Abstract
Several patient groups undergoing small‐diameter (<6 mm) vessel bypass surgery have limited autologous vessels for use as grafts. Tissue‐engineered vascular grafts (TEVG) have been suggested as an alternative, but the ideal TEVG remains to be generated, and a systematic overview and meta‐analysis of clinically relevant studies is lacking. We systematically searched PubMed and Embase databases for (pre)clinical trials and identified three clinical and 68 preclinical trials ([>rabbit]; 873 TEVGs) meeting the inclusion criteria. Preclinical trials represented low to medium risk of bias, and binary logistic regression revealed that patency was significantly affected by recellularization, TEVG length, TEVG diameter, surface modification, and preconditioning. In contrast, scaffold types were less important. The patency was 63.5%, 89%, and 100% for TEVGs with a median diameter of 3 mm, 4 mm, and 5 mm, respectively. In the group of recellularized TEVGs, patency was not improved by using smooth muscle cells in addition to endothelial cells nor affected by the endothelial origin, but seems to benefit from a long‐term (46–240 hours) recellularization time. Finally, data showed that median TEVG length (5 cm) and median follow‐up (56 days) used in preclinical settings are relatively inadequate for direct clinical translation. In conclusion, our data imply that future studies should consider a TEVG design that at least includes endothelial recellularization and bioreactor preconditioning, and we suggest that more standard guidelines for testing and reporting TEVGs in large animals should be considered to enable interstudy comparisons and favor a robust and reproducible outcome as well as clinical translation.
Keywords: Revascularization, Bypass, Tissue‐engineered vascular grafts, Patency, Meta‐analysis
Significance Statement.
Small‐diameter blood vessel bypass surgery is primarily performed using autologous arteries or veins, yet several patient groups have limited grafts, and therefore substantial effort is put into bioengineering small diameter tissue‐engineered vascular grafts (TEVGs). This study provides the first‐ever systematical status of TEVGs and has performed a meta‐analysis of factors contributing to TEVG patency in clinically relevant settings such as large animals. Thus, in an unbiased fashion, this study determines critical parameters for in vivo patency of bioengineered TEVGs, and also pinpoints the need for more standard guidelines for testing and reporting TEVGs in large animals. The data will thus bring the field a novel unbiased status that hopefully will accelerate clinical translation of tissue engineered grafts in the near future.
Introduction
Reperfusing ischemic tissue may be performed by endovascular or bypass surgery. As such, coronary artery bypass grafting includes connecting the internal mammarial artery to the coronary artery distally for the occluded coronary vessel or, alternatively, insertion of an autologous vein or artery from ascending aorta to this coronary segment. Similarly, in relation to peripheral artery disease, autologous veins are used to redirect blood around the affected vessel in the legs. However, wound infection in the graft donor site is a frequent complication, and former use of the patient's veins for bypasses, dialysis, varicose disease with or without removal of the saphenous veins, as well as patients with hypoplastic v. saphena magna or damages from previous thrombophlebitis have limited graft material. Synthetic grafts such as Dacron and Teflon, which have been used since 1950s, works relatively well for vascular grafts with a large diameter; however, the success for small‐diameter synthetic vessels (<6 mm) is lacking 1. The blood flow in smaller vascular grafts, higher thrombogenecity of the graft material as well as poor homogeneity in the compliance of the native vessel and the graft are all factors contributing to the low patency of synthetic grafts with a diameter ≤6 mm 1, 2, 3, 4, 5, 6. Likewise, the use of allografts as an alternative remains complicated and often gives rise to immune responses. Thus, novel sources of blood vessels are highly demanded for bypass surgery in various pathological states.
Already in 1986, Weinberg and Bell succeeded in bioengineering a multilayered artery with physiological properties resembling that of a native vessel 7. Although having low burst strength, this work kick‐started the field of tissue‐engineered vascular grafts (TEVG), providing an alternative source for small‐diameter blood vessel grafts in bypass surgery. Yet, clinical translation of small‐diameter TEVG (from now on referred to as TEVG) is still restricted to a few studies 8, 9, 10, and the ideal TEVG with long‐term patency remains to be developed. As summarized and discussed in many excellent reviews 1, 11, 12, 13, multiple approaches are used to generate TEVGs from autologous cells grown on synthetic or biological scaffolds with or without modifications, but also acellular conduits have been rigorously tested. But how far are researchers indeed from generating TEVG products that at least meet the 60% patency obtained after 10 years in humans when using autologous grafts 14, and which approaches contribute positively to the long‐term patency of TEVGs?
