Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is the treatment of choice for a large number of malignant and nonmalignant (inherited) diseases of the hematopoietic system. Nevertheless, non‐HLA identical transplantations are complicated by a severe T‐cell immunodeficiency associated with a high rate of infection, relapse and graft‐versus‐host disease. Initial recovery of T‐cell immunity following HSCT relies on peripheral expansion of memory T cells mostly driven by cytokines. The reconstitution of a diverse, self‐tolerant, and naive T‐cell repertoire, however, may take up to 2 years and crucially relies on the interaction of T‐cell progenitors with the host thymic epithelium, which may be altered by GvHD, age or transplant‐related toxicities. In this review, we summarize current concepts to stimulate reconstitution of a peripheral and polyclonal T‐cell compartment following allogeneic transplantation such as graft manipulation (i.e., T‐cell depletion), transfusion of ex vivo manipulated donor T cells or the exogenous administration of cytokines and growth factors to stimulate host‐thymopoiesis with emphasis on approaches which have led to clinical trials. Particular attention will be given to the development of cellular therapies such as the ex vivo generation of T‐cell precursors to fasten generation of a polyclonal and functional host‐derived T‐cell repertoire. Having been tested so far only in preclinical mouse models, clinical studies are now on the way to validate the efficacy of such T‐cell progenitors in enhancing immune reconstitution following HSCT in various clinical settings. stem cells translational medicine 2019;00:1–8
Keywords: Cellular therapy, Hematopoietic stem cell transplantation, Immunodeficiency, Immune reconstitution, T‐cell, Thymus, Hematologic malignancies
Significance Statement.
Prolonged T‐cell immunodeficiency following allogeneic transplantation is a major clinical problem leading to high rate of infectious complications and disease relapse. The present article discusses current strategies targeted to enhance immune reconstitution post‐HSCT. In particular, this review emphasizes the importance of cellular therapies such as the injection of ex vivo generated T‐cell progenitors to accelerate immune reconstitution following transplantation as this approach confers a polyclonal host repertoire without the risk of alloreactivity.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is the standard treatment for many malignant and nonmalignant hematopoietic disorders. Complications include conditioning regimen‐related toxicities, infections, relapse, and graft‐versus‐host disease (GvHD).
Conventional T‐cell depletion of the graft by CD34+ cell selection, post‐transplant immunosuppression or use of immediate post‐transplant cyclophosphamide (PTCY) in case of T‐cell replete HSCT have enabled HSCT from partially human leukocyte antigen (HLA)‐mismatched or even haploidentical donors to become a valid therapeutic option for patients lacking an HLA‐matched sibling or matched unrelated donor 1, 2, 3. In recent years, advances in graft handling such as partial depletion of certain T‐cell subsets and ex vivo manipulation of donor T cells have further improved the feasibility of haploidentical HSCT 4, 5. However, despite improving transplant‐related toxicity such as reducing the risk of GvHD, all these approaches negatively impact on restoration of immunity; the profound immunodeficiency following this type of HSCT remains one of the major challenges exposing patients to infectious complications and relapse.
After allogeneic HSCT, innate immunity normally recovers within weeks to few months after allogeneic HSCT, whereas the reconstitution of an adaptive immune response is a much longer process 6, 7. In particular, qualitative and quantitative reconstitution of a functional T‐cell compartment may take up to 2 years, although it is of key importance due to the critical role of T cells in the defense against opportunistic pathogens and as mediators of graft‐versus‐tumor immune responses. It is therefore not surprising, that early immune recovery following HSCT is associated with lower risk of infection and relapse rates resulting in a lower incidence of transplant‐related complications and more favorable outcome for both pediatric and adult patients 8, 9, 10. In particular, the early acquisition of a CD4+ T‐cell compartment following HSCT (defined as >50 per microliter CD4 T cells at day 100 or >200 per microliter CD4+ CD45RA+ naive T cells at 6 months post‐transplantation) is associated with faster clearance of viremia and improvement of overall survival 11, 12, 13. Conversely, the presence of infection seems to have a negative effect on the regeneration of T‐cell immunity following allogeneic HSCT 12.
Besides infection, T‐cell reconstitution following HSCT may be influenced by several other factors such as the presence of GvHD (damage of the thymic epithelium and priming of alloreactive T cells), leukemia relapse and several pretransplant parameters (e.g., HLA mismatch, conditioning regimen, graft type, infectious status of donor and recipient) 14, 15, 16. In addition, age‐related changes such as thymic involution and disrupted thymic architecture lead to a decreased thymic output 15, 16.
In light of the above, strategies to boost T‐cell reconstitution following allogeneic HSCT are urgently needed in order to re‐enforce immune competence against pathogens or malignant cells especially in the lymphodepleted setting. The present article will discuss current concepts and future perspectives to enhance/accelerate post‐transplant T‐cell reconstitution and thymus function with a particular focus on cellular therapies such as the use of T‐cell precursors to fasten the regeneration of a polyclonal T‐cell immunity following allogeneic HSCT.
T Cell Differentiation in Humans
In mice and humans, the production of T cells follows a defined series of differentiation steps starting from pluripotent hematopoietic stem cells in the bone marrow, which then give rise first to multipotent progenitors and subsequently to multilymphoid (multipotent) progenitors (MLP) before entering the circulation. Once released into the periphery, they become committed to the lymphoid pathway and susceptible to homing signals from the thymus, the crucial organ for coordinating the differentiation and education of a functional and polyclonal T‐cell repertoire. Within the thymus, MLPs commit to T‐cell differentiation under the influence of Notch signals provided by the thymic epithelium 17. Differentiation steps include the sequential acquisition of CD7 (early thymic precursor, ETP), CD5 (pro‐T), and CD1a (pre‐T) surface markers as well as the progressive loss of surface CD34. At the molecular level, T lineage commitment is marked by the gradual downregulation of early lymphoid development and B‐cell genes (e.g., IKAROS, MYB, and PAX5) as well as the induction of crucial T‐lineage differentiation genes, such as BCL11B, IL7RA, and TCF7 18.
