Abstract
Aim of the present study was to investigate whether body weight (BW) in broilers is associated with functional modular genes. To this end, first a GWAS for BW was conducted using 6,598 broilers and the high density SNP array. The next step was to search for positional candidate genes and QTLs within strong LD genomic regions around the significant SNPs. Using all positional candidate genes, a network was then constructed and community structure analysis was performed. Finally, functional enrichment analysis was applied to infer the functional relevance of modular genes. A total number of 645 positional candidate genes were identified in strong LD genomic regions around 11 genome-wide significant markers. 428 of the positional candidate genes were located within growth related QTLs. Community structure analysis detected 5 modules while functional enrichment analysis showed that 52 modular genes participated in developmental processes such as skeletal system development. An additional number of 14 modular genes (GABRG1, NGF, APOBEC2, STAT5B, STAT3, SMAD4, MED1, CACNB1, SLAIN2, LEMD2, ZC3H18, TMEM132D, FRYL and SGCB) were also identified as related to body weight. Taken together, current results suggested a total number of 66 genes as most plausible functional candidates for the trait examined.
Subject terms: Genomics, Animal breeding
Introduction
Body weight (BW) is an economically important trait for the broiler industry. This trait also presents considerable biological interest as it is a typical complex (polygenic) trait. To date, the ChickenQTLdb1 contains over 7,812 QTL/SNP associations of which 3,582 are related to growth. Several genome wide association studies (GWAS) have already been performed for growth traits (e.g.2,3) in the species. The development of the chicken 600k SNP array4 facilitates efficient screening for causal loci and genes with relevance to target traits due to the uniform coverage across chromosomes and the inclusion of markers within coding regions. Despite the large number of findings by GWAS, understanding of the genetic architecture of BW in chicken remains limited5, since only a small number of positional candidate genes are confirmed as truly functionally relevant to the trait (e.g. HDAC2 and GNPDA26,7). The use of various Bioinformatics tools such as gene enrichment analysis8, pathway analysis9 and gene network analysis10 can tackle this problem and aid in identifying the most promising functional candidate genes for the trait under study. Moreover, applications such as GeneMANIA11 that is based on the guilt-by-association (GBA) principle12 may also facilitate the identification of true causative genetic variants. The GBA principle states that gene products, which are protein interaction partners, tend to be functionally related13. Furthermore, genes in protein–protein interaction networks (PPINs) are organized into densely linked clusters i.e. communities or modules14. Modules present a structurally independent gene sub-network with more interior connections and consist of proteins which have the same or similar biological function(s)15. Modules could be further distinguished in protein complexes and in dynamic functional modules. Protein complexes are formed by several proteins which interact at the same place and time while dynamic functional modules are composed of few proteins participating in a specific cellular function not necessarily at the same place and time16. Moreover, functional modules consist of one or multiple protein complexes participating in a common biological process17. Since modules do not emerge by chance, they can reveal interactions with biological importance within large PPINs16,18. The module-based approach has already been used to cluster genes into functional groups and to predict protein functions19. Investigation of functional modules has mainly been focused on human diseases such as obesity20, breast cancer21,22, coronary artery disease23 and asthma24. Apart from human, functional modules have been identified in other species as well, such as in Mus musculus for discrete and rhythmic forelimb movements in motor cortex25 and in Gallus gallus for muscle development and intramuscular fat accumulation at different post-hatching ages26.
Driven from findings in other species and traits, aim of the present study was first to investigate whether body weight in broilers is associated with functional modules and second to propose novel candidate genes for the trait in question.
Results
Significant SNPs and positional candidate genes
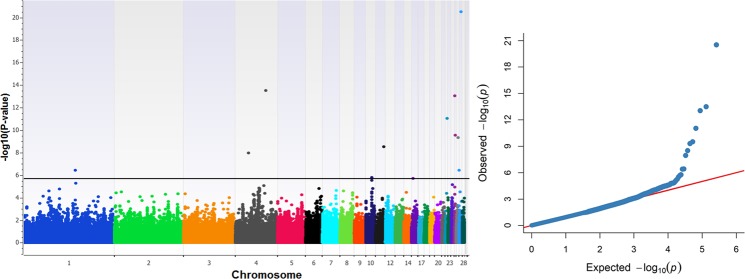
Figure 1 shows the Q-Q plot of the expected and the observed p values (−log10 p values) of all SNPs. The genomic inflation factor (λ) was also estimated as high as 0.93. According to Kang et al.27, λ values that lie outside of the conservative 95% confidence interval (0.992 to 1.008) denote dependency of SNPs. However, as the Q-Q plot clearly shows, there is no evidence of any systematic bias due to population structure or analytical approach in our case. As Yang et al.28 emphasize in their paper, it is reasonable to expect deviation(s) of λ from 1 for purely polygenic traits such as that examined here in the absence of any systematic bias. The Q-Q plot also shows that some SNPs depart from the expected probability and thus might be associated with the trait. These SNPs are also displayed in Fig. 1 in a form of a Manhattan plot.
Figure 1.
Manhattan plot (left) and quantile-quantile plot (right) for BW. Manhattan plot shows the −log10 (observed p-values) of the genome-wide SNPs (y-axis) across the 28 autosomes (x-axis), and the horizontal line denotes the genome-wide significant threshold. With regard to the Q-Q plot, the y-axis represents the observed −log10 (p-values) and the x-axis shows the expected −log10 (p-values). Manhattan plot was constructed with SNP & Variation Suite (version 8.8.1) software (Golden Helix: http://www.goldenhelix.com) while Q-Q plot with the CMplot package (https://github.com/YinLiLin/R-CMplot) in R (http://www.r-project.org/).
Specifically, there were 12 SNPs detected, across nine autosomes (1, 4, 10, 11, 15, 22, 25, 26 and 27) reaching genome-wide significance (FDR p-value < 0.05). A detailed description of the significant (lead) SNPs is provided in Table 1. Table 2 displays the extent of genomic regions displaying strong LD (D′ > 0.8) around the lead markers that were searched for positional candidate genes. Note that marker rs312758346 (GGA25) was omitted here as LD levels around this marker were below the threshold LD value (D′ < 0.8). In total, 645 positional candidate genes were identified within the searched genomic regions (Supplementary Table S1). From the candidate genes, n = 15 were microRNAs with 13 of them (MIR6672, MIR1720, MIR7-2, MIR3529, MIR1571, MIR1560, MIR1785, MIR6662, MIR7454, MIR10A, MIR6663, MIR1735 and MIR6547) published in the miRBase database (http://www.mirbase.org/) for the species. Moreover, 190 candidate genes were unannotated (LOC) resulting in a total number of 455 annotated positional candidate genes. The maximum number of candidate genes (n = 192) was identified in a region spanning 998.5 kb (average D′ = 0.98) around marker rs315329074 on GGA27. At the other extreme, the smallest number of candidate genes was identified for rs316794400 within a narrow region spanning 26.6 kb (average D′ = 0.96) on GGA22. Six out of the 11 lead markers were located within annotated genes i.e. SLAIN2 (GGA4), ZC3H18 (GGA11), TMEM132D (GGA15), F-KER (GGA25), LEMD2 (GGA26) and CACNB1 (GGA27).
Table 1.
