Abstract
The present study was aimed to investigate the effects of 3D8 scFv-secreting Probiotic Lactobacillus reuteri (L. reuteri) on growth performance, inflammatory responses, and intestinal microbial flora in chickens. To this end, a total of 14 healthy wild-type chickens were divided into two experimental groups. Each group was orally administrated with a daily dose of 109 colony-forming units (CFU) of 3D8 scFv-producing L. reuteri or wild-type (WT) for 35 days. Administration of L. reuteri/3D8 scFv significantly improved the body weight of chickens when compared to L. reuteri/WT group. The bacterial taxonomic composition of the fecal microbiota was determined by pyrosequencing of 16S rRNA gene amplicons. Firmicutes, Actinobacteria, and Proteobacteria were dominant phyla in two experimental groups. However, in 3D8 L. reuteri treatment groups at genus level, the Lactobacillus was highly abundant, being represented by 18.12%. In addition, serum levels of primary cytokines such as IL-6, IL-8, TNF-α, IFN-γ, IL-4, and IGF1 were markedly reduced in the probiotic L. reuteri 3D8 group. In summary, our results indicate that the administration of L. reuteri expressing 3D8 scFv has a modulatory effect on inflammatory responses, improves weight gain while not affecting the common microbial composition of the chicken intestine.
Keywords: Lactobacillus reuteri, 3D8 scFv, Chicken, Oral administration receive
Introduction
Due to the rapid development of the poultry industry, high accumulation densities and high-yield requirements have induced broiler chickens to have higher immunological stress reactions and increased the hazard of disease outbreaks in commercial poultry flocks (Wu et al. 2018). Grievous stress conditions are a major epidemiological factor that disturb immune homeostasis and induce immunological stress responses, followed by inflammation of intestine and mortality. Hence, immunological stress indirectly causes huge economic burden due to production losses and higher expenses for treatment and disease prevention. Considering the economic impact, our investigation involved exploring alternative substances that could modulate the chicken immune system and reduce the immune stress conditions, which is of great significance. Previously, Mappley et al. (2013) reported that Lactobacillus-based probiotics are well known to protect against infection by common enteric pathogens in the livestock industry. Similarly, Balamuralikrishnan et al. (2017)reported that bacterial probiotics are most commonly used in poultry because of their ability to sporulate.
Lactobacillus is a predominant genus of Gram-positive lactic acid bacteria in the avian alimentary tract that possesses nonsporulating, catalase-negative, and acid-tolerant characteristics and is devoid of cytochromes (Hill et al. 2005). Previous literature has reported that Lactobacillus paracasei-(L. paracasei) secreting 3D8 single-chain variable fragment (3D8 scFv) provides a basis for the development of ingestible antiviral probiotics that are active against gastrointestinal viral infection (Hoang et al. 2015). However, many studies have focused on the impact of probiotic administration on the normal microbiota. As some alterations of the microbial population are connected with intestinal disorders similar to inflammatory disease and obesity, it is important to know whether probiotics could alter the composition or activity of the host microbiota (Fiocchi 1998; Ley 2010). Along with this imperative, there is a consequential desire to carry out similar studies using different known probiotic microorganisms to increase our knowledge of the impact of novel Lactobacillus strains on the normal microbiota, to evaluate the efficiency with which Lactobacillus species deliver 3D8 scFv in the gut of chickens, and to enumerate their effect on metabolic processes after excretion.
Earlier findings of our laboratory showed the nonspecific nuclease activity of 3D8 scFv towards DNA and RNA (Byun et al. 2017). The 3D8 scFv protein purified from Escherichia coli was subsequently shown to penetrate the cytosol of HeLa cells via caveolae-mediated endocytosis. As an illustration, the treatment of porcine kidney cells with 3D8 scFv confers resistance against classical swine fever viral infection (Jun et al. 2010). Lee et al. (2014) suggest the antiviral effects of 3D8 scFv against DNA viruses in a human cell line and in mice that are based on the detection of DNase activity in the nucleus and RNase activity in the cytoplasm. Hence, 3D8 scFv antiviral activity against various viral infections is broad spectrum, and our study primarily aimed to investigate the prospect of using Lactobacillus reuteri (L. reuteri) (GenBank accession number CP029615) as a delivery system for 3D8 scFv to enhance the growth performance and microbial composition production of healthy chickens. L. reuteri strains were isolated from chicken small intestine and identified based on the 16 sRNA gene sequencing (Kim et al. 2018). The Lactobacillus species carrying 3D8 scFv was administered orally to chickens and tested for its beneficial and functional effects. To further explore the effect of the Lactobacillus strain, we measured growth performance, cytokine levels, and bacterial communities from feces of chickens.
