| Extraction method/solvent | Dried biomass (g) | Total extract (mg) | Yield (extract/dried biomass) (%) | Aspect of the extract | Color |
|---|---|---|---|---|---|
| Maceration (methanol 100 mL) | 10 | 1.55 | 15.5 | Viscous | Brown |
| Soxhlet (dicholoromethane 750 mL) | 50 | 0.24 | 0.48 | Smooth | Light brown |
Antimicrobial assay
The bacterial strains were grown overnight at 37 °C in nutrient agar, while C. albicans was grown in Sabouraud agar. The inoculum for the assays was prepared by inoculating three to five colonies from an agar plate culture into 10 mL of nutrient broth, and then incubated at 37 °C for 24 h. After growing, the microbial suspension was standardized with a sterile saline solution to a turbidity equivalent to 0.5 McFarland scale (108 CFU/mL for bacteria and 106 CFU/mL for C. albicans). For the filamentous fungi, mycelial plugs of 5 mm in diameter were taken from pre-cultures grown from 4 to 7 days, and then placed them into test tubes containing 10 mL of physiological sterile water. After vortexing, the absorbance was standardized between 0.08 and 0.12 optical density (OD) by a UV–Vis spectrophotometer at 625 nm. If necessary, the turbidity was decreased by adding more physiological sterile water or increased by adding more mycelial plugs. The antimicrobial activity of the crude extracts was assayed using the agar well-diffusion method on Mueller Hinton medium (MHA). For this, 0.1 mL of bacterial, yeast or fungal spore suspension was spread on the surface of MHA evenly. Wells of 6 mm in diameter were punched into the agar and filled with 25 μL of the crude extracts of the desert truffle T. arenaria. The plates were first kept at 4 °C for at least 2 h to allow the diffusion of any antibacterial metabolites, and then incubated at 37 °C for 24 h for the bacteria, 48 h for the yeast, and 4 days for the filamentous fungi. The antibacterial and antifungal activities of the extracts of T. arenaria were compared with those of the antibiotics oxacillin, ampicillin, ciprofloxacin, and azithromycin, which are the antibiotics usually employed against pathogenic strains. All experiments were carried out in triplicate. The antimicrobial activity was determined by measuring the zones of inhibition (ZOI) (Neggaz et al. 2015).
Thin layer chromatography
About 2 µg of the T. arenaria extracts were loaded onto TLC plates (Merck). The plates were developed in different solvent systems (Table 2) to separate the phenol compounds of the extracts and compared with standards (i.e., quercetin, gallic acid). The developed plate was air dried and visualized under a UV light at 254 and 366 nm, since according to Mansar-Benhamza et al. (2013) phenolic compounds are active molecules absorbing UV radiation at 366 nm. The Rf (retention factor) value of the bands was determined according to the following formula:
Table 2.
Solvent systems used in thin layer chromatography (TLC) of Terfezia arenaria extracts (Mansar-Benhamza et al. 2013)
| Solvent systems | Abbreviation | (v/v/v) |
|---|---|---|
| Chloroform/methanol | CM | (8/2) |
| Ethyl acetate/methanol/water | AME | (7/1.5/1.5) |
| Acetate/n-butanol | AB | (9/1) |
| Ethyl acetate/acetic acid/water | AAE | (8/1/1) |
| Ethyl acetate/diethyl ether | AE | (2/8) |
| Dichloromethane/methanol | DM | (8/2) |
| Dichloromethane/ethyl acetate | DA | (9/1) |
| Ethyl acetate/formic acid/water | AFE | (8/1/1) |
The revelation was made according to spot coloring, since there is a close relationship between the fluorescence and the chemical structure of the substances (Seghiri et al. 2009).
Determination of total phenolic content
The total phenolic compounds of the T. arenaria extracts were quantitatively determined with the Folin–Ciocalteu reagent according to the method of Waterman (1994). In this method, 0.1 mL of extract diluted tenfold with deionized water (to obtain an absorbance in the range of the prepared calibration curve) was transferred to a test tube and mixed with 0.25 mL of Folin–Ciocalteu reagent (previously diluted tenfold with deionized water). The mixture was left at room temperature for 2 min. Then 1.25 mL of 20% sodium carbonate solution was added to the mixture, mixed gently, shaken thoroughly, and 0.5 mL of water was added. The mixture was left to stand for 40 min and the blue color developed was measured at 725 nm using a UV–Vis spectrophotometer (Shimadzu). A calibration curve of gallic acid (ranging from 20 to 200 μg/mL) was used as a standard. The total phenolic content was expressed as milligram of gallic acid equivalents per gram of extract.