Genome-wide significant SNPs (FDR p-value < 0.05) for BW.
| SNP ID | GGA | Position (bp)1 | −log10(p-value) | FDR p-value |
|---|---|---|---|---|
| rs13923872 | 1 | 112,741,685 | 6.415 | 0.0112 |
| rs312691174 | 4 | 29,074,989 | 7.948 | 0.00037 |
| rs15608447 | 4 | 66,885,210 | 13.489 | 4.25E-09 |
| rs318199727 | 10 | 13,536,548 | 5.763 | 0.04111 |
| rs318098582 | 11 | 18,651,449 | 8.513 | 0.00012 |
| rs317945754 | 15 | 3,557,083 | 5.677 | 0.04594 |
| rs316794400 | 22 | 4,594,855 | 11.033 | 6.07E-07 |
| rs317288536 | 25 | 976,833 | 13.035 | 8.05E-09 |
| rs312758346 | 25 | 2,412,866 | 9.517 | 1.59E-05 |
| rs317627533 | 26 | 4,597,439 | 9.313 | 2.12E-05 |
| rs314452928 | 27 | 104,022 | 6.398 | 0.0105 |
| rs315329074 | 27 | 4,528,275 | 20.513 | 8.05E-16 |
1Positions are based on Gallus gallus-5.0 genome assembly.
Table 2.
Number of positional candidate genes and QTL/associations within the searched genomic regions (±maximum distance of the farest SNP being in strong LD (D′ >0.8) with the lead SNP; D′: average D′ values within the searched genomic region).
| SNP ID | GGA | Position (bp)1 | Searched genomic range around ‘lead’ SNP (±bp) | D′ | Number of positional candidate genes | Number of QTL/associations |
|---|---|---|---|---|---|---|
| rs13923872 | 1 | 112,741,685 | 613,054 | 0.91 | 33 | 20 |
| rs312691174 | 4 | 29,074,989 | 650,472 | 1 | 16 | 14 |
| rs15608447 | 4 | 66,885,210 | 718,407 | 0.88 | 36 | 36 |
| rs318199727 | 10 | 13,536,548 | 737,906 | 0.83 | 33 | 11 |
| rs318098582 | 11 | 18,651,449 | 300,257 | 0.81 | 27 | 9 |
| rs317945754 | 15 | 3,557,083 | 935,183 | 0.99 | 20 | 21 |
| rs316794400 | 22 | 4,594,855 | 26,589 | 0.96 | 7 | 1 |
| rs317288536 | 25 | 976,833 | 1,004,513 | 0.83 | 176 | — |
| rs317627533 | 26 | 4,597,439 | 773,988 | 0.9 | 93 | 6 |
| rs314452928 | 27 | 104,022 | 140,067 | 0.94 | 12 | 3 |
| rs315329074 | 27 | 4,528,275 | 998,553 | 0.98 | 192 | 65 |
1Positions are based on Gallus gallus-5.0 genome assembly.
Reported QTL/associations
Table 2 shows the number of published QTL/associations reported within the searched genomic regions. A total of 186 QTL/associations related to growth traits or carcass traits (carcass weight, abdominal fat percentage, breast muscle percentage and average daily gain) were identified within the searched regions. QTL/associations were distributed across eight chromosomes (1, 4, 10, 11, 15, 22, 26 and 27) and a detailed description of the reported QTL can be found in Supplementary Table S2. Note that the searched region around rs317288536 (GGA25) is not reported to harbor any QTL/association (Table 2). Furthermore, the only QTL reported on GGA22 as well as two additional QTL on GGA26 and GGA27 could not be remapped in Gallus gallus−5.0 by the Genome remapping service tool from NCBI database. Nevertheless, based on the Gallus gallus-4 genome assembly, the searched regions around rs316794400, rs317627533 and rs314452928 overlapped with three QTL (IDs: 95429, 30883 and 55944). The maximum number (n = 65) of QTL/associations was reported around rs315329074 (GGA27) and the minimum number (n = 1) around rs316794400 (GGA22). Nine out of the 12 lead SNPs on autosomes 1, 4, 10, 11, 15, 26 and 27 lie within 96 out of the 186 growth-related QTL (Supplementary Table S2). In addition, nearly all reported QTL on the searched regions on GGA4 (n = 49/50) and GGA11 (n = 9/9) contain at least one of the lead markers (rs312691174, rs15608447 and rs318098582 respectively).
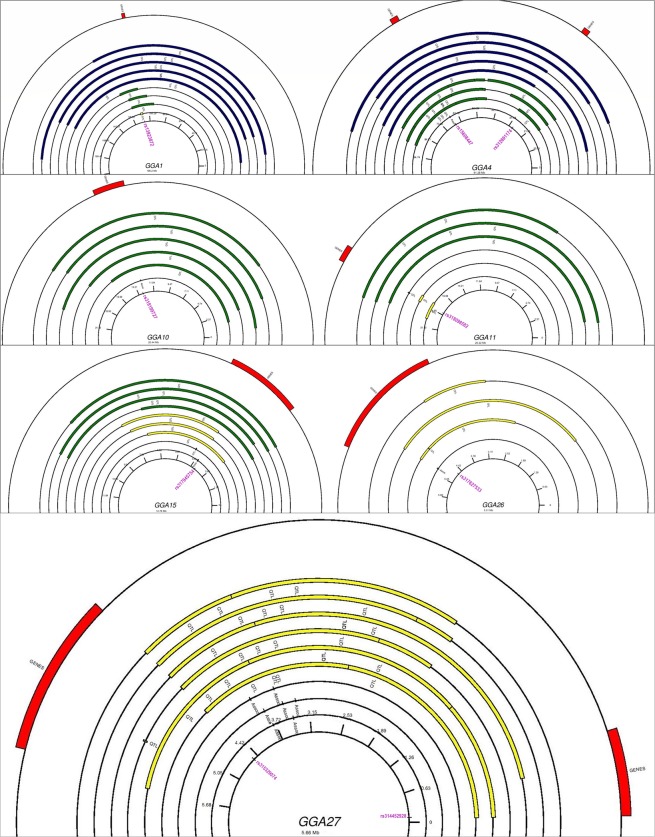
We further sought to examine the locations of the positional candidate genes in the relation to the positions of the reported QTL. These results are illustrated in Fig. 2 in forms of circular maps for seven autosomes (1, 4, 10, 11, 15, 26 and 27). On GGA1, all 33 candidate genes (around rs13923872) are lying in a genomic region spanning from 2421 to 196203 kb where 17 relevant QTL have been reported. On GGA4, all 16 candidate genes around rs312691174 are located within a region spanning from 4965 to 91268 kb, where all 14 published QTL reside. On the same autosome, all genes (n = 36) around the second significant marker (rs15608447) are located in a region spanning from 4965 to 91268 kb where 35 QTL relevant to body weight, liver weight, carcass weight, total white fat weight have been reported. On GGA10, the region spanning from 693 to 20423 kb around the ‘lead’ marker (rs318199727) harbours all the 33 candidate genes and overlaps with 6 growth related QTL. On GGA11, in a region spanning from 953 to 20209 kb around rs318098582 there have been 7 reported QTL and 27 candidate genes identified. On GGA15, in a region spanning from 1932 to 10689 kb around rs317945754), 6 QTL related to growth traits (visceral fat weight, abdominal fat weight and breast muscle weight) are reported and 20 candidate genes were identified. Moreover, on GGA26 (rs317627533), 64 out of the 93 candidate genes lie in a narrow region (1264 to 4918 kb) where QTL associated with growth traits such as body weight and shank weight, are reported. On GGA27 in a regions spanning from 55 to 4520 kb around rs314452928, 2 related QTL were identified including 7 out of the 12 candidate genes. All 192 genes around the second marker (rs315329074) on GGA27 were located within one published QTL spanning 3788 to 5630 kb that has been associated with thigh percentage. In total, 428 out of the 462 positional candidate genes (genes on GGA22 and GGA25 were not included here) were located within regions with reported QTL/associations.