Materials and methods
Construction of recombinant L. reuteri expressing 3D8 scFv
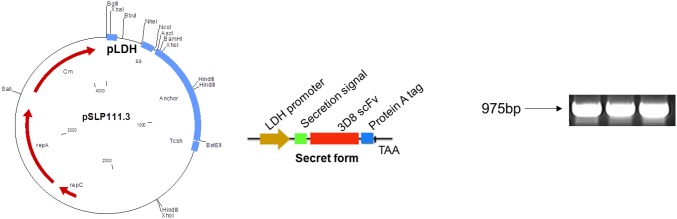
The pSLP111.3 expression vector for Lactobacillus was provided by Dr. Jos Seegers (Falcobio, Netherland). To induce 3D8 scFv protein expression, codon-optimized 3D8 scFv was cloned into pSLP-LDH that was slightly modified from the original vector as previously described (Hoang et al. 2015) (Fig. 1). Wild-type (WT) L. reuteri (SKKU-OGDONS-01) was cultured at 37 °C for 2–3 h before transformation. At the early log phase, L. reuteri was harvested and washed twice using PBS. 3D8 scFv expression vectors were transformed into L. reuteri by electroporation using a Bio-Rad Gene Pulser Xcell electroporator (Bio-Rad Laboratories, Hercules, CA, USA). After electroporation, transformed L. reuteri was incubated anaerobically on MRS plates containing chloramphenicol.
Fig. 1.
Cloning strategy for cell wall secretion of 3D8 scFv via the pSLP-LDH expression vector in Lactobacillus reuteri (a). L. reuteri designed to secrete 3D8 scFv (b). PLDH Lactobacillus dehydrogenase promoter, Ss SlpA secretion signal, Pro A protein A tag to prevent fusion of 3D8 scFv with the cell wall-anchoring domain of PrtP (anchor sequence). For 3D8 scFv protein expression, a codon-optimized 3D8 scFv gene (975 bp) was cloned into the pSLP-LDH vector (c)
Assessment of 3D8 scFv expression in recombinant L. reuteri
To analyze the expression level of 3D8 scFv protein in recombinant L. reuteri, a recombinant L. paracasei ATCC 334 strain (Hoang et al. 2015) that had previously been confirmed for protein expression was used (Bioneer Co, Daejeon, Republic of Korea) according to the manufacturer’s protocols. Quantitative real-time PCR to analyze the expression level of 3D8 scFv in transformed L. reuteri was conducted with SYBR Premix Ex Taq (TaKaRa, Otsu, Shinga, Japan) and a Rotor-Gene Q system (Qiagen, Chadstone, Victoria, Australia). The universal 16S rRNA gene as an internal control and 3D8 scFv gene were amplified with the indicated primers: 16S rRNA (forward 5′-CAYRCCGTAAACGATGARTGCTA-3′; reverse 5′-TAAGGTTCTTCGCGTWGCWTC-3′) and 3D8 scFv (forward 5′-GGCAGTATCTGCTGGTGAGA-3′; reverse 5′-CAGTGCCTGAACCACTACCA-3′) (Fig. 2).
Fig. 2.
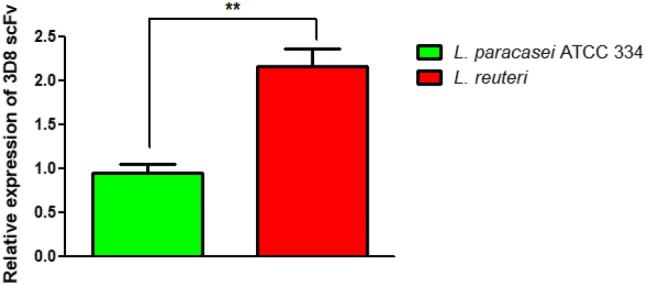
The expression level of 3D8 scFv protein in recombinant L. reuteri and recombinant L. paracasei ATCC 334 strains. The expression level of 3D8 scFv in transformed L. reuteri was determined with SYBR Premix Ex Taq by real-time RT-PCR
Experimental design, birds and husbandry
All experiments were performed following an approved animal-use document and according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the National Institute of Animal Science (Approval No: 2018-273), South Korea. A total of 14 normal, healthy 10-week-old male wild-type chickens were used for a 35-day feeding trial period. Chickens were housed individually in a 38 × 50 × 40 cm wire cage and provided a 16 h light program with 8 h of dark/day. During the experimental period, the chickens were observed daily to measure the mortality rate. All chickens were identified using individual tags; water and feed were supplied ad libitum. Chickens were randomly separated into two experimental treatment groups. Chickens were oral administrated 109 colony-forming units (CFU) (per chicken per day) of L. reuteri/WT and L. reuteri/3D8 using a syringe and blunt-end catheter. The basal diet was formulated to exceed the nutritional requirement of the chickens according to the NRC (1994) experimental recommendations.