Herein, we set out to combine systematic literature searches, risk of bias assessments, and meta‐analysis of selected parameters to provide a status on TEVG in clinically relevant settings such as large animals. With this we provide, to the best of our knowledge, the first meta‐data on multiple factors influencing TEVG patency as well as considerations on how to proceed with generating the ideal TEVG.
Materials and Methods
Search Strategy
The systematic literature search and design was performed in agreement with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines 15. One person (I.S.) well‐trained in the literature search performed the searches and initial screening in the electronic databases PubMed and Embase from inception to January 1, 2018. The search strategy was based on the three key terms “tissue engineering,” “vascular grafts,” and “in vivo evaluation,” and was composed of both indexed subject headings (MeSH and Emtree terms in PubMed and Embase, respectively) as well as related free text terms. No database limits were imposed on the search in PubMed, whereas in Embase only the publication type “Article” was chosen for further processing. All retrieved items were imported to EndNote and duplicates were removed before further processing. The complete search strategy can be found in Supporting Information Figure S1.
Study Selection
Retrieved studies were screened against the inclusion criteria using the systematic data management program Covidence 16, first based on title and abstract (I.S.), and secondly by a full‐text screening (independently by I.S. and H.J.B.). Overall, inclusion criteria (Fig. 1 and Supporting Information Fig. S2) embraced studies on tissue engineering of tubular small diameter (≤6 mm inner diameter assessed before implantation) 1, 17, 18 blood vessels and their evaluation as interposition grafts in large animals (>rabbit). The small‐diameter criterion was met if: the inner diameter was disclosed and was ≤6 mm; the inner diameter could be inferred from pictures to be ≤6 mm; or the study defined small diameter as no more than ≤6 mm and claimed to deal with small‐diameter grafts. If information regarding analyzed parameters such as follow‐up time, patency, or distribution of individuals among experimental groups were absent or unclear to us, the study authors (only for studies <20 years) were contacted for clarification. If information remained insufficient or still lacking, the study was excluded. Two studies could be partly included despite insufficient information and/or no author response 19, 20. Nine studies were completely excluded (Supporting Information Fig. S3). Only primary studies published in peer‐reviewed journals in English language were eligible; others were excluded (Supporting Information Fig. S4).
Figure 1.
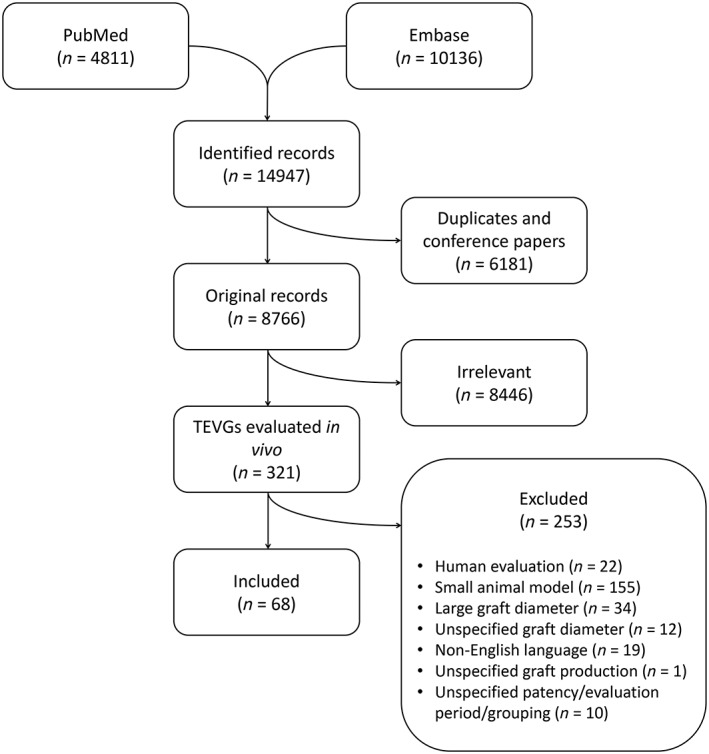
Flowchart of the literature search and exclusion criteria for meta‐analysis. See Materials and Methods for details.