Figure 2.
Circular chromosome maps for seven autosomes presenting combined data of reported QTL (n = 183) and positional candidate genes (n = 462). Blue color represents the extent of large sized QTL (50–196.2 Mb), green color the medium sized QTL (5–50 Mb) and the yellow color is indicative of the small QTL (0–5 Mb). Red color indicates the starting and ending positions of positional candidate genes. The position(s) of the significant SNPs (labeled in purple color) is also given. The figure was constructed using GenomeVx87.
Detection of community structure
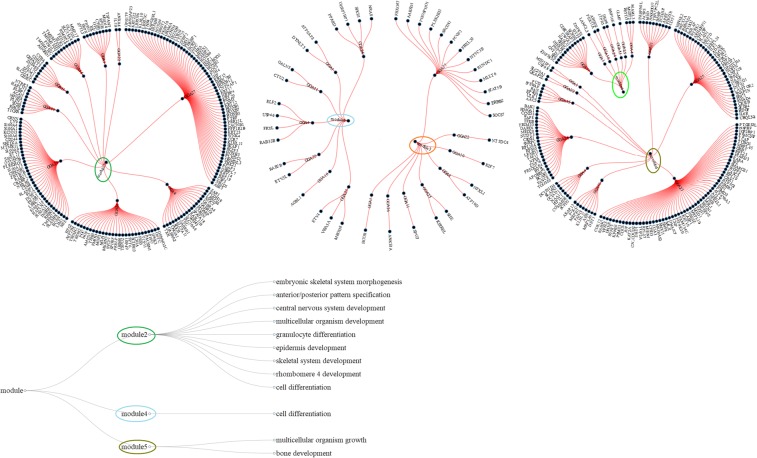
A network including 402 genes (nodes) and 5294 interactions (edges) was generated. Note that for APOA1BP and LOH11CR2A genes the homologous human gene descriptions (NAXE and VWA5A) were used, respectively. Community structure analysis detected 5 modules, formed by 401 genes (see Supplementary Table S3). One more module was also detected but this was consisted by only one gene (NIPAL1). Thus it cannot be considered as a typical module. Note that this gene network had a strong community structure as indicated by the high (0.59) estimated modularity value29. Distribution of the 401 genes across the 5 modules is displayed in Fig. 3. Module_2 consisted of 187 genes, module_3 of 22 genes, module_4 of 18 genes, module_5 of 152 genes and module_6 of 22 genes.
Figure 3.
Network modules along with the significantly enriched developmental processes per module. The five modules are presented in the three radial networks (on the top) as circles/ellipses with different color together with their member genes and the corresponding chromosomes. The diagonal network at the bottom provides the significantly enriched developmental processes per module. Figure was constructed using the data.tree and networkD3 packages in R (http://www.r-project.org/).
Functional enrichment analysis per module
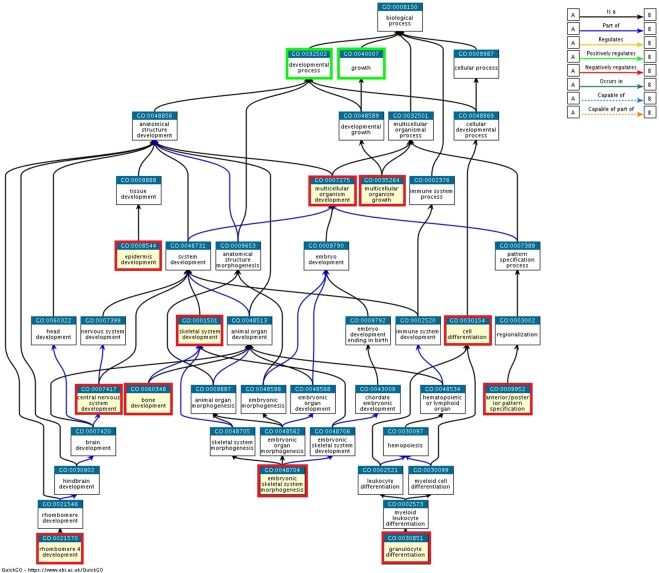
Four (module_ID: 2–5) out of the five modules exhibited enriched GO BPs while only three modules were associated with developmental processes according to QuickGO. Specifically, in module_2, a total number of 21 enriched GO BPs (Supplementary Table S4) and 78 participating genes were identified. According to QuickGO (see Figs 3 and 4), 9 out of the 21 GO BPs were related to development with 42 member genes (Supplementary Table S4). In the same module, 8 genes belonging to the homeobox B family genes along with MDFI were found to be enriched in embryonic skeletal system morphogenesis (GO:0048704). In module_3, none of the enriched GO BP terms were related to development (Supplementary Table S4). In module_4, three significantly enriched BPs (Supplementary Table S4) were identified in 7 member genes. Here, the only GO BP term that was associated with development through QuickGO was cell differentiation (GO:0030154) with 4 member genes (PPARD, ELF2, ETV3L and ETV4, Fig. 4). A total number of 29 GO BPs were found as significantly enriched in module_5 (Supplementary Table S4). Here, two development related processes i.e. multicellular organism growth (GO:0035264) and bone development (GO:0060348) were identified by QuickGO (Fig. 4) with 6 involved genes (KAT2A, SP2, ANKRD11, RARA, BGLAP and AKAP13).
Figure 4.
GO hierarchical structure for the eleven significantly enriched BPs (denoted with red color) associated with developmental process/growth term (denoted with green color). This GO tree was created and extracted by QuickGO86.
Functional candidate genes
An exhaustive list, including 66 modular genes, of the most plausible candidate genes for BW is provided in Table 3. From these genes, 52 were participating in enriched developmental processes, 7 were growth related genes that were not enriched to any developmental process and 7 were growth related genes identified in previous studies. These 66 modular genes were distributed across 7 chromosomes (GGA4, GGA10, GGA11, GGA15, GGA25, GGA26 and GGA27) with 47 of them detected in module_2. The KRT (keratins) family and B cluster of HOX (homeobox) family genes were also included here.
Table 3.
