Abstract
The authors report a case of a germinoma of the brain in the child with symptoms restricted to central nervous system. Ten-year-old girl presented initially with sight deterioration, learning difficulties, abnormal behavior, polydipsia, and polyuria. Brain magnetic resonance examination revealed T2 hyperintensity of the corpus callosum, anterior commissure, and caudate nuclei. Brain biopsy revealed extensive macrophage infiltration. Given these results and positive antinuclear antibodies in the blood, immunosuppressive and immunomodulatory treatment was implemented but it was not effective. The patient developed progressive quadriparesis, sleep disturbances, and dementia. Second brain biopsy was performed and it revealed germinoma cells. Chemotherapy was administered, but the girl died due to disseminated intravascular coagulation syndrome. The reported case shows an unusual coexistence of germinoma with prominent inflammation in the brain and highlights the importance of brain biopsy in such complex cases.
Keywords: germ cell tumor, diabetes insipidus, brain biopsy
Germinoma is one of the intracranial germ cell tumors. Intracranial germ cell tumors occur in 2% to 4% pediatric brain tumors1–7 and are more frequently diagnosed in Japan, Taiwan, and Korea (11%-15% of children brain tumors). There is a strong male predominance: 1.8:1 in patients with intracranial germinomas.1–3,5–7 The World Health Organization recognizes 5 histological subtypes of these tumors: germinoma, teratoma, choriocarcinoma, yolk sac tumor, and embryonal carcinoma. They can also present as mixed type of tumor.5,7 Among these neoplasms, germinoma is the most common one.4,7 Thay can be divided into pure germinomas developing in older children and germinomas with syncytiotrophoblastic giant cells.2–4,7 Most commonly, they grow as midline lesions around the third ventricle, especially pineal gland and the neurohypophyseal region of the brain. Suprasellar tumors are more common in females (75%), and pineal region tumors are more specific for males (70%).1,3,4,7 In about 5% of patients, germinomas emerge both in pineal and suprasellar region and are called dual intracranial germinoma.4,6,7 Germinomas localized in off-midline structures such as tumors of basal ganglia, thalami and internal capsule comprise 5% to 10% of intracranial germ cell tumors. Tumors localized in both midline and off-midline structures are very rare.6,7 Other classification divides germ cell tumors into nonsecreting and secreting with an elevated cerebrospinal fluid α-fetoprotein and/or β-human chorionic gonadotropin levels. These laboratory abnormalities do not always correlate with elevated serum levels of α-fetoprotein and/or β-human chorionic gonadotropin.4,6,7 Germinoma may present significant diagnostic problems, as it can mimic other tumors or inflammatory diseases.
Case Report
Our patient was a hitherto healthy 10-year-old girl, who presented with visual acuity deterioration and behavioral problems such as learning difficulties and tendency to lie reported by her parents for 3 months. She also developed polydipsia and polyuria. She had a family history of epilepsy and inflammatory diseases of retina and thyroid gland. In this period, she was admitted to the local hospital 2 times because of episodes of fever with dehydration and delusions. Electroencephalogram disclosed focal changes. Despite no infection confirmation, antibiotics were used, but fever and elevated white blood cells count did not respond to therapy. She was also diagnosed with optic nerve atrophy. After the second episode, she was transferred to our hospital for further investigation. On admission, the patient presented with tawny skin, decreased visual acuity, discrete nystagmus, and brisk tendinous reflexes. Laboratory examination showed elevated platelet count, lactate dehydrogenase, low urine weight, and positive antinuclear antibodies (ANA) in the serum. Her parents reported progressive memory loss, polydipsia, polyphagia, and polyuria. Further survey revealed paroxysmal tachycardia on electrocardiograph, abnormal visual evoked potentials, and serum hypernatremia. Brain magnetic resonance imaging (MRI) showed T2 hyperintensity of the corpus callosum, anterior commissure, and caudate nuclei and reduced volume of the basal ganglia. Abnormal contrast enhancement of the optical chiasm and slight enlargement of the lateral ventricles was also visible (Figure 1). Given that her growth rate was reduced. Endocrinological examinations revealed reduced level of insulin-like growth factor 1. Cortisol, adrenocorticotropic hormone, and thyroid hormones levels were normal. She was diagnosed with diabetes insipidus and treated with desmopressin with some improvement in polydipsia and polyuria symptoms. Antineuronal antibodies in serum were not present. In the detailed laboratory workout, no metabolic disorder was found. Cerebrospinal fluid was positive for ANA and there were also oligoclonal bands present, as well as slightly elevated β-human chorionic gonadotropin (1.22 mIU/mL, norm: <0.1 mIU/mL). Flow cytometry of cerebrospinal fluid showed numerous but normal appearing lymphocytes and macrophages.