Growth performance and determination of cytokine levels by ELISA
In this experiment, we first checked the body-weight gain in chickens every week and recorded the body-weight gain individually. At the end of the experiment, we collected serum samples to determine cytokine levels by enzyme-linked immunosorbent assay (ELISA) experiments. Serum concentrations of IL-6, IL-Iβ, IL-8, TNF-α, IFN-γ, IL-4, and IGF1 were determined using commercial chicken ELISA kits (Genorise Scientific, Glen Mils, USA, and Wuhan Abebio Science, Co., Ltd., Wuhan city, China).
Sample preparation and 16S ribosomal RNA gene sequencing
At the end of the experiment, fecal samples were collected from chickens, and genomic DNA was extracted using an Extra Master™ Fecal DNA extraction kit according to the manufacturer’s protocol (Epicentre, Madison, WI, USA). An Illumina 16S rRNA sequencing library was prepared for the V3 and V4 regions, and paired-end sequencing using a 2 × 300 bp paired-end protocol was conducted on the MiSeq platform (Illumina, San Diego, CA, USA) at Macrogen (Seoul, Republic of Korea). Sequencing reads of samples were assigned to a specific sample by their endemic bar codes. PCR primer sequences, barcodes, and linkers were then removed from the original sequence analysis. For the subsequent analysis, the pyrosequencing reads were selected based on a quality-filtering process with reads containing more than 300 base pairs and with an average quality score of more than 25. The EzTaxon-e database was used with the BLAST search tool to perform taxonomic alignment of high-quality bacterial sequence reads. Sequences that could not be matched to the EzTaxon-e database, which was set at a 97% identity species level, were used in a second-order UCHIME program to identify unmatched sequences. Operational taxonomic units (OTUs) were generated using a CD-HIT program at a similarity level of 97% according to protocol procedures designated by (Edgar 2010). Microbial community richness and diversity indexes were analyzed by the Shannon–Weaver diversity index, and Chao1, Shannon, and Good’s library coverage were determined by the mothur program.
Statistical analysis
Data analyses were conducted using GraphPad Prism statistical software (GraphPad Software version 5.03). Student’s t test was used for supplementation group comparison, and a P-value less than 0.05 was considered statistically significant. The data are presented as mean ± SE or SD.
Result
Growth performance and cytokine levels in serum
To analyze the expression level of 3D8 scFv in recombinant L. reuteri and recombinant L. paracasei ATCC 334 strain, qPCR experiment was performed. As expected, the relative expression of 3D8 scFv in L. reuteri was significantly higher than the L. paracasei ATCC 334 (Fig. 2).
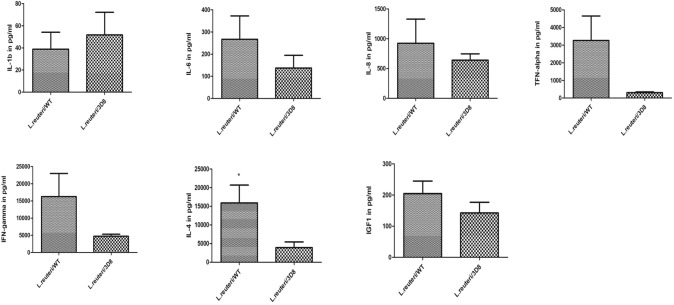
All the chickens were in very good health during the 5-week experimental period. Oral administration of probiotics L. reutrti expression of 3D8scFv showed improved body-weight gain compared with that in the wild-type L. reuteri (Fig. 3). In addition, chicken serum levels of the primary cytokines IL-6, IL-8, TNF-α, IFN-γ, IL-4, and IGF1 were markedly reduced in the 3D8 L. reuteri administration experimental group. However, IL-1β levels in the serum of chickens were profoundly increased by the oral administration of L. reuteri/3D8 (Fig. 4).
Fig. 3.