Determination of total flavonoid content
The aluminum chloride colorimetric method was modified from the procedure reported by Lamaison et al. (1991). Quercetin was used to make the calibration curve. For this, 400 μg of quercetin was dissolved in 10 mL of ethanol, and then diluted from 2.5 to 40 μg/mL. The diluted standard solutions (0.5 mL) were separately mixed with 0.5 mL of 95% ethanol and 1 mL of 2% aluminum chloride (AlCl3). After incubation at room temperature for 10 min, the absorbance of the reaction mixture was measured at 430 nm with a UV–Vis spectrophotometer (Shimadzu). For the determination of the flavonoid content in the samples, 0.5 mL of the crude extracts (100 µg/mL) was used and the total flavonoid content was expressed as milligram of quercetin equivalents per gram of extract.
Statistical analysis
Excel (Microsoft Corporation, USA) was used for the statistical analysis. Data are presented as mean ± SD.
Results
The identification and the macroscopic and microscopic characterization of the desert truffles, based on the morphological characteristics of their ascocarps and asci using the Meltzer’s staining technique, revealed that they belonged to the specie T. arenaria, locally called brown truffle (Fig. 1, Table 3). On the outside, the peridium presented an outer shell, whose thickness varied from 0.5 to 1 mm and had a color from pale yellow to brown. The flesh (gleba) was white, fleshy, and with small veins (furrows), pale yellow in color, and the number of spores ranged from six to eight with a diameter of 19 μm (Figs. 2, 3).
Table 3.
Macroscopic and microscopic characteristics of Terfezia arenaria
| Color of the fruiting bodies | Dark brown color, with a peduncle at the base |
| Diameter of the ascocarp | 62.8 mm |
| Surface configuration | Smooth; glabrous |
| Peridium | Thickness from 0.5 to 1 mm; color from pale yellow to brown; plectenchymatic |
| Gleba | White with pale yellow veins |
| Spores | 6–8 warty spores |
| Asci | Subrounded with dimension 78 × 69 μm |
| Ascospores | Spherical in shape 19 × 19 μm |
Fig. 2.
Macroscopic and microscopic observation of Terfezia arenaria: a fruity body dimension (mm); b ascospore dimension; c diameters of asci and ascospores desiccated at Gx 400; d diameters of fresh asci and ascospores at Gx 400
Fig. 3.
Staining of the asci of Terfezia arenaria by Melzer’s reagent at different times and magnifications (optical microscope): a at Gx100 after 10 min; b at Gx100 after 24 h; c at Gx40 after 48 h
Antimicrobial activities
The antibacterial and antifungal activities of different concentrations of T. arenaria extracts, obtained by two extraction methods (i.e., maceration with methanol and Soxhlet with dichloromethane), were tested against different sensitive microorganisms by the diffusion agar method. For the extracts obtained by the maceration method with methanol, it was found that the concentrations of 25 and 50 mg/mL showed low antibacterial activity against the strain Pseudomonas aeruginosa and no antibacterial activity against the rest of the tested bacteria (i.e., Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, and Salmonella typhimurium). For an extract concentration of 100 mg/mL, low antimicrobial activities against all the tested bacteria were noted. The same trend was found for an extract concentration of 200 mg/mL but with higher antimicrobial activities (Table 4). The strain S. typhimurium was found to be resistant to all the extract concentrations tested. As for the antifungal activities, all the extract concentrations (i.e., 25, 50, 100, and 200 mg/mL) presented antifungal activities against all the tested strains (i.e., Aspergillus niger, Penicillium sp., and Candida albicans), especially against the yeast C. albicans (Table 4). The highest inhibition diameter was obtained for 200 mg/mL of the extract against the fungus A. niger (22 mm) followed by the yeast C. albicans (18 mm) (Table 5).
Table 4.
In vitro antibacterial activity of Terfezia arenaria extracts by the diffusion disc method
| Method | Concentration (mg/mL) | Inhibition zone diameter (mm + SD) | ||||
|---|---|---|---|---|---|---|
| S. aureus | E. faecalis | E. coli | P. aeruginosa | S. typhimurium | ||
| Maceration (methanol extract) | 25 | n.a. | n.a. | n.a. | 6.8 ± 0.2 | n.a. |
| 50 | n.a. | n.a. | n.a. | 6.9 ± 0.4 | n.a. | |
| 100 | 7.0 ± 0.4 | 6.3 ± 0.7 | 6.8 ± 0.6 | 6.6 ± 0.7 | n.a. | |
| 200 | 9.3 ± 0.5 | 11 ± 0.8 | 10 ± 0.7 | 10.6 ± 0.9 | n.a. | |
| Soxhlet (dichloromethane extract) | 25 | 10.4 ± 0.6 | 10.2 ± 0.7 | 10.5 ± 0.9 | 8.0 ± 0.8 | n.r. |
| 50 | 10.7 ± 0.7 | 10.5 ± 0.5 | 11.8 ± 0.5 | 10 ± 0.7 | n.r. | |
| 100 | 11 ± 0.8 | 11 ± 0.7 | 14 ± 0.9 | 11 ± 0.5 | n.r. | |
SD standard deviation of three determinations, n.a. no activity (no IZD), n.r. not recommended
Table 5.