Figure 1.
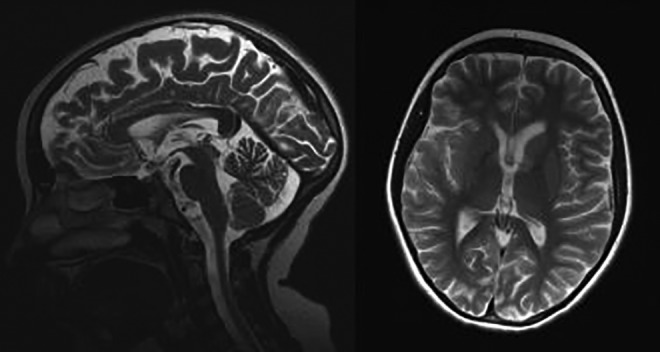
Brain magnetic resonance imaging (MRI) of the patient performed at the age 10 years. T2-weighted images. A, Abnormal, high signal intensity of the anterior part of the corpus callosum is seen. B, The volume of the basal ganglia is asymmetric and reduced with the hyperintense part of the left head of the caudate nuclei.
In the following weeks, the neurological status of the patient kept deteriorating. Follow-up brain MRIs disclosed progression of the lesions, and in follow-up, cerebrospinal fluid showed β-human chorionic gonadotropin level higher than in the previous examination (3.96 mIU/mL, norm: <0.1 mIU/mL). At that time, elevated angiotensin-converting enzyme in serum was found, and she was suspected for having neurosarcoidosis. The patient received methylprednisolone followed with prednisone, together with azathioprine. Despite the treatment, the symptoms of the disease progressed, and within months the girl became blind and tetraparetic. She underwent brain biopsy, but nonspecific activation of macrophages and no malignant cells were found (Figure 2). Glial fibrillary acidic protein and CD68 were positive, Ki-67 was negative. Steroids were withdrawn and immunomodulatory therapy with mycophenolate mofetil, cyclophosphamide, and eventually rituximab were administered without any improvement. Repeated flow cytometry of the cerebrospinal fluid did not confirm the presence of neoplastic cells. At this stage, there were ANA and double-stranded DNA antinuclear antibodies positive in the serum. Alpha-fetoprotein in both serum and cerebrospinal fluid was negative, and β-human chorionic gonadotropin was slightly elevated in both (in serum 0.53, norm: <0.1, in cerebrospinal fluid: 9.76, norm: <0.1). Test for John Cunningham Virus was negative.
Figure 2.
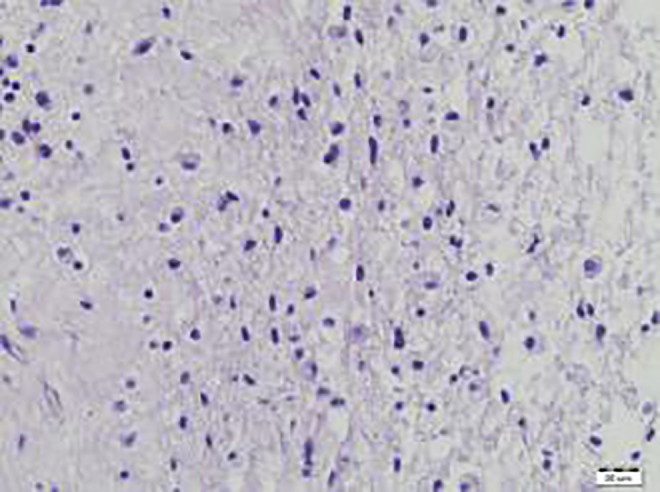
First stereotactic biopsy: no neoplastic cells. Numerous macrophages visible.