List of 66 most plausible candidate genes for BW according to the following criteria: modular genes participating in enriched developmental processes, growth related modular genes not significantly enriched to any developmental process and growth related modular genes reported in previous studies.
| Criterion | Gene | Description | Module_ID | GGA |
|---|---|---|---|---|
| modular genes participating in enriched developmental processes | BTG2 | BTG anti-proliferation factor 2 | module_2 | 26 |
| ZAR1 | zygote arrest 1 | module_2 | 4 | |
| MEOX1 | mesenchyme homeobox 1 | module_2 | 27 | |
| KRT14 | keratin 14 | module_2 | 27 | |
| KRT15 | keratin 15 | module_2 | 27 | |
| TXK | TXK tyrosine kinase | module_2 | 4 | |
| CSF3 | colony stimulating factor 3 | module_2 | 27 | |
| ACAN | aggrecan | module_2 | 10 | |
| HOXB1 | homeobox B1 | module_2 | 27 | |
| HOXB2 | homeobox B2 | module_2 | 27 | |
| HOXB3 | homeobox B3 | module_2 | 27 | |
| HOXB4 | homeobox B4 | module_2 | 27 | |
| HOXB5 | homeobox B5 | module_2 | 27 | |
| HOXB6 | homeobox B6 | module_2 | 27 | |
| HOXB7 | homeobox B7 | module_2 | 27 | |
| HOXB8 | homeobox B8 | module_2 | 27 | |
| HOXB9 | homeobox B9 | module_2 | 27 | |
| HOXB13 | homeobox B13 | module_2 | 27 | |
| MDFI | MyoD family inhibitor | module_2 | 26 | |
| NES | nestin | module_2 | 25 | |
| TBX21 | T-box 21 | module_2 | 27 | |
| IGFBP4 | insulin like growth factor binding protein 4 | module_2 | 27 | |
| PRELP | proline and arginine rich end leucine rich repeat protein | module_2 | 26 | |
| HAPLN2 | hyaluronan and proteoglycan link protein 2 | module_2 | 25 | |
| HAPLN3 | hyaluronan and proteoglycan link protein 3 | module_2 | 10 | |
| GABRA4 | gamma-aminobutyric acid type A receptor alpha4 subunit | module_2 | 4 | |
| BCAN | brevican | module_2 | 25 | |
| NHLH1 | nescient helix-loop-helix 1 | module_2 | 25 | |
| ZBTB7B | zinc finger and BTB domain containing 7B | module_2 | 25 | |
| FZD10 | frizzled class receptor 10 | module_2 | 15 | |
| TCP11 | t-complex 11 | module_2 | 26 | |
| PIWIL1 | piwi like RNA-mediated gene silencing 1 | module_2 | 15 | |
| SPDEF | SAM pointed domain containing ETS transcription factor | module_2 | 26 | |
| ZFPM1 | zinc finger protein, FOG family member 1 | module_2 | 11 | |
| CBFA2T3 | CBFA2/RUNX1 translocation partner 3 | module_2 | 11 | |
| KRT17 | keratin 17 | module_2 | 27 | |
| CRABP2 | cellular retinoic acid binding protein 2 | module_2 | 25 | |
| SH2D2A | SH2 domain containing 2 A | module_2 | 25 | |
| NR1D1 | nuclear receptor subfamily 1 group D member 1 | module_2 | 27 | |
| STX2 | syntaxin 2 | module_2 | 15 | |
| TEC | tec protein tyrosine kinase | module_2 | 4 | |
| ETV3 | ETS variant 3 | module_2 | 25 | |
| PPARD | peroxisome proliferator activated receptor delta | module_4 | 26 | |
| ELF2 | E74 like ETS transcription factor 2 | module_4 | 4 | |
| ETV3L | ETS variant 3 like | module_4 | 25 | |
| ETV4 | ETS variant 4 | module_4 | 27 | |
| KAT2A | lysine acetyltransferase 2A | module_5 | 27 | |
| RARA | retinoic acid receptor alpha | module_5 | 27 | |
| BGLAP | bone gamma-carboxyglutamate protein | module_5 | 25 | |
| SP2 | Sp2 transcription factor | module_5 | 27 | |
| ANKRD11 | ankyrin repeat domain 11 | module_5 | 11 | |
| AKAP13 | A-kinase anchoring protein 13 | module_5 | 10 | |
| growth related modular genes not significantly enriched to any developmental process | GABRG1 | gamma-aminobutyric acid type A receptor gamma1 subunit | module_2 | 4 |
| NGF | nerve growth factor | module_2 | 26 | |
| APOBEC2 | apolipoprotein B mRNA editing enzyme catalytic subunit 2 | module_5 | 26 | |
| STAT5B | signal transducer and activator of transcription 5B | module_3 | 27 | |
| STAT3 | signal transducer and activator of transcription 3 | module_5 | 27 | |
| SMAD4 | SMAD family member 4 | module_5 | 25 | |
| MED1 | mediator complex subunit 1 | module_5 | 27 | |
| growth related modular genes reported in previous studies | CACNB1 | calcium voltage-gated channel auxiliary subunit beta 1 | module_2 | 27 |
| SLAIN2 | SLAIN motif family member 2 | module_5 | 4 | |
| LEMD2 | LEM domain containing 2 | module_5 | 26 | |
| ZC3H18 | zinc finger CCCH-type containing 18 | module_5 | 11 | |
| TMEM132D | transmembrane protein 132D | module_2 | 15 | |
| FRYL | FRY like transcription coactivator | module_4 | 4 | |
| SGCB | sarcoglycan beta | module_2 | 4 |
Discussion
Results of the present study have shown that a typical quantitative trait such as that examined here is associated with modular genes exhibiting functional relevance to developmental processes. This means that application of functional enrichment analysis on modular genes can facilitate the identification of true causative genes for the trait under study. Following this approach, a total number of 52 functional candidate genes could be identified in the present study. Example genes that fall in this category were the following: BTG2, ZAR1, MEOX1, KRT14, KRT15, TXK, CSF3, ACAN, HOXB, MDFI, NES, IGFBP4, PRELP, PPARD, ELF2, KAT2A, RARA and BGLAP. Specifically, BTG2, ZAR1, MEOX1, KRT14 and KRT15 have been reported to participate in cerebellar development30, development of follicular oocytes31, somite differentiation32, keratinocytes proliferation33 and pigmentation of muscle tissues34, in chickens, respectively. TXK (TXK tyrosine kinase) has been reported as BW related gene35 while CSF3 (colony stimulating factor 3) has been described as a myelomonocytic growth factor in the species36. ACAN (aggrecan) is essential for cartilage formation during development in chicken and mouse mutants37 and the HOX B cluster genes are expressed in chick embryonic development38. The MDFI (MyoD family inhibitor) tumor suppressor gene is known to have a negative effect on myogenic regulatory factors39 while NES (nestin) is known as a neural progenitor cell marker during central nervous system development and a marker protein for neovascularization40. Furthermore, IGFBP4 (insulin like growth factor binding protein 4) is required for the adipose tissue development41 while PRELP (proline and arginine rich end leucine rich repeat protein) is highly expressed in cartilage, basement membranes, and bone development42. PPARD (peroxisome proliferator activated receptor delta) is a critical gene for normal adipose development and lipid homeostasis43 while ELF2 (E74 like ETS transcription factor 2) plays a key role in the development of lymphocytes44. KAT2A (lysine acetyltransferase 2A) is necessary for growth and differentiation of craniofacial cartilage and bone in zebrafish and mice45, RARA (retinoic acid receptor alpha) affects the hippocampal development46 and finally BGLAP is produced by osteoblasts shaping new bones in chickens47.
