Abstract
Caries progression seems to follow universal, predictable rates, depending largely on the caries severity in populations: the higher the caries severity, the higher the progression rates. Quantification of these rates would allow prediction of future caries increments. Our aim was to describe caries progression rates in the primary and permanent dentition in Western populations (not in lesions) of children and adolescents. Therefore, we systematically searched MEDLINE-PubMed, Embase, CINAHL, and the Cochrane library for studies reporting caries progression data. Eligibility criteria were reporting empirical data from at least 2 full-mouth dental caries examinations in a closed cohort during a follow-up of at least 3 y, a first examination after 1974, a second examination before the age of 22 y, caries assessed as dentine caries (d3/D3), and caries reported in dmfs/DMFS (decayed, missing, and filled surfaces), dmft/DMFT (decayed, missing, and filled teeth), or caries-free participants. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, we described the results for the primary and permanent dentition in a systematic review, performed a meta-analysis for the caries incidence rate in the permanent dentition, and conducted multivariate, hierarchical meta-regression analyses for the caries incidence rate and the increments in DMFS and DMFT. Of the 6,343 unique studies retrieved, 43 studies (56,376 participants) were included for systematic review and 32 for meta-analyses (39,429 participants). The annual decline in caries-free children in the permanent dentition ranged from 0.8% to 10.2%. The annual increment ranged from 0.07 to 1.77 in DMFS and from 0.06 to 0.73 in DMFT. The pooled caries incidence rate was 0.11 (0.09–0.13) per person-year at risk. Meta-regression analyses showed that the methods of individual studies influenced pooled caries incidence rates and increments in DMFS and DMFT. This should be taken into account in planning and evaluation of oral health care services. However, the caries incidence rate is promising for prediction of future caries increments in populations.
Keywords: epidemiology, DMF index, incidence, longitudinal studies, child, adolescent
Introduction
Dental caries is one of the most prevalent chronic diseases in the world, affecting 60% to 90% of schoolchildren (World Health Organization 2012). There are disparities in caries onset and caries progression rates between and within populations. This is due to differences in behavioral and sociodemographic conditions that interact with the etiology of dental caries (Fejerskov 2004).
Nevertheless, progression of dental caries seemed to follow predictable rates that depended largely on the caries severity in a population; the higher the caries severity, the higher the progression rates (Broadbent et al. 2013). Other studies also described fixed patterns of caries progression and suggested that these were universal, for both the permanent and the primary dentition (Massler et al. 1954; Sheiham and Sabbah 2010).
If these patterns are indeed universal, it would be possible to predict future caries increments in a population. This would have several advantages. As well as improving the planning of oral health services and targeted use of preventive care (Sheiham and Sabbah 2010), it would also indicate which improvements in oral health are achievable. While many studies have described the incidence, prevalence, and progression of caries, we do not know of systematic reviews or meta-analyses reporting on caries progression from pooled findings of longitudinal studies. In this research, we first systematically review studies reporting on follow-up in Western (or comparable) populations of children and adolescents for annual progression of caries in the primary and permanent dentition. Second, using meta-analyses, we provide an estimate for the caries incidence rate in the permanent dentition. Third, using meta-regression methods we assess the impact of study methods to explore the possible bias in reported caries progression rates.
Methods
We defined progression rate as the mean caries increment in a population (not in a caries lesion) during a certain time period. From there, we defined caries progression in a population as 1) the annual decline in the percentage of caries-free children and adolescents; 2) the annual increment in decayed, missing, and filled surfaces (dmfs/DMFS); and 3) the annual increment in decayed, missing, and filled teeth (dmft/DMFT). We defined the latter as caries incidence rate. This is the number of participants acquiring a first dentine lesion divided by the total time that all participants were caries free during follow-up of a population of caries-free persons (the so-called population at risk).
Our scope was limited to dentine lesions (d3/D3) in children and adolescents up to age 21 y. We chose dentine lesions, as this is the stage at which restorative interventions are generally indicated (Lenkkeri et al. 2012; Hall-Scullin et al. 2017), and the DMF index, as this has been the leading index in research for decades (Larmas 2010; Ekbäck et al. 2016). Since patterns are longitudinal, we searched for cohort studies with at least 2 examinations in the primary or permanent dentition of the same participants. It takes a relatively long period for enamel lesions to develop into dentine lesions (Mejàre et al. 2004). To observe caries progression, the minimum follow-up period was set at 3 y (Ekbäck et al. 2016). The study methods and reporting were based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al. 2009); the PRISMA checklist is provided in Appendix Table 14.
Search Strategy
We systematically searched MEDLINE-PubMed, Embase, CINAHL, and the Cochrane library (on April 16, 2018) for publications describing the longitudinal development of caries in cohorts of children and adolescents. Search terms were “child*” and “adolescen*.” Search terms on dental caries and follow-up included the MeSH terms “tooth demineralization,” “DMF index,” “disease progression,” “incidence,” and “cohort studies.” The full search strategy for MEDLINE-PubMed is described in Table 1.
Table 1.
Search Strategy MEDLINE-PubMed.
| Search | Query |
|---|---|
| #4 | Search #1 AND #2 AND #3 |
| #3 | Search (“Disease Progression”[Mesh] OR prognos*[tiab] OR progres*[tiab] OR incidence[Mesh] OR incidence[tiab] OR “cohort studies”[Mesh] OR cohort*[tiab] OR follow-up[tiab] OR prospect*[tiab] OR longitudinal[tiab]) |
| #2 | Search (“Tooth Demineralization”[Mesh] OR (tooth[tiab] AND demineralization[tiab]) OR caries[tiab] OR carious[tiab] OR “DMF Index”[Mesh] OR DMF[tiab]) |
| #1 | Search (child*[tw] OR adolescen*[tw]) |
The search results were imported in EndNote (X8, Clarivate Analytics), and duplications were removed using the Bramer method (Bramer 2014). Caries progression has declined since the introduction of fluoridated toothpastes. As the use of fluoridated toothpastes became common practice from the mid-1970s (ten Cate 2013) and we opted for a follow-up period of at least 3 y, we excluded studies published before 1978 (1975 + 3 years). Subsequently, we imported the remaining references in the online systematic review software from Covidence (Veritas Health Innovation) for title and abstract screening.