In vitro antifungal activities of Terfezia arenaria extracts by the diffusion disc method
| Method | Concentration (mg/L) | Inhibition zone diameter (mm ± SD) | ||
|---|---|---|---|---|
| A. niger | Penicillium sp. | C. albicans | ||
| Maceration (methanol extract) | 25 | 6.4 ± 0.3 | 6.5 ± 0.6 | 16 ± 0.7 |
| 50 | 6.4 ± 0.4 | 6.5 ± 0.5 | 17 ± 0.9 | |
| 100 | 7.8 ± 0.5 | 7.0 ± 0.3 | 17.2 ± 0.8 | |
| 200 | 22 ± 0.8 | 9.2 ± 0.4 | 18 ± 0.9 | |
| Soxhlet (dichloromethane extract) | 25 | 17 ± 0.9 | 10.5 ± 0.5 | 15.5 ± 0.8 |
| 50 | 18 ± 0.8 | 11 ± 0.6 | 19 ± 0.9 | |
| 100 | 21 ± 0.7 | 11.5 ± 0.4 | 17.5 ± 0.7 | |
SD standard deviation of three determinations
Regarding the T. arenaria extracts obtained by the Soxhlet method with dichloromethane, they presented strong antibacterial activities against all the tested bacteria (i.e., S. aureus, E. faecalis, E. coli, and P. aeruginosa) for all the extract concentrations tested (i.e., 25, 50, and 100 mg/mL). The highest inhibition diameter was obtained for 100 mg/mL of the extract against E. coli (14 mm) (Table 4). Also, high antifungal activities for all the tested extract concentrations (i.e., 25, 50, and 100 mg/mL) against all the tested strains (i.e. A. niger, Penicillium sp., and C. albicans), especially against the fungus A. niger followed by the yeast C. albicans, were observed (Table 5). The highest inhibition diameter was obtained for 100 mg/mL of the extract against the fungus A. niger (21 mm) (Table 5).
Analysis of T. arenaria extracts
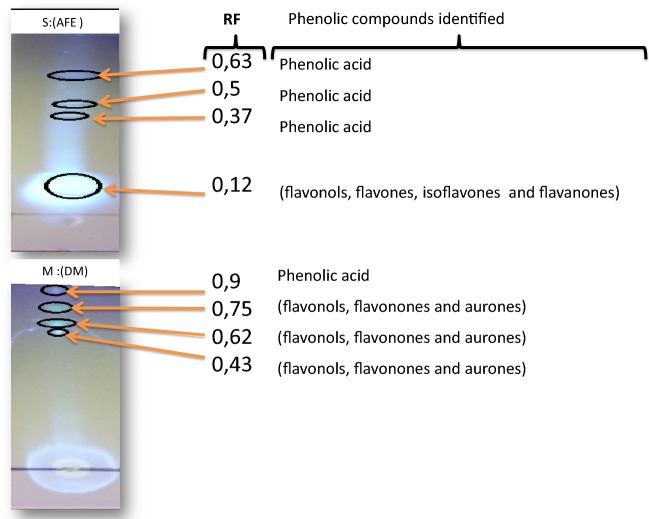
The observation of the TLC plates under UV at 366 nm showed spots of different colors (blue, fluorescent white blue, green, and red) which may correspond to several classes of secondary metabolites, e.g., phenolic compounds (Fig. 4). The composition of the T. arenaria extract obtained by the maceration method showed a less complex composition than that obtained by the Soxhlet one and, therefore, its separation was easier.
Fig. 4.