After commitment to the T‐cell lineage, progenitors undergo rearrangements of the δ, γ, and α/β T‐cell receptor (TCR) loci leading to either the production of γδ TCR+ T cells (<5% of the peripheral T‐cell compartment) or the production of CD4+ CD8+ TCRαβ+ double positive (DP) cells. These cells then undergo positive and negative selection processes orchestrated, respectively, by the cortical and medullary thymic microenvironments in order to produce a polyclonal, HLA‐restricted and self‐tolerant αβ+ T‐cell repertoire 19.
T‐Cell Reconstitution in Allogeneic Transplantation
In any type of HSCT, T‐cell reconstitution occurs through two distinct pathways. The early post‐transplant period is marked by peripheral expansion of either donor T cells in the graft or recipient T cells that survive conditioning. This cytokine‐dependent expansion occurs predominantly in the CD8+ memory T‐cell population and leads to a contraction and skewing of the TCR repertoire, thus producing an ineffective immune response 20. In contrast, complete reconstitution of the T‐cell repertoire relies on a second pathway, in which de novo production and education of naive T cells in the thymus of the recipient takes place. During this process, the thymus is seeded by rare MLPs present in the graft or arising from donor hematopoietic stem cells. T‐cell development is subsequently tightly regulated by the bi‐directional crosstalk between thymic stromal cells and developing thymocytes. Conditioning regimens, infections, chronic inflammation, GvHD, and age may damage or alter the thymic epithelium and thereby hamper the reconstitution of a functional T‐cell repertoire 12, 16, 17.
In CD34+ selected haploidentical transplantation, this first wave of homeostatically expanded CD8+ T cells is virtually absent and T‐cell reconstitution completely relies on the production of a naive T‐cell repertoire. Especially CD4+ T cells, which in general reconstitute later than CD8+ T cells, are dependent on thymic generation of CD4+ CD45RA+ naive T cells, which also explains the reported inversion of CD4/CD8 ratios following HSCT 21. TCR excision circles (TRECs), a surrogate parameter for production of thymic derived naive T cells, remain low until 3–6 months following allogeneic HSCT 20. Even in young patients (i.e., most of those transplanted for severe combined immunodeficiency, naive T cells appear in the blood after 3–6 months 22, 23 post‐HSCT. A period of 6–12 months is required to achieve CD4+ cell counts that provide protective immunity. More complete restoration of the overall T‐cell compartment (i.e., naive T cells exhibiting a polyclonal TCR repertoire) is an even longer process and may take up to 2 years 6, 7.
Clinical therapies to stimulate T‐cell production or expansion can be divided into two categories: (a) direct restoration of the peripheral T‐cell compartment and (b) enhancement of a naive T‐cell production by the host thymic environment (Fig. 1).
Figure 1.
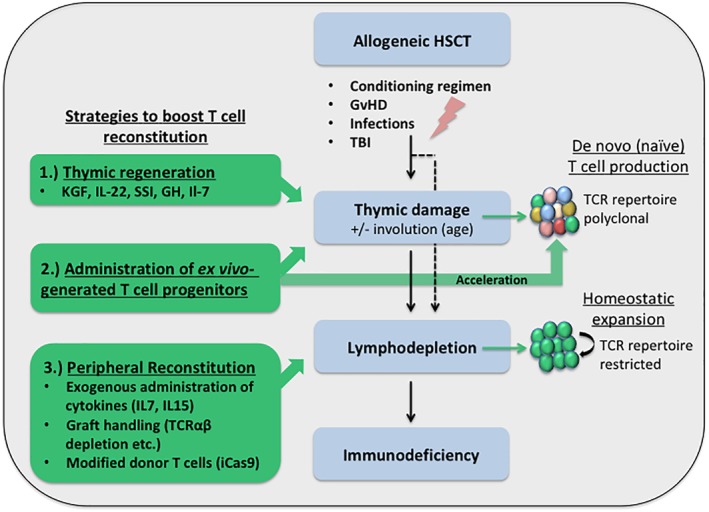
Strategies to accelerate T‐cell reconstitution following allogeneic‐hematopoietic stem cell transplantation (HSCT). Immunological consequences of allogeneic HSCT are depicted in blue boxes. The differential strategies to overcome immunodeficiency following allogeneic‐HSCT are shown on the left in green. Their respective impact on the reconstitution of the T‐cell repertoire is shown on the right side of the diagram.
Restoring the Peripheral T‐Cell Compartment
Homeostatic expansion of donor‐derived T cells in the peripheral blood after HSCT has been demonstrated to rely on the presence of cytokines such as interleukin‐7 (IL‐7) 24. IL‐7 is primarily produced by thymic epithelial cells and its receptor (IL7R‐α) is expressed at all stages of lymphocyte development 25. The importance of IL‐7 signals for proliferation and survival of lymphocytes is highlighted by the fact that the presence of mutations in the IL‐7R α chain leads to severe combined immunodeficiency syndromes characterized by the complete absence of T lymphocytes 26. In addition, aberrant expression of IL‐7R is frequently found in precursor T‐ and B‐cell leukemia 27, 28 and IL‐7 serum levels increase during stages of T‐cell depletion 29. Results on exogenous administration of IL‐7 following allogeneic transplantation in mice and nonhuman primates have been controversial with some demonstrating an effect on thymopoiesis and functional T‐cell recovery 30, 31, 32, and others proving no effect on thymic regeneration 33.
