Abstract
Magnetic stimulation (MS) is a novel approach for treating urinary incontinence (UI), but its applicability remains unclear. This systematic review and meta-analysis were conducted to evaluate the effects of MS treatment on UI. A literature search was performed in EMBASE, PubMed and Cochrane Library (from May 2018 to August 2018), and all randomized control trials (RCTs) published in English were screened to determine whether they met the inclusion criteria. A manual search of the reference lists of the retrieved studies was also performed. Eleven studies involving 612 patients were included in this review. According to the results of the meta-analysis, MS therapy relieved UI symptoms evaluated using the International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF) score (mean difference [MD] −3.03, 95% CI −3.27 to −2.79). In addition, the frequency of UI in the MS treatment group was also alleviated compared with sham group (MD −1.42, 95% CI −2.15 to −0.69). Finally, MS treatment improved the quality of life of patients with UI (standardized mean difference [SMD] −1.00, 95% CI −1.24 to −0.76). Our meta-analysis preliminarily indicates that MS treatment is an effective therapeutic modality for patients with UI. Nevertheless, additional large, high quality RCTs with a longer follow-up period that use consistent stimulation methods and analyse comparable outcomes are required to validate the efficacy.
Subject terms: Urinary incontinence, Urological manifestations
Introduction
Urinary incontinence (UI), which severely decrease the quality of life (QoL) and sexual function of patients1, presents as the complaint of any involuntary loss of urine2. Stress urinary incontinence (SUI), which accounts for over half of UI cases, is the symptomatic complaint of involuntary leakage upon effort, exertion, sneezing or coughing3. Meanwhile, urgency urinary incontinence (UUI) involves involuntary leakage accompanied by or immediately preceded by a sudden, compelling and uncontrolled desire to pass urine. As the term suggests, mixed urinary incontinence is defined as the combination of SUI and UUI. These three types of UI are the most common forms based on the symptoms, but other types of UI also exist (e.g., continuous UI, nocturnal enuresis, insensible incontinence, and neurogenic UI).
UI is a chronic condition that poses a substantial financial burden on individuals4 and society5. Most epidemiological studies reported a prevalence of any type of UI ranging from approximately 25% in young adults to 45% in older women6,7. A prospective longitudinal study including 1081 urban Swedish women revealed that the overall prevalence of UI increased from 15% in 1991 to 28% in 20078. In this context of an increasing prevalence of UI, susceptible populations require more careful management by health practitioners.
The current initial treatment for all types of UI includes lifestyle interventions, physical therapies, scheduled voiding regimes, behavioural therapies and medication3. In particular, pelvic floor muscle training (PFMT) is recommended as a first line therapy for SUI, and PFMT combined with either bladder training or antimuscarinics are advocated for UUI3. Other adjunct therapeutic modalities, such as electrical stimulation (ES), vaginal devices and urethral inserts are the second-line options for both patients with SUI and UUI9. Magnetic stimulation (MS) treatment is a novel approach to provide noninvasive, passive stimulation to the sacral roots or the pelvic floor. This new form of conservative therapy for UI was approved by the United States Food and Drug Administration in 199810. Pulsed magnetic fields are generated by an electrified coil that induces a flow of ions to form eddy currents when the excitable tissue is exposed to a magnetic field with a sufficient intensity11. Therefore, MS depolarizes the motor nerve to produce an action potential that ultimately triggers muscle contractions. Due to the advantages of the lack of an internal probe and requirement for supervision and the ability of magnetic fields to pass through clothing, MS is a very convenient, acceptable and hospitable modality that has attracted increasing attention.
However, the applicability of MS for UI remains unclear9. Thus, we conducted this systematic review and meta-analysis to evaluate the effects of MS therapy on UI.
Results
Study identification and characteristics
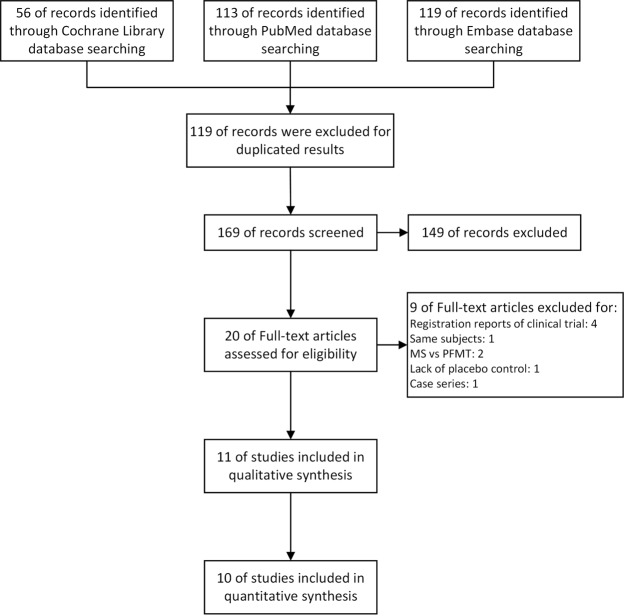
The process used to select the studies included in this article is summarized in Fig. 1. No disagreement occurred between the two reviewers regarding the inclusion of the selected randomized control trials (RCTs). Eleven articles were finally included in this quantitative synthesis (612 patients)12–22, and no additional study was identified by searching manual search of the reference lists of these articles. This review identified 3 publications16,17,23 that were based on the same RCT24. We only extracted unduplicated and useful data from 2 of these studies16,17.
Figure 1.
Flow diagram of the systematic review and meta-analysis.