Data Extraction
All data were extracted independently by I.S. and H.J.B., and then repeated independently by I.S. and E.B.H. Any disagreements were solved by discussion between the disagreeing parties and by involving D.C.A. to solve the controversy. For each graft, data were extracted (see Supporting Information datasheet) on animal model, graft diameter (mm), follow‐up (days [1 month = 30 days, 1 year = 365 days]), the addition of surface modification (conjugated molecules, Supporting Information Fig. S5), recellularization (cellular/acellular), preconditioning (yes/no), length (cm), publication year, the scaffold type (natural, synthetic, hybrid [of natural and synthetic], self‐assembly cellular sheet, decellularized, in‐body tissue architecture, and combinations of these; Supporting Information Fig. S6), and primary patency at the final evaluation time point. A patent vessel is defined as an open vessel (any degree of partial obstruction is still defined as patent as in general for the field). A second and a third round of data extraction were performed independently by E.B.H. and D.C.A. to retrieve information on type of circulation (artery, venous, arterio‐venous), antithrombotic treatment (type and duration), mechanical properties (methods used and strength; Supporting Information Fig. S7), cell type according to origin (blood, vessel, bone marrow, and whether cells were endothelial or smooth muscle cells and of progenitor/stem cell (blood, bone marrow, other) or mature/differentiated (vessel) origin as well as details on cell culturing (media and culture time) and recellularization time (Supporting Information Fig. S8). Again, any disagreements were rechecked and solved. Information on the patency of the grafts corresponding to each study is represented in two ways: the patency for each study group in percentage (of patent grafts in the group) as reported by the authors of the study; and each graft separately, patency indicated as either patent or occluded (i.e., binary). The latter indication of patency was used for the regression analysis, as it considers the number of grafts in the study groups.
Quality Assessment
All studies were evaluated for risk of bias using a customized tool (Supporting Information Fig. S9), which was developed based on the recommendations of the Agency for Healthcare Research and Quality 21. Using this tool, both study design, execution and reporting are assessed in a total of 10 categories, including the animal model chosen for in vivo evaluation (dog < sheep, goat, pig < nonhuman primate; i.e., the more similar to human physiology, the better); whether the length of the implanted graft was stated (not reported < reported as interval or approximation < reported as exact measurement); the choice of control (use of control in another incomparable individual and location or no control < use of control in the same location but in another comparable individual < use of control in the same individual and equivalent contralateral location); the consistency of treatment (highly inconsistent treatment and/or outcome assessment within or between groups < slight differences in treatment and/or outcome assessment within or between groups of little or no consequence < consistent treatment and outcome assessment within and between groups); the length of the evaluation period (divided into three intervals based on previous observations of pathophysiological events occurring in the graft post implantation 20 and subsequent expected time graft failure 13: [<1 month] < [1–9 months] < [>9 months]); the method for validation of the graft patency (another method than angiography and ultrasonography < either angiography or ultrasonography < both angiography and ultrasonography), and whether the graft quality was evaluated by other methods including mechanical and histological evaluation (no assessment of grafting success besides patency < assessment of one parameter of grafting success besides patency < assessment of at least two parameters of grafting success besides patency); the incident of unexpected events or treatments and whether such may affect the outcome of the study (unintended or concurrent interventions that can heavily influence results < unintended or concurrent interventions that might slightly influence results, or that are accounted for in the results < no unintended or concurrent interventions); the existence of drop‐outs of the study and whether such was included (if possible) in the overall evaluation (loss of participants, without inclusion of drop‐out data in the synthesis of results < loss of participants with partial inclusion of drop‐out data in the synthesis of results < no loss of participants, or loss of participants with inclusion of drop‐out data in the synthesis of results); and finally, whether the authors reported any conflict of interest (no reported < present < absent). The studies were rated independently (I.S. and E.B.H.) in each category as low risk, medium risk, or high risk of bias, with a corresponding score of low (1), medium (2), or high (3) risk of bias, respectively. The results are visualized by a heatmap using the Euclidean distance measure to compute the distances. The order of the risk of bias categories is according to total sum with the highest scoring. The difference in patency between the group of studies scoring low, medium, or high risk of bias was tested using Dunn's test of multiple comparison (with p‐values adjusted using the Benjamini–Hochberg method 22) following Kruskal–Wallis tests for each parameter, and the patency distribution was plotted as a box‐and‐whiskers plot. The patency was here defined as the reported percentage for each group comprising the studies and tested against high risk of bias.