Interleukin 15 (IL‐15), another member of the common γ chain cytokine family, has equally been shown to improve homeostatic proliferation of T cells and reconstitution of T‐cell immunity in mice 34. However, both IL‐7 and IL‐15 act primarily on expansion of the CD8+ T‐cell compartment 35, and have been shown to enhance acute GvHD even following T‐cell‐depleted allogeneic transplantation in mice due to their impact on proliferation of circulating alloreactive T cells 36, 37. Phase I trials of recombinant human IL‐7 (r‐hIL‐7) in patients with solid tumors or HIV infection reported an increase of peripheral CD4 and CD8 T cells as well as an expansion of recent thymic emigrants (RTEs), naive and central memory, but not effector T cells 38, 39, 40. In contrast, the administration of r‐hIL‐7 in a phase I clinical trial including 12 patients receiving T‐cell‐depleted allogeneic HSCT for malignant hematologic disorders revealed an effect on the expansion of effector memory T cells only 41.
With reference to cellular therapies targeting the peripheral T‐cell compartment, manipulation of the stem cell graft and donor‐lymphocyte infusions (DLIs) historically provide a well‐established way to influence T‐cell immunity and antitumor effects following allogeneic transplantation. However, it is well‐known that the administration of nonmanipulated donor lymphocytes is complicated by high rates of GvHD 37, 42. On the other hand, nonspecific T‐cell depletion of allogeneic grafts is complicated by high rates of graft rejection, delayed engraftment, and higher risk of relapse due to the loss of graft‐versus‐leukemia (GvL) effects 43, 44, 45, 46.
To overcome this imbalance, advances in graft‐handling and optimization of conventional DLI infusions use depletion of alloreactive T‐cell subsets and/or enrichment of tumor‐directed lymphocytes with the aim of protecting preferred T‐cell subsets to maintain graft‐versus‐tumor and antiviral effects of T‐cell deplete grafts while reducing the risk of GvHD in T‐cell replete grafts.
Recent advances in graft‐handling strategies include the selective depletion of TCRα/β lymphocytes allowing for enrichment of a (donor‐derived) γ/δ innate‐like population conferring an improved anti‐infective and antitumor response in an HLA‐nonrestricted manner. This approach is currently being tested in several clinical trials in the haploidentical transplantation setting 47, 48, 49, 50.
CD45RA+ naive alloreactive T cells previously unexposed to allogeneic stimuli have been shown to be responsible for GvH reactions 51. Consequently, strategies to deplete the CD45RA+ naive T‐cell subset in the graft via magnetic beads have been developed allowing for the preservation of functional memory T cells specific for viral and fungal antigens 52. Clinical trials have shown a slight reduction in the incidence of chronic GvHD and rapid T‐cell recovery following transplantation of these grafts. 53, 54. However, more studies are needed to prove the safety and efficacy of this approach.
Modification of DLIs such as the deletion of alloreactive T cells from the donor lymphocyte graft via immunotoxins, reagents reacting with activation markers such as CD25 55, 56 or a special photodepletion technique (ATIR101, Kiadis) represent a other options to reduce alloreactivity while sparing antitumor T cells to preserve GvL functions 57. Currently, the safety and efficiency of such modified donor T‐cell infusions is being evaluated in comparison to the use of PTCY in the haploidentical transplantation setting (HATCY study NCT02999854 and NCT01827579).
In recent years, another interesting approach to strengthen the peripheral T‐cell compartment and thus reduce infection‐related mortality after allogeneic transplantation has been the administration of donor T cells armed with an inducible suicide gene (either HSV‐TK or iCas9) 58, 59. In the case of CD19‐transduced iCas9 T cells the coexpression of CD3 and CD19 surface markers allows for traceability in vivo. More importantly, alloreactive iCas9 T cells can be depleted in vivo upon injection of the small‐molecule drug AP1903. Compared with HSV‐TK modified cells, the iCas9 safety switch is less immunogenic and allows for faster T‐cell elimination in the case of GvHD 59. The safety and efficacy of iCas9 cell infusion in the haploidentical transplantation setting has been tested in two clinical trials. A phase I–II clinical trial in 112 pediatric patients transplanted with haploidentical, TCR α/β depleted grafts for malignant (leukemia) and nonmalignant hematologic disorders is currently ongoing (NCT02065869). Both trials report expansion and persistence of CD3+ CD19+ iCas9 cells over time in the host accompanied by detection of efficient antiviral responses (EBV, CMV, HHV6, VZV, and BKV) in infected patients and a fastened recovery of endogenous CD3+ CD19− T cells 59.
In addition to graft‐handling procedures and modified DLIs, post‐transplant cellular therapy approaches also include the adoptive transfer of virus specific T cells (VST). Initially, the manufacture of VST products included time‐consuming strategies such as ex vivo stimulation/culture to expand T cells targeting one or multiple viruses in a single product 60, 61. However, viral infection in recipients of allogeneic transplantation represents a medical emergency in which antiviral therapy needs to be initiated immediately. Therefore, recent studies have focused on rapid manufacturing strategies such as the direct selection of donor‐derived VSTs via leukapheresis on the basis of their binding of viral peptide/HLA tetramers or dissociable streptamers 62. An alternative, HLA unrestricted strategy is based on secretion of interferon‐γ (IFN‐γ) by VSTs after short‐term stimulation with peptide antigens followed by magnetic enrichment of IFN‐γ secreting cells 63, 64. Because a major reason for failure is lack of immunity to the infecting virus in a naive donor, more recent studies have infused closely matched third‐party VSTs. Such VST have been proven to be effective against EBV, CMV, adeno‐virus, and recently also HHV‐6 and BK‐virus infections without causing GvHD (response rates between 70% and 90%) 65. The advantages and disadvantages of each approach are summarized in recent reviews 66, 67 and will not be discussed here.