Specifically, 6 papers from 5 different RCTs reported outcomes for patients with SUI12–17; 3 RCTs compared the effects of MS and a sham device on patients with UUI18–20; 1 RCT21 focused on patients with MUI and the last RCT22 included patients with all types of UI. Overall, 612 participants were enrolled, and 343 patients were assigned to the active MS group and 269 patients were allocated to the sham MS group. Two studies confirmed the type of UI with only a urodynamic examination14,15, and 3 studies employed a urodynamic examination and voiding diary to determine the diagnosis19,21,22. Five RCTs confirmed the eligibility of the participants based on a voiding diary12,13,16–18,20. The detailed characteristics of the selected studies are summarized in Table 1.
Table 1.
Characteristics of studies included in the meta-analysis.
| Study | Jadad score | Type of UI | Diagnosis method | Group | Sample size | Age, mean (SD) | Length of intervention period and frequency | Location | Protocols | Additional therapy | Outcome measures and results | Instrument or questionnaire used | Follow-up period |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fujishiro 2000 | 0 | SUI | Voiding diary | Active | 31 | 58 (37–79)a | Only once | Sacral roots | 30-min stimulation repetition of 15 Hz in 5-s per minute | None | 1. Maximum urethral closure pressure; 2. Frequency of UI; 3. QoL; 4. Objective cureb; 5. Improved incontinencec | 1. Cystometry; 2. 3-day urinary diary; 3. 1-hour pad test | 1 week |
| Sham | 31 | Use sham stimulating coil | |||||||||||
| Manganotti 2007 | 0 | SUI | Voiding diary | Active | 10 | 50.1 (2.86) | Three sessions per week for 2 weeks | Sacral roots | 15-min stimulation repetition cycle of 15 Hz in 3 s per minute | None | 1. QoL; 2. Severity of SUI; 3. Stress pad test | 1. KHQ; 2. SEAPI-QMM Incontinence Classification System; 3. 1-hour pad test | 1 month |
| Sham | 10 | The magnetic coil was positioned over the sacrum in a vertical position | |||||||||||
| Gilling 2009 | 4 | SUI | Urodynamic examination | Active | 35 | 54.0 (2.0) | Three treatment sessions per week for 6 weeks | Pelvic floor | 1. 10-min stimulation at 10 Hz; 2. 3-min rest; 3. 10-min stimulation at 50 Hz | Low intensity home-based PFMT | 1. Frequency of UI; 2. Stress pad test; 3. PFM strength; 4. QoL; 5. ALPP | 1. 3-day urinary diary; 2. CVM score; 3. I-QoL; 4. KHQ; 5. Urodynamic test; 6. Perineometer | 6-month |
| Sham | 35 | 54.8 (2.2) | A thin deflective aluminium plate inserted in the chair | ||||||||||
| Yamanishi 2017 | 4 | SUI | Urodynamic examination | Active | 18 | NA | One session per week for 10 weeks | Pelvic floor | 20-min stimulation repetition cycle of 50 Hz in a 5-s “on” 5-s “off” pulsing manner | NA | 1. Frequency of UI; 2. Severity of UI; 3. Stress pad test; 4. QoL; 5. ALPP | 1. 7-day urinary diary; 2. 24-h pad test; 3. ICIQ-UI SF score; 4. ICIQ-LUTSqol; 5. Urodynamic test | 10-week |
| Sham | 12 | 1 Hz in 5-s on/5-s off cycles, with a maximum output of ≤42% of the active stimulation | |||||||||||
| Lim 2017 | 5 | SUI | Voiding diary, ICIQ-UI SF score | Active | 60 | 51.8 (10.0) | Two sessions per week for 2 months | Pelvic floor and sphincter muscles | 20-min stimulation repetition cycle of 50 Hz in an 8-s “on” 4-s “off” pulsing manner | None | 1. Frequency of UI; 2. Objective cured; 3. Subjective cure; 4. Stress pad test; 5. PFM function; 6. Severity of UI | 1. Urinary diary; 2. ICIQ-UI SF score; 3. Perineometer; 4. PGI-I; 5. ICIQ-LUTSqol; 6. 1-hour pad test | 14-month |
| Sham | 60 | 52.7 (7.8) | The magnetic coil was tilted 22 degrees down | ||||||||||
| Fujishiro 2002 | 0 | UUI | Voiding diary | Active | 22 | 61.3 (8.3) | Only once | Sacral roots | 30-min stimulation repetition of 15 Hz in 5-s per minute | None | 1. Maximum urethral closure pressure; 2. Frequency of UI; 3. QoL | 1. 3-day urinary diary; 2. Cystometry | 1 week |
| Sham | 15 | 62.7 (8.9) | Use sham stimulating coil | ||||||||||
| Suzuki 2007 | 2 | UUI | Urodynamic examination, Voiding diary | Active | 20 | 65.2 (13.1) | One session per week for 10 weeks | Pelvic floor | 10 Hz with a pulse width of 300 μs for 20-min | None | 1. Frequency of UI; 2. Severity of UI; 3. QoL | 1. 7-day urinary diary; 2.ICIQ-UI SF score; 3. ICIQ-LUTSqol; 4. Urodynamic test | 24-week |
