Abstract
Universal screening of all newly diagnosed colorectal cancer tumors can identify individuals at high risk for Lynch syndrome (LS), a hereditary cancer syndrome predisposing carriers to increased risk of colorectal, endometrial, and other cancers. To inform planning of a universal tumor screening program for LS in our jurisdiction, we undertook online surveys of Canadian pathologists and genetic counselors to describe existing tumor screening programs. Online surveys were hosted on SurveyMonkey between October 2016 and March 2017. Fifty-three pathologists and 66 genetic counselors completed surveys (total n = 119). While attitudes towards tumor screening were positive, considerable variability was observed in the existence of tumor screening, test ordering criteria, and practices. Most respondents indicated consent was not obtained for tumor screening nor were educational materials provided to patients; however, opting out of additional mutation testing in the event of a positive tumor screen was endorsed. Results add to the growing literature on providers’ perspectives on population-based tumor screening programs and inform ways to offer these. Findings highlight the need to develop methods of patient education that allow meaningful opt-out decisions. The variability we observed also suggests the need for national standards and guidance on tumor screening for LS.
Keywords: Lynch syndrome, Universal tumor screening, Colorectal, Informed consent, Survey
Introduction
Lynch syndrome (LS), a genetic syndrome associated with an increased risk of colorectal (CRC), endometrial, and other cancers, accounts for 3–5% of all CRC cases, making it the most common form of hereditary CRC (Hampel et al. 2006; Lynch et al. 2009; Moreira et al. 2012; Weissman et al. 2011). Men with LS have an average lifetime risk for CRC of 54–74%; the comparable risk for women is 30–52%. Women with LS also have a 31–66% lifetime risk for endometrial cancer (Barrow et al. 2008; Stoffel et al. 2009; Watson et al. 2008). CRC is the second most common cancer in Canada, accounting for 13% of all cancers in 2017 (Canadian Cancer Society 2017). The incidence of CRC is highest in Newfoundland and Labrador (NL); further, the province has the highest rate of familial CRC in the world (i.e., families with high CRC burden, but often without a known mutation) (Woods et al. 2010).
Identifying individuals at risk for LS is important as surveillance reduces morbidity and mortality. At-risk individuals can receive more frequent screening at younger ages than what is recommended for the general population (Jarvinen et al. 2000; Jarvinen et al. 2009; National Comprehensive Cancer Network 2012). Nonetheless, some high-risk individuals remain unidentified and uninformed about prevention strategies (Tomiak et al. 2014). Amsterdam criteria and Bethesda guidelines rely mainly on family history and age of onset to identify patients with CRC who should be evaluated for LS (Rodriguez-Bigas et al. 1997; Umar et al. 2004; Vasen et al. 1991, 1999). These methods are not always sensitive enough to identify at-risk individuals as not all patients meet these criteria and family history information is not always available. Some patients who meet the criteria are not properly diagnosed (Cross et al. 2013; Hampel et al. 2005; Hunter et al. 2015).
Genetic screening alternatives
LS can present with variable age of onset, symptoms, and outcomes; as such, traditional screening criteria are not sensitive enough to identify all individuals at risk, particularly in women with endometrial cancer (Cohen 2014; Hampel et al. 2005; Kalloger et al. 2012; South et al. 2009; Tomiak et al. 2014). Thus, its diagnosis is defined in genetic terms (Hampel et al. 2005; Lynch et al. 2009; South et al. 2009). LS is associated with deleterious mutations in the DNA mismatch repair (MMR) genes MLH1, MLH2, MSH6, and PMS2 (Hampel et al. 2006; Lynch et al. 2009; Moreira et al. 2012). Screening newly diagnosed colorectal or endometrial tumors with immunohistochemistry (IHC) stains for the MMR genes and/or microsatellite instability (MSI) testing could better identify patients with LS (Cohen 2014; Frolova et al. 2015; Kalloger et al. 2012; South et al. 2009; Tomiak et al. 2014).
MSI testing uses variability in lengths of microsatellite markers as a proxy for defects in MMR genes. Normally identical between cells, microsatellites are stretches of DNA in which a short sequence is repeated several times. Defective MMR causes microsatellites to become unstable and thus vary in length between cells. MSI screening compares microsatellite lengths between abnormal and normal tissue. Typically, a panel of five microsatellite markers is used, with instability in two or more signifying a positive or MSI-high result (Chubak et al. 2011; De la Chapelle 2003; Hampel 2009). MSI is not diagnostic of LS, but suggestive of an MMR mutation. MSI has a sensitivity of 89% for MLS1 and MSH2 mutations and 77% for the MSH6 mutation (EGAPP Working Group 2009).
An alternative to MSI screening is the use of IHC on resected tumor tissue. This antibody-based test uses chemical detection methods to visualize proteins directly on tissues under a microscope. In the case of LS, loss of one or more of the MMR proteins from a patient’s tumor suggests the possibility of mutation in the associated MMR genes (Chubak et al. 2011). The sensitivity of IHC as an indication of MMR mutation is 83%, while the specificity is 90% (Chubak et al. 2011; South et al. 2009). Because of its integration into routine testing, IHC has been utilized as a cost-effective, low-level triage tool for LS screening (Colling et al. 2015; Frolova et al. 2015; Kalloger et al. 2012).