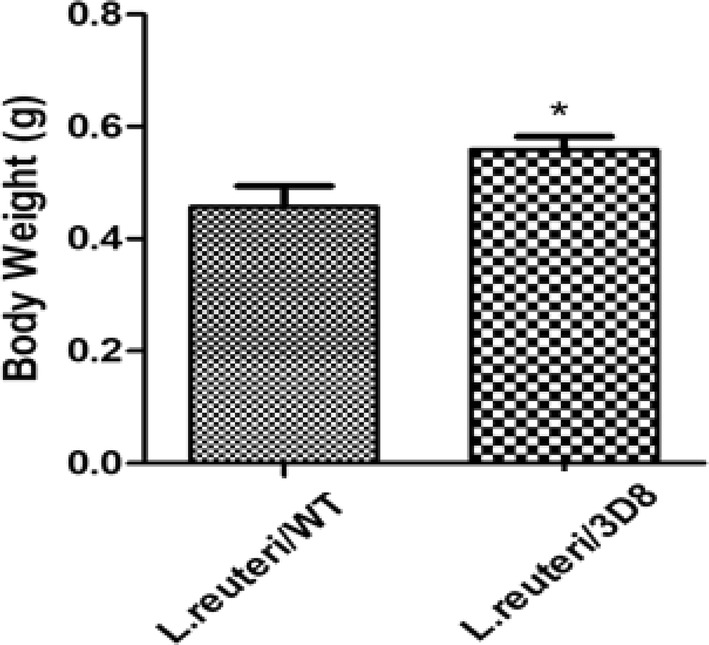
Effect of L. reuteri secreting 3D8 scFv on growth performance traits in chickens. Statistical comparisons were made between chickens that received WT or 3D8 scFv-expressing L. reuteri administration once/day for 35 days. The results are expressed as the mean ± SE or SD
Fig. 4.
Effects of L. reuteri secreting 3D8 scFv on serum cytokine levels in chickens. Statistical comparisons were made between chickens that received WT or 3D8 scFv-expressing L. reuteri once/day for 35 days. The results are expressed as mean ± SE or SD
Bacterial taxonomic composition
This molecular bacterial typing represents 269 bacterial species, of which 176 species were found to be unique. Of the 269 bacterial species, 148 and 121 species were identified from the WT-and 3D8-producing L. reuteri groups, respectively, indicating the existence of differential microbial communities between the two groups. The 176 unique bacterial species mostly comprise members of the phylum Firmicutes, followed by those of Proteobacteria and Bacteroidetes. Out of the 176 unique bacterial species, 93 species were commonly present, including 22 Lactobacillus species in two treatment groups. However, 28 and 55 species were specific to the WT L. reuteri and 3D8 L. reuteri oral administration groups, respectively. When compared with the 3D8 L. reuteri treatment group, the WT L. reuteri group exclusively contained Lactobacillus kitasatonis and Lactobacillus rodentium. We examined the bacterial taxonomic abundance in chicken fecal samples after oral administration of L. reuteri was showed differences at the phylum and genus levels (Figs. 5 and 6).
Fig. 5.
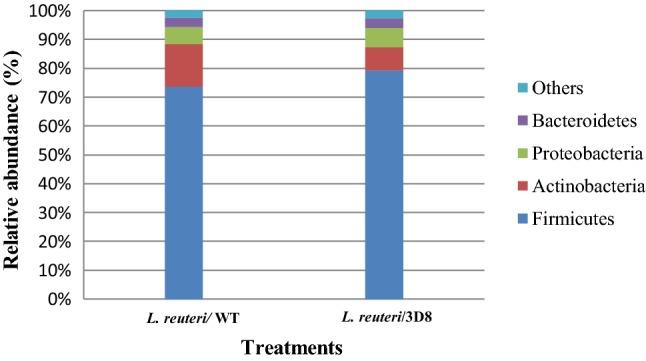
Bacterial taxonomic composition for the fecal microbiota of the chickens that received L. reuteri dietary treatment at the phylum level, in which each bar in the stacked bar charts represents the classifications of the total sequences
Fig. 6.
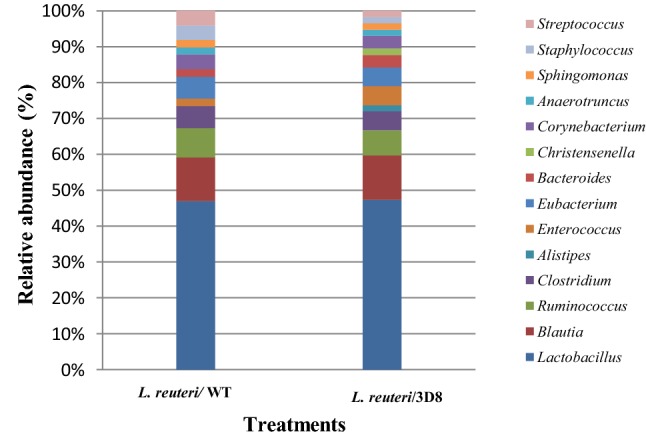
Bacterial taxonomic composition from the fecal microbiota of the L. reuteri dietary supplementation groups at the genus level, in which each bar in the stacked bar charts represents the classifications of the total sequences
The fecal microbial data for the two experimental groups were analyzed to determine the percentage of relative abundance (taxon reads/total reads in a sample). At the phylum level, Firmicutes (79.08%), Proteobacteria (8.05%), Actinobacteria (6.71%), and Bacteroidetes (3.35%) showed differences in abundance between the L. reuteri/3D8 oral administration group and the L. reuteri/WT group. Determination of the microbiota composition in the L. reuteri/3D8 group revealed that the chicken fecal microbiota was composed mostly of the phylum Firmicutes. Interestingly, Lactobacillus species were more abundant in the WT L. reuteri treatment group (19%) than in the L. reuteri/3D8 group (18.12%). Furthermore, continuous administration of L. reuteri the abundance of Bacteroides, Enterococcus, and Eubacterium was increased, while that of Blautia, Ruminococcus, and Clostridium was decreased, in the 3D8 L. reuteri treatment group (Fig. 6).