| Sham | 19 | 71.4 (12.6) | 1 Hz in 5-s on/5-s off manner with a maximum output of ≤20% of the active stimulation | ||||||||||
| Yamanishi 2014 | 2 | UUI | Voiding diary | Active | 94 | 64.1 (13.9) | Two sessions per week for 6 weeks | Pelvic floor | 10 Hz with a pulse width of 300 μs for 25-min | None | 1. Frequency of UI; 2. Severity of UI; 3. QoL | 1. 7-day urinary diary; 2. OABSS; 3. IPSS QoL | None |
| Sham | 49 | 67.2 (13.0) | 1 Hz in 5-s on/5-s off manner with a maximum output of ≤20% of the active stimulation | ||||||||||
| But 2005 | 3 | MUI | Urodynamic examination, voiding diary | Active | 23 | 54.0 (28–70)a | Daily use for 2 months | Pelvic floor | 18.5 Hz continuous stimulation | None | Severity of UI | Urodynamic test | None |
| Sham | 16 | Inactive stimulation | |||||||||||
| But 2003 | 3 | UI | Urodynamic examination, voiding diary | Active | 30 | 55.8 (34–78)a | Daily use for 2 months | Pelvic floor | 10 Hz with a pulse width of 55 μs for daily | None | 1. Frequency of UI; 2. Severity of UI; 3. PFM strength | 1. Volume-voided chart; 2. Visual analog scale; 3. Flowmetry; 4. Perineometer | None |
| Sham | 22 | Inactive stimulation |
Abbreviations: UI, urinary incontinence; SUI, stress urinary incontinence; UUI, urgency urinary incontinence; MUI, mixed urinary incontinence; ICIQ-UI SF, Incontinence Questionnaire-Urinary Incontinence Short Form; SD, standard deviation; PFMT, pelvic floor muscle training; NA, not available; QoL, quality of life; PFM, pelvic floor muscle; ALLP, abdominal leak-point pressure; KHQ, King’s Health Questionnaire; CVM, circumvaginal muscle; I-QOL, Urinary Incontinence Quality of Life; ICIQ-LUTSqol, International Consultation on Incontinence Questionnaire-Lower Urinary Tract Symptom Quality of Life; OABSS, overactive bladder symptom score; IPSS, International Prostate Symptom Score. aMean (range). bNo incontinence noted in the voiding diary and leaking of less than 1 gm. cFrequency of incontinence or leaking volume on the pad test decreased by more than 50% compared with the baseline level. dLeakage less than 1 gm on the 1-hour pad test.
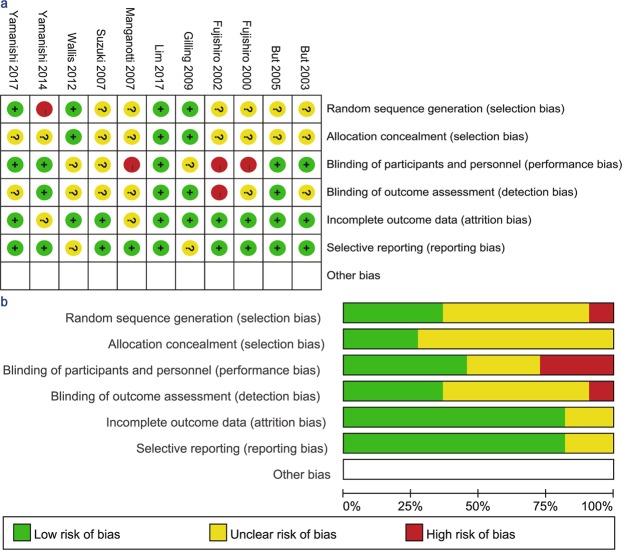
Only 2 RCTs14,16,17 reported detailed methods for both random sequence generation and allocation concealment. Six studies15–17,20–22 had a low risk of performance bias via blinding of participants and personnel, and the remaining studies had an unclear or relatively high risk of performance bias. Outcome assessments were blinded in 5 studies. In addition, only 2 studies had an unclear risk of attrition bias and 1 article had an unclear risk of reporting bias. The risk of bias graph and summary are illustrated in Fig. 2. Over half of the included studies14–17,21,22 were defined as high quality (3–5 points) according to the Jadad scale (Table 1).
Figure 2.
(a) Risk of bias summary: review authors’ judgements about each risk of bias item for each included study. (b) Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages for all included studies.
Remarkably, significant differences in the frequencies, durations and treatment periods of stimulation were observed, ranging from 10 Hz to 50 Hz, 15 minutes to daily usage, and one single session to 10 weeks, respectively. In addition, the modality used in the sham group was also inconsistent (Table 1).
Study outcomes
UI symptoms
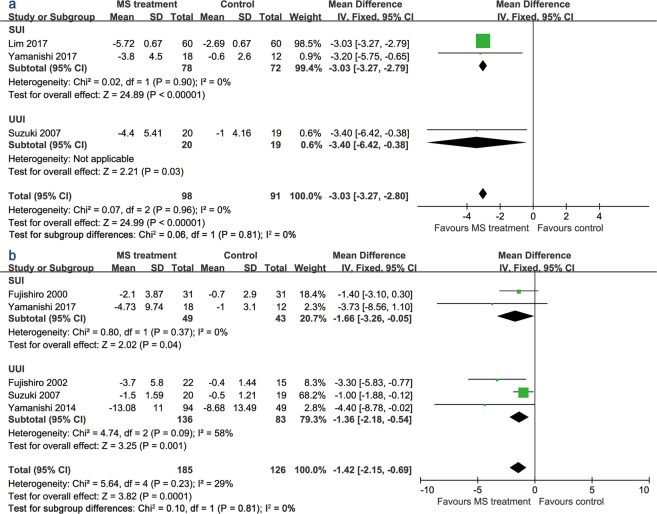
Three trials15,17,19 provided data about UI symptoms using the International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-UI SF) score. High scores on this questionnaire indicated worse symptoms of UI. MS therapy relieved UI symptoms when evaluated using the ICIQ-UI SF score (Fig. 3a). The mean difference (MD) was −3.03 (95% CI −3.27 to −2.79). The treatment period of all studies was at least 2 months. The effect of the MS treatment on UI frequency is illustrated in Fig. 3b. Three studies15,19,20 assessed UI frequency with a voiding diary for 1 week, and 2 studies12,18 used a diary for 3 days with a short treatment period (single session). The UI frequency of the MS treatment group was alleviated compared with the sham group (MD −1.42, 95% CI −2.15 to −0.69).