Eligibility Criteria
We had 9 inclusion criteria: a closed cohort and results reported for complete cases; a follow-up of at least 3 y; at least 2 examinations of dental caries; first examination after 1974; a second caries examination before the age of 22 y; caries assessed as dentine caries (d3/D3); caries reported in decayed, missing, and filled surfaces or teeth (dmfs or dmft for the primary dentition; DMFS or DMFT for the permanent dentition) or in number or percentage of caries-free participants; the results presented full-mouth examinations; and the publication was written in English, Dutch, or German. Prospective, retrospective, cohort, and intervention studies were all supposed eligible, but cross-sectional and case-control studies were not. Studies were also excluded if no abstracts or full texts were available and if the results of a cohort concerned participants in an age range of more than 3 y.
Study Selection
Two researchers (R.H. and N.A.E.A.) independently screened titles and abstracts to verify whether the eligibility criteria for full-text reading were met. If the eligibility was unclear, the full text was read.
Full texts were reviewed independently for confirmation of eligibility (by R.H. and N.A.E.A.). Studies were excluded if they did not make a distinction between dentine and enamel caries. If a study did not describe whether they included only dentine caries, we looked into the full text of the reference of the diagnostic criteria for a description. We assumed that studies using the World Health Organization criteria included dentine caries. We excluded studies that reported results as a graph, had unclear groups, or had deviant counts of missing surfaces. Reviews and systematic reviews were evaluated for their relevance and were used to identify additional studies that had not yet been found in our search. All disagreements on inclusion of studies were discussed and resolved by mutual agreement. Hence, results are based on full agreement.
Quality Assessment of Study Methods
The quality of study methods for the included publications was assessed independently by the same 2 investigators using the scoring form shown in Appendix Table 1. This form consisted of items on the relevance of evidence and risk of bias. An item only could have been met if it had been described in the study.
The items to assess the relevance of evidence were chosen to reflect the current situation in Western countries. There were 4 items regarding the relevance of evidence: 1) the results applied to a cohort of children or adolescents spanning an age range of no more than 2 y (to ensure less spreading in the variable “age at baseline”; children in 1 school grade were regarded as covering a 2-y age range), 2) the cohort had used fluoridated toothpaste and/or their drinking water had been sufficiently fluoridated (>1.0 ppm) for the entire study period, 3) the cohort had had regular access to oral health care services (whether preventive, restorative, or both) throughout the study period, and 4) the study had been conducted in Western Europe, North America, Australia, or New Zealand. If none or 1 of the above items were met, the relevance of evidence was low; if 2 were met, it was moderate; and if 3 or 4 were met, it was high.
Three dichotomous (yes/no) items were used to assess the risk of bias: 1) the number of dropouts, 2) the reasons for dropping out, and 3) the investigator had been blinded to the clinical history of the participants and/or blinded to the group allocation in case of interventions. The risk of bias was considered high if none of the items were met, moderate if 1 or 2 items were met, and low if all 3 items were met. Studies with insufficient quality (i.e., a low relevance of evidence and/or a high risk of bias) were excluded. Disagreements on the quality of study methods were discussed until a consensus was reached.
Data Collection
A data extraction form was developed that comprised the following 9 variables: number of years of follow-up; use of bitewing radiographs (no or not described/yes); age at baseline (age at the start of the study); year in which the study started; collective preventive intervention (no/yes); risk of bias (low/moderate); relevance of evidence (high/moderate); dmfs, dmft, DMFS, and DMFT scores with standard deviations at baseline and follow-up; and the percentage of caries-free participants at baseline and follow-up. Preventive interventions that might have influenced caries progression were recorded if they had been collectively provided to all or part of the study population and were considered as additional to care as usual in general dental practices. One investigator (RH) extracted the data from the included studies. The data were checked by the other investigator (NAEA) and initial disagreements were resolved by consensus discussion. If different publications reported data on the same cohort, we extracted the data from the publication with the highest quality.
Data Processing
The year in which the study started, together with the number of years of follow-up, was used to classify the decade in which the study had been performed (1970s, 1980s, 1990s, 2000s, or 2010s). We took the decade that covered the largest part of the study period. For example, a study that started in 1996 and lasted 4 y was classified as having been conducted in the 1990s. If the year of the start of the study was not provided, we guessed the decade based on the year of publication and the number of years of follow-up.
Some publications presented only results for subgroups. These results were clustered by calculating weighted means. For the DMF indices, the standard deviations (SDs) were also pooled.
As we expected higher caries progression rates in populations with higher caries severity at the start of the study (baseline), we determined categories for baseline caries experience (low, moderate, high) in the permanent dentition. The classification of the studies was based on the mean DMFS and/or DMFT of their participants at baseline. We assumed that the DMFS and DMFT were 0 at age 5 y with a linear increment until adulthood. At age 12 y, caries experience was considered low with a DMFS <1.5 or a DMFT <1, moderate with a DMFS between 1.5 and 3.5 or a DMFT between 1 and 2.3, and high with a DMFS >3.5 or DMFT >2.3 (based on Broadbent et al. 2013). Drawing lines from the 0 at age 5 y through the limit values at age 12 y enabled us to plot the included studies for the determination of the baseline caries experience. These indices were also used to assess the baseline caries experience for studies reporting on caries-free survival as Sheiham and Sabbah (2010) described a predictable relationship between mean DMFS/DMFT and caries prevalence in a population. We checked this, and our data confirmed this relationship; the higher the baseline DMFS or DMFT, the lower the percentage of caries-free participants.