However, the search for modular genes that are exclusively enriched in functionally relevant terms has not proved to be efficient in identifying all true functional candidate genes. This finding may be fairly supported by the fact that 7 more genes (GABRG1, NGF, APOBEC2, STAT5B, STAT3, SMAD4 and MED1) that despite having well documented relevance to development were found to be enriched in other but developmental GO BP terms. Specifically, GABRG1(gamma-aminobutyric acid type A receptor gamma1 subunit) is reported as a BW related gene35 and NGF (nerve growth factor) is a regulator of the somite survival and axial rotation during early chicken embryo development48. APOBEC2 (apolipoprotein B mRNA editing enzyme catalytic subunit 2) is known as a critical regulator and maintainer of muscle development in mammals and might affect muscle development in chickens49. In the species, STAT5B (signal transducer and activator of transcription 5B) is associated with growth50. STAT3 (signal transducer and activator of transcription 3) plays a central role in development51, SMAD4 (SMAD family member 4) is a central mediator of the transforming growth factor β signaling pathway which affects among others the cell growth52 and finally MED1 (mediator complex subunit 1) has a key role in mammary epithelial cell growth53.
The list with the most plausible candidate genes for the trait was, however, not exhausted in the previous two categories since 7 more genes (CACNB1, SLAIN2, LEMD2, ZC3H18, TMEM132D, FRYL and SGCB) with well documented implication to BW, were completely omitted in any enrichment analysis. Most interestingly, five of the above genes (CACNB1, SLAIN2, LEMD2, ZC3H18 and TMEM132D) contained lead SNPs. CACNB1 (calcium voltage-gated channel auxiliary subunit beta 1) has been reported to affect skeletal muscle development54 in mice. SLAIN2 (SLAIN motif family member 2) is necessary for the normal structure of microtubule cytoskeleton as it controls the microtubule growth during interphase55. LEMD2 (LEM domain containing 2) participates in nuclear structure organization56 and plays an important role in mouse embryonic development by regulating various signaling pathways such as MAPK (mitogen-activated protein kinase) and AKT (also known as Protein Kinase B)57. ZC3H18 (zinc finger CCCH-type containing 18) participates in RNA degradation58 and affects mRNA metabolism59. Finally, TMEM132D (transmembrane protein 132D) may function as a tumor suppressor gene60. Finally, both FRYL (FRY like transcription coactivator) and SGCB (sarcoglycan beta) have been associated with growth61,62 in the species.
The two aforementioned gene lists underline the potential limitations of a cluster based method such as that used here to assess the biological properties of the candidate gene sets. Specifically, these limitations relate to i) grouping of similar terms into a cluster and evaluating the enrichment of functional clusters instead of each individual term within the clusters and ii) the evaluation of the identified term clusters separately, while not taken into consideration the relationships between clusters63.
Apart from functional enrichment analysis, other analyses such as pathway analysis, gene network analysis and GBA gene prioritization analysis could also assist in identifying true causative genetic variants for the trait under study. For instance, in a previous study64, the use of GBA gene prioritization analysis on 1,012 positional candidate genes revealed 248 functional candidate genes for the same trait. However, fixed genomic regions (of 1 Mb) around the lead genomic markers were used in that study. A final interesting result of the present study was the discovery of 15 microRNAs within the 645 candidate genes for the trait under investigation. One of these, i.e. MIR10A has been reported as significant for feed intake in broilers65. MIR10A together with MIR10B have been reported to inhibit the development of human, mouse and rat granulosa cells during folliculogenesis66. Finally, MIR7-2 has been reported as genomic locus for peroxisome proliferator activated receptor regulation67 and may have a functional role in hepatic lipid homeostasis. MicroRNAs have emerged as important regulators of gene expression post-transcriptionally and in Gallus gallus are known to play crucial roles in various biological processes such as the accumulation of abdominal fat68 and the lipid metabolism69.
In conclusion, the present GWAS revealed a large number of genomic regions and genes implicated in the genetic architecture of a complex trait such as the BW that fully complies with the Fisher’s infinitesimal model of inheritance. Exploitation of both community structure and functional enrichment analyses highlighted 3 modules as related to development. Current findings also indicated 52 modular genes participating in developmental processes and 14 more modular genes related to BW. Finally, the present study proposed 66 functional candidate genes for BW, some of which are novel and some identified candidates in previous studies.
Methods
Ethics statement
All animals included in this study were not subjected to any invasive procedures.
Data and quality control
In total, n = 6,727 broilers (n = 3,735 males and n = 2,992 females) from a grand-grandparent (GGP) commercial line with records on BW at 35 days of age were made available by Aviagen Ltd. Phenotypic records for BW ranged from 1,130 to 2,630 g with an average of 1840.2 g (SD = 194 g). Animals were genotyped using the 600k Affymetrix® Axiom® high density genotyping array4 resulting in a total number of 578,815 SNPs. Only autosomal SNPs (n = 547,705) were considered. Quality control was performed first at a sample and second at a marker level. At a sample level, 72 females and 57 males were excluded due to call rate <0.99 and autosomal heterozygosity outside the 1.5 IQR (inter-quartile range) resulting in a number of n = 6,598 samples. At a marker level, a number of 285,717 SNPs were excluded due to: call rate <0.99, MAF (minor allele frequency) <0.01 and linkage disequilibrium (LD) r2 values greater than 0.99 within windows of 1 Mb inter-marker distance(s). A total of 6,598 samples and 262,067 SNPs were retained for GWAS. Quality control was performed using the SNP & Variation Suite software (version 8.8.1) of Golden Helix (http://www.goldenhelix.com).
Statistical analysis
A multi-locus mixed-model (MLMM) stepwise regression with forward inclusion and backward elimination70 of SNPs was employed to identify genome-wide significant markers associated with the trait. The following statistical model was applied to the data:
where y is the n x 1 vector of phenotypic values of BW for n broilers, X is the n x 55 matrix of fixed effects: sex (2 classes), hatch (36 classes) and mating group (17 classes), β is the 55 × 1 vector of corresponding coefficients of fixed effects, w is the vector with elements of 0, 1, and 2 for the homozygote of the minor allele, heterozygote, and homozygote of the major allele, α is the vector of the fixed effect for the minor allele of the candidate SNP to be tested for association, Z is the incidence matrix relating observations to the polygenic random effects, u is the vector of polygenic random effects and e is the vector of random residuals.
The random effects were assumed to be normally distributed with zero means and the following covariance structure:
where and are the polygenic and error variance components, I is the nxn identity matrix, and G is the nxn genomic relationship matrix (GRM71) with elements of pairwise relationship coefficient using the 262,067 SNPs. Τhe genomic relationship coefficient between two individuals j and k, was estimated as follows:
where xij and xik represent the number (0, 1, or 2) of the minor allele for the ith SNP of the jth and kth individuals, and pi is the frequency of the minor allele71.
Statistically significant markers were selected at the optimal step of the MLMM stepwise regression according to extended Bayesian Information Criterion (eBIC72). P-values of these SNPs were then corrected for multiple comparisons using the false-discovery rate (FDR73) correction method. Here, a cut-off FDR p-value less than 0.0574 was considered as significant. The FDR p-value of 0.05 states that, among all observed results, 5% would be false positives.
A Quantile-quantile (Q-Q) plot was also used to analyze the extent to which the observed distribution of the test statistic followed the expected (null) distribution. This plot along with the estimation of the genomic inflation factor (λ) was done to assess potential systematic bias due to population structure or to the analytical approach28. This analysis was performed using the SNP & Variation Suite (version 8.8.1) software (Golden Helix: http://www.goldenhelix.com).