An emerging consensus—universal tumor screening
There is growing interest in screening all newly diagnosed cases of CRC for LS, a process referred to as “universal screening” (Frolova et al. 2015; Hampel et al. 2005; Hampel 2009; Kalloger et al. 2012). In 2009, the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (EWG) recommended that all newly diagnosed patients with CRC, regardless of age or family history, should undergo tumor screening for an MMR defect; those testing positive should be offered genetic counseling and testing (EGAPP Working Group 2009).
Since this recommendation, there has been a movement towards universal screening of all newly diagnosed CRC tumors as a feasible mechanism to identify individuals who could have LS (Bellcross et al. 2015; Colling et al. 2015; Frolova et al. 2015; Mvundura et al. 2010; Peres 2010; South et al. 2009). While there is debate as to which screening method (MSI or IHC) is preferred, and whether informed consent for screening is needed, consensus is emerging that tumor-based screening protocols identify a higher percentage of CRC patients with LS than age or family history alone (Bellcross et al. 2012; EGAPP Working Group 2009). The Lynch Syndrome Screening Network (LSSN) was created in the USA in 2011 to promote universal tumor screening of CRC tumors (Mange et al. 2015). As of 2015, 67 member institutions were screening CRC tumors; 18 others were planning to implement universal screening. A recent survey of American genetic counselors reported about the half of whose institutions had a routine tumor screening protocol for LS (Cohen 2014). Canada lacks a standardized and integrated approach to the identification of patients with LS, with variable existence of universal tumor screening programs across the country. The result is heterogeneity in current practices of test initiation and subsequent patient management (Bombard et al. 2017; Kalloger et al. 2012). For example, Kalloger et al. (2012) reported that while a majority of Canadian pathologists in their survey (78.9%) stated they had access to MMR IHC, almost 22% were unaware or uncertain of their access and only 58% could access MSI testing (representing 31 centers in Canada). Only nine centers across Canada had implemented an integrated approach to screening for LS, typically in academic centers (Kalloger et al. 2012).
Key stakeholders’ perceptions
Despite the emerging consensus for universal tumor screening, there is no current standard of care regarding the process of LS screening, informed consent protocols, or follow-up procedures for those whose tumors test positive (Bombard et al. 2017; Hampel et al. 2008; Kalloger et al. 2012; Mange et al. 2015). As part of planning a population-based program of universal screening in Ontario, Canada, a recent qualitative study of healthcare professionals revealed support for the program. However, there were a range of complex, and sometimes contradictory views on consent for tumor testing. Most favored an opt-out approach for reflex testing for LS (Bombard et al. 2017). This and similar studies describing early experiences with universal tumor screening (Kidambi et al. 2016; Marquez et al. 2013; Schneider et al. 2016) are suggesting optimal ways to design and deliver these programs. In our jurisdiction, universal tumor screening might be particularly important. NL has the highest incidence of CRC in Canada, along with the highest CRC mortality rate (Canadian Cancer Society 2017; Parfrey et al. 2017). Compared to other population-based studies, NL also has the highest rate of familial colon cancer worldwide (Green et al. 2007). Recent statistics from the Canadian Cancer Society revealed NL had the highest stage specific, age-standardized incidence rate of CRC of around 20 per 100,000 for stage II and III colon cancer (Canadian Cancer Society 2018). With a population of just over 528,000 people, CRC represents a significant public health burden for the province.
To inform discussion about a universal tumor screening program for LS in our jurisdiction, we undertook online surveys of Canadian pathologists and genetic counselors, two key stakeholder groups in such a program. A third phase surveyed local patients; those data will be reported separately. Here, we aim to contribute to the evidence base: (Barrow et al. 2008) describing the existence of universal tumor screening programs in Canada and (Beamer et al. 2012) exploring attitudes towards universal screening and the need (or not) for informed consent through the perspectives of Canadian pathologists and genetic counselors.
Materials and methods
Ethics approval was obtained from the Health Research Ethics Board (Ref no. 16.062), St. John’s, NL, Canada.
Survey development
Tumor screening practices across Canada were explored via two online, descriptive surveys (one for pathologists containing 36 items, and one for genetic counselors comprised of 38 items). A key goal was to describe what programs currently exist, tumors screened and method used for screening, as well as informed consent protocols. We found only one previous study (Kalloger et al. 2012) that reported on tumor screening programs for LS in Canada. A survey of genetic counselors in the USA (Cohen 2014) was also obtained and with permission, used as an additional guide to the creation of current survey items. An interest for our group was to measure attitudes about informed consent for tumor testing, as well as attitudes towards a testing program more generally. These items were drafted by our team for use in the current study.
Survey content areas
The survey began by asking if there was a routine tumor screening program at the respondent’s institution, and if so, when it began, and what tumors were routinely screened and with what criteria. Items then asked about the type of screening test used (IHC, MSI or both) and the ordering healthcare professional. The next section measured consent protocols and whether educational materials were provided to patients about tumor screening. Respondents with tumor screening programs were also asked about barriers to implementing a universal screening protocol, how barriers were overcome, and whether they had advice for those centers that were planning a similar program. Respondents who indicated that at the start of the survey they did not have a universal tumor screening program at their center were directed to items exploring possible barriers to a screening program and what might be helpful in implementing such a program. The final sections measured attitudes towards universal tumor screening and respondent demographics.