Major caveats of graft‐manipulation and post‐transplantation approaches are the development of a skewed TCR repertoire and the potential induction of a memory phenotype in expanded donor T cells all of which lead to the absence of a polyclonal host immunity. Furthermore, for the iCas9 system, AP1903‐induced deletion of the donor T cells in the case of GvHD immediately resets the immune status of the recipient. As a consequence, such transfer of donor‐derived immunity provides rather limited or only very specific protection (in the case of VSTs) against infections and may be insufficient to control the significant risk of relapse in the malignant setting.
Stimulating Thymic Regeneration and T‐Cell Output Post‐Transplantation
As mentioned above, attempts to provide modified donor immunity within the first months following transplantation result in a donor HLA‐restricted and skewed TCR repertoire. Whereas this might be sufficient with reference to antitumor effects in some cases, the absence of specific immunity against infections still harbors a significant risk. Especially in haploidentical transplantations particular attention should be given to the analysis of early immune‐reconstitution within the first 6 months following transplantation of HSCT, as a total number of >300 CD3 T cells at 3 months has been identified as a crucial milestone for survival by reduction of nonrelapse mortality 22.
Therefore, strategies to accelerate the reconstitution of a naive, self‐tolerant, and polyclonal host‐derived T‐cell repertoire following allogeneic transplantation by stimulating thymic niche and increasing thymic output (RTEs) are needed.
Several factors directly impact on proliferation of TECs, thymus homing potential of lymphoid progenitors and thymus cellularity after thymic injury in mice thereby enhancing T‐cell differentiation and thymic output, such as keratinocyte growth factor (KGF), sex‐steroid inhibition by luteinizing hormone‐releasing hormone‐agonists and the administration of growth hormone 68, 69, 70, 71. Mechanisms include direct activation of the FgfR2‐IIIb receptor on TECs and subsequent upregulation of NFk‐B and Wnt/BMP pathways for KGF, as well as upregulation of CCL25 and DLL4 for sex‐steroid inhibition 72, 73. Currently, clinical trials in the U.S. are evaluating the effect of Leuprolide alone or in combination with KGF on immune reconstitution in 118 adult patients undergoing allogeneic transplantation for hematologic malignancies (NCT01338987 and NCT01746849).
Recent data in mouse models of allogeneic transplantation have also established the role of interleukin‐22 (IL‐22) and RankL/lymphotoxin α signaling provided by innate cell populations such as lymphoid tissue inducer cells in the regeneration of the thymic microenvironment and T‐cell differentiation following thymic epithelial damage. Interestingly, exogenous administration of RankL was effective independently of age, making it also potentially applicable for older patients 74, 75, 76. The clinical impact of these factors has yet to be determined, especially in light of the potential side effects such as osteoporosis in the case of RankL 77.
Ex Vivo Generated T‐Cell Progenitors to Accelerate Reconstitution of T‐Cell Immunity Following Allogeneic HSCT
As mentioned earlier, T‐cell differentiation and selection in the host thymus is dependent on the continuous seeding of the latter by lymphoid progenitors. In the case of allogeneic HSCT, these progenitors derive from donor hematopoietic stem cells, which have to populate the bone‐marrow of the host to become MLPs before homing to the thymus via the periphery 78. Even in a healthy individual, this process is quite long lasting and might be hampered in the setting of allogeneic transplantation leading to prolonged post‐transplantation immunodeficiency. In particular, the CD4+ T‐cell compartment may take up to 2 years to recover 6, 7.
Hence, adoptive transfer of in vitro generated human T lymphoid precursors (HTLPs) provides a novel and promising approach to accelerate T‐cell reconstitution after HSCT. In this case, ex vivo culture conditions mimic the initial steps of lymphoid and T‐cell commitment normally occurring in the bone marrow and in the cortico‐medullary junction of the thymus.
Considering the known role of Notch signaling in T‐cell lineage differentiation in mice and humans, we have implemented a feeder cell‐free culture system for hematopoietic stem cells based on the immobilized Notch ligand DL‐4 and a cocktail of cytokines known to induce T‐cell differentiation and expansion in hematopoietic stem cells (for details on the experimental protocol, see Fig. 2) 79. This system allows for the in vitro generation of large amounts of HTLPs from different CD34+ hematopoietic stem cell sources. In recent years, we have extended and improved this protocol to differentiate and expand not only neonatal cord blood (CB) but also adult CD34+ cells from granulocyte‐colony stimulation factor (G‐CSF)‐mobilized peripheral blood (mPB) 80. mPB is currently the main source of HSPCs in allogeneic HSCT, as adult HSPCs are available in large quantity and exhibit several advantages over CB grafts in the clinical setting. Our culture system, therefore, exhibits major advantages over other recently described cell culture systems 81, which are restricted to the use of CB HSPCs and less efficient.
Figure 2.
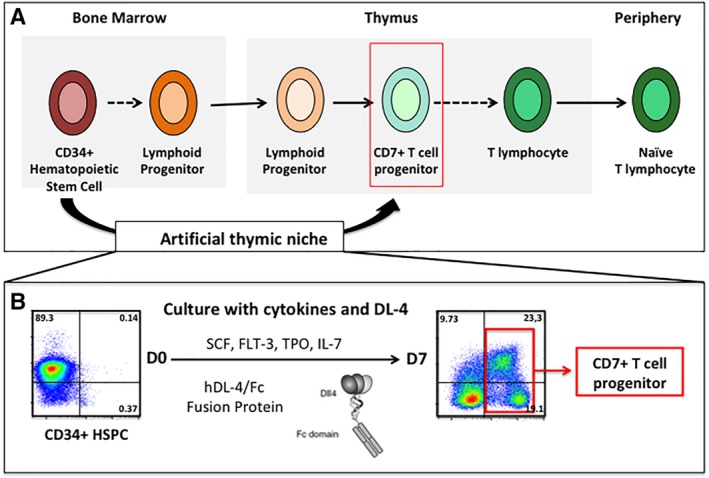
Ex vivo generation of human T lymphoid progenitors (HTLPs). (A): Human T‐cell development. (B): Experimental protocol for the 7‐day generation of CD7+ T‐cell precursors (=HTLPs) from hematopoietic stem and precursor cells (HSPCs) by coculture with an immobilized Notch ligand DL4 and a cocktail of cytokines. Specifically, CD34+ hematopoietic stem and progenitor cells are seeded on day 0 in culture wells coated with immobilized DL‐4/Fc fusion protein and Retronectin. After addition of cytokines (IL7, TPO, FLT‐3, and SCF) cells are cultured for 7 days with medium change at day 3. HTLPs on day 7 display an early ETP/pro‐T differentiation phenotype as shown by expression of CD7 and downregulation of CD34.