Detection of candidate genomic regions with strong LD
We first estimated LD levels around each lead i.e. significant SNP. We then searched for genomic regions with strong LD around the lead SNPs defined as the maximum distance between the lead and the last SNP with D′ ≥ 0.875. Note that, the D′, instead of the r2 LD measurement, was preferably used here as the first one is reported to be independent76 or less dependent77 on MAF. All LD calculations were performed using the SNP & Variation Suite (version 8.8.1) software (Golden Helix: http://www.goldenhelix.com).
Identification of reported QTL and positional candidate genes
Next, we searched for growth/fatness related QTL in the ChickenQTLdb1 and positional candidate genes in the NCBI database78,79, within the strong LD genomic regions. Positions of QTL were remapped from Gallus gallus 4 to Gallus _gallus-5.0 assembly using the Genome Remapping Service from NCBI database80.
Detection of community structure and functional module characterization
A gene network using all positional candidate genes was first constructed integrating the available Homo sapiens genes database (updated 17/3/2017) via the GeneMANIA V.3.4.1 plug-in11 in Cytoscape V3.6.0 (http://cytoscape.org/ 81). The gene network was built according to 7 types of interaction terms i.e. co-expression, co-localization, genetic interaction, pathway, physical interaction, predicted and shared protein domains. The automatic weighting method for network construction was also used while the number of related genes was set to zero.
Detection of community structure i.e. the appearance of densely interconnected nodes (modules) was then performed using the Girvan and Newman’s clustering algorithm29 via the GLay82 of clusterMaker83 plugin in Cytoscape81. This algorithm identifies modules within networks by repetitively removing edges with the highest “betweeness” i.e. edges between modules with higher values of betweeness rather than edges within modules. The strength of the network division into modules was also quantified using the modularity measure29. Typically, modularity values ranging from 0.3 to 0.7 are indicative of strong community structure29.
Modular genes were then subjected to GO Biological Process (BP) term enrichment analysis using the DAVID functional annotation tool (https://david.ncifcrf.gov/, version 6.8)84. The Homo sapiens species was also selected for the input gene list and as whole genome background for enrichment analysis. The following settings were used during this analysis: an EASE score (a modified Fisher exact p-value85) cut-off = 0.05 and a minimum number of genes per GO BP term = 2. GO biological processes with p-values lower than 0.05 were considered as significantly enriched. The QuickGO86 web-based tool was subsequently used to examine each resulting significantly enriched GO BP through browsing the hierarchical structure in the GO annotation database. GO BPs associated with developmental process or growth parent term(s) were considered as functionally relevant to the trait under study.
Supplementary information
Author Contributions
E.T. performed all the analyses and drafted the main manuscript text. A.K.R., G.M. and S.A. conducted data collection and preparation and contributed to writing the manuscript. A.L.H.T. assisted in the search and identification of Q.T.L. and candidate genes and contributed to writing the manuscript. A.K. conceived and supervised the study and drafted the final version of the manuscript. All authors read and approved the final manuscript.
Data Availability
The data that support the findings of this study are available from Aviagen Ltd. but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Aviagen Ltd.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-45520-5.
References
- 1.Chicken QTL Database. Available at: https://www.animalgenome.org/cgi-bin/QTLdb/GG/download?tmpname=mapDwnLd&file=cM. (Accessed: 3rd September 2017).
- 2.Xie L, et al. Genome-Wide Association Study Identified a Narrow Chromosome 1 Region Associated with Chicken Growth Traits. PLoS One. 2012;7:e30910. doi: 10.1371/journal.pone.0030910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Goor A, et al. Identification of quantitative trait loci for body temperature, body weight, breast yield, and digestibility in an advanced intercross line of chickens under heat stress. Genet. Sel. Evol. 2015;47:96. doi: 10.1186/s12711-015-0176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kranis A, et al. Development of a high density 600K SNP genotyping array for chicken. BMC Genomics. 2013;14:59. doi: 10.1186/1471-2164-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pettersson ME, Carlborg Ö. Dissecting the genetic architecture of complex traits and its impact on genetic improvement programs: lessons learnt from the Virginia chicken lines. Rev. Bras. Zootec. 2010;39:256–260. doi: 10.1590/S1516-35982010001300028. [DOI] [Google Scholar]
- 6.Shahjahan M, et al. Identification of Histone Deacetylase 2 as a Functional Gene for Skeletal Muscle Development in Chickens. Asian-Australasian J. Anim. Sci. 2016;29:479–486. doi: 10.5713/ajas.15.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouyang H, et al. Identification, expression and variation of the GNPDA2 gene, and its association with body weight and fatness traits in chicken. PeerJ. 2016;4:e2129. doi: 10.7717/peerj.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Campos MA, Espinal-Enríquez J, Hernández-Lemus E. Pathway Analysis: State of the Art. Front. Physiol. 2015;6:383. doi: 10.3389/fphys.2015.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Aronow BJ, Jegga AG. Disease candidate gene identification and prioritization using protein interaction networks. BMC Bioinformatics. 2009;10:73. doi: 10.1186/1471-2105-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montojo J, et al. GeneMANIA Cytoscape plugin: fast gene function predictions on the desktop. Bioinformatics. 2010;26:2927–2928. doi: 10.1093/bioinformatics/btq562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker MG, Volkmuth W, Sprinzak E, Hodgson D, Klingler T. Prediction of gene function by genome-scale expression analysis: prostate cancer-associated genes. Genome Res. 1999;9:1198–203. doi: 10.1101/gr.9.12.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliver S. Guilt-by-association goes global. Nature. 2000;403:601–602. doi: 10.1038/35001165. [DOI] [PubMed] [Google Scholar]
- 14.Liu G, Wang H, Chu H, Yu J, Zhou X. Functional diversity of topological modules in human protein-protein interaction networks. Sci. Rep. 2017;7:16199. doi: 10.1038/s41598-017-16270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terentiev AA, Moldogazieva NT, Shaitan KV. Dynamic proteomics in modeling of the living cell. Protein-protein interactions. Biochemistry. (Mosc). 2009;74:1586–607. doi: 10.1134/S0006297909130112. [DOI] [PubMed] [Google Scholar]