Both surveys were reviewed extensively by an advisory group consisting of three pathologists, a genetic counselor, a gynecologic oncologist, a patient, as well as the study team. Although the surveys were quite similar, the one for genetic counselors included additional items to probe issues of follow-up in the event of an abnormal tumor screen. Participants were given ample opportunity to provide open comments throughout (pathologist survey in Supplemental file).
Survey administration
Online surveys and responses were hosted on SurveyMonkey and were accessible between October 2016 and March 2017.
Participant recruitment
All Canadian pathologists and genetic counselors were eligible to complete surveys. In this paper, we focus in the main on those respondents who indicated they had a tumor screening program at their institution. However, attitude items are presented for all respondents. A separate paper will present barriers endorsed by those respondents without a tumor screening program, while comparing facilitators endorsed by those with a program. We advertised the survey link through regular communication channels (e.g., list servers, emails, or newsletters) of the Canadian Association of Pathology (CAP), the Canadian Association of Genetic Counselors (CAGC), and the Canadian Immunohistochemistry Quality Control (cIHc). Survey links were advertised on at least six separate occasions. Members of the study team, as well as our advisory panel, also sent e-mail invitations to individual pathologists or genetic counselors through their professional networks.
Survey analysis
Descriptive statistics (counts, percentages) are used to describe characteristics of tumor testing programs in Canada. Differences in attitudes towards tumor testing and informed consent between pathologists and genetic counselors were explored using the Mann–Whitney U test with a significance level of p < .05. Qualitative data generated in response to open items were analyzed using a descriptive approach (Sandelowski 2000, 2010) that compared responses across surveys to index and identify emerging themes using constant comparison.
Results
Fifty-three pathologists and 66 genetic counselors completed the surveys (total n = 119). Survey invites were received by 472 pathologists through the CAP, giving a response rate of approximately 11%. At the beginning of the project, the CAGC advised there were about 330 genetic counselors in their membership (assuming all received the invite, the response rate for genetic counselors was 66/330 or 20%). There were noticeable gaps in demographic item response. Only seven genetic counselors completed demographic information. All seven were female; four practiced in ON, with three from Western Canada. Most were practicing for 11–15 years; four identified academic medical center as their place of practice, three a community hospital, and one a diagnostic laboratory. Thirty-two pathologists completed demographic items: 16 from Western Canada and 14 from ON and QB, with two from Atlantic Canada. There were roughly equal numbers of men and women (n = 16), and most had been in practice for greater than 15 years (~ 29%). Almost half practiced in academic medical centers (41.5%), with almost 25% practicing in community hospitals; the remainder identified private practice or diagnostic lab.
Existence of tumor screening programs
Over half of the respondents indicated there was a routine tumor screening program at their institution (57.6% of genetic counselors, n = 38, and 54.7% of pathologists, n = 29). Nearly all indicated colorectal tumors were routinely screened, while screening of endometrial tumors occurred somewhat less routinely (Table 1).
Table 1.
Tumors indicated as screened
| Tumors screened | Genetic counselors (n = 29) | Pathologists (n = 27) |
|---|---|---|
| Colorectal | 93% | 100% |
| Endometerial | 86% | 48% |
| Other | 25% | 22% |
Numbers do not add to the total numbers who indicated there was a tumor program in their institution (38 genetic counselors and 29 pathologists) due to missing responses
“Other” tumors cited were brain, gastric, ovarian clear cell, pancreas, and sebaceous cancers. Open comments revealed variability in tumor screening:
Most sebaceous tumors, some upper tract urothelial carcinomas; some other less common Lynch tumor types (e.g., gastric) if there is a suggestive family history. Some endometrial carcinomas are screened as well. For all of these, the decision whether to do MMR immunohistochemistry is made on a case by case basis depending on factors like age and family history, and there are no strict criteria for when to test as there are for colorectal cancers. -Pathologist
These tumors are routinely screening at some hospitals in our region and in some other provincial health regions, but it’s not consistent. –Genetic Counselor
While age was the most common clinical criterion for tumor screening, here too, there was variation in response (Table 2).
Table 2.
Number of genetic counselors and pathologists indicating different age criteria for tumor testing
| Colon tumors | Endometrial tumors | |
|---|---|---|
| Genetic counselors (n = 26) | ||
| < 50 years | 7 | 2 |
| < 60 years | 7 | 10 |
| < 70 years | 6 | 1 |
| Test all tumors | 6 | 9 |
| Pathologists (n = 24) | ||
| < 50 years | 7 | 4 |
| < 60 years | 9 | 2 |
| < 70 years | 4 | 0 |
| Test all tumors | 4 | 5 |
Open comments revealed further criteria for tumor screening:
Other tumors associated with Lynch can also be screened if requested. –Genetic Counselor
There is no official system for colon cancer that I’m aware of, but some will get screening ordered by pathology or the surgeon if the diagnosis is ‘young’ or if there are characteristics of the tumor that suggest Lynch syndrome (mucinous pathology, for example). I wouldn’t necessarily call the endometrial cancers ‘routinely screened’ but the gynecologic oncologists who treat them are well aware of the utility of screening for Lynch syndrome, and may initiate screening based on family history and/or tumor pathology, even if the diagnosis isn’t younger than 60. –Genetic counselor
Histologic features that suggest MSI; family history suggestive of other cancers that suggest Lynch syndrome. –Pathologist
More than 50% mucinous differentiation, signet ring differentiation, medullary features or significant numbers of tumor infiltrating lymphocytes (especially if right sided). –Pathologist
Type of screening test used and ordering professional
IHC was the screening method reported by 68% of genetic counselors for colorectal tumors, with 75% endorsing it as the screening method for endometrial tumors. Minorities indicated both IHC and MSI tests were used to screen colorectal (32%) and endometrial tumors (18%). All pathologists who answered this question (n = 27) reported that IHC was used for screening colorectal tumors, with 67% indicating IHC was also used as the initial screen for endometrial tumors. No pathologists indicated either MSI alone, or in conjunction with IHC, for screening of either colon or endometrial tumors. Most respondents noted that screening was done in house, with only three genetic counselors and two pathologists indicating no.