After 7 days of culture, HTLPs exhibit an early ETP/pro‐T differentiation stage as indicated by the surface expression of the T‐cell precursor marker CD7 and the concomitant downregulation of CD34 (Fig. 2). In addition, HTLPs express low levels of CD5 and no CD1a. Importantly, HTLPs express intracellular CD3e, GATA3, and Bcl11b, three factors implicated in T‐cell differentiation and physiologically expressed at the ETP/pro‐T‐cell stages. Transcriptional analysis of HTLPs revealed the expression of T lineage related genes, thymus homing and crosstalk genes as well as early lymphoid commitment genes. As expected, at this point of differentiation, HTLPs do not harbor any TCR rearrangements.
Using our HTLP culture system together with the OP9 DL‐1 stromal cell line in vitro, we were able to demonstrate that the kinetics of appearance of DP cells and mature T cells from HTLPs is accelerated by 3 weeks in comparison to noncultured HSCs. The putative thymus homing potential of HTLPs was confirmed in vivo upon transplantation into nonirradiated newborn NSG mice. Human thymic engraftment was greatly accelerated occurring at only 4 weeks in the mice injected with day 7 adult HTLPs and persisting thereafter (as compared with 12 weeks after injection of uncultured CD34+ selected HSCs). Active human thymopoiesis was further demonstrated by the presence of human CD4+ CD8+ DP cells and enlarged thymic lobes as compared with recipients of uncultured adult HSPCs 80. This data provided further evidence of the ability of in vitro‐generated HTLP to accelerate T‐cell reconstitution in vivo.
Based on this preclinical work, we have initiated a phase I/II clinical study evaluating the safety and efficacy of HTLP injection to accelerate immune reconstitution after haploidentical HSCT in SCID patients (EudraCT N°: 2018‐001029‐14). In this situation, the major obstacle to a successful outcome is the long‐lasting T‐cell immunodeficiency 82, 83.
The intended cellular therapy consists of the injection of in vitro‐committed T‐cell precursors (HTLPs) capable of accelerating the production of a mature and polyclonal T‐cell wave following haploidentical transplantation. Theoretically, once injected in vivo, these HTLPs should be capable of migrating to the thymus where they undergo further T‐cell differentiation and selection and interact with the thymic epithelium. The putative interaction between injected HTLPs and TECs will help to quickly restore a proper thymus architecture 16, which in turn will support not only T‐cell differentiation of HTLPs but also differentiation of MLPs generated from the noncultured primary CD34+ graft. Due to the fact that HTLPs do not harbor any TCR rearrangements at the time of injection they are susceptible to thymic maturation and selection processes in the host, which will allow for the generation of a polyclonal and self‐tolerant T‐cell repertoire without increasing the risk of GvHD. The injection of HTLPs directly after transplantation is expected to shorten the time required to get >300 CD3+ T cells per microliter in peripheral blood, a threshold below which the patients are at high risk of viral reactivation 22.
If successful in pediatric patients, administration of T‐cell progenitors to enhance immune reconstitution may also become available to adult patients with relapsed malignant diseases.
Conclusion
Despite numerous advances in graft‐handling and conditioning, delayed immune reconstitution still remains a major issue after partially HLA‐ mismatched HSCT because of its consequences in terms of relapses and infections. Various strategies are being explored and are at different stages of development, among which treatments by cytokines aiming at improving thymopoiesis or mature T‐cell based and T‐cell progenitor based cellular therapies. They all present advantages and disadvantages and deserve a rigorous comparison in the various indications before their inclusion in the conventional HSCT procedure either alone or in combination.
Author Contributions
L.S., M.C., I.A.: manuscript writing; final approval of the manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
This study was supported by the French National Institute of Health and Medical Research (INSERM), a European Research Council grant (ERC Regenerative Therapy, 269037), a European Union FP7 grant (CELL‐PID, 261387), a European Union H2020 grant (SCIDNet, 666908), Imagine Institute, and a public grant overseen by the French National Research Agency (ANR) as part of the program “Investissements d'Avenir” (reference: ANR‐10‐IAHU‐01).