Figure 3.
Forest plots comparing the changes in (a) the ICIQ-UI SF score and (b) UI frequency between the active and sham groups.
Cure rates and incontinence improvement
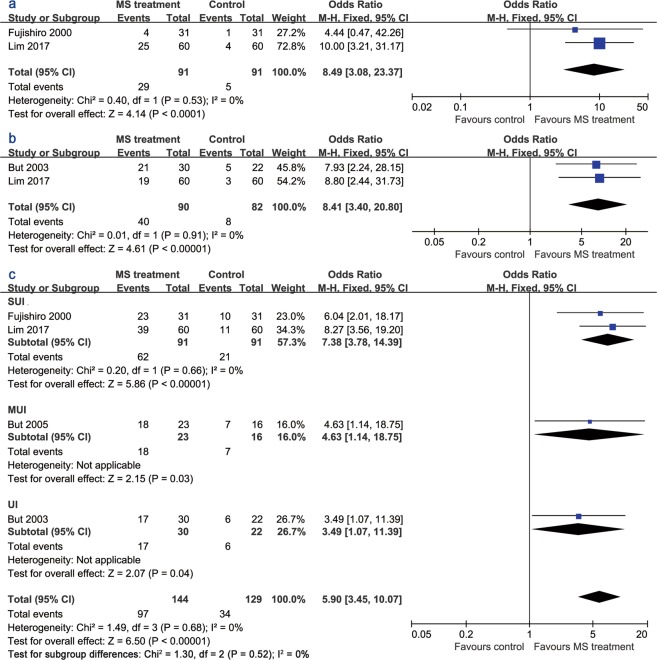
The pooled data for an objective cure (leakage less than 1 gm on the 1-hour pad test) rate was derived from patients with SUI (Fig. 4a). Patients who received active MS treatment were more likely to be continent when assessed using the pad test (odds ratio [OR] 8.49, 95% CI 3.08 to 23.37). Likewise, the pooled subjective cure rate was significantly higher in the active stimulation group (OR 8.41, 95% CI 3.40 to 20.80) (Fig. 4b). Furthermore, more patients in the MS treatment group exhibited an improvement in incontinence symptoms (OR 5.90, 95% CI 3.45 to 10.07) (Fig. 4c).
Figure 4.
Forest plots comparing (a) the objective cure rate, (b) subjective cure rate, and (c) the incontinence improvement outcome between the active and sham groups.
Stress pad test and urination
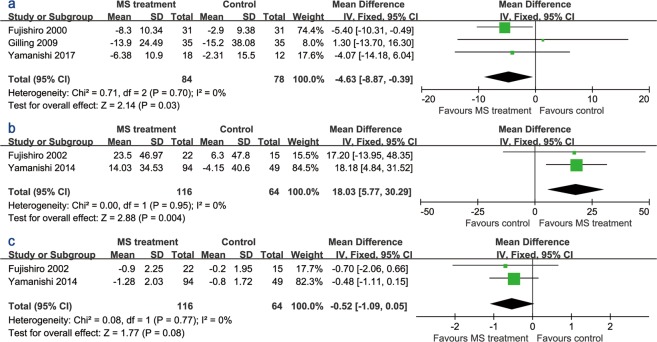
Three studies of patients with SUI12,14,15 provided detailed data from the stress pad test. Compared to the sham group, active stimulation lessened the UI symptoms (MD −4.63, 95% CI −8.87 to −0.39) assessed using the stress pad test (Fig. 5a). However, Fujishiro, et al.12 used the 1-hour pad test, and other two studies adopted the 24-hour pad test. Active stimulation also improved the process of urination in patients with UUI. As illustrated in Fig. 5b,c, MS treatment increased the mean urine volume per void compared with the sham group (MD 18.03, 95% CI 5.77 to 30.29), although the improvement in the micturition number was not statistically significant (MD −0.52, 95% CI −1.09 to 0.05).
Figure 5.
Forest plots comparing the changes in (a) the stress pad test, (b) mean urine volume per void, and (c) micturition number between the active and sham groups.