- 16.Spirin V, Mirny LA. Protein complexes and functional modules in molecular networks. Proc. Natl. Acad. Sci. USA. 2003;100:12123–8. doi: 10.1073/pnas.2032324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu H, et al. Integrated analysis of multiple data sources reveals modular structure of biological networks. Biochem. Biophys. Res. Commun. 2006;345:302–309. doi: 10.1016/j.bbrc.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Ning X, Zhang X-S. Identification of functional modules in a PPI network by clique percolation clustering. Comput. Biol. Chem. 2006;30:445–451. doi: 10.1016/j.compbiolchem.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Sharan R, Ulitsky I, Shamir R. Network-based prediction of protein function. Mol. Syst. Biol. 2007;3:88. doi: 10.1038/msb4100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jagannadham J, Jaiswal HK, Agrawal S, Rawal K. Comprehensive Map of Molecules Implicated in Obesity. PLoS One. 2016;11:e0146759. doi: 10.1371/journal.pone.0146759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma P, Bhattacharyya DK, Kalita J. Disease biomarker identification from gene network modules for metastasized breast cancer. Sci. Rep. 2017;7:1072. doi: 10.1038/s41598-017-00996-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallett RM, et al. Identification and evaluation of network modules for the prognosis of basal-like breast cancer. Oncotarget. 2015;6:17713–24. doi: 10.18632/oncotarget.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lempiäinen H, et al. Network analysis of coronary artery disease risk genes elucidates disease mechanisms and druggable targets. Sci. Rep. 2018;8:3434. doi: 10.1038/s41598-018-20721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma A, et al. A disease module in the interactome explains disease heterogeneity, drug response and captures novel pathways and genes in asthma. Hum. Mol. Genet. 2015;24:3005–3020. doi: 10.1093/hmg/ddv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hira R, Terada S-I, Kondo M, Matsuzaki M. Distinct Functional Modules for Discrete and Rhythmic Forelimb Movements in the Mouse Motor Cortex. J. Neurosci. 2015;35:13311–13322. doi: 10.1523/JNEUROSCI.2731-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, et al. Protein Profiles for Muscle Development and Intramuscular Fat Accumulation at Different Post-Hatching Ages in Chickens. PLoS One. 2016;11:e0159722. doi: 10.1371/journal.pone.0159722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang HM, et al. Variance component model to account for sample structure in genome-wide association studies. Nat. Genet. 2010;42:348–354. doi: 10.1038/ng.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, et al. Genomic inflation factors under polygenic inheritance. Eur. J. Hum. Genet. 2011;19:807–12. doi: 10.1038/ejhg.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman MEJ, Girvan M. Finding and evaluating community structure in networks. Phys. Rev. E. 2004;69:026113. doi: 10.1103/PhysRevE.69.026113. [DOI] [PubMed] [Google Scholar]
- 30.Kamaid A, Giráldez F. Btg1 and Btg2 gene expression during early chick development. Dev. Dyn. 2008;237:2158–2169. doi: 10.1002/dvdy.21616. [DOI] [PubMed] [Google Scholar]
- 31.Sun C, et al. Promising Loci and Genes for Yolk and Ovary Weight in Chickens Revealed by a Genome-Wide Association Study. PLoS One. 2015;10:e0137145. doi: 10.1371/journal.pone.0137145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reijntjes S, Stricker S, Mankoo BS. A comparative analysis of Meox1 and Meox2 in the developing somites and limbs of the chick embryo. Int. J. Dev. Biol. 2007;51:753–759. doi: 10.1387/ijdb.072332sr. [DOI] [PubMed] [Google Scholar]
- 33.Couteaudier M, et al. Derivation of keratinocytes from chicken embryonic stem cells: Establishment and characterization of differentiated proliferative cell populations. Stem Cell Res. 2015;14:224–237. doi: 10.1016/j.scr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Tarique T, et al. Identification of genes involved in regulatory mechanism of pigments in broiler chickens. Genet. Mol. Res. 2014;13:7201–7216. doi: 10.4238/2014.September.5.6. [DOI] [PubMed] [Google Scholar]
- 35.Gu X, et al. Genome-Wide Association Study of Body Weight in Chicken F2 Resource Population. PLoS One. 2011;6:e21872. doi: 10.1371/journal.pone.0021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibson MS, Kaiser P, Fife M. Identification of Chicken Granulocyte Colony-Stimulating Factor (G-CSF/CSF3): The Previously Described Myelomonocytic Growth Factor Is Actually CSF3. J. Interf. Cytokine Res. 2009;29:339–344. doi: 10.1089/jir.2008.0103. [DOI] [PubMed] [Google Scholar]
- 37.Bou-Gharios G, Liu K, Li I, De Val S. Three new functionally conserved cis-regulatory elements in the Acan gene. Osteoarthr. Cartil. 2014;22:S143–S144. doi: 10.1016/j.joca.2014.02.267. [DOI] [Google Scholar]
- 38.Gouveia A, Marcelino HM, Gonçalves L, Palmeirim I, Andrade RP. Patterning in time and space: HoxB cluster gene expression in the developing chick embryo. Cell Cycle. 2015;14:135. doi: 10.4161/15384101.2014.972868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, et al. DNA methylation of CMTM3, SSTR2, and MDFI genes in colorectal cancer. Gene. 2017;630:1–7. doi: 10.1016/j.gene.2017.07.082. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki S, Namiki J, Shibata S, Mastuzaki Y, Okano H. The Neural Stem/Progenitor Cell Marker Nestin Is Expressed in Proliferative Endothelial Cells, but Not in Mature Vasculature. J. Histochem. Cytochem. 2010;58:721–730. doi: 10.1369/jhc.2010.955609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maridas DE, DeMambro VE, Le PT, Mohan S, Rosen CJ. IGFBP4 Is Required for Adipogenesis and Influences the Distribution of Adipose Depots. Endocrinology. 2017;158:3488–3500. doi: 10.1210/en.2017-00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rucci N, et al. The glycosaminoglycan-binding domain of PRELP acts as a cell type-specific NF-kappaB inhibitor that impairs osteoclastogenesis. J. Cell Biol. 2009;187:669–83. doi: 10.1083/jcb.200906014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiue Y-L, Chen L-R, Tsai C-J, Yeh C-Y, Huang C-T. Emerging roles of peroxisome proliferator-activated receptors in the pituitary gland in female reproduction. Biomarkers Genomic Med. 2013;5:1–11. doi: 10.1016/j.gmbhs.2013.04.008. [DOI] [Google Scholar]
- 44.Guan FHX, et al. The antiproliferative ELF2 isoform, ELF2B, induces apoptosis in vitro and perturbs early lymphocytic development in vivo. J. Hematol. Oncol. 2017;10:75. doi: 10.1186/s13045-017-0446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sen R, et al. Kat2a and Kat2b Acetyltransferase Activity Regulates Craniofacial Cartilage and Bone Differentiation in Zebrafish and Mice. J. Dev. Biol. 2018;6:27. doi: 10.3390/jdb6040027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang H, et al. Changes in the expression and subcellular localization of RARα in the rat hippocampus during postnatal development. Brain Res. 2008;1227:26–33. doi: 10.1016/j.brainres.2008.06.073. [DOI] [PubMed] [Google Scholar]
- 47.Jiang S, Cheng HW, Hester PY, Hou J-F. Development of an enzyme-linked immunosorbent assay for detection of chicken osteocalcin and its use in evaluation of perch effects on bone remodeling in caged White Leghorns. Poult. Sci. 2013;92:1951–1961. doi: 10.3382/ps.2012-02909. [DOI] [PubMed] [Google Scholar]
- 48.Manca A, et al. Nerve growth factor regulates axial rotation during early stages of chick embryo development. Proc. Natl. Acad. Sci. 2012;109:2009–2014. doi: 10.1073/pnas.1121138109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, et al. APOBEC2 mRNA and protein is predominantly expressed in skeletal and cardiac muscles of chickens. Gene. 2014;539:263–269. doi: 10.1016/j.gene.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Zhao XH, et al. Single nucleotide polymorphism in the STAT5b gene is associated with body weight and reproductive traits of the Jinghai Yellow chicken. Mol. Biol. Rep. 2012;39:4177–4183. doi: 10.1007/s11033-011-1202-7. [DOI] [PubMed] [Google Scholar]