Discussion
The genus Lactobacillus comprises nonpathogenic Gram-positive residents of livestock intestinal microbiota widely used as probiotics. Although the behavior of this genus is not fully understood, using these beneficial bacteria in both humans and livestock animals is an area of thorough research (Callaway et al. 2008; Brisbin et al. 2010). Previously, Angel et al. (2005) and Awad et al. (2009) reported these bacteria to improve production constraints and reduce food-borne pathogens. However, they treated animals with various members of the Lactobacillus genus shown to influence various aspects of the immune response. The current data from this study showed that the oral administration of 3D8 L. reuteri treatment group significantly improved body-weight gain in the overall experiment. Our results were in agreement with Hofacre et al. (2003) and Zulkifli et al. (2000) who observed that Lactobacillus administration in chicken feed improved average daily gain (ADG). Previous study indicates that sex affects performance traits of chicken. Benyi et al. (2015) reported that growth performance and morality rate is higher in male chicken compared to female chicken. In agreement with these studies we used male chicken for this experiment. Similarly, Liu et al. (2005) suggested that dietary supplementation with transformed L. reuteri Pg4 improved body-weight gain during the growing phase of the chicken.
A previous study by Jia et al. (2010) reported that the fecal microbiota plays an important role in host health in the small and large intestines, including the energy intake from diet, growth performance, response to gastrointestinal diseases, and improved immune function. Lactobacillus administration provided several health benefits ensuing from improved digestion of chickens (Deeth and Tamime 1981). In the present study, Firmicutes (79.08%), Proteobacteria (8.05%), Actinobacteria (6.71%), and Bacteroidetes (3.35%) were the most common phyla identified in chicken fecal samples from 3D8 L. reuteri administration group. These results are consistent with Lamendella et al. (2011) who reported that the most abundant major phyla in chicken fecal are Firmicutes and Bacteroidetes. Similarly, Yan et al. (2015) have reported that Firmicutes and Proteobacteria maximum bacterial taxonomic composition for the fecal microbiota from the L. reuteri treatment group at the phylum level. In addition, Montalban-Arques et al. (2015) previously reported that L. reuteri supplementation has many physiological effects on birds, including microbial interference, antimicrobial effects, and dietary supplementation as well as reduction in serum cholesterol and antitumor effects. Lactobacillus species are commonly found in the chicken small intestine, and bacterial density generally quickly improves animal growth performance (Konstantinov et al. 2004; Hill et al. 2005). Microbial communities in the small and large intestines play an important role in managing the host’s health, including the energy intake from food, immune system function, generation of important metabolites, and the response to gastrointestinal diseases (Barnes et al. 1972; Lu et al. 2003). The present data may indicate that the 3D8 scFv secreted by L. reuteri to chicken using oral administration increases beneficial intestinal microflora and improves body-weight gain. Yu et al. (2007) have reported that inclusion of L. reuteri Pg4 in the diet can increase the balance of gut health and microflora, and improve crypt depth and villous height, thereby improving chicken health. Lactobacillus species administration is known to stimulate the secretion of mucus, promote growth of intestinal microflora (Estienne et al. 2005; Yu et al. 2008). The effective clarification has been proposed to restore and expand the microbial balance in the intestine and improve growth promotion of the host.