QoL score
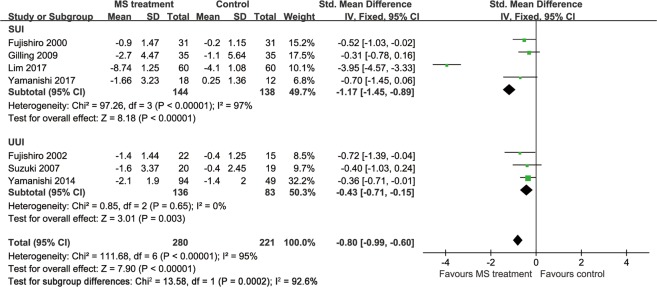
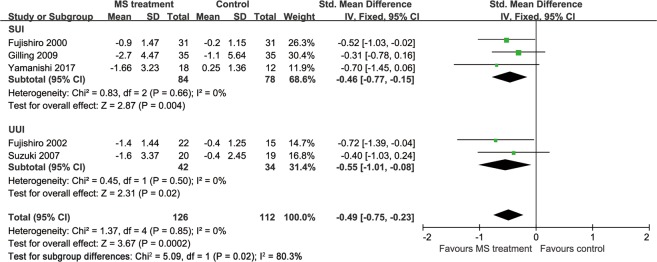
Six studies provided data showing that the MS modality improved the QoL of patients with UI (Fig. 6). Specifically, 4 studies14,15,17,19 provided these data using the International Consultation on Incontinence Questionnaire-Lower Urinary Tract Symptom Quality of Life (ICIQ-LUTSqol) score25, one study used the International Prostate Symptom Score (IPSS) QoL20 and the last 2 studies12,18 did not provide adequate information from the questionnaire. High scores on all these questionnaires indicated a poor quality of life. Hence, the MS treatment improved the QoL of patients with UI (standardized mean difference [SMD] −0.80, 95% CI −0.99 to −0.60), but the I2 test (95%) revealed heterogeneity. A subsequent influence analysis revealed that the studies conducted by Lim, et al.17 and Yamanishi, et al.20 had the greatest influence (Fig. 7). A significant improvement in the QoL was also observed in the active stimulation group (SMD −1.00, 95% CI −1.24 to −0.76) after omitting these studies, and no heterogeneity existed (Fig. 8).
Figure 6.
Forest plot comparing the change in the QoL score between the active and sham groups.
Figure 7.
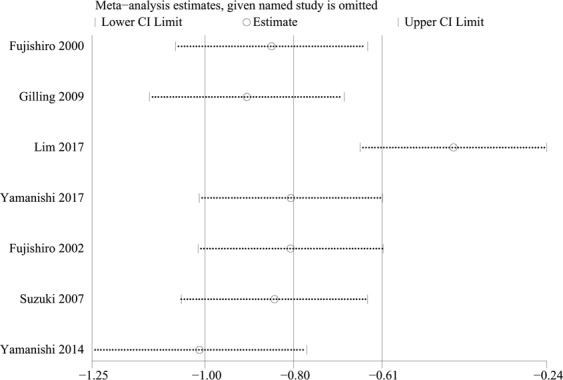
Results of the influence analysis.
Figure 8.
Forest plot comparing the change in the QoL score after omitting studies.
Discussion
In the present meta-analysis, the UI questionnaire, the frequency of UI, objective and subjective cure rates, the stress pad test, urination condition, and QoL were used to evaluate the efficacy of MS therapy. The active MS treatment decreased UI symptoms (ICIQ-UI SF score, MD −3.03, 95% CI −3.27 to −2.79), alleviated the UI frequency (MD −1.42, 95% CI −2.15 to −0.69), and improved incontinence (OR 5.90, 95% CI 3.45 to 10.07), urination (mean urine volume per void, MD 18.03, 95% CI 5.77 to 30.29) and the QoL of patients with UI (SMD −1.00, 95% CI −1.24 to −0.76) in this meta-analysis.
The ICIQ-SF, an internationally applicable questionnaire, is an effective and validated tool used to quantify the symptoms of UI. Lim, et al.17, Yamanishi, et al.15 and Suzuki, et al.19 all reported that the MS treatment relieved UI symptoms, according to the ICIQ-UI SF score. Recently, a systematic review26 including 45 RCTs of surgical or non-surgical interventions for SUI published between January 2015 and July 2017 indicated that the ICIQ-UI SF was used in 18 RCTs, which was the most commonly adopted subjective measurement. More trials using the ICIQ-UI SF will generate more standardized and comparable results in the future, which will strengthen the conclusions of this meta-analysis.
The urinary diary and stress pad test were more objective than the questionnaire in effectively assessing the ability of the MS treatment to alleviate UI symptoms. The reduction in UI frequency was statistically significant in both patients with SUI and UUI (Fig. 3b). The recommended 1-hour stress pad test was conducted as described below. The patient started without voiding and wore a weighed pad. The subject drank 500 ml of a sodium-free liquid within a short period (max. 15 minutes), and then sat or rested 30 minutes. After patient completed a series of physical exercises for 30 minutes, the pad was removed and weighed27. Although the pooled data showed a significant improvement in urine loss was significantly improved in active stimulation group, Gilling, et al.14 and Yamanishi, et al.15 did not report a significantly greater improvement in the active stimulation group than in the sham group, because they used the 24-hour pad test. Although the ICI recommendation states that pad testing is optional for the routine evaluation of UI and the 24-hour pad test was suggested, several studies have questioned the reliability and reproducibility of the pad test. Simons, et al.28 assessed the repeatability of the 1-hour pad test in 56 incontinent women and observed significant differences between two tests performed at intervals of 3 to 10 days (MD 9.7 g, 95% CI −66 to 46). Henderson, et al.29 also concluded that the 24-hour pad test had no significant predictive ability to diagnose SUI. Only less than 10% of urologists routinely perform this test, and several testing protocols with varying recording times exist30. Hence, additional studies are required to establish optimal protocols for this test in clinical research and daily care.