The decline in caries-free participants and increment in DMFS/DMFT was based only on the first and last measurements. We therefore ignored any reported intermediate measurements and assumed a relatively linear progression. This was checked and confirmed by plotting the data from included studies with repeated measures. The declines/increments were calculated by subtracting the results at baseline from the results at follow-up. If the SDs of the increments were not reported, the SDs were pooled from the SDs at baseline and follow-up. For the meta-analysis and meta-regression analyses, the SDs were recalculated into standard errors.
Caries incidence rate is the number of caries-free participants who developed dental caries (events) during follow-up, divided by the “healthy time” (at risk) for all participants together in that study (Ekbäck et al. 2016). Events were defined as persons getting a first cavity (caries lesion D3). The number of events was calculated by subtracting the number of caries-free participants at follow-up from those at baseline. We used the constant hazard assumption to derive the total person-years at risk. For participants without caries at the end of follow-up, their person-years at risk is the sum of their individual years of follow-up. For participants with caries during follow-up (events), their person-years at risk is half of the sum of their individual years of follow-up. We summed both to calculate the total person-time of follow-up of the population. We then calculated the caries incidence rate by dividing the total number of events by the total person-time of follow-up (time at risk) of the population.
Meta-Analysis and Meta-Regression Analyses
These analyses were carried out for the permanent dentition. In the analyses of the caries incidence rate, we included only studies that reported caries-free survival as well as the mean DMFS or DMFT, as these indices were needed to determine baseline caries experiences (see data processing). In the analyses of increments in DMFS and DMFT, only studies that reported measures for data distribution were included.
The meta-analysis of the caries incidence rate was performed with the package “metamean” from R software (3.3; Development Core Team). We used a random-effects model weighted by total person-years. A forest plot was made to show the estimated effect across studies. Then we performed multivariate, hierarchical, linear meta-regression analyses using the R package “metaphor.” The random-effects model assumption was used to explore the impact of covariates on the pooled caries incidence rate and DMFS and DMFT increments. First, all covariates, notably study design features, were categorized into 4 hierarchical groups based on an a priori expectation of effect on the estimates. Group 1 consisted of the use of bitewing radiographs and age at baseline, group 2 of caries experience at baseline, group 3 of decade and preventive intervention, and group 4 of risk of bias and relevance of evidence. Next, the meta-regressions were conducted starting with crude analyses on follow-up years. Subsequently, we added group by group to the analyses.
Results
Study Selection
We identified 12,580 records through database searching. Seventy-seven publications met our inclusion criteria and were assessed for quality. Nine studies were judged to be of high quality (relevance of evidence high and risk of bias low), 6 studies were of moderate quality (relevance of evidence and risk of bias both moderate), 5 had moderate relevance of evidence and a low risk of bias, and 27 studies had high relevance of evidence and moderate risk of bias. The quality of 30 studies was insufficient, as they had either low relevance of evidence or a high risk of bias. These studies were excluded. Appendix Table 2 provides a list of these studies.
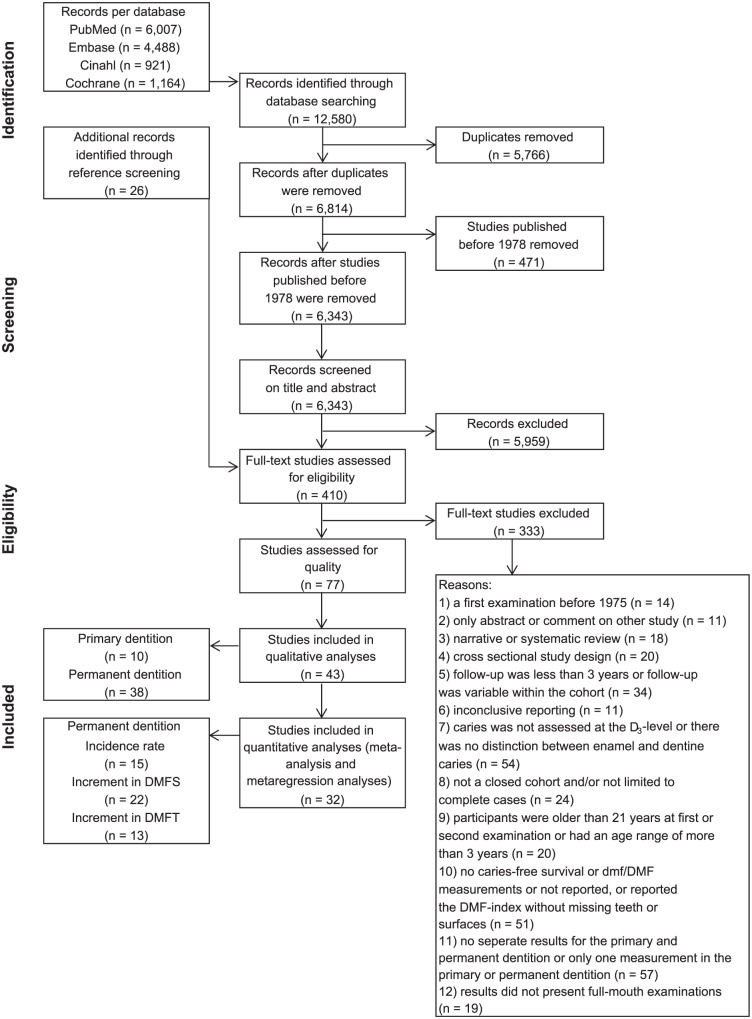
Eventually, 47 publications were found to be eligible for inclusion in this systematic review. However, as 4 of them presented the same cohorts as other included studies (Appendix Table 3), we ultimately included 43 studies in the descriptive analyses in this systematic review together, including 56,376 participants. Of these 43 studies, 32 (including 39,429 participants) reported for the permanent dentition on caries-free survival data and DMFT data, DMFS data, or both in a manner to allow for their inclusion in the meta-analysis and meta-regression analyses. Figure 1 shows the flow diagram of the search and selection process.
Figure 1.