Tumor screens were most often ordered by pathologists, but were sometimes automatic (Table 3). Little clarification was provided on “automatic.” For example, open comments included “it should be automatic through pathology,” “automatic for endometrial,” or “routine screening.”
Table 3.
Professional who typically ordered the tumor screening test
| Tumor screen ordered by | Genetic counselors (n = 28) | Pathologists (n = 25) |
|---|---|---|
| Pathologist | 6 | 19 |
| Surgeon | 2 | 0 |
| Genetic counselor | 4 | 1 |
| No one, it is automatic | 10 | 2 |
| Do not know | 4 | 0 |
| Other (e.g., “screening ordered by various people”; “often it’s oncology, but pathology and surgeons will too”) | 2 | 3 (e.g., medical oncologist, “from multiple sources”) |
Informed consent for tumor screening
While close to 20% of genetic counselors indicated both verbal and written consents were received from patients, consent was not normally obtained for routine tumor screening (Table 4).
Table 4.
Type of consent obtained for tumor screening
| Consent | Genetic counselors (% endorsing) | Pathologists (% endorsing) |
|---|---|---|
| Verbal | 11 | 4 |
| Written | 0 | 4 |
| Both | 18.5 | 4 |
| None | 52 | 68 |
| Do not know | 18.5 | 20 |
Total number of responding pathologists, 25. Total number of responding genetic counselors, 27
Respondents who indicated consent was obtained were asked who obtains the consent at their institution in an open item. In the main, comments indicated genetic counselors were more likely to obtain consent.
If done by a genetic counselor, verbal and/or written consent. If done by anyone other than a genetic counselor, no consent is obtained. –Pathologist
If the genetics clinic orders it, verbal consent. If oncologists or others order it, I’m unsure. –Genetic counselor
The type of tumor screening also influenced verbal or written consent:
Until recently, if the testing was ordered by a genetic counselor, then verbal and written consent was obtained. If the IHC was automatically ordered, neither was obtained. We have recently switched to verbal consent to order BRAF or IHC. However, we still require a release of tissue form for MLH1 promoter hypermethylation testing since this testing is done out of province. –Pathologist
It all depends on what is being ordered, as this is not a consistent process for colon as to who does what – it varies! If it’s in [city], we take verbal consent. We have to get a release of tissue signed if the tumor is outside [city]. –Genetic counselor
Opting out of tumor screening
Generally, respondents explained that opting out of tumor screening was not possible:
Not for routine screening initiated at the pathology department. –Genetic counselor
Patients are unaware that screening takes place until the diagnosis is released. Our interpretation is that MMR testing represents biological subtyping of all Lynch-associated tumors, regardless of whether the MMR status is epigenetic or germline, hence is required for complete and precise diagnosis. –Pathologist
In the event of an abnormal screen, however, most respondents suggested patients could opt out of further testing:
The initial immunohistochemical screening, if positive, is reported to the clinician who discusses the results with the patients. Consent for further screening is then pursued, in conjunction with referral to a geneticist. –Pathologist
Cannot opt out of the universal screening, but if they are referred to provincial cancer genetics for reflex testing, they can choose not to proceed. –Genetic counselor
I think they can refuse. Genetics is notified about all abnormal results and we contact them. They certainly refuse to meet with Genetics, we have had that happen. –Genetic Counselor
Provision of information to patients
Very few respondents reported educational materials were available for patients about tumor screening, and most did not know (Table 5).
Table 5.