She was suspected as having histiocytosis but CD1a marker was negative and she did not fulfill diagnostic criteria for this neoplasm. The patient neurological status was very severe: She was blind, unable to speak, tetraparetic with pyramidal, and extrapyramidal signs. Eighteen months after the onset of the symptoms, the follow-up serum examination revealed elevated α-fetoprotein level and brain MRI showed significant progression with new focal lesions with contrast enhancement suggesting neoplastic disease (Figure 3). The patient had another brain biopsy in which pure germinoma cells were found (Figure 4). She received chemotherapy with visible response on MRI (Figure 5); however, she developed serious adverse events: pancytopenia and renal failure followed with fatal disseminated intravascular coagulation. On autopsy, significant brain atrophy and no evidence of tumor were found (Figures 6 and 7).
Figure 3.
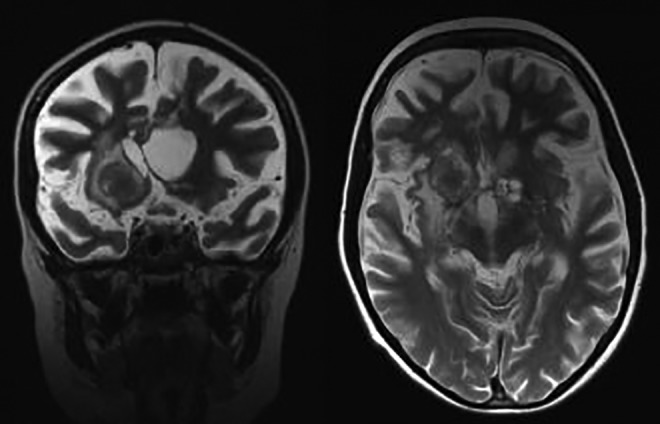
Magnetic resonance imaging (MRI) examination performed 18 months later, after rituximab administration. T2-weighted images. Focal hyperintense lesion in the right cerebral hemisphere and small cystic lesions on the left side. Note diffuse cerebral atrophy. Progression of lesions.
Figure 4.
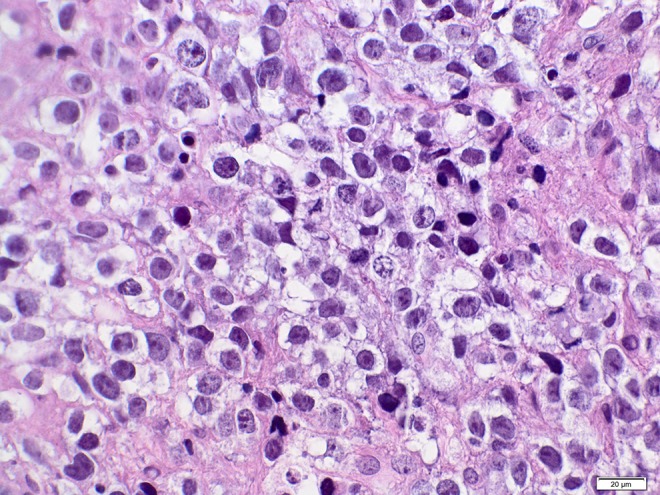
Second brain biopsy: H&E staining. Germinoma with large, anaplastic.
Figure 5.