Flow diagram of the study selection for inclusion in the systematic review, meta-analysis, and meta-regression analyses. DMFS, decayed, missing, and filled surfaces; DMFT, decayed, missing, and filled teeth.
Primary Dentition
The percentage of caries-free children increased in some studies. These studies were excluded as they provided cross-sectional results for the prevalence of dental caries in the primary dentition. Six studies remained that described the percentage of caries-free children (d3) in the primary dentition. The annual decline in percentage of caries-free children ranged from 3.8% to 12.2%. Seven studies reporting on dmfs and 4 studies reporting on dmft were included. The annual increments ranged from −1.0 to 1.0 in dmfs and from −0.3 to 0.3 in dmft (Table 2). These inconsistent increments were caused by exfoliation of teeth. Study characteristics and results per study are provided in Appendix Tables 4 to 6.
Table 2.
Results of Caries Progression in the Primary and Permanent Dentition: Annual Decline in Percentage of Caries-Free Children and Annual Increments in dmfs/DMFS/dmft/DMFT (d3/D3).
| Characteristic | No. of Studies | No. of Persons | Range |
|---|---|---|---|
| Annual decline in percentage of caries-free children and adolescents | |||
| Primary dentitiona | 6 | 3,318 | 3.8% to 12.2% |
| Permanent dentitionb | 18 | 12,017 | 0.8% to 10.2% |
| Baseline caries experience | |||
| Lowc | 9 | 4,811 | 4.3% to 10.2% |
| Moderated | 4 | 928 | 3.7% to 8.3% |
| Highe | 2 | 5,029 | 0.8% to 2.2% |
| Annual increment in dmfs (primary dentition) and DMFS (permanent dentition) | |||
| Primary dentitionf | 7 | 5,896 | −1.0 to 1.0 |
| Permanent dentition | 26 | 22,803 | 0.07 to 1.77 |
| Baseline caries experience | |||
| Lowg | 8 | 3,735 | 0.07 to 0.53 |
| Moderateh | 13 | 16,473 | 0.23 to 1.23 |
| Highi | 5 | 2,595 | 0.44 to 1.77 |
| Annual increment in dmft (primary dentition) and DMFT (permanent dentition) | |||
| Primary dentitionj | 4 | 1,734 | −0.3 to 0.3 |
| Permanent dentition | 14 | 25,206 | 0.06 to 0.73 |
| Baseline caries experience | |||
| Lowk | 7 | 4,121 | 0.06 to 0.34 |
| Moderatel | 6 | 16,705 | 0.24 to 0.73 |
| Highm | 1 | 4,380 | 0.39 to 0.39 |
DMFS, decayed, missing, and filled surfaces; DMFT, decayed, missing, and filled teeth.
Holt 1995; Karjalainen et al. 2001; Pienihäkkinen et al. 2004; van Rijkom et al. 2004; Vermaire et al. 2014; Tickle et al. 2017.
Assessments of categories for caries experience at baseline were based on the mean DMFS or DMFT at baseline. Three studies did not report DMFS/DMFT measures: van Palenstein Helderman et al. 2001; Ekbäck et al. 2016; Basha et al. 2017.
Virtanen 2001; van Rijkom et al. 2004; Sánchez-Pérez et al. 2010; Lenkkeri et al. 2012; Masood et al. 2012; Vermaire et al. 2014; Peres et al. 2016; Heinemann et al. 2017; Li et al. 2017.
Margolis et al. 1994; Holt 1995; Petersen et al. 2004; van Rijkom et al. 2004; Sánchez-Pérez et al. 2010; Vermaire et al. 2014; Tickle et al. 2017.
Virtanen 2001; Petersen et al. 2004; van Rijkom et al. 2004; Truin and van ‘t Hof 2005; Tai et al. 2009; Sánchez-Pérez et al. 2010; Lenkkeri et al. 2012; Vermaire et al. 2014.
Ruiken et al. 1986; Isogangas et al. 1993; Sköld et al. 1994; Heidmann and Poulsen 1997; Morgan et al. 1998; Alanen et al. 2000; Sköld et al. 2001; Bruno-Ambrosius et al. 2005; Källestål 2005; David et al. 2006; Heyduck et al. 2006; Foster Page and Thomson 2012; Holmén et al. 2013.
Virtanen 2001; Tai et al. 2009; Sánchez-Pérez et al. 2010; Masood et al. 2012; Peres et al. 2016; Heinemann et al. 2017; Li et al. 2017.
Permanent Dentition
Eighteen studies described the decline in caries-free participants for the permanent dentition. The annual decline ranged from 0.8% to 10.2%. This decline was lower in populations with higher caries experiences at baseline. Twenty-six studies reporting on DMFS and 14 studies reporting on DMFT were included. The annual increments ranged from 0.07 to 1.77 in DMFS and from 0.06 to 0.73 in DMFT (Table 2). These increments were higher in populations with higher caries experiences at baseline. Study characteristics and results per study are provided in Appendix Tables 7 to 9.
Meta-Analysis and Meta-Regression Analyses
For the primary dentition, we did not perform meta-regression analyses. The reported dmfs/dmft in the included studies were not comparable due to the nonstandardized approaches of exfoliated teeth.
For the permanent dentition, 15 studies were included in the meta-analysis and meta-regression analysis of survival data and 22 and 13 studies in the meta-regression analyses of the increments in DMFS and DMFT, respectively.
In the meta-analysis, we found a caries incidence rate of 0.11 (0.09–0.13) per person-year at risk (Fig. 2). In the subsequent meta-regression analyses, we explored the uncertainties around this incidence rate (Table 3). The caries incidence rate was constant over time as the unadjusted regression coefficient for follow-up was 0. Adjusting for the covariates did not affect the estimate. Only in the last step, when risk of bias and relevance of evidence were added, the estimate changed to −0.02. So, the pooled caries incidence rate of 0.11 is probably an overestimation.
Figure 2.