Number of respondents reporting whether educational materials about tumor screening are provided to patients
| Education materials? | Genetic counselors | Pathologists |
|---|---|---|
| Yes | 5 | 3 |
| No | 13 | 9 |
| Do not know | 9 | 12 |
Total number of responding pathologists, 24. Total number of responding genetic counselors, 27
Very few respondents indicated they knew when these were provided, but at the time of surgery seemed to be most common:
Genetic counselors provide an information sheet about IHC and Lynch syndrome. No other providers hand out educational material to my knowledge. –Pathologist
The surgeon and/or his or her nurse; however, I have no idea if this is actually happening. We created a patient information sheet and provided it for their use. –Genetic counselor
We worked on an information sheet to be included in our surgery package. –Genetic counselor
Attitudes towards tumor testing and informed consent
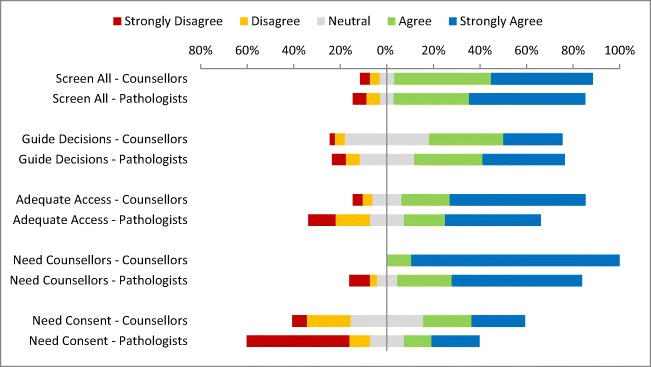
Most respondents (both those whose institutions had a tumor program and those who did not) had a favorable attitude towards the use of universal tumor screening to identify individuals at risk for LS and to inform chemotherapy decisions (Fig. 1). There were no differences in attitudes between pathologists and genetic counselors towards the use of universal screening to identify high-risk individuals (Mann–Whitney U = 788.5, n1 = 34, n2 = 48, p = .78) or to guide treatment decisions (Mann–Whitney U = 734, n1 = 34, n2 = 48, p = .52). Pathologists placed less emphasis on the need for informed consent for tumor testing (Mann–Whitney U = 568, n1 = 34, n2 = 48, p < .05) and the need for genetic counseling following a positive tumor test than did genetic counselors (Mann–Whitney U = 523.5, n1 = 34, n2 = 48, p < .05).
Fig. 1.
Genetic counselor (n = 48) and pathologist (n = 34) responses to attitude items. Screen all—“The use of universal tumour screening of all patients with colon cancer to identify families with Lynch syndrome (LS).” Guide decisions—“The use of universal tumour screening of all patients with colon cancer to guide adjuvant chemotherapy decisions.” Adequate access—“Following a positive tumour test, patients identified at high risk of LS have adequate access to genetic counselling at your institution”. Need counselors—“The need for genetic counselling following a positive tumour test when testing is used to identify potential families with Lynch syndrome.” Need consent—“The need for informed consent for tumour screening to identify potential families with Lynch syndrome”
Discussion
Identifying patients at high risk for CRC due to an inherited disposition remains a public health challenge. Universal tumor screening could better identify high-risk patients, potentially reducing cancer burden (EGAPP Working Group 2009; Hampel 2005) and is becoming standard of care in some jurisdictions (Bellcross et al. 2012; EGAPP Working Group 2009; Mange et al. 2015). In Canada, some provinces are planning tumor screening programs (Bombard et al. 2017) and there are calls for a national consensus statement and guidelines (Kalloger et al. 2012). Amid this growing interest, our study provides information on current practices and attitudes in Canada.
While response rates were low, the current survey revealed variability in the existence of tumor screening programs across Canada, as well as test ordering criteria and practices, consistent with an earlier survey of pathologists in 2012 (Kalloger et al. 2012). Just over half of our respondents reported a tumor screening program in their jurisdiction. Colorectal tumors were routinely screened, while screening of endometrial tumors occurred somewhat less routinely. IHC was most often indicated as the screening method of choice. Age seemed to be the most common criterion used for screening initiation, though even here, there was variability among age cutoffs for testing, and other criteria were noted in open comments (e.g., family history). A variety of health care professionals were reported to order tumor screens, including pathologists, surgeons, and oncologists, but often tests were ordered automatically or respondents indicated they did not know who ordered the initial screen.
Since the EGAPP recommendations, several centers in the USA have reported on their institutional experiences with universal tumor screening (Hill et al. 2015; Kidambi et al. 2016; Marquez et al. 2013), and these reports also document variation in the type of testing, test ordering patterns, and access to testing, with testing performed less often than recommended in public and community hospital settings (Beamer et al. 2012; Karlitz et al. 2015). Higher rates of screening in academic settings and urban centers could be explained by a greater concentration of specialists who are more familiar with hereditary cancer syndromes or a greater availability of genetic counselors (Karlitz et al. 2015). Our findings and others (e.g., Kalloger et al. 2012) highlight potential patient disparities in access to testing and suggest the need for standardized screening protocols and national guidelines. An integrated, multidisciplinary approach to tumor screening has been shown to achieve the highest detection rate of LS (Sanchez et al. 2008; Wright et al. 2011), indicating a need in Canada to increase accessibility to tumor screening and integrate and coordinate clinical resources to ensure appropriate patient management (Kalloger et al. 2012). A recent qualitative study highlighted the need for a comprehensive approach in Canada to not only increase awareness and visibility of LS among patients and providers, but also to ensure adequate resources for genetic services, coordination of care, and ongoing patient management (Bombard et al. 2017). As in that study, our findings reveal strong support for universal tumor screening in Canada as indicated by the high levels of agreement on attitude items.
While genetic counselors in our survey were more likely to agree consent was needed for tumor screening than pathologists, in the main, both groups indicated consent was not normally obtained in their settings or they did not know if it was. Regarding an opt-out for screening, most respondents indicated it was unavailable due to the automatic nature of tumor screening, but supported patients opting out of further mutation testing if an initial screening test was positive. These results are very consistent with prior research and current practice across Canada (Bombard et al. 2017; Kalloger et al. 2012) and the USA (Beamer et al. 2012; Cohen 2014; Williams and Williams 2011). For example, only 14% of 29 National Cancer Institute cancer centers offered an opt-out from tumor screening and most did not provide educational materials to patients (Beamer et al. 2012). Most of our respondents also indicated patient education materials were not normally provided to patients about tumor screening, though if they were, genetic counselors provided them.