Interleukins are composed of cytokines, an important component of the immune system. According to Tayal and Kalra (2008), cytokines exhibit an important function, and pathological disorders occur in response to imbalances in cytokine production. Th1 cells primarily produce IFN-γ, TNF-α, IL-6, and IL-2, which are identified as pro-inflammatory cytokines. However, Th2 cells secrete anti-inflammatory cytokines such as IL-4 and IL-8 (Romagnani 1995). A lack of activated AP-1 may result in reduced levels of TNF-α gene expression and diminished pro-inflammatory cytokine secretion. Previously, Lamprecht et al. (2012) reported that IL-6 and TNF-α have independent pathways and that IL-6 had no influence after Lactobacillus supplementation, IL-6 cytokines were not affected by chronic exercise, and they mildly decreased gut barrier function. In addition, Meyer et al. (2007) described that probiotic supplementation promoted increased IL-1β production in chicken serum compared with that of chickens receiving a basal diet. In our data, IL-1β cytokine levels increased more in the L. reuteri/3D8 group than they did in the L. reuteri/WT experimental group. Our findings from this study showed that the chicken serum levels of the primary cytokines IL-6, IL-8, TNF-α, IFN-γ, IL-4, and IGF1 were reduced after oral administration of L. reuteri/3D8, indicating that 3D8 could regulate the humoral immunity of chickens. IFN-γ plays an important role in the activation of macrophages for nitric oxide production and in the conversion of native T cells to Th1 cells (Schoenborn and Wilson 2007; He et al. 2011; Sun et al. 2018). In addition, Deng-sheng reported that dietary Yucca schidigera supplementation improved immune status, including decreasing IL-4 production, in broilers (Sun et al. 2018). Scanes (2009) and Boschiero et al. (2013) reported that IGF1 is involved in growth hormone secretion and regulation. IGF1 is known as one of more predominant hormones necessary to support normal growth in chicken.
Huang and Lee (2018) reported the possibility of cinnamaldehyde regulating the expression of pro-inflammatory mediators in the nuclear factor kappa B (NFkB) signaling pathways, inflammation, and the immune response in chickens. Brisbin et al. (2010) reported that Lactobacillus species reduce antigen-specific IFN-γ production by chicken spleen mononuclear cells. Although the fundamental mechanism for this phenomenon is unknown, it can be speculated that the decrease in IFN-γ production by the splenocytes of chicken-fed Lactobacillus species may reflect a selective decrease in Th1 cell activation. In the present study, the decreased cytokine levels produced in L. reuteri/3D8-administered chickens are likely related to the anti-inflammatory potential of 3D8 scFv through the repression of T cell immune responses. Together these results indicate that improved body-weight gain of the chicken is due to the synergetic effect of increased efficiency of digestion and immune homeostasis due to L. reuteri expressing 3D8 scFv administration.
Conclusion
In summary, our result indicates that administration of L. reuteri expressing 3D8 scFv reduces inflammatory responses thereby improves the growth performance of the chickens. Moreover, these observations suggest that administration of L. reuteri expressing 3D8 scFv would not affect the microbial composition in the chicken intestine. Therefore, addition of the 3D8 scFv-expressing recombinant L. reuteri as a feed additive might be a best solution to improve growth performance and health of the chickens which will improve the profits of livestock industry.
Acknowledgements
This study was supported by the 2018–2020 RDA Fellowship Program of the National Institute of Animal Science, Rural Development Administration, Republic of Korea. This work was carried out with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01328303)” of the Rural Development Administration, Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Angel R, Dalloul RA, Doerr J. Performance of broiler chickens fed diets supplemented with a direct-fed microbial. Poult Sci. 2005;84(8):1222–1231. doi: 10.1093/ps/84.8.1222. [DOI] [PubMed] [Google Scholar]
- Awad W, Ghareeb K, Abdel-Raheem S, Böhm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult Sci. 2009;88(1):49–56. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- Balamuralikrishnan B, Lee SI, Kim IH. Dietary inclusion of different multi-strain complex probiotics on performance in broilers. Br Poult Sci. 2017;58(1):83–86. doi: 10.1080/00071668.2016.1257112. [DOI] [PubMed] [Google Scholar]