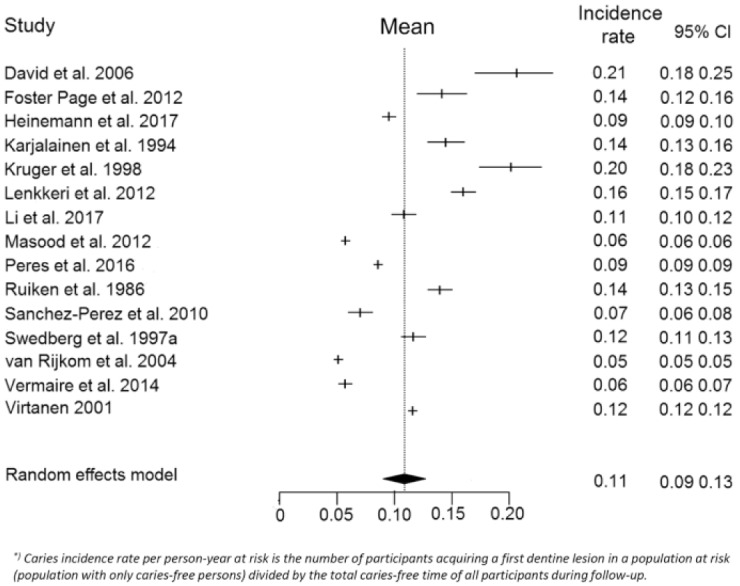
Forest plot of the caries incidence rate per person-year at risk (D3) in the permanent dentition and the 95% confidence interval (CI). The caries incidence rate (first caries events [D3] per person-year at risk) could be recalculated for 15 studies. These reported on 1,995 caries events for a total of 10,768 participants with a total follow-up time of 22,292 person-years. The data were pooled using a random-effects model, because the reported caries incidence rates showed marked heterogeneity (I2 of 100%). The studies were weighted by the number of total person-years. The weight of the studies ranged from 6.5% to 6.7%, and the median was 6.7%.
Table 3.
Results of the Hierarchical, Multivariable Meta-Regression Analyses of the Relationship between the Caries Incidence Rate in the Permanent Dentition per Person-Year at Risk (D3) and Follow-Up.
| Caries Incidence Rate of Permanent Dentition (n = 15 Studies, n = 10,768 Persons) | |
|---|---|
| Per person-year at risk (D3)a,b | Ruiken et al. 1986; Karjalainen et al. 1994; Swedberg, Fredén, and Norén 1997; Kruger et al. 1998; Virtanen 2001; van Rijkom et al. 2004; David et al. 2006; Sánchez-Pérez et al. 2010; Foster Page and Thomson 2012; Lenkkeri et al. 2012; Masood et al. 2012; Vermaire et al. 2014; Peres et al. 2016; Heinemann et al. 2017; Li et al. 2017 |
| Per year of follow-up, β (95% confidence interval) | −0.00 (−0.01 to 0.01)c
0.00 (−0.00 to 0.01)d 0.00 (−0.00 to 0.01)e −0.00 (−0.02 to 0.02)f −0.02 (−0.05 to 0.01)g |
Caries incidence rate per person-year at risk is the number of participants acquiring a first dentine lesion in a population at risk (population with only caries-free persons) divided by the total caries-free time of all participants during follow-up.
Tables of the output are available in Appendix Table 10.
Not adjusted.
Adjusted for group1 (group 1: bitewings + age at baseline).
Adjusted for groups 1 and 2 (group 2: caries experience at baseline).
Adjusted for groups 1, 2, and 3 (group 3: decade + preventive intervention).
Adjusted for groups 1, 2, 3, and 4 (group 4: relevance of evidence + risk of bias).
The unadjusted increment in DMFS per year of follow-up was 0.43 (Table 4). Adjusting for the various groups indicated that the estimate of the annual increment in DMFS ranged from 0.36 to 0.64 and was affected by covariates.
Table 4.
Results of the Hierarchical, Multivariable Meta-Regression Analyses of the Relationships between the Increments in DMFS/DMFT (D3) and Follow-Up.
DMFS, decayed, missing, and filled surfaces; DMFT, decayed, missing, and filled teeth.
Tables of the output are available in Appendix Table 11.
Not adjusted.
Adjusted for group1 (group 1: bitewings + age at baseline).
Adjusted for groups 1 and 2 (group 2: caries experience at baseline).
Adjusted for groups 1, 2, and 3 (group 3: decade + preventive intervention).
Adjusted for groups 1, 2, 3, and 4 (group 4: relevance of evidence + risk of bias).
The unadjusted increment in DMFT per year of follow-up was 0.18 (Table 4). After adjusting for groups 1 and 2, adding group 3 (decade and preventive intervention) showed a large decrease of the estimate to 0.07. Adding group 4 (relevance of evidence and risk of bias) led to a remarkable, negative increment of −0.04. The estimate of the annual increment in DMFT was highly influenced by covariates.
Appendix Tables 10 to 12 provide the full output of the meta-regression analyses, and Appendix Table 13 gives a description of the covariates used in these analyses.
Discussion
This study revealed that the caries incidence rate of the permanent dentition is a promising caries progression rate in populations of children and adolescents as its use is rather new and it seems fairly stable. Ekbäck et al. (2016) described that the caries incidence rate is rarely used in oral health studies but well known in medical epidemiology. Hence, they suggested to report caries progression in longitudinal studies in incidence rates. We found a pooled caries incidence rate of 0.11 (0.09−0.13) per person-year at risk (Fig. 2). The uncertainties around our estimate were small, although it was somewhat influenced by the risk of bias and relevance of evidence of the included studies. The meaning of this caries incidence rate is that per year, 11 persons will develop dentine caries for the first time (newly diseased) per 100 persons who were caries free at the beginning of that year.