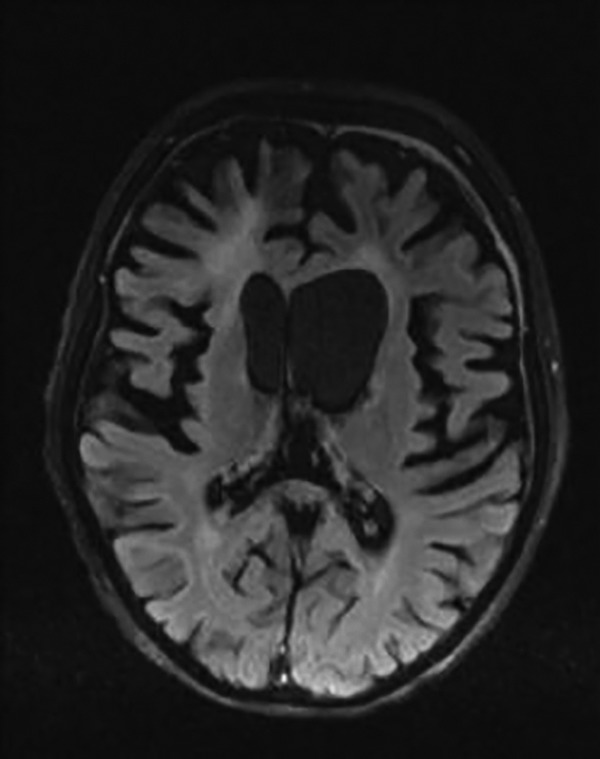
Brain magnetic resonance imaging (MRI) performed after second chemotherapy course. Axial FLAIR image. Regression of the right focal lesion and the cerebral atrophy in both gray and white matter more prominent in the front parts of the brain. Hyperintense signal of the frontal white matter. Chronic, subdural hematoma in the frontal regions bilaterally.
Figure 6.
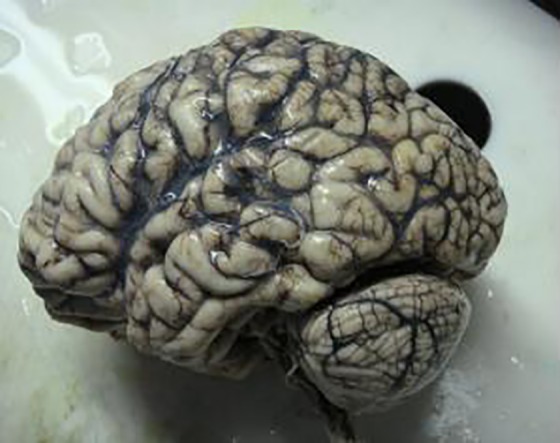
Autopsy. Brain of the patient with visible atrophic changes.
Figure 7.
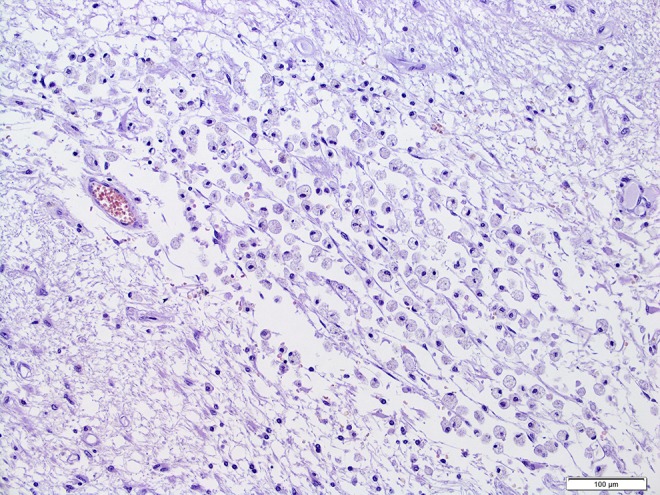
Post-autopsy histopathology. Necrotic tissue with numerous macrophages and no presence of neoplastic cells.
Discussion
Intracranial germinomas are rare and may create diagnostic difficulties, especially if laboratory tumor biomarkers are not significantly elevated. In the reported case, the laboratory findings and the first brain biopsy were inconclusive. The authors presume that germinoma was the first pathology in the patient and produced extensive brain inflammation that masked small tumor for a long time. However, the authors cannot exclude that intensive immunosuppressive and immunomodulatory treatment influenced the natural history of germinoma or even promoted its development.