The issue of consent and informed decision-making in the context of tumor screening is important if we are to optimize the uptake of screening so that its public health benefits can be achieved. Much depends here on whether universal screening is treated as a matter of public health policy, or as a matter of individual patient choice. Informed choice generally plays a lesser role in a public health model as the perceived overall benefit to society justifies universal participation. However, in a clinical setting, patient choice is paramount such that the right of the individual to participate trumps any perceived overall utility. In the current example, the notion of no opt-out for universal screening is consistent with a public health model, while the need for consent for further testing is more consistent with a clinical choice model. The upshot is that we should not be all that concerned about getting consent at the initial screening stage, but should be more concerned about it when the initial screening reveals a potential clinical issue. Of course, when health care is funded publicly, as it is in Canada, the right of an individual to refuse further testing and/or to refuse results from previous testing could be mitigated.
Studies have shown that when provided with an opt-out, almost half of high-risk patients declined confirmatory mutation testing or did not receive their results (Ward et al. 2013). Findings such as these raise questions about the optimal way to facilitate informed decisions in this context and provide meaningful information to patients and their families (Manne et al. 2010). More broadly, findings highlight unresolved ethical issues in the context of tumor screening programs (Chubak et al. 2011; EGAPP Working Group 2009; Kalloger et al. 2012). Further, our findings revealed somewhat different attitudes towards consent among genetic counselors and pathologists, where the former were more likely to agree consent was needed. These differences of opinion will need to be resolved in determining a consent governance mechanism for population-based tumor screening programs and in ensuring the interdepartmental collaboration and communication necessary to implement these programs. With growing interest in universal screening for endometrial tumors (Ferguson et al. 2014; Mills et al. 2014), key issues regarding consent, adequate funding for genetic services and coordination of patient care are even more pressing in the context of universal tumor screening programs.
Limitations
The response rates were low in the current study and the use of SurveyMonkey to host the surveys did not allow an accurate determination of response rate. Many respondents did not complete demographic items, limiting our ability to adequately describe our sample or conduct analyses with these items. We do not know why response rate was low as numerous efforts were made to send the survey invite via national organizations and personal networks. Our panel reviewed both surveys and determined they were not overly long or taxing. It may be that online survey methods are not acceptable to Canadian genetic counselors and pathologists, or it may be that these professionals did not have time to complete the survey or did not see it as relevant to their work. Research fatigue could also be an issue as the relatively small number of genetic counselors and pathologists in general may result in an inordinate number of requests by researchers on a variety of genetic conditions. Future research will have to try a different recruitment method if findings are to be reflective of national practice, which we believe is critical in the development of national guidelines. Research with patients is also urgently needed to ensure their preferences and positions are incorporated into the development of tumor screening programs. To that end, we are undertaking a provincial survey in our jurisdiction of patients who have had colon cancer; those data will be reported separately.
Despite these limitations, our results add to the growing literature on providers’ perspectives on population-based tumor screening programs in Canada and inform ways to offer these. Genetic counselors and pathologists evinced positive attitudes towards tumor screening, endorsing an opt-out model following the initial screening test. However, the lack of patient education materials currently used in Canada highlights a critical need to develop flexible methods of patient education to allow meaningful opt-out decisions. The variability we observed also suggests the need for national standards and guidance on tumor screening for LS.
Acknowledgements
We are grateful to respondents who completed surveys and to Nic Fairbridge of the Health Research Unit, Memorial University, for assistance with data analysis.
Funding information
Funding for this project was provided through a grant to Etchegary from the Dean’s Innovation Fund, Faculty of Medicine, Memorial University.
Compliance with ethical standards
Ethics approval was obtained from the Health Research Ethics Board (Ref No. 16.062), St. John’s, NL, Canada. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (Canadian Cancer Society 2017). Informed consent was obtained from all patients for being included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Barrow E, Alduaij W, Robinson L, Shenton A, Clancy T, Lalloo F, Hill J, Evans DG. Colorectal cancer in HNPCC: cumulative lifetime incidence, survival and tumour distribution. A report of 121 families with proven mutations. Clin Genet. 2008;74:233–242. doi: 10.1111/j.1399-0004.2008.01035.x. [DOI] [PubMed] [Google Scholar]