In our meta-analysis, the MS treatment led to better objective and subjective cure rates. Additionally, the process of micturition was improved in patients who received active stimulation. Continence is only maintained when the intra-urethral closure pressure exceeds the intravesical pressure both at rest and during periods of increased intra-abdominal pressure31. The voiding of urine, which is controlled by reflex mechanisms within the automatic and somatic nervous systems, is also influenced by supraspinal inputs from the central nervous system32. As mentioned above, MS is characterized by noninvasive, passive magnetic waves that stimulate the sacral roots or the pelvic floor with the final effect of muscle contraction. For this reason, magnetic stimulation offers an opportunity for patients who may not be motivated to perform regular PFMT for conservative management. Notably, the stimulation is nonspecific, and magnetic waves are not significantly attenuated by the interaction with the tissue. Thus, in addition to the pelvic floor and sacral roots, other muscles, nerves, and even the uterus may react to the stimulation, although most patients tolerate the treatment well.
The QoL is vital for patients with UI, because UI symptoms have been shown to reduce QoL similarly to severe chronic diseases, such as stroke, arthritis and chronic kidney disease33,34. An investigation reported an overall prevalence of UI of up to 43.5% in perimenopausal women. Furthermore, few of the affected women sought medical treatment, which in turn had a serious impact on their QoL. In our meta-analysis, patients with UI who received active MS had a better QoL than the sham group. However, 2 studies did not provide detailed information from the QoL questionnaires. The ICIQ-LUTSqol, which is also named King’s Health Questionnaire (KHQ), is a high-quality tool used to assess the impact of LUTS on health-related QoL. Recently, Krhut, et al.35 included 391 incontinent women and 81 continent volunteers to explore the correlation between incontinence severity and QoL. Even mild urinary leakage significantly reduces the QoL (significantly higher KHQ score). Moreover, a linear correlation between incontinence severity and QoL is not observed.
Our review has several limitations. First, according to the International Consultation on Incontinence and European Association of Urology recommendation, 5 domains of interest should be reported in clinical trials, including patient observations, quantification of symptoms, clinician observations (anatomical, functional, and compliance), QoL, and socioeconomic outcomes3,9,36. Unfortunately, only one RCT24 planned to report all five domains. Second, the inconsistent protocols for the use of active MS and sham devices, as well as different types of UI, may lead to heterogeneity, although most pooled data were homogeneous. Third, the inconsistent definitions, varying outcome measurements, short length of follow-up, and lack of information about patients who were lost to follow-up may be a potential source of bias. Finally, all included RCTs investigated a relatively small sample size, and data were not sufficient to perform a further analysis such as pelvic floor muscle strength and pressure.
Based on the results of our meta-analysis, MS treatment potentially represents an effective therapeutic modality for patients with UI, as evidenced by the reduced UI symptoms, alleviated UI frequency, increased cure rate, improved micturition, and better QoL. In particular, patients with UI who may not be motivated to conduct regular PFMT can also be treated conservatively with this method. Nevertheless, further large-scale RCTs should be performed to determine consistent intervention protocols and standardize the outcome measurements to generate comparable data. Additionally, a longer follow-up period and a cost-effectiveness analysis will provide more evidence to validate the effects of MS treatment.
Methods
Study identification
The present systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist37. Literature databases, including EMBASE, PubMed and Cochrane library, were searched for all RCTs published in English, and the final search was conducted from May 2018 to August 2018. The Boolean operator “and” was used to combine the search themes. The first theme was magnetic stimulation therapy and expanded versions of the Medical Subject Headings (MeSH) terms magnetic field therapy or electromagnetic therapy. The last theme was urinary incontinence, combined with the expanded versions of the MeSH terms stress urinary incontinence or urge urinary incontinence. The publication language was restricted to English.
Inclusion and exclusion criteria
The potentially relevant articles were independently reviewed by two authors, who reached a consensus on all disagreements. Inclusion criteria were: (1) RCTs evaluating the efficacy of active MS versus sham MS as a treatment for urinary incontinence; (2) study population aged 18 years or older with symptoms of stress urinary incontinence, urgent urinary incontinence or mixed urinary incontinence. Accordingly, studies were excluded based on the following criteria: (1) head-to-head studies of MS versus other modalities (e.g., PFMT and ES); (2) studies including patients who were pregnant; presented with pelvic organ prolapse, severe cardiac/cerebrovascular disorders, urinary tract infection, or a history of pelvic surgery; used medications that may affect urinary incontinence; or received other ongoing treatment for urinary incontinence; (3) abstracts, comments, reviews, conference papers, case reports, meta-analyses and other irrelevant studies. When more than one study included duplicate data from the same population, we only selected the study reporting useful information. Reference lists of selected articles were also examined.
Data extraction and outcomes
The following data were extracted from each study, if available, using a Microsoft® Excel worksheet: first author’s name, country, year of publication, the number of patients, intervention method, follow-up time, patients’ ages and other characteristics, and the outcomes of urinary incontinence. Dichotomous data were extracted into two-by-two tables. For continuous data, available summary estimates for each group (means and changes in means) and measures of variability (standard deviation [SD]) were extracted. Data were collected by Qing He and the precision of the records was verified by Kaiwen Xiao.
Evaluation of study quality
Two reviewers independently evaluated the quality of all selected studies, and the final result was recorded after a discussion between these reviewers. The methodological quality of all RCTs was evaluated using the Jadad score38 and Cochrane risk of bias assessment tool.