- 51.Johnston PA, Grandis JR. STAT3 SIGNALING: Anticancer Strategies and Challenges. Mol. Interv. 2011;11:18–26. doi: 10.1124/mi.11.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao M, Mishra L, Deng C-X. The role of TGF-β/SMAD4 signaling in cancer. Int. J. Biol. Sci. 2018;14:111–123. doi: 10.7150/ijbs.23230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasegawa N, et al. Mediator Subunits MED1 and MED24 Cooperatively Contribute to Pubertal Mammary Gland Development and Growth of Breast Carcinoma Cells. Mol. Cell. Biol. 2012;32:1483–1495. doi: 10.1128/MCB.05245-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen F, et al. Neuromuscular synaptic patterning requires the function of skeletal muscle dihydropyridine receptors. Nat. Neurosci. 2011;14:570–577. doi: 10.1038/nn.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Vaart B, et al. SLAIN2 links microtubule plus end–tracking proteins and controls microtubule growth in interphase. J. Cell Biol. 2011;193:1083–1099. doi: 10.1083/jcb.201012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brachner A, Reipert S, Foisner R, Gotzmann J. LEM2 is a novel MAN1-related inner nuclear membrane protein associated with A-type lamins. J. Cell Sci. 2005;118:5797–810. doi: 10.1242/jcs.02701. [DOI] [PubMed] [Google Scholar]
- 57.Tapia O, Fong LG, Huber MD, Young SG, Gerace L. Nuclear Envelope Protein Lem2 is Required for Mouse Development and Regulates MAP and AKT Kinases. PLoS One. 2015;10:e0116196. doi: 10.1371/journal.pone.0116196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giacometti S, et al. Mutually Exclusive CBC-Containing Complexes Contribute to RNA Fate. Cell Rep. 2017;18:2635–2650. doi: 10.1016/j.celrep.2017.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winczura K, et al. Characterizing ZC3H18, a Multi-domain Protein at the Interface of RNA Production and Destruction Decisions. Cell Rep. 2018;22:44–58. doi: 10.1016/j.celrep.2017.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iwakawa R, et al. Expression and clinical significance of genes frequently mutated in small cell lung cancers defined by whole exome/RNA sequencing. Carcinogenesis. 2015;36:616–621. doi: 10.1093/carcin/bgv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang GX, et al. Genome-wide association study of growth traits in the Jinghai Yellow chicken. Genet. Mol. Res. 2015;14:15331–15338. doi: 10.4238/2015.November.30.10. [DOI] [PubMed] [Google Scholar]
- 62.Pampouille E, et al. Mapping QTL for white striping in relation to breast muscle yield and meat quality traits in broiler chickens. BMC Genomics. 2018;19:202. doi: 10.1186/s12864-018-4598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun D, Liu Y, Zhang X-S, Wu L-Y. CEA: Combination-based gene set functional enrichment analysis. Sci. Rep. 2018;8:13085. doi: 10.1038/s41598-018-31396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tarsani E, Kranis A, Maniatis G, Kominakis A. Investigating the functional role of 1,012 candidate genes identified by a Genome Wide Association Study for body weight in broilers. Proc. World Congr. Genet. Appl. to Livest. Prod. Species-Avian. 2018;1:564. [Google Scholar]
- 65.Yuan J, et al. Genome-wide association studies for feed intake and efficiency in two laying periods of chickens. Genet. Sel. Evol. 2015;47:82. doi: 10.1186/s12711-015-0161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiajie T, Yanzhou Y, Hoi-Hung AC, Zi-Jiang C, Wai-Yee C. Conserved miR-10 family represses proliferation and induces apoptosis in ovarian granulosa cells. Sci. Rep. 2017;7:41304. doi: 10.1038/srep41304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singaravelu R, et al. MicroRNA-7 mediates cross-talk between metabolic signaling pathways in the liver. Sci. Rep. 2018;8:361. doi: 10.1038/s41598-017-18529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang HY, et al. Integrated analysis of microRNA and mRNA expression profiles in abdominal adipose tissues in chickens. Sci. Rep. 2015;5:16132. doi: 10.1038/srep16132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H, et al. Systematic analysis of the regulatory functions of microRNAs in chicken hepatic lipid metabolism. Sci. Rep. 2016;6:31766. doi: 10.1038/srep31766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Segura V, et al. An efficient multi-locus mixed-model approach for genome-wide association studies in structured populations. Nat. Genet. 2012;44:825–830. doi: 10.1038/ng.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.VanRaden PM. Efficient Methods to Compute Genomic Predictions. J. Dairy Sci. 2008;91:4414–4423. doi: 10.3168/jds.2007-0980. [DOI] [PubMed] [Google Scholar]
- 72.Chen J, Chen Z. Extended Bayesian information criteria for model selection with large model spaces. Biometrika. 2008;95:759–771. doi: 10.1093/biomet/asn034. [DOI] [Google Scholar]
- 73.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 74.Qu H-Q, Tien M, Polychronakos C. Statistical significance in genetic association studies. Clin. Invest. Med. 2010;33:E266–70. doi: 10.25011/cim.v33i5.14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yi G, et al. Genome-wide association study dissects genetic architecture underlying longitudinal egg weights in chickens. BMC Genomics. 2015;16:746. doi: 10.1186/s12864-015-1945-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hedrick PW. Gametic disequilibrium measures: proceed with caution. Genetics. 1987;117:331–41. doi: 10.1093/genetics/117.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McRae AF, et al. Linkage disequilibrium in domestic sheep. Genetics. 2002;160:1113–22. doi: 10.1093/genetics/160.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gallus gallus Annotation Release 103. Available at: https://www.ncbi.nlm.nih.gov/genome/annotation_euk/Gallus_gallus/103/. (Accessed: 3rd September 2017).
- 79.Gallus_gallus-5.0 in NCBI assembly data base. Available at: https://www.ncbi.nlm.nih.gov/assembly/GCF_000002315.4/. (Accessed: 3rd September 2017)
- 80.Coordinate remapping service: NCBI. Available at: https://www.ncbi.nlm.nih.gov/genome/tools/remap. (Accessed: 3rd September 2017)
- 81.Shannon P, et al. Genome Res. 2003. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks; pp. 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Su G, Kuchinsky A, Morris JH, States DJ, Meng F. GLay: community structure analysis of biological networks. Bioinformatics. 2010;26:3135–3137. doi: 10.1093/bioinformatics/btq596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morris JH, et al. clusterMaker: a multi-algorithm clustering plugin for Cytoscape. BMC Bioinformatics. 2011;12:436. doi: 10.1186/1471-2105-12-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dennis G, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:R60. doi: 10.1186/gb-2003-4-9-r60. [DOI] [PubMed] [Google Scholar]
- 85.Hosack DA, et al. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4:R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huntley RP, et al. QuickGO: a user tutorial for the web-based Gene Ontology browser. Database (Oxford). 2009;2009:bap010. doi: 10.1093/database/bap010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Conant GC, Wolfe KH. GenomeVx: simple web-based creation of editable circular chromosome maps. Bioinformatics. 2008;24:861–862. doi: 10.1093/bioinformatics/btm598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Aviagen Ltd. but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Aviagen Ltd.