We were also able to pool the increments in DMFS and DMFT. The meaning of the results found in these analyses was inconclusive; the uncertainties around the estimates were increased by the diversity of the study methods of the studies involved. Nevertheless, the linear increments we found are consistent with the findings of Broadbent et al. (2013), as were our findings that higher baseline caries experiences precede higher progression rates.
Some aspects of this review warrant further attention.
First, there were wide variations in study methods. For future research, adequate study designs and standardized methods of data collection are desirable. Harmonization of study designs can contribute to reduction of the uncertainties that the meta-regression analyses demonstrated. The inclusion of both intervention and observational studies might cause some confusion. Our aim was to find data on caries progression in studies with follow-up for cohorts with or without a collective, uniform preventive intervention. We corrected for such interventions in the meta-regression analyses if they had been provided to all or part of the study population (regardless of individual indications) and were considered additional to care as usual. Yet, we found that this did not explain the variation in outcomes. This might be due to the fact that the cohort participants could also have received preventive interventions as most of them had access to regular dental care.
Second, the filled component of the DMF index was probably influenced by lesion thresholds of dentists to intervene restoratively. These thresholds vary between countries and decades (Innes et al. 2017). Caries progression rates would have been higher in situations where it is common practice to “drill” in earlier stages of the caries process.
Third, the assessment of dental caries is complex and methods for assessment were varied, such as use of bitewing radiographs and drying of teeth. This would have resulted in differences in the diagnosis of dental caries. However, these differences were probably reduced as the included studies used the same methods for the baseline and follow-up measurements.
Fourth, meta-analyses were not possible for the primary dentition as a result of the limited number of included studies and the inconsistent results due to exfoliated teeth. A follow-up of 3 y might not have been necessary for the primary dentition, since caries lesions in primary teeth generally progress faster than in permanent teeth. To avoid the exfoliation problem in the primary dentition, it could be considered to follow up from ages 3 to 5 y. However, this precludes insights into caries progression in children ages 5 to 12 y. A solution is not to ignore past caries experience in exfoliated teeth in longitudinal studies. This can be achieved by calculating the total number of decayed, extracted, and filled primary surfaces or teeth ever observed in a participant at baseline and at follow-up (Ruff 2018).
Finally, another source of bias may have been the inclusion of studies with only results of complete cases (i.e., the results at baseline and at follow-up were for the same participants). This might have caused a selective follow-up. Nonetheless, we needed complete cases to determine the number of events for the caries incidence rates.
Our findings for the permanent dentition provide indications for caries progression rates in populations. These rates could be used for planning, targeted use of preventive care, and evaluation of (preventive) oral health care services. They provide a starting point for further research. They could also be used by general dental practitioners for reflections on the caries progression rates in their patient populations. And last but not least, they emphasize the importance of preventing caries at early ages as progression rates for DMFS/DMFT were higher in populations with higher baseline caries experiences.
Conclusions
In this systematic review, we described caries progression rates in the primary and permanent dentition. Pooled caries progression rates were not achievable for the primary dentition due to the limited number of included studies and the nonstandardized approaches of exfoliated teeth. For the permanent dentition, our pooled findings on caries progression in populations were a caries incidence rate of 0.11 (0.09–0.13) per person-year at risk, an increment in DMFS of 0.43 per year of follow-up, and an increment in DMFT of 0.18 per year of follow-up. So far, the caries incidence rate measure rarely has been used in longitudinal oral health research but seemed fairly stable and therefore most promising. When using our progression rates for the prediction of caries increments, caution is justified because these measures were influenced by methods of the studies included. For better insight into caries progression rates in populations and usefulness for policy makers, more standardization of measuring and study methods in (epidemiological) research is essential.
Author Contributions
R. Hummel, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; N.A.E. Akveld, contributed to design, data acquisition, analysis, and interpretation, critically revised the manuscript; J.J.M. Bruers, W.J.M. van der Sanden, G.J.M.G. van der Heijden, contributed to design and data interpretation, critically revised the manuscript; N. Su, contributed to data analysis and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034519847953 for Caries Progression Rates Revisited: A Systematic Review by R. Hummel, N.A.E. Akveld, J.J.M. Bruers, W.J.M. van der Sanden, N. Su and G.J.M.G. van der Heijden in Journal of Dental Research
Acknowledgments
We thank Tessa van Geijn for her help with the revision of the final draft of the manuscript.
Footnotes
A supplemental appendix to this article is available online.
The authors received no financial support and declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iD: R. Hummel  https://orcid.org/0000-0003-4275-3361
https://orcid.org/0000-0003-4275-3361
References
- Alanen P, Isokangas P, Gutmann K. 2000. Xylitol candies in caries prevention: results of a field study in Estonian children. Community Dent Oral Epidemiol. 28(3):218–224. [DOI] [PubMed] [Google Scholar]
- Basha S, Mohamed RN, Swamy HS, Ramamurthy PH, Sexena V. 2017. Caries incidence among obese adolescents: a 3-year prospective study. Oral Health Prev Dent. 15(1):65–71. [DOI] [PubMed] [Google Scholar]
- Bramer W. 2014. Manual on deduplicating in EndNote—the Bramer method [accessed 5 Apr 2019]. https://www.researchgate.net/publication/284515937_Manual_on_Deduplicating_in_EndNote_-_the_Bramer_method.