Statistical analysis
We used RevMan version 5.3 software (Cochrane Collaboration, Oxford, UK) to perform the meta-analysis. The efficacy of the MS treatment was assessed by calculating the OR, MD and SMD, along with the corresponding 95% confidence interval (CI), for the comparison between active stimulation and sham stimulation. The pooled value was calculated using the Z test. In addition, if p < 0.05, the difference was considered statistically significant. The heterogeneity among studies was evaluated using the Cochrane Q statistic (significance level of p ≤ 0.10) and the inconsistency (I2) test. If heterogeneity was observed, the influence analysis (Stata 15.0, Stata Corp, College Station, Texas) was used to identify the study with the greatest influence on the pooled data. If heterogeneity still existed after omitting this study, the random effects model was used to generate the most conservative estimate.
Acknowledgements
This study was supported by Grant No. 81770703, No. 81470927 and No. 81770673 from the National Natural Science Foundation of China, and Grant No. ZY2016104 plus No. ZY2017310 from 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University.
Author Contributions
Dr. Qing He and Dr. Kaiwen Xiao contributed equally to this study, had full access to all the study data and take responsibility for the integrity of the data and the accuracy of the results. They contributed to the study concept and design, data collection, quality assessment, data analysis and writing the manuscript. Dr. Liao Peng and Dr. Junyu Lai were responsible for identifying relevant studies and creating the figures. Professor Hong Li and Dr. Deyi Luo were responsible for editing the manuscript and procuring funding. Professor Kunjie Wang, the corresponding author, managed the project development, guided the writing of the manuscript and procured funding.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qing He and Kaiwen Xiao contributed equally.
References
- 1.Abrams P, Smith AP, Cotterill N. The impact of urinary incontinence on health-related quality of life (HRQoL) in a real-world population of women aged 45–60 years: results from a survey in France, Germany, the UK and the USA. Bju International. 2015;115:143–152. doi: 10.1111/bju.12852. [DOI] [PubMed] [Google Scholar]
- 2.Haylen BT, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. International urogynecology journal. 2010;21:5–26. doi: 10.1007/s00192-009-0976-9. [DOI] [PubMed] [Google Scholar]
- 3.Abrams P, et al. Fourth international consultation on incontinence recommendations of the international scientific committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourology and Urodynamics. 2010;29:213–240. doi: 10.1002/nau.20870. [DOI] [PubMed] [Google Scholar]
- 4.Subak LL, et al. High costs of urinary incontinence among women electing surgery to treat stress incontinence. Obstetrics and gynecology. 2008;111:899–907. doi: 10.1097/AOG.0b013e31816a1e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irwin DE, et al. The economic impact of overactive bladder syndrome in six Western countries. BJU Int. 2009;103:202–209. doi: 10.1111/j.1464-410X.2008.08036.x. [DOI] [PubMed] [Google Scholar]
- 6.Irwin DE, et al. Population-Based Survey of Urinary Incontinence, Overactive Bladder, and Other Lower Urinary Tract Symptoms in Five Countries: Results of the EPIC Study. European Urology. 2006;50:1306–1315. doi: 10.1016/j.eururo.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Hunskaar S, et al. Epidemiology and natural history of urinary incontinence in women. Urology. 2003;62:16–23. doi: 10.1016/S0090-4295(03)00755-6. [DOI] [PubMed] [Google Scholar]
- 8.Wennberg AL, et al. A longitudinal population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in women. European Urology. 2009;55:783–791. doi: 10.1016/j.eururo.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Lim R, Lee SWH, Tan PY, Liong ML, Yuen KH. Efficacy of electromagnetic therapy for urinary incontinence: A systematic review. Journal of Urology. 2016;195:1833–1833. doi: 10.1016/j.juro.2016.03.065. [DOI] [PubMed] [Google Scholar]
- 10.Galloway NTM, et al. Extracorporeal magnetic innervation therapy for stress urinary incontinence ⋆. Urology. 2000;56:82–86. doi: 10.1016/S0090-4295(00)00686-5. [DOI] [PubMed] [Google Scholar]
- 11.Quek P. A critical review on magnetic stimulation: what is its role in the management of pelvic floor disorders? Current opinion in urology. 2005;15:231–235. doi: 10.1097/01.mou.0000172395.54643.4d. [DOI] [PubMed] [Google Scholar]
- 12.Fujishiro T, et al. Magnetic stimulation of the sacral roots for the treatment of stress incontinence: an investigational study and placebo controlled trial. The Journal of urology. 2000;164:1277–1279. doi: 10.1016/S0022-5347(05)67155-8. [DOI] [PubMed] [Google Scholar]
- 13.Manganotti P, et al. Repetitive magnetic stimulation of the sacral roots for the treatment of stress incontinence: a brief report. Europa medicophysica. 2007;43:339–344. [PubMed] [Google Scholar]
- 14.Gilling PJ, et al. A double-blind randomized controlled trial of electromagnetic stimulation of the pelvic floor vs sham therapy in the treatment of women with stress urinary incontinence. BJU Int. 2009;103:1386–1390. doi: 10.1111/j.1464-410X.2008.08329.x. [DOI] [PubMed] [Google Scholar]