References
- 1. Klein OR, Buddenbaum J, Tucker N et al. Nonmyeloablative haploidentical bone marrow transplantation with post‐transplantation cyclophosphamide for pediatric and young adult patients with high‐risk hematologic malignancies. Biol Blood Marrow Transplant 2017;23:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bacigalupo A, Dominietto A, Ghiso A et al. Unmanipulated haploidentical bone marrow transplantation and post‐transplant cyclophosphamide for hematologic malignanices following a myeloablative conditioning: An update. Bone Marrow Transplant 2015;50:S37–S39. [DOI] [PubMed] [Google Scholar]
- 3. Busca A, Locatelli F, Flonta SE et al. In vivo T‐cell depletion with pretransplant low‐dose antithymocyte globulin is associated with reduced transplant‐related mortality and improved clinical outcome in patients receiving allogeneic hematopoietic stem cell transplantation from unrelated and partially matched related donors. Am J Hematol 2011;86:214–217. [DOI] [PubMed] [Google Scholar]
- 4. Robinson TM, O'Donnell PV, Fuchs EJ et al. Haploidentical bone marrow and stem cell transplantation: Experience with post‐transplantation cyclophosphamide. Semin Hematol 2016;53:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sengsayadeth S, Savani BN, Blaise D et al. Haploidentical transplantation: Selecting optimal conditioning regimen and stem cell source. Semin Hematol 2016;53:111–114. [DOI] [PubMed] [Google Scholar]
- 6. Storek J, Geddes M, Khan F et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol 2008;30:425–437. [DOI] [PubMed] [Google Scholar]
- 7. Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Curr Opin Hematol 2012;19:324–335. [DOI] [PubMed] [Google Scholar]
- 8. Clave E, Lisini D, Douay C et al. Thymic function recovery after unrelated donor cord blood or T‐cell depleted HLA‐haploidentical stem cell transplantation correlates with leukemia relapse. Front Immunol 2013;4:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bartelink IH, Belitser SV, Knibbe CAJ et al. Immune reconstitution kinetics as an early predictor for mortality using various hematopoietic stem cell sources in children. Biol Blood Marrow Transplant 2013;19:305–313. [DOI] [PubMed] [Google Scholar]
- 10. Storek J, Gooley T, Siadak M et al. Allogeneic peripheral blood stem cell transplantation may be associated with a high risk of chronic graft‐versus‐host disease. Blood 1997;90:4705–4709. [PubMed] [Google Scholar]
- 11. Admiraal R, de Koning CCH, Lindemans CA et al. Viral reactivations and associated outcomes in the context of immune reconstitution after pediatric hematopoietic cell transplantation. J Allergy Clin Immunol 2017;140:1643–1650 e1649. [DOI] [PubMed] [Google Scholar]
- 12. de Koning C, Admiraal R, Nierkens S et al. Human herpesvirus 6 viremia affects T‐cell reconstitution after allogeneic hematopoietic stem cell transplantation. Blood Adv 2018;2:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haddad E, Logan BR, Griffith LM et al. SCID genotype and 6‐month posttransplant CD4 count predict survival and immune recovery. Blood 2018;132:1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hick RW, Gruver AL, Ventevogel MS et al. Leptin selectively augments thymopoiesis in leptin deficiency and lipopolysaccharide‐induced thymic atrophy. J Immunol 2006;177:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krenger W, Blazar BR, Hollander GA. Thymic T‐cell development in allogeneic stem cell transplantation. Blood 2011;117:6768–6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaudhry MS, Velardi E, Malard F et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation: Time to T up the thymus. J Immunol 2017;198:40–46. [DOI] [PubMed] [Google Scholar]
- 17. Koch U, Fiorini E, Benedito R et al. Delta‐like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J Exp Med 2008;205:2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rothenberg EV, Zhang J, Li L. Multilayered specification of the T‐cell lineage fate. Immunol Rev 2010;238:150–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rothenberg EV, Kueh HY, Yui MA et al. Hematopoiesis and T‐cell specification as a model developmental system. Immunol Rev 2016;271:72–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Toubert A, Glauzy S, Douay C et al. Thymus and immune reconstitution after allogeneic hematopoietic stem cell transplantation in humans: Never say never again. Tissue Antigens 2012;79:83–89. [DOI] [PubMed] [Google Scholar]
- 21. Huttunen P, Taskinen M, Siitonen S et al. Impact of very early CD4(+)/CD8(+) T cell counts on the occurrence of acute graft‐versus‐host disease and NK cell counts on outcome after pediatric allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer 2015;62:522–528. [DOI] [PubMed] [Google Scholar]
- 22. Heimall J, Cowan MJ. Long term outcomes of severe combined immunodeficiency: Therapy implications. Expert Rev Clin Immunol 2017;13:1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Touzot F, Moshous D, Creidy R et al. Faster T‐cell development following gene therapy compared with haploidentical HSCT in the treatment of SCID‐X1. Blood 2015;125:3563–3569. [DOI] [PubMed] [Google Scholar]
- 24. Schluns KS, Kieper WC, Jameson SC et al. Interleukin‐7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol 2000;1:426–432. [DOI] [PubMed] [Google Scholar]
- 25. Alpdogan O, van den Brink MR. IL‐7 and IL‐15: Therapeutic cytokines for immunodeficiency. Trends Immunol 2005;26:56–64. [DOI] [PubMed] [Google Scholar]