There are few hypotheses on how germ cell tumors appear in central nervous system. According to Teilum germ cell hypothesis, germ cell progenitors mismigrate during early embryogenesis and remain in midline structures. When local environment develops favorable conditions they may proliferate and form tumors.4,7 Embryonic cell theory says that a mismigrational pluripotent embryonic cell gives rise to germ cell tumors. Some scientists believe that only pure germinomas arise from germ cells and other germ cell tumors develop secondary to misfolding and misplacement of embryonic cells into the lateral mesoderm. Such cells may become entrapped in different brain regions.4,7 There are also some reports showing that germ cell tumors in fact do not originate from germ cell progenitors but from neural stem cells with overexpression of Oct4 gene. Oct4 promotes pluripotency. However, none of these hypotheses is widely accepted and it is still not clarified how those neoplasms occur in extragonadal localization.3,7,8 In patients with intracranial GCT mutations in the KIT/RAS signaling pathway (50% of cases) and the AKT/mTOR pathway (19% of cases) were found.4
The clinical signs of germinoma depend on its localization. Tumors developing in suprasellar region produce symptoms that can develop slowly. These symptoms include hormonal deficiencies, psychiatric changes, and visual loss associated with optic chiasma compression when tumor expands more dorsally. Diabetes insipidus is a common symptom for suprasellar tumors. Parinaud syndrome may appear when tumor is present in posterior third ventricular region. When tumors are bifocal or multifocal, symptoms may overlap.4–7,9 Tumors located in pineal region usually develop clinical symptoms earlier in time, as they may produce increased intracranial pressure, diplopia, tremor, altered pupillary reflexes, hormonal disturbances, seizures, and ataxia. Diabetes insipidus can also occur. In our patient, nonspecific symptoms such as progressive behavioral changes and visual loss preceded diabetes insipidus and thermoregulation disturbances. Together with reduced growth rate they implied hypothalamus and pituitary disorder.
The laboratory biomarkers of germ cell tumors include elevated α-fetoprotein and β-human chorionic gonadotropin in both serum and cerebrospinal fluid. However, they are not specific and can be present also in embryonal cell carcinoma, teratoma, yolk sac tumor, and choriocarcinoma. In pure germinomas, high β-human chorionic gonadotropin level is rare and elevated α-fetoprotein level is not observed.2,4,10 In the reported case, the initial levels of α-fetoprotein and β-human chorionic gonadotropin were slightly abnormal and then increased over time. Eventually they were high, which is not a typical finding in a pure germinoma. It cannot be excluded that the biopsy did not represent the whole tumor and in fact it was composed of other pathological cells. This hypothesis could not be verified because in the autopsy, performed after chemotherapy there was no neoplasm.
Tumor biopsy is still a gold standard in germinoma diagnostics. In the reported case, first biopsy revealed no neoplastic cells and abundant infiltration of macrophages. Pure germinoma are typically infiltrated by T lymphocytes that generate the 2-cell pattern in histopathology. Likely, in our case T helper lymphocytes attracted macrophages to migrate into lesion. This finding, however, together with oligoclonal bands and lymphocytes as well as macrophages present in cerebrospinal fluid led to the initial misdiagnosis of inflammatory disease. Recurrent fevers and positive ANA antibodies supported the diagnosis. Moreover, our patient cerebrospinal fluid cytology didn’t reveal neoplastic cells, probably because there were no metastases.4 There are reports on germinoma mimicking inflammatory process,11–13 sometimes with clinical improvement after anti-inflammatory drugs administration.12 On the other hand, lymphocytic reaction seen in germinomas is thought to be protective and reduction in lymphocytic infiltration with for example steroids might induce tumor growth.14 It cannot be excluded that such phenomenon was at least partly associated with the deterioration of the clinical status of our patient after immunomodulatory agents.