- Beamer L, Grant M, Espenschied C, et al. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow up of abnormal results. J Clin Oncol. 2012;30:1058–1063. doi: 10.1200/JCO.2011.38.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellcross C, Bedrosian S, Daniels E, et al. Implementing screening for Lynch syndrome among patients with newly diagnosed colorectal cancer: summary of a public health/clinical collaborative meeting. Genet Med. 2012;14:152–162. doi: 10.1038/gim.0b013e31823375ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombard Y, Rozmovits L, Sorvari A, Daly C, Carroll JC, Kennedy E, Rabeneck L, Baxter NN. Universal tumor screening for Lynch syndrome: healthcare providers’ perspectives. Genet Med. 2017;19(5):568–574. doi: 10.1038/gim.2016.150. [DOI] [PubMed] [Google Scholar]
- Canadian Cancer Society (2017). Canadian Cancer Statistics 2017. Special topic: Pancreatic cancer http://www.cancer.ca/en/cancer-information/cancer-101/canadian-cancer-statistics-publication/?region=on; Accessed December 5, 2017
- Canadian Cancer Society (2018). Canadian Cancer Statistics: a 2018 special report on cancer incidence by stage.. http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2018-EN.pdf?la=en; Accessed August 4, 2018
- Chubak B, Heald B, Sharp R. Informed consent to microsatellite instability and immunohistochemistry screening for Lynch syndrome. Genet Med. 2011;13:356–360. doi: 10.1097/GIM.0b013e31820aee09. [DOI] [PubMed] [Google Scholar]
- Cohen S. Current Lynch syndrome tumor screening practices: a survey of genetic counselors. J Genet Couns. 2014;23:38–47. doi: 10.1007/s10897-013-9603-5. [DOI] [PubMed] [Google Scholar]
- Colling R, Church DN, Carmichael J, Murphy L, East J, Risby P, Kerr R, Chetty R, Wang LM. Screening for Lynch syndrome and referral to clinical genetics by selective mismatch repair protein immunohistochemistry testing: an audit and cost analysis. J Clin Pathol. 2015;68:1036–1039. doi: 10.1136/jclinpath-2015-203083. [DOI] [PubMed] [Google Scholar]
- Cross D, Rahm A, Kauffman T, CERGEN Study Team et al. Underutilization of Lynch syndrome screening in a multisite study of patients with colorectal cancer. Genet Med. 2013;15:933–940. doi: 10.1038/gim.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Chapelle A. Microsatellite instability. N Engl J Med. 2003;349:209–210. doi: 10.1056/NEJMp038099. [DOI] [PubMed] [Google Scholar]
- EGAPP Working Group Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11:35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S, Aronson M, Pollett A, et al. Performance characteristics of screening strategies for Lynch syndrome in unselected women with newly diagnosed endometrial cancer who have undergone universal germline mutation testing. Cancer. 2014;120:3932–3939. doi: 10.1002/cncr.28933. [DOI] [PubMed] [Google Scholar]
- Frolova A, Babb S, Zantow E, et al. Impact of an immunohistochemistry-based universal screening protocol for Lynch syndrome in endometrial cancer on genetic counseling and testing. Gynecol Oncol. 2015;137:7–13. doi: 10.1016/j.ygyno.2015.01.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, Green J, Buehler S, et al. Very high incidence of familial colorectal cancer in Newfoundland: a comparison with Ontario and 13 other population-based studies. Familial Cancer. 2007;6:53–62. doi: 10.1007/s10689-006-9104-x. [DOI] [PubMed] [Google Scholar]
- Hampel H. Genetic testing for hereditary colorectal cancer. Surg Oncol Clin N Am. 2009;18:687–703. doi: 10.1016/j.soc.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Frankel W, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, Comeras I, la Jeunesse J, Nakagawa H, Westman JA, Prior TW, Clendenning M, Penzone P, Lombardi J, Dunn P, Cohn DE, Copeland L, Eaton L, Fowler J, Lewandowski G, Vaccarello L, Bell J, Reid G, de la Chapelle A. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66:7810–7818. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- Hampel H, Frankel W, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A, Sumra K, Russell M, et al. A single institution experience in compliance with universal screening for Lynch syndrome in colorectal cancer. J Gastrointest Surg. 2015;19:543–550. doi: 10.1007/s11605-014-2687-x. [DOI] [PubMed] [Google Scholar]
- Hunter J, Zepp J, Gilmore M, et al. Universal tumor screening for Lynch syndrome: assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015;121:3281–3290. doi: 10.1002/cncr.29470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen H, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. GastroEnterology. 2000;118:829–834. doi: 10.1016/S0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- Jarvinen H, Renokonen-Sinisalo L, Aktan-Collan K, et al. Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol. 2009;27:4793–4797. doi: 10.1200/JCO.2009.23.7784. [DOI] [PubMed] [Google Scholar]
- Kalloger S, Ghassan A, Mulligan A, et al. Use of mismatch repair immunohistochemistry and microsatellite instability testing: exploring Canadian practices. Am J Surg Pathol. 2012;36:560–569. doi: 10.1097/PAS.0b013e31823f3b28. [DOI] [PubMed] [Google Scholar]
- Karlitz J, Hsieh M, Liu Y, et al. Population-based Lynch syndrome screening by microsatellite instability prior to colon surgery. Am J Gastroenterol. 2015;110:948–955. doi: 10.1038/ajg.2014.417. [DOI] [PubMed] [Google Scholar]