Thin layer chromatography (TLC) of Terfezia arenaria extracts obtained for both methods (i.e., Soxhlet and maceration). The spots were visualized under UV at 254 nm. S Soxhlet method, M maceration method, AFE ethyl acetate/formic acid/water, DM dichloromethane/methanol
The total phenol content and the total flavonoid content for the dichloromethane extract (i.e., Soxhlet method) and the methanol extract (i.e., maceration method) of T. arenaria are displayed in Table 6. The former was found to be 55.02 and 48.99 μg GAE/mg for the Soxhlet and the maceration extracts, respectively. This slight difference (about 11%) can be related to the Soxhlet extraction that was carried out at a higher temperature than the maceration technique (45 and 25 °C, respectively). The total flavonoid content obtained for the maceration extract was about 72% higher than those obtained for the Soxhlet extract (Table 6). This might be due to methanol which is more polar than dichloromethane, thereby affecting the solubility of flavonoids. It has been reported that flavonoid solubility is strongly affected by the nature of both the solvent and the flavonoid structure (Chebil et al. 2007).
Table 6.
Total phenol and flavonoid contents of Terfezia arenaria crude extracts
| Soxhlet (dichloromethane extract) | Maceration (methanol extract) | |
|---|---|---|
| Total polyphenol content (μg GAE/mg extract) | 55.02 | 48.99 |
| Total flavonoid content (μg QE/mg extract) | 5.68 | 9.79 |
Gallic acid equivalents, QE quercetin equivalents
Discussion
The morphological and microscopic examination of the mature Terfez’s gleba showed that it was formed by a large number of mycelia and contained between six and eight ascospores. The Meltzer’s reagent staining allowed identifying the specimen as Terfezia areneria as well as the warty spore’s plectenchymatic peridium (Neggaz et al. 2015).
Two extraction techniques (i.e., maceration with methanol and Soxhlet with dichloromethane) were used to select the best one from the point of view of yield of extraction and active biomolecules. The yields of extraction using the Soxhlet method with dichloromethane and the maceration method with methanol for T. arenaria were 0.48% and 15% [(grams of extract/grams of T. arenaria powder) × 100], respectively. Hence, the highest yield was obtained for the maceration extraction with methanol (i.e., 15%). This value is similar to that obtained by Neggaz et al.(2015) who obtained a yield of 13.1% for Terfezia claveryi extraction by maceration with methanol.
Terfezia arenaria extracts showed a significant inhibitory effect on the growth of most of the microbial strains tested, especially for the Soxhlet extracts. Thus, it was found that T. arenaria extracts obtained by the Soxhlet method with dichloromethane presented a significant antibacterial and antifungal (yeast and filamentous fungi) activity. Thus, the dichloromethane extract was found to be effective against all the tested bacteria, yeast, and filamentous fungi. These results agree with those reported by Neggaz et al. (2015) who found that the Soxhlet extract of T. claveryi showed greater antimicrobial activities against Gram-positive, Gram-negative, and yeast than that of the maceration extract.
The composition of the T. arenaria extracts obtained by the maceration method presented less phenolic compounds than that obtained by the Soxhlet method. This is due to the difference in solvent diffusion into the powder of the plants in the maceration step and possibly to the nature of the solvents used for extraction (Naczk and Shahidi 2004).
Conclusions
From the present research study, morphological, microscopical, and Meltzer’s staining showed that the collected specimens belonged to the T. arenaria species. This species was identified for the first time in the South East region of Algeria (Djanet Illizi City). This study showed that the extracts of T. arenaria exhibited antimicrobial activity against pathogenic bacteria, yeast, and filamentous fungi. It is worthy to point out that the antifungal potential against the yeast Candida albicans was stronger than in previous reported studies.
The best bioactive compound extraction yield was achieved when the maceration method with methanol was used compared to the Soxhlet extraction with dichloromethane. The molecular studies of the 28 s regions of the collected desert truffles, their purification and chemical characterization, and their production of bioactive compounds will be the future direction of this study.
Acknowledgements
Authors acknowledge the contribution of Dr. Peter Elias Kidibule of Universidad Autónoma de Madrid (UAM), Spain, as well as volunteers for completion of this study.
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interest.