Standard treatment of germinoma includes radiotherapy and chemotherapy. Surgery is rarely performed. The outcome of germinoma is relatively good: 90% survival rate after 5 years and 80% after 20 years following radiotherapy were reported.4,7 In our patient, chemotherapy resulted in total tumor remission; however, the patient developed fatal adverse events. It cannot be excluded that the production of the inflammatory response to neoplasm, together with the preceding intensive immunosuppressive treatment resulted in an increased patient’s vulnerability to toxicity of chemotherapy.
In conclusion, our case illustrates the need for extensive clinical workout toward germ cell tumors in patients presenting neurological symptoms of brain midline lesion, especially when symptoms of diabetes insipidus are present.
Footnotes
Authors’ Contribution: All authors contributed to conception and design; acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Joo-Young Kim MD, Jeonghoon P. Understanding the treatment strategies of intracranial germ cell tumors: Focusing on radiotherapy. J Korean Neurosurg Soc. 2015;57(5):315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fukushima S, Otsuka A, Suzuki T, et al. Mutually exclusive mutations of KIT and RAS are associated with KIT mRNA expression and chromosomal instability in primary intracranial pure germinomas. Acta Neuropathol. 2014;127(6):911–925. [DOI] [PubMed] [Google Scholar]
- 3. Thakar S, Furtado SV, Ghosal N, Hegde AS. Intracranial germ cell tumor mimicking granulomatous inflammation. Neurol India. 2013;61(4):433–434. [DOI] [PubMed] [Google Scholar]
- 4. Takami H, Fukushima S, Fukuoka K, et al. Human chorionic gonadotropin is expressed virtually in all intracranial germ cell tumors. J Neurooncol. 2015;124(1):23–32. [DOI] [PubMed] [Google Scholar]
- 5. Tan C, Scotting PJ. Stem cell research points the way to the cell of origin for intracranial germ cell tumours. J Pathol. 2013;229(1):4–11. [DOI] [PubMed] [Google Scholar]
- 6. Gupta R, Songara A. Management of dual intracranial germinoma by radiotherapy alone. J Pediatr Neurosci. 2015;10(1):38–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Battum P, Huijberts MS, Heijckmann AC, Wilmink JT, Nieuwenhuijzen Kruseman AC. Intracranial multiple midline germinomas: is histological verification crucial for therapy? Neth J Med. 2007;65(10):386–389. [PubMed] [Google Scholar]
- 8. Echevarría ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: a review. Oncologist. 2008;13(6):690–699. [DOI] [PubMed] [Google Scholar]
- 9. National Cancer Institute. PDQ® Childhood Central Nervous System Germ Cell Tumors Treatment. Bethesda, MD: National Cancer Institute. Date last modified; 2015. http://www.cancer.gov/types/brain/hp/child-cns-germ-cell-treatment-pdq. Accessed October 22, 2015. [Google Scholar]
- 10. Wanggou S, Jiang X, Li Q, et al. HESRG: a novel biomarker for intracranial germinoma and embryonal carcinoma. J Neurooncol. 2012;106(2):251–259. [DOI] [PubMed] [Google Scholar]
- 11. Mascalchi M, Roncaroli F, Salvi F, Frank G. Transient regression of an intracranial germ cell tumour after intravenous steroid administration: a case report. J Neurol Neurosurg Psychiatry. 1998;64(5):670–672. doi:10.1136/jnnp.64.5.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manoj PR, Saad AF, Doughty KE, et al. Intracranial germinoma. Proc (Bayl Univ Med Cent). 2015;28(1):43–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Strowd RE, Burger P, Holdhoff M, et al. Steroid-responsive intracranial germinoma presenting as Holmes’ tremor: importance of a tissue diagnosis. J Clin Neurosci. 2015;22(5):911–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Loto MG, Danilowicz K, González Abbati S, Torino R, Misiunas A. Germinoma with involvement of midline and off-midline intracranial structures. Case Rep Endocrinol. 2014;2014:936937. [DOI] [PMC free article] [PubMed] [Google Scholar]