Regression and Exploratory Data Analysis
A binary logistic regression model was fitted using patency (patent and occluded coded numerically as 1 and 0, respectively) as the dependent variable. For the regression analysis, we treated variables that can be used as interventions in the graft production in a sensible manner as candidate explanatory variables: length (continuous), diameter (continuous), recellularization (binary), scaffold type (categorical), surface modification (binary), and preconditioning (binary). For further details of each of these explanatory variables, see above. For model selection, (a) backward elimination, (b) forward selection, (c) bidirectional elimination, and (d) exhaustive searches for the best subsets of explanatory variables was performed using the Akaike information criterion and the Bayesian information criterion, respectively. Remarkably, these approaches to model selection all suggested the same subset of explanatory variables, supporting the final choice of model: length, diameter, recellularization, surface modification, and preconditioning. Prior to fitting the model, listwise deletion was used to eliminate 142 observations with missing values, since we assume that the probability of missing data on length or diameter does not depend on the values of any of the explanatory variables. Notably, since the proposed regression model is logistic, listwise deletion yields consistent estimates of the regression coefficients and their standard errors, even if the probability of missing data on length or diameter depends on the value of patency 23. The proposed final model assuming a binomial distribution of the dependent variable was compared with the quasi‐binomial model estimating an additional dispersion parameter (Supporting Information Fig. S10) to confirm that the standard errors were similar.
As an exploratory analysis, a Pearson's chi‐squared test with Yates' continuity correction was used to test the null hypothesis that the patency/occlusion of a graft is independent of recellularization. Similarly, a Pearson's chi‐squared test with Yates' continuity correction was used to test the null hypothesis that the patency/occlusion of a graft is independent of cell type. Likewise, for diameter (pooled in three bins, [2 mm, 4 mm), [4 mm, 5 mm), and [5 mm, 6 mm]), scaffold type, and type of conjugated molecule, a Pearson's chi‐squared test with Yates' continuity correction was performed, and in case the overall chi‐squared test was significant, it was followed by a post hoc analysis using Fisher's exact test with Benjamini–Hochberg adjusted p‐values for testing significance of differences among all pairs. For duration of recellularization, the data was pooled in two bins, short‐term (0.16–6 hours) and long‐term (46–240 hours), and Pearson's chi‐squared test with Yates' continuity correction was performed. For testing the effect of anticoagulation factors on patency, the Wilcoxon rank sum test was used on the reported patency (in percentage) for each group of grafts composing the study. For mechanical testing, the reported strength for each of the three most frequently used methods was plotted against the reported patency (in percentage) for each study. The term “significant” refers to statistical significance at the .05 level, unless otherwise stated. For all statistical analyses the R software version 3.3.3 was used 24.
Results
Included Studies and Study Characteristics
A total of 14,947 studies were retrieved from the two databases PubMed and Embase (Fig. 1 and Supporting Information Fig. S1). Exclusion of duplicates and then systematic screening of title and abstract using the Covidence platform revealed 321 studies, which was finally limited by thorough text reading to 68 studies based on inclusion criteria 19, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91. These studies represented a total of 873 grafts: 258 occluded and 615 patent grafts at the end of the evaluation period. An unambiguous description of parameters, including animal model, scaffold type, cell treatment, scaffold surface modifications, graft diameter and length, evaluation period, and patency, are listed for each study in Supporting Information Figure 11. These specific parameters reflect main variables suggested and discussed in the field to affect in vivo patency of small‐diameter bioengineered blood vessels 1, 13. Approximately half of the studies were