References
- Attia WY, El-Naggar RE, Bawadekji A, Al Ali M. Evaluation of some in vitro anti-carcinogenic activities of polysaccharides extracted from Ascomata of the desert truffle Terfezia claveryi Chatin. J Appl Environ Biol Sci. 2018;8:152–159. [Google Scholar]
- Bassah G (1999) Contribution à l’étude de la symbiose mycorhiziennes entre deux espèces de Terfez: Terfezia claveryi, Terfezia boudieri avec le pin d’Alpe (Pinus halepensis Mill) dans la région du Djelfa. M.Sc. Thesis. Institut National Agronomique El-Harach, Algeria
- Bouchet R, Siebert E. Proton conduction in acid doped polybenzimidazole. Solid State Ionics. 1999;118:287–299. doi: 10.1016/S0167-2738(98)00466-4. [DOI] [Google Scholar]
- Bradai L (2006) Contribution à l’étude bioécologique de la truffe blanche de désert (Terfezia sp) cas de la région de Oued Mya (Ouargla). M.Sc. Thesis. University Kasdi Merbah Ouargla, Algeria
- Chatin A. La Truffe. J.E. Baillière et fils, Paris: Botanique de la Truffe et des plantes Truffières; 1892. [Google Scholar]
- Chebil L, Humeau C, Anthoni J, Dehez F, Engasser JM, Ghoul M. Solubility of flavonoids in organic solvents. J Chem Eng Data. 2007;52:1552–1556. doi: 10.1021/je7001094. [DOI] [Google Scholar]
- Cole GM, Lim GP, Yang F, Teter B, Begum A, Ma Q, Harris-White MC, Frautschy A. Prevention of Alzheimer’s disease: omega-3 fatty acid and phenolic antioxidant interventions. Neurobiol Aging. 2005;26:S133–S136. doi: 10.1016/j.neurobiolaging.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Dib-Bellahouel S, Fortas Z. Activity of the desert truffle Terfezia boudieri Chatin, against associated soil microflora. Afr J Microbiol Res. 2014;8:3008–3016. doi: 10.5897/AJMR2014.6881. [DOI] [Google Scholar]
- Gouzi F, Prefaut C, Abdellaoui A, Vuillemin A, Molinari N, Ninot G, Hayot M. Evidence of an early physical activity reduction in chronic obstructive pulmonary disease patients. Arch Phys Med Rehabil. 2011;92:1611–1617. doi: 10.1016/j.apmr.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Hamza A, Zouari N, Zouari S, Jdir H, Zaidi S, Gtari M, Neffati M. Nutraceutical potential, antioxidant and antibacterial activities of Terfezia boudieri Chatin, a wild edible desert truffle from Tunisia arid zone. Arab J Chem. 2016;9:383–389. doi: 10.1016/j.arabjc.2013.06.015. [DOI] [Google Scholar]
- Josserand A (1983) Apport de l’immunofluorescence à l’étude écologique des germes nitrifiants (genre Nitrobacter). Ph.D. Thesis, University of Lyon, France
- Khanaqa A. Truffle production in the Kingdom of Saudi Arabia-potential and limitation. J Appl Bot Food Qual. 2006;80:14–18. [Google Scholar]
- Lamaison JL, Petitjean-Freyet C, Carnat A. Lamiacees medicinales à proprietes antioxydantes, sources potentielles d’acide rosmarinique. Pharm Acta Helv. 1991;66:185–188. [PubMed] [Google Scholar]
- Mansar-Benhamza L, Djerrou Z, Pacha H. Evaluation of anti-hyperglycemic activity and side effects of Erythraea centaurium (L) Pers in rats. Afr J Biotechnol. 2013;12:6980–6985. [Google Scholar]
- Nazck M, Shahidi F. Extraction and analysis of phenolics in food. J Chromatogr A. 2004;1054:95–111. doi: 10.1016/S0021-9673(04)01409-8. [DOI] [PubMed] [Google Scholar]
- Neggaz S, Fortas Z, Chenni M, El Abed D, Ramli B, Kambouche N. In vitro evaluation of antioxidant, antibacterial and antifungal activities of Terfezia claveryi. Chatin Phytothérapie. 2015;16:20–26. doi: 10.1007/s10298-015-0993-4. [DOI] [Google Scholar]
- Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78:559–569. doi: 10.1093/ajcn/78.3.559S. [DOI] [PubMed] [Google Scholar]
- Seghiri R, Boumaza O, Mekkiou R, Benayache S, Mosset P, Quintana J, Benayache F. A flavonoid with cytotoxic activity and other constituents from Centaurea africana. Phytochem Lett. 2009;2:114–118. doi: 10.1016/j.phytol.2009.03.002. [DOI] [Google Scholar]
- Tadja A (1996) Etude écologique de deux espèces de Terfezia du sud Oued Algérie Essai de leur mycorhization sur trois espèces céréalières. M.Sc. Thesis Institut National Agronomique El-Harrach, Algeria
- Trappe JM. The orders, families and genera of Hypogeous ascomycotina (truffles and their relatives) Mycotaxon. 1979;9:297–340. [Google Scholar]
- Wasser S. Medicinal mushroom science: history, current status, future trends, and unsolved problems. Int J Med Mushrooms. 2010;12:1–16. doi: 10.1615/IntJMedMushr.v12.i1.10. [DOI] [PubMed] [Google Scholar]
- Waterman PG. Analysis of phenolic plant metabolites. Oxford: Blackwell Scientific Publications; 1994. [Google Scholar]