- Broadbent JM, Foster Page LA, Thomson WM, Poulton R. 2013. Permanent dentition caries through the first half of life. Br Dent J. 215(7):E12. [DOI] [PubMed] [Google Scholar]
- Bruno-Ambrosius K, Swanholm G, Twetman S. 2005. Eating habits, smoking and toothbrushing in relation to dental caries: a 3-year study in Swedish female teenagers. Int J Paediatr Dent. 15(3):190–196. [DOI] [PubMed] [Google Scholar]
- David J, Raadal M, Wang NJ, Strand GV. 2006. Caries increment and prediction from 12 to 18 years of age: a follow-up study. Eur Arch Paediatr Dent. 7(1):31–37. [DOI] [PubMed] [Google Scholar]
- Ekbäck G, Ordell S, Palmetun-Ekbäck M, Ekbäck G, Unell L, Johansson AK. 2016. Reporting dental caries disease in longitudinal studies—a suggestion. Swed Dent J. 40(2):173–179. [PubMed] [Google Scholar]
- Fejerskov O. 2004. Changing paradigms in concepts on dental caries: consequences for oral health care. Caries Res. 38(3):182–191. [DOI] [PubMed] [Google Scholar]
- Forgie AH, Paterson M, Pine CM, Pitts NB, Nugent ZJ. 2000. A randomised controlled trial of the caries-preventive efficacy of a chlorhexidine-containing varnish in high-caries-risk adolescents. Caries Res. 34(5):432–439. [DOI] [PubMed] [Google Scholar]
- Foster Page LA, Thomson WM. 2012. Caries prevalence, severity, and 3-year increment, and their impact upon New Zealand adolescents’ oral-health-related quality of life. J Public Health Dent. 72(4):287–294. [DOI] [PubMed] [Google Scholar]
- Hall-Scullin E, Whitehead H, Milsom K, Tickle M, Su TL, Walsh T. 2017. Longitudinal study of caries development from childhood to adolescence. J Dent Res. 96(7):762–767. [DOI] [PubMed] [Google Scholar]
- Hanachowicz L. 1984. Caries prevention using a 1.2% sodium monofluorophosphate dentifrice in an aluminium oxide trihydrate base. Community Dent Oral Epidemiol. 12(1):10–16. [DOI] [PubMed] [Google Scholar]
- Heidmann J, Poulsen S. 1997. Comparative three-year caries protection from an aluminum-containing and a fluoride-containing toothpaste. Caries Res. 31(2):85–90. [DOI] [PubMed] [Google Scholar]
- Heinemann F, Ifland S, Heinrich-Weltzien R, Schüler IM. 2017. Influence of fissure sealants on dental health of elementary school children in Weimar—a longitudinal observational study under real-life conditions. Gesundheitswesen. 79(3):195–202. [DOI] [PubMed] [Google Scholar]
- Heyduck C, Meller C, Schwahn C, Splieth CH. 2006. Effectiveness of sealants in adolescents with high and low caries experience. Caries Res. 40(5):375–381. [DOI] [PubMed] [Google Scholar]
- Holmén A, Strömberg U, Magnusson K, Twetman S. 2013. Tobacco use and caries risk among adolescents—a longitudinal study in Sweden. BMC Oral Health. 13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RD. 1995. The pattern of caries in a group of 5-year-old children and in the same cohort at 9 years of age. Community Dent Health. 12(2):93–99. [PubMed] [Google Scholar]
- Innes NPT, Schwendicke F. 2017. Restorative thresholds for carious lesions: systematic review and meta-analysis. J Dent Res. 96(5):501–508. [DOI] [PubMed] [Google Scholar]
- Isogangas P, Mäkinen KK, Tiekso J, Alanen P. 1993. Long-term effect of xylitol chewing gum in the prevention of dental caries: a follow-up 5 years after termination of a prevention program. Caries Res. 27(6):495–498. [DOI] [PubMed] [Google Scholar]
- Julihn A, Ekbom A, Modéer T. 2009. Maternal overweight and smoking: prenatal risk factors for caries development in offspring during the teenage period. Eur J Epidemiol. 24(12):753–762. [DOI] [PubMed] [Google Scholar]
- Källestål C. 2005. The effect of five years’ implementation of caries-preventive methods in Swedish high-risk adolescents. Caries Res. 39(1):20–26. [DOI] [PubMed] [Google Scholar]
- Karjalainen S, Eriksson AL, Ruokola M, Toivonen A. 1994. Caries development after substitution of supervised fluoride rinses and toothbrushings by unsupervised use of fluoride toothpaste. Community Dent Oral Epidemiol. 22(6):421–424. [DOI] [PubMed] [Google Scholar]
- Karjalainen S, Söderling E, Sewón L, Lapinleimu H, Simell O. 2001. A prospective study on sucrose consumption, visible plaque and caries in children from 3 to 6 years of age. Community Dent Oral Epidemiol. 29(2):136–142. [DOI] [PubMed] [Google Scholar]
- Kruger E, Thomson WM, Poulton R, Davies S, Brown RH, Silva PA. 1998. Dental caries and changes in dental anxiety in late adolescence. Community Dent Oral Epidemiol. 26(5):355–359. [DOI] [PubMed] [Google Scholar]
- Larmas M. 2010. Has dental caries prevalence some connection with caries index values in adults? Caries Res. 44(1):81–84. [DOI] [PubMed] [Google Scholar]
- Lenkkeri AM, Pienihäkkinen K, Hurme S, Alanen P. 2012. The caries-preventive effect of xylitol/maltitol and erythritol/maltitol lozenges: results of a double-blinded, cluster-randomized clinical trial in an area of natural fluoridation. Int J Paediatr Dent. 22(3):180–190. [DOI] [PubMed] [Google Scholar]
- Li LW, Wong HM, McGrath CP. 2017. Longitudinal association between obesity and dental caries in adolescents. J Pediatr. 189:149–154. [DOI] [PubMed] [Google Scholar]
- Margolis MQ, Hunt RJ, Vann WF, Jr, Stewart PW. 1994. Distribution of primary tooth caries in first-grade children from two nonfluoridated US communities. Pediatr Dent. 16(3):200–205. [PubMed] [Google Scholar]