- Kidambi T, Lee R, Terdiman J, Day L. Successful implementation of Lynch syndrome screening in a safety net institution. J Community Genet. 2016;7:255–260. doi: 10.1007/s12687-016-0270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch H, Lynch P, Lanspa S, Snyder C, Lynch F, Boland C. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1–18. doi: 10.1111/j.1399-0004.2009.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mange S, Bellcross C, Cragun D, Duquette D, Gorman L, Hampel H, Jasperson K. Creation of a network to promote universal screening for Lynch syndrome: the Lynch Syndrome Screening Network. J Genet Couns. 2015;24:421–427. doi: 10.1007/s10897-014-9770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne S, Meropol N, Weinberg D, et al. Facilitating informed decisions regarding microsatellite instability testing among high-risk individuals diagnosed with colorectal cancer. J Clin Oncol. 2010;28:1366–1372. doi: 10.1200/JCO.2009.25.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez E, Geng Z, Pass S, Summerour P, Robinson L, Sarode V, Gupta S. Implementation of routine screening for Lynch syndrome in university and safety-net health system settings: successes and challenges. Genet Med. 2013;15(12):925–932. doi: 10.1038/gim.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills A, Liou S, Ford J, et al. Lynch syndrome screening should be considered for all patients with newly diagnosed endometrial cancer. Am J Surg Pathol. 2014;38(11):1501–1509. doi: 10.1097/PAS.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira L, Balaguer F, Lindor N, de la Chapelle A, Hampel H, Aaltonen LA, Hopper JL, le Marchand L, Gallinger S, Newcomb PA, Haile R, Thibodeau SN, Gunawardena S, Jenkins MA, Buchanan DD, Potter JD, Baron JA, Ahnen DJ, Moreno V, Andreu M, Ponz de Leon M, Rustgi AK, Castells A, EPICOLON Consortium Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308:1555–1565. doi: 10.1001/jama.2012.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mvundura M, Grosse S, Hampel H, Palomaki G. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med. 2010;12:93–104. doi: 10.1097/GIM.0b013e3181cd666c. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (2012). Clinical practice guidelines in oncology: colorectal cancer screening (v2.2012). http://www.tri-kobe.org/nccn/guideline/colorectal/english/colorectal_screening.pdf. Accessed September 1, 2015 [DOI] [PubMed]
- Parfrey P, Dicks E, Parfrey O, et al. Evaluation of a population-based approach to familial colorectal cancer. Clin Genet. 2017;91:672–682. doi: 10.1111/cge.12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres J. To screen or not to screen for Lynch syndrome. J Natl Cancer Inst. 2010;102:1382–1384. doi: 10.1093/jnci/djq372. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Bigas M, Boland C, Hamilton S, et al. A National Cancer Institute Workshop on hereditary nonpolyposis colorectal cancer syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89:1758–1762. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- Sanchez J, Vogel J, Kalady M, et al. Identifying Lynch syndrome: we are all responsible. Dis Colon Rectum. 2008;51:1750–1756. doi: 10.1007/s10350-008-9414-1. [DOI] [PubMed] [Google Scholar]
- Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23:334–340. doi: 10.1002/1098-240X(200008)23:4<334::AID-NUR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Sandelowski M. What’s in a name? Qualitative description revisited. Res Nurs Health. 2010;33:77–84. doi: 10.1002/nur.20362. [DOI] [PubMed] [Google Scholar]
- Schneider J, Davis J, Kauffman T, et al. Stakeholder perspectives on implementing a university Lynch syndrome screening program: a qualitative study of early barriers and facilitators. Genet Med. 2016;18(2):152–161. doi: 10.1038/gim.2015.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South C, Yearsley M, Martin E, et al. Immunohistochemistry staining for the mismatch repair proteins in the clinical care of patients with colorectal cancer. Genet Med. 2009;11:812–817. doi: 10.1097/GIM.0b013e3181b99b75. [DOI] [PubMed] [Google Scholar]
- Stoffel E, Mukherjee B, Raymond V, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. GastroEnterology. 2009;137:1621–1627. doi: 10.1053/j.gastro.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiak E, Samson A, Spector N, Mackey M, Gilpin C, Smith E, Jonker D, Allanson J, Asmis T. Relfex testing for Lynch syndrome: if we build it, will they come? Lessons learned from the uptake of clinical genetic services by individuals with newly diagnosed colorectal cancer. Familial Cancer. 2014;13:75–82. doi: 10.1007/s10689-013-9677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umar A, Boland C, Terdiman J, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasen H, Mecklin J, Khan P, Lynch H. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34:424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- Vasen H, Watson P, Mecklin J, Lynch H. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. GastroEnterology. 1999;116:1453–1456. doi: 10.1016/S0016-5085(99)70510-X. [DOI] [PubMed] [Google Scholar]
- Ward R, Hicks S, Hawkins N. Population-based molecular screening for Lynch syndrome: implications for personalized medicine. J Clin Oncol. 2013;31:2554–2562. doi: 10.1200/JCO.2012.46.8454. [DOI] [PubMed] [Google Scholar]
- Watson P, Vasen H, Mecklin J, et al. The risk of extra-colonic, extra endometrial cancer in the Lynch syndrome. Int J Cancer. 2008;123:444–449. doi: 10.1002/ijc.23508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman S, Bellcross C, Bittner C, et al. Genetic counseling considerations in the evaluation of families for Lynch syndrome—a review. J Genet Couns. 2011;20:5–19. doi: 10.1007/s10897-010-9325-x. [DOI] [PubMed] [Google Scholar]
- Williams J, Williams M. Informed consent and immunohistochemistry screening for Lynch syndrome. Genet Med. 2011;13:848–849. doi: 10.1097/GIM.0b013e318228efc8. [DOI] [PubMed] [Google Scholar]
- Woods M, Younghusband B, Parfrey P, et al. The genetic basis of colorectal cancer in a population-based incident cohort with a high rate of familial disease. Gut. 2010;59:1369–1377. doi: 10.1136/gut.2010.208462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D, Arnold J, Parry B, et al. Immunohistochemistry to detect hereditary polyposis colorectal cancer in young patients: the 7-year Auckland experience. Dis Colon Rectum. 2011;54:552–558. doi: 10.1007/DCR.0b013e31820e3265. [DOI] [PubMed] [Google Scholar]