- Barnes EM, Mead GC, Barnum DA, Harry EG. The intestinal flora of the chicken in the period 2–6 weeks of age, with particular reference to the anaerobic bacteria. Br Poult Sci. 1972;13(3):311–326. doi: 10.1080/00071667208415953. [DOI] [PubMed] [Google Scholar]
- Benyi K, Tshilate TS, Netshipale AJ, Mahlako KT. Effects of genotype and sex on the growth performance and carcass characteristics of broiler chickens. Trop Anim Health Prod. 2015 doi: 10.1007/s11250-015-0850-3. [DOI] [PubMed] [Google Scholar]
- Boschiero C, Jorge EC, Ninov K, Nones K, et al. Association of IGF1 and KDM5A polymorphisms with performance, fatness and carcass traits in chickens. J Appl Genet. 2013;54:103–112. doi: 10.1007/s13353-012-0129-6. [DOI] [PubMed] [Google Scholar]
- Brisbin JT, Gong J, Parvizi P, Sharif S. Effects of lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin Vaccine Immunol. 2010;17(9):1337–1343. doi: 10.1128/CVI.00143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun SJ, Yuk SS, Jang YJ, Choi H, Jeon MH, Erdene-Ochir TO, Kwon JH, Noh JY, Sun Kim J, Gyu Yoo J, et al. Transgenic chickens expressing the 3D8 single chain variable fragment protein suppress avian influenza transmission. Sci Rep. 2017;7(1):5938. doi: 10.1038/s41598-017-05270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway TR, Edrington TS, Anderson RC, Harvey RB, Genovese KJ, Kennedy CN, Venn DW, Nisbet DJ. Probiotics, prebiotics and competitive exclusion for prophylaxis against bacterial disease. Anim Health Res Rev. 2008;9(2):217–225. doi: 10.1017/S1466252308001540. [DOI] [PubMed] [Google Scholar]
- Deeth HC, Tamime AY. Yogurt: nutritive and therapeutic aspects. J Food Protect. 1981;44(1):78–86. doi: 10.4315/0362-028X-44.1.78. [DOI] [PubMed] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Estienne M, Hartsock T, Harper A. Effects of antibiotics and probiotics on suckling pig and weaned pig performance. Intern J Appl Res Vet Med. 2005;3:303–308. [Google Scholar]
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115(1):182–205. doi: 10.1016/S0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- He H, Genovese KJ, Kogut MH. Modulation of chicken macrophage effector function by TH1/TH2 cytokines. Cytokine. 2011;53(3):363–369. doi: 10.1016/j.cyto.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Hill JE, Hemmingsen SM, Goldade BG, Dumonceaux TJ, Klassen J, Zijlstra RT, Goh SH, Van Kessel AG. Comparison of ileum microflora of pigs fed corn-, wheat-, or barley-based diets by chaperonin-60 sequencing and quantitative PCR. Appl Environ Microbiol. 2005;71(2):867–875. doi: 10.1128/AEM.71.2.867-875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang PM, Cho S, Kim KE, Byun SJ, Lee TK, Lee S. Development of Lactobacillus paracasei harboring nucleic acid-hydrolyzing 3D8 scFv as a preventive probiotic against murine norovirus infection. Appl Microbiol Biotechnol. 2015;99(6):2793–2803. doi: 10.1007/s00253-014-6257-7. [DOI] [PubMed] [Google Scholar]
- Hofacre C, Beacorn T, Collett S, Mathis GJJo APR. Using competitive exclusion, mannan-oligosaccharide and other intestinal products to control necrotic enteritis. J Appl Poult Res. 2003;12(1):60–64. doi: 10.1093/japr/12.1.60. [DOI] [Google Scholar]
- Huang CM, Lee TT. Immunomodulatory effects of phytogenics in chickens and pigs—a review. Asian-Australas J Anim Sci. 2018;31(5):617–627. doi: 10.5713/ajas.17.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Frantz N, Khoo C, Gibson GR, Rastall RA, McCartney AL. Investigation of the faecal microbiota associated with canine chronic diarrhoea. FEMS Microbiol Ecol. 2010;71(2):304–312. doi: 10.1111/j.1574-6941.2009.00812.x. [DOI] [PubMed] [Google Scholar]
- Jun HR, Pham CD, Lim SI, Lee SC, Kim YS, Park S, Kwon MH. An RNA-hydrolyzing recombinant antibody exhibits an antiviral activity against classical swine fever virus. Biochem Biophys Res Commun. 2010;395(4):484–489. doi: 10.1016/j.bbrc.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Kim D, Cho MJ, Cho S, Lee Y, Byun SJ, Lee S. Complete genome sequence of Lactobacillus reuteri SKKU-OGDONS-01, isolated from a chicken’s small intestine. Microbial Resour Announc. 2018;7(20):1251-18. doi: 10.1128/MRA.01251-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinov SR, Awati A, Smidt H, Williams BA, Akkermans AD, de Vos WM. Specific response of a novel and abundant Lactobacillus amylovorus-like phylotype to dietary prebiotics in the guts of weaning piglets. Appl Environ Microbiol. 