- Masood M, Yusof N, Hassan MI, Jaafar N. 2012. Assessment of dental caries predictors in 6-year-old school children—results from 5-year retrospective cohort study. BMC Public Health. 12:989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massler M, Pindborg JJ, Mohammed C. 1954. A compilation of epidemiologic studies in dental caries. Am J Public Health Nations Health. 44(10):1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejàre I, Stenlund H, Zelezny-Holmlund C. 2004. Caries incidence and lesion progression from adolescence to young adulthood: a prospective 15-year cohort study in Sweden. Caries Res. 38(2):130–141. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MV, Campain AC, Adams GG, Crowley SJ, Wright FA. 1998. The efficacy and effectiveness of a primary preventive dental programme in non-fluoridated areas of Victoria, Australia. Community Dent Health. 15(4):263–271. [PubMed] [Google Scholar]
- Peres MA, Sheiham A, Liu P, Demarco FF, Silva AE, Assunção MC, Menezes AM, Barros FC, Peres KG. 2016. Sugar consumption and changes in dental caries from childhood to adolescence. J Dent Res. 95(4):388–394. [DOI] [PubMed] [Google Scholar]
- Petersen PE, Peng B, Tai B, Bian Z, Fan M. 2004. Effect of a school-based oral health education programme in Wuhan City, Peoples Republic of China. Int Dent J. 54(1):33–41. [DOI] [PubMed] [Google Scholar]
- Pienihäkkinen K, Jokela J, Alanen P. 2004. Assessment of caries risk in preschool children. Caries Res. 38(2):156–162. [DOI] [PubMed] [Google Scholar]
- Poulsen VJ. 1987. Caries risk children in the Danish child dental service. Scand J Prim Health Care. 5(3):169–175. [DOI] [PubMed] [Google Scholar]
- Ruff RR. 2018. Total observed caries experience: assessing the effectiveness of community-based caries prevention. J Public Health Dent. 78(4):287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiken R, König K, Truin GJ, Plasschaert F. 1986. Longitudinal study of dental caries development in Dutch children aged 8–12 years. Community Dent Oral Epidemiol. 14(1):53–56. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pérez L, Irigoyen ME, Zepeda M. 2010. Dental caries, tooth eruption timing and obesity: a longitudinal study in a group of Mexican schoolchildren. Acta Odontol Scand. 68(1):57–64. [DOI] [PubMed] [Google Scholar]
- Schmoeckel J, Santamaría RM, Splieth CH. 2015. Long-term caries development in schoolchildren and the role of educational status. Quintessence Int. 46(5):409–415. [DOI] [PubMed] [Google Scholar]
- Sheiham A, Sabbah W. 2010. Using universal patterns of caries for planning and evaluating dental care. Caries Res. 44(2):141–150. [DOI] [PubMed] [Google Scholar]
- Sköld L, Sundquist B, Eriksson B, Edeland C. 1994. Four-year study of caries inhibition of intensive Duraphat application in 11-15-year-old children. Community Dent Oral Epidemiol. 22(1):8–12. [DOI] [PubMed] [Google Scholar]
- Sköld UM, Lindvall AM, Rasmusson CG, Birkhed D, Klock B. 2001. Caries incidence in adolescents with low caries prevalence after cessation of weekly fluoride rinsing. Acta Odontol Scand. 59(2):69–73. [DOI] [PubMed] [Google Scholar]
- Swedberg Y, Fredén H, Norén JG. 1997. Caries extreme groups among adolescents, leaving organised dental care in Goteborg, Sweden. Swed Dent J. 21(6):221–226. [PubMed] [Google Scholar]
- Swedberg Y, Fredén H, Norén JG, Johnsson T. 1997. On longitudinal caries index data. A comparison study between cohort and cross-sectional attempts. Swed Dent J. 21(5):205–211. [PubMed] [Google Scholar]
- Tai BJ, Jiang H, Du MQ, Peng B. 2009. Assessing the effectiveness of a school-based oral health promotion programme in Yichang City, China. Community Dent Oral Epidemiol. 37(5):391–398. [DOI] [PubMed] [Google Scholar]
- ten Cate JM. 2013. Contemporary perspective on the use of fluoride products in caries prevention. Br Dent J. 214(4):161–167. [DOI] [PubMed] [Google Scholar]
- Tickle M, O’Neill C, Donaldson M, Birch S, Noble S, Killough S, Murphy L, Greer M, Brodison J, Verghis R, Worthington HV. 2017. A randomized controlled trial of caries prevention in dental practice. J Dent Res. 96(7):741–746. [DOI] [PubMed] [Google Scholar]
- Truin GJ, van ‘t Hof MA. 2005. Professionally applied fluoride gel in low-caries 10.5-year-olds. J Dent Res. 84(5):418–421. [DOI] [PubMed] [Google Scholar]
- van Palenstein Helderman WH, Mikx FH, van ‘t Hof MA, Truin G, Kalsbeek H. 2001. The value of salivary bacterial counts as a supplement to past caries experience as caries predictor in children. Eur J Oral Sci. 109(5):312–315. [DOI] [PubMed] [Google Scholar]
- van Rijkom HM, Truin GJ, van ‘t Hof MA. 2004. Caries-inhibiting effect of professional fluoride gel application in low-caries children initially aged 4.5–6.5 years. Caries Res. 38(2):115–123. [DOI] [PubMed] [Google Scholar]
- Vermaire JH, Poorterman JH, van Herwijnen L, van Loveren C. 2014. A three-year randomized controlled trial in 6-year-old children on caries-preventive strategies in a general dental practice in the Netherlands. Caries Res. 48(6):524–533. [DOI] [PubMed] [Google Scholar]
- Virtanen JI. 2001. Changes and trends in attack distributions and progression of dental caries in three age cohorts in Finland. J Epidemiol Biostat. 6(4):325–329. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2012. Oral Health information sheet [accessed 5 Apr 2019]. https://www.who.int/oral_health/publications/en/.
- Zimmer S, Robke FJ, Roulet JF. 1999. Caries prevention with fluoride varnish in a socially deprived community. Community Dent Oral Epidemiol. 27(2):103–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034519847953 for Caries Progression Rates Revisited: A Systematic Review by R. Hummel, N.A.E. Akveld, J.J.M. Bruers, W.J.M. van der Sanden, N. Su and G.J.M.G. van der Heijden in Journal of Dental Research