- 26. Puel A, Ziegler SF, Buckley RH et al. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet 1998;20:394–397. [DOI] [PubMed] [Google Scholar]
- 27. Brown VI, Fang J, Alcorn K et al. Rapamycin is active against B‐precursor leukemia in vitro and in vivo, an effect that is modulated by IL‐7‐mediated signaling. Proc Natl Acad Sci USA 2003;100:15113–15118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoda A, Yoda Y, Chiaretti S et al. Functional screening identifies CRLF2 in precursor B‐cell acute lymphoblastic leukemia. Proc Natl Acad Sci USA 2010;107:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guimond M, Veenstra RG, Grindler DJ et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat Immunol 2009;10:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Storek J, Gillespy T 3rd, Lu H et al. Interleukin‐7 improves CD4 T‐cell reconstitution after autologous CD34 cell transplantation in monkeys. Blood 2003;101:4209–4218. [DOI] [PubMed] [Google Scholar]
- 31. Fry TJ, Christensen BL, Komschlies KL et al. Interleukin‐7 restores immunity in athymic T‐cell‐depleted hosts. Blood 2001;97:1525–1533. [DOI] [PubMed] [Google Scholar]
- 32. Lai L, Zhang M, Song Y et al. Recombinant IL‐7/HGFbeta hybrid cytokine enhances T cell recovery in mice following allogeneic bone marrow transplantation. PLoS One 2013;8:e82998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andre‐Schmutz I, Bonhomme D, Yates F et al. IL‐7 effect on immunological reconstitution after HSCT depends on MHC incompatibility. Br J Haematol 2004;126:844–851. [DOI] [PubMed] [Google Scholar]
- 34. Sauter CT, Bailey CP, Panis MM et al. Interleukin‐15 administration increases graft‐versus‐tumor activity in recipients of haploidentical hematopoietic SCT. Bone Marrow Transplant 2013;48:1237–1242. [DOI] [PubMed] [Google Scholar]
- 35. Tan JT, Ernst B, Kieper WC et al. Interleukin (IL)‐15 and IL‐7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med 2002;195:1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blaser BW, Roychowdhury S, Kim DJ et al. Donor‐derived IL‐15 is critical for acute allogeneic graft‐versus‐host disease. Blood 2005;105:894–901. [DOI] [PubMed] [Google Scholar]
- 37. Thiant S, Moutuou MM, Leboeuf D et al. Homeostatic cytokines in immune reconstitution and graft‐versus‐host disease. Cytokine 2016;82:24–32. [DOI] [PubMed] [Google Scholar]
- 38. Rosenberg SA, Sportes C, Ahmadzadeh M et al. IL‐7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T‐regulatory cells. J Immunother 2006;29:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sportes C, Hakim FT, Memon SA et al. Administration of rhIL‐7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med 2008;205:1701–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Levy Y, Lacabaratz C, Weiss L et al. Enhanced T cell recovery in HIV‐1‐infected adults through IL‐7 treatment. J Clin Invest 2009;119:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perales MA, Goldberg JD, Yuan J et al. Recombinant human interleukin‐7 (CYT107) promotes T‐cell recovery after allogeneic stem cell transplantation. Blood 2012;120:4882–4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kolb HJ, Mittermüller J, Clemm C et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 1990;76:2462–2465. [PubMed] [Google Scholar]
- 43. Bordignon C, Keever CA, Small TN et al. Graft failure after T‐cell‐depleted human leukocyte antigen identical marrow transplants for leukemia: II. in vitro analyses of host effector mechanisms. Blood 1989;74:2237–2243. [PubMed] [Google Scholar]
- 44. Reisner Y, Kapoor N, Kirkpatrick D et al. Transplantation for acute leukaemia with HLA‐A and B nonidentical parental marrow cells fractionated with soybean agglutinin and sheep red blood cells. Lancet 1981;2:327–331. [DOI] [PubMed] [Google Scholar]
- 45. Horowitz MM, Gale RP, Sondel PM et al. Graft‐versus‐leukemia reactions after bone marrow transplantation. Blood 1990;75:555–562. [PubMed] [Google Scholar]
- 46. Urbano‐Ispizua A, Rozman C, Pimentel P et al. The number of donor CD3(+) cells is the most important factor for graft failure after allogeneic transplantation of CD34(+) selected cells from peripheral blood from HLA‐identical siblings. Blood 2001;97:383–387. [DOI] [PubMed] [Google Scholar]
- 47. Wolfgang Bethge SM, Niederwieser D, Meisel R et al. First results of a prospective multicenter phase I/II clinical trial in adult patients using TCRalpha/beta and CD19 depleted haploidentical stem cell transplantation following reduced intensity conditioning. ASH Abstracts 2017;2017. [Google Scholar]
- 48. Feuchtinger T, Heiko‐Manuel T, Schumm M et al. Transplantation of TcRαβ/CD19 depleted stem cells from haploidentical donors in children: Current results. Blood 2013;122:692. [Google Scholar]
- 49. Locatelli F, Merli P, Pagliara D et al. Outcome of children with acute leukemia given HLA‐haploidentical HSCT after alphabeta T‐cell and B‐cell depletion. Blood 2017;130:677–685. [DOI] [PubMed] [Google Scholar]
- 50. Bertaina, A.P., D .; Pende, D. ; Rutella, S. , Falco, M. , Bauquet, A. et al. Beta+ T Cells and of CD19+ B Cells from the Graft Translates Into Rapid Engraftment, Absence of Visceral Graft‐Versus‐Host Disease and Low Transplant‐Related Mortality in Children with Acute Leukemia Given HLA‐Haploidentical Hematopoietic Stem Cell Transplantation, 2013 ASH Annual Meeting (2013).