- 15.Yamanishi T, et al. Effects of magnetic stimulation on urodynamic stress incontinence refractory to pelvic floor muscle training in a randomized sham-controlled study. Lower urinary tract symptoms. 2017 doi: 10.1111/luts.12197. [DOI] [PubMed] [Google Scholar]
- 16.Lim R, Liong ML, Leong WS, Karim Khan NA, Yuen KH. Pulsed Magnetic Stimulation for Stress Urinary Incontinence: 1-Year Followup Results. The Journal of urology. 2017;197:1302–1308. doi: 10.1016/j.juro.2016.11.091. [DOI] [PubMed] [Google Scholar]
- 17.Lim R, Liong ML, Leong WS, Khan NAK, Yuen KH. Effect of pulsed magnetic stimulation on quality of life of female patients with stress urinary incontinence: an IDEAL-D stage 2b study. International urogynecology journal. 2018;29:547–554. doi: 10.1007/s00192-017-3439-8. [DOI] [PubMed] [Google Scholar]
- 18.Fujishiro T, et al. Magnetic stimulation of the sacral roots for the treatment of urinary frequency and urge incontinence: an investigational study and placebo controlled trial. The Journal of urology. 2002;168:1036–1039. doi: 10.1097/01.ju.0000025868.08859.9e. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki T, et al. Randomized, double-blind, sham-controlled evaluation of the effect of functional continuous magnetic stimulation in patients with urgency incontinence. Neurourol Urodyn. 2007;26:767–772. doi: 10.1002/nau.20423. [DOI] [PubMed] [Google Scholar]
- 20.Yamanishi T, Homma Y, Nishizawa O, Yasuda K, Yokoyama O. Multicenter, randomized, sham-controlled study on the efficacy of magnetic stimulation for women with urgency urinary incontinence. International journal of urology: official journal of the Japanese Urological Association. 2014;21:395–400. doi: 10.1111/iju.12289. [DOI] [PubMed] [Google Scholar]
- 21.But I, Faganelj M, Sostaric A. Functional magnetic stimulation for mixed urinary incontinence. The Journal of urology. 2005;173:1644–1646. doi: 10.1097/01.ju.0000157336.87781.32. [DOI] [PubMed] [Google Scholar]
- 22.But I. Conservative treatment of female urinary incontinence with functional magnetic stimulation. Urology. 2003;61:558–561. doi: 10.1016/S0090-4295(02)02249-5. [DOI] [PubMed] [Google Scholar]
- 23.Lim R, Liong ML, Leong WS, Khan NAK, Yuen KH. Patients’ perception and satisfaction with pulsed magnetic stimulation for treatment of female stress urinary incontinence. International urogynecology journal. 2018;29:997–1004. doi: 10.1007/s00192-017-3425-1. [DOI] [PubMed] [Google Scholar]
- 24.Lim R, Liong ML, Leong WS, Khan NA, Yuen KH. Magnetic stimulation for stress urinary incontinence: study protocol for a randomized controlled trial. Trials. 2015;16:279. doi: 10.1186/s13063-015-0803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to assess the quality of life of urinary incontinent women. British journal of obstetrics and gynaecology. 1997;104:1374–1379. doi: 10.1111/j.1471-0528.1997.tb11006.x. [DOI] [PubMed] [Google Scholar]
- 26.Lim R, Liong ML, Leong WS, Yuen KH. Which outcome measures should be used in stress urinary incontinence trials? BJU Int. 2018;121:805–810. doi: 10.1111/bju.14121. [DOI] [PubMed] [Google Scholar]
- 27.Abrams P, Blaivas JG, Stanton SL, Andersen JT. The standardisation of terminology of lower urinary tract function. World Journal of Urology. 1989;6:233–245. doi: 10.1007/bf00328107. [DOI] [PubMed] [Google Scholar]
- 28.Simons AM, Yoong WC, Buckland S, Moore KH. Inadequate repeatability of the one-hour pad test: the need for a new incontinence outcome measure. BJOG: An International Journal of Obstetrics & Gynaecology. 2001;108:315–319. doi: 10.1111/j.1471-0528.2001.00069.x. [DOI] [PubMed] [Google Scholar]
- 29.Henderson JW, et al. A Randomized Comparative Study Evaluating Various Cough Stress Tests and 24-Hour Pad Test with Urodynamics in the Diagnosis of Stress Urinary Incontinence. The Journal of urology. 2018;199:1557–1564. doi: 10.1016/j.juro.2017.11.073. [DOI] [PubMed] [Google Scholar]
- 30.Krhut J, et al. Pad weight testing in the evaluation of urinary incontinence. Neurourol Urodyn. 2014;33:507–510. doi: 10.1002/nau.22436. [DOI] [PubMed] [Google Scholar]
- 31.Allen C, Keane D. Pathophysiology of urinary incontinence. Reviews in Gynaecological Practice. 2005;5:65–70. doi: 10.1016/j.rigp.2004.08.001. [DOI] [Google Scholar]
- 32.Shah AP, et al. Continence and micturition: an anatomical basis. Clinical anatomy (New York, N.Y.) 2014;27:1275–1283. doi: 10.1002/ca.22388. [DOI] [PubMed] [Google Scholar]
- 33.Horng SS, et al. The epidemiology of urinary incontinence and it’s influence on quality of life in Taiwanese middle-aged women. Neurourol Urodyn. 2013;32:371–376. doi: 10.1002/nau.22302. [DOI] [PubMed] [Google Scholar]
- 34.Schultz SE, Kopec JA. Impact of chronic conditions. Health reports. 2003;14:41–53. [PubMed] [Google Scholar]
- 35.Krhut Jan, Gärtner Marcel, Mokris Jan, Horcicka Lukas, Svabik Kamil, Zachoval Roman, Martan Alois, Zvara Peter. Effect of severity of urinary incontinence on quality of life in women. Neurourology and Urodynamics. 2018;37(6):1925–1930. doi: 10.1002/nau.23568. [DOI] [PubMed] [Google Scholar]
- 36.Thüroff JW, et al. [EAU Guidelines on Urinary Incontinence] European Urology. 2011;59:387–400. doi: 10.1016/j.eururo.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jadad AR, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]