2004;70(7):3821–3830. doi: 10.1128/AEM.70.7.3821-3830.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamendella R, Domingo JW, Ghosh S, Martinson J, Oerther DB. Comparative fecal metagenomics unveils unique functional capacity of the swine gut. BMC Microbiol. 2011;11:103. doi: 10.1186/1471-2180-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht M, Bogner S, Schippinger G, Steinbauer K, Fankhauser F, Hallstroem S, Schuetz B, Greilberger JF. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J Int Soc Sports Nutr. 2012;9(1):45. doi: 10.1186/1550-2783-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Yu J, Cho S, Byun SJ, Kim DH, Lee TK, Kwon MH, Lee S. A nucleic-acid hydrolyzing single chain antibody confers resistance to DNA virus infection in hela cells and C57BL/6 mice. PLoS Pathog. 2014;10(6):e1004208. doi: 10.1371/journal.ppat.1004208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26(1):5–11. doi: 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- Liu JR, Yu B, Lin SH, Cheng KJ, Chen YC. Direct cloning of a xylanase gene from the mixed genomic DNA of rumen fungi and its expression in intestinal Lactobacillus reuteri. FEMS Microbiol Lett. 2005;251(2):233–241. doi: 10.1016/j.femsle.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Lu J, Idris U, Harmon B, Hofacre C, Maurer JJ, Lee MD. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl Environ Microbiol. 2003;69(11):6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mappley LJ, Tchorzewska MA, Nunez A, Woodward MJ, Bramley PM, La Ragione RM. Oral treatment of chickens with Lactobacillus reuteri LM1 reduces Brachyspira pilosicoli-induced pathology. J Med Microbiol. 2013;62(Pt 2):287–296. doi: 10.1099/jmm.0.051862-0. [DOI] [PubMed] [Google Scholar]
- Meyer AL, Elmadfa I, Herbacek I, Micksche M. Probiotic, as well as conventional yogurt, can enhance the stimulated production of proinflammatory cytokines. J Hum Nutr Diet. 2007;20(6):590–598. doi: 10.1111/j.1365-277X.2007.00807.x. [DOI] [PubMed] [Google Scholar]
- Montalban-Arques A, De Schryver P, Bossier P, Gorkiewicz G, Mulero V, Gatlin DM, 3rd, Galindo-Villegas J. Selective manipulation of the gut microbiota improves immune status in vertebrates. Front Immunol. 2015;6:512. doi: 10.3389/fimmu.2015.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Biology of human T H 1 and T H 2 cells. J Clin Immunol. 1995;15(3):121–129. doi: 10.1007/BF01543103. [DOI] [PubMed] [Google Scholar]
- Scanes CG. Perspectives on the endocrinology of poultry growth and metabolism. Gen Comp Endocrinol. 2009;163:24–32. doi: 10.1016/j.ygcen.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Schoenborn JR, Wilson CBJ. Regulation of interferon-γ during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- Sun DS, Shi BL, Tong MM, Yan SM. Improved performance and immunological responses as a result of dietary Yucca schidigera extract supplementation in broilers. Italian J Anim Sci. 2018;17(2):511–517. doi: 10.1080/1828051X.2017.1358593. [DOI] [Google Scholar]
- Tayal V, Kalra BS. Cytokines and anti-cytokines as therapeutics—an update. Eur J Pharmacol. 2008;579(1–3):1–12. doi: 10.1016/j.ejphar.2007.10.049. [DOI] [PubMed] [Google Scholar]
- Wu QJ, Zheng XC, Wang T, Zhang TY. Effects of dietary supplementation with oridonin on the growth performance, relative organ weight, lymphocyte proliferation, and cytokine concentration in broiler chickens. BMC Vet Res. 2018;14(1):34. doi: 10.1186/s12917-018-1359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Xin Z, Minh HAL, Ruurd TZ, Michael GG. Reutericyclin producing Lactobacillus reuteri modulates development of fecal microbiota in weanling pigs. Front Microbial. 2015;28(6):762. doi: 10.3389/fmicb.2015.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Liu J, Chiou M, Hsu Y, Chiou PJA. The effects of probiotic Lactobacillus reuteri Pg4 strain on intestinal characteristics and performance in broilers. Asian-Australas J Anim Sci. 2007;20(8):1243–1251. doi: 10.5713/ajas.2007.1243. [DOI] [Google Scholar]
- Yu HF, Wang AN, Li XJ, Qiao SY. Effect of viable Lactobacillus fermentumon the growth performance, nutrient digestibility and immunity of weaned pigs. J Anim Feed Sci. 2008;17:61–69. doi: 10.22358/jafs/66470/2008. [DOI] [Google Scholar]
- Zulkifli I, Abdulllah N, Azrin NM, Ho YW. Growth performance and immune response of two commercial broiler strains fed diets containing Lactobacillus cultures and oxytetracycline under heat stress conditions. Br Poult Sci. 2000;41(5):593–597. doi: 10.1080/713654979. [DOI] [PubMed] [Google Scholar]