- 51. Shlomchik WD. Graft‐versus‐host disease. Nat Rev Immunol 2007;7:340–352. [DOI] [PubMed] [Google Scholar]
- 52. Teschner D, Distler E, Wehler D et al. Depletion of naive T cells using clinical grade magnetic CD45RA beads: A new approach for GVHD prophylaxis. Bone Marrow Transplant 2014;49:138–144. [DOI] [PubMed] [Google Scholar]
- 53. Bleakley M, Heimfeld S, Loeb KR et al. Outcomes of acute leukemia patients transplanted with naive T cell‐depleted stem cell grafts. J Clin Invest 2015;125:2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Touzot F, Neven B, Dal‐Cortivo L et al. CD45RA depletion in HLA‐mismatched allogeneic hematopoietic stem cell transplantation for primary combined immunodeficiency: A preliminary study. J Allergy Clin Immunol 2015;135:e1301–e1303. [DOI] [PubMed] [Google Scholar]
- 55. Andre‐Schmutz I, Le Deist F, Hacein‐Bey‐Abina S et al. Immune reconstitution without graft‐versus‐host disease after haemopoietic stem‐cell transplantation: A phase 1/2 study. Lancet 2002;360:130–137. [DOI] [PubMed] [Google Scholar]
- 56. Amrolia PJ, Muccioli‐Casadei G, Huls H et al. Adoptive immunotherapy with allodepleted donor T‐cells improves immune reconstitution after haploidentical stem cell transplantation. Blood 2006;108:1797–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mielke S, Nunes R, Rezvani K et al. A clinical‐scale selective allodepletion approach for the treatment of HLA‐mismatched and matched donor‐recipient pairs using expanded T lymphocytes as antigen‐presenting cells and a TH9402‐based photodepletion technique. Blood 2008;111:4392–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ciceri F, Bonini C, Stanghellini MTL et al. Infusion of suicide‐gene‐engineered donor lymphocytes after family haploidentical haemopoietic stem‐cell transplantation for leukaemia (the TK007 trial): A non‐randomised phase I–II study. Lancet Oncol 2009;10:489–500. [DOI] [PubMed] [Google Scholar]
- 59. Zhou X, Dotti G, Krance RA et al. Inducible caspase‐9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood 2015;125:4103–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rooney CM, Ng CYC, Loftin S et al. Use of gene‐modified virus‐specific T lymphocytes to control Epstein‐Barr‐virus‐related lymphoproliferation. Lancet 1995;345:9–13. [DOI] [PubMed] [Google Scholar]
- 61. Walter EA, Greenberg PD, Gilbert MJ et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T‐cell clones from the donor. N Engl J Med 1995;333:1038–1044. [DOI] [PubMed] [Google Scholar]
- 62. Peggs KS, Tholouli E, Chakraverty R et al. CMV; IMPACT: Results of a randomized controlled trial of immuno‐prophylactic adoptive cellular therapy following sibling donor allogeneic HSCT. Blood 2014;124:1109. [Google Scholar]
- 63. Peggs KS, Thomson K, Samuel E et al. Directly selected cytomegalovirus‐reactive donor T cells confer rapid and safe systemic reconstitution of virus‐specific immunity following stem cell transplantation. Clin Infect Dis 2011;52:49–57. [DOI] [PubMed] [Google Scholar]
- 64. Creidy R, Moshous D, Touzot F et al. Specific T cells for the treatment of cytomegalovirus and/or adenovirus in the context of hematopoietic stem cell transplantation. J Allergy Clin Immunol 2016;138:920.e3–924.e3. [DOI] [PubMed] [Google Scholar]
- 65. Tzannou I, Papadopoulou A, Naik S et al. Off‐the‐shelf virus‐specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein‐Barr virus, and adenovirus infections after allogeneic hematopoietic stem‐cell transplantation. J Clin Oncol 2017;35:3547–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bollard CM, Heslop HE. T cells for viral infections after allogeneic hematopoietic stem cell transplant. Blood 2016;127:3331–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barrett AJ, Prockop S, Bollard CM. Virus‐specific T cells: Broadening applicability. Biol Blood Marrow Transplant 2018;24:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rossi SW, Jeker LT, Ueno T et al. Keratinocyte growth factor (KGF) enhances postnatal T‐cell development via enhancements in proliferation and function of thymic epithelial cells. Blood 2007;109:3803–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Goldberg GL, Sutherland JS, Hammet MV et al. Sex steroid ablation enhances lymphoid recovery following autologous hematopoietic stem cell transplantation. Transplantation 2005;80:1604–1613. [DOI] [PubMed] [Google Scholar]
- 70. Dudakov JA, Goldberg GL, Reiseger JJ et al. Sex steroid ablation enhances hematopoietic recovery following cytotoxic antineoplastic therapy in aged mice. J Immunol 2009;183:7084–7094. [DOI] [PubMed] [Google Scholar]
- 71. Alpdogan O, Muriglan SJ, Kappel BJ et al. Insulin‐like growth factor‐I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation 2003;75:1977–1983. [DOI] [PubMed] [Google Scholar]
- 72. Williams KM, Lucas PJ, Bare CV et al. CCL25 increases thymopoiesis after androgen withdrawal. Blood 2008;112:3255–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Velardi E, Tsai JJ, Holland AM et al. Sex steroid blockade enhances thymopoiesis by modulating Notch signaling. J Exp Med 2014;211:2341–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dudakov JA, Hanash AM, Jenq RR et al. Interleukin‐22 drives endogenous thymic regeneration in mice. Science 2012;336:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dudakov JA, Mertelsmann AM, O'Connor MH et al. Loss of thymic innate lymphoid cells leads to impaired thymopoiesis in experimental graft‐versus‐host disease. Blood 2017;130:933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lopes N, Vachon H, Marie J et al. Administration of RANKL boosts thymic regeneration upon bone marrow transplantation. EMBO Mol Med 2017;9:835–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jabbar S, Drury J, Fordham JN et al. Osteoprotegerin, RANKL and bone turnover in postmenopausal osteoporosis. J Clin Pathol 2011;64:354–357. [DOI] [PubMed] [Google Scholar]
- 78. Glauzy S, André‐Schmutz I, Larghero J et al. CXCR4‐related increase of circulating human lymphoid progenitors after allogeneic hematopoietic stem cell transplantation. PLoS One 2014;9:e91492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Reimann C, Six E, Dal‐Cortivo L et al. Human T‐lymphoid progenitors generated in a feeder‐cell‐free Delta‐like‐4 culture system promote T‐cell reconstitution in NOD/SCID/gammac(−/−) mice. Stem Cells 2012;30:1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Simons L, Ma K, de Chappedelaine C et al. Generation of adult human T‐cell progenitors for immunotherapeutic applications. J Allergy Clin Immunol 2018;141:1491–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shukla S, Langley MA, Singh J et al. Progenitor T‐cell differentiation from hematopoietic stem cells using Delta‐like‐4 and VCAM‐1. Nat Methods 2017;14:531–538. [DOI] [PubMed] [Google Scholar]
- 82. Buckley RH. Transplantation of hematopoietic stem cells in human severe combined immunodeficiency: Longterm outcomes. Immunol Res 2011;49:25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Neven B, Leroy S, Decaluwe H et al. Long‐term outcome after hematopoietic stem cell transplantation of a single‐center cohort of 90 patients with severe combined immunodeficiency. Blood 2009;113:4114–4124. [DOI] [PubMed] [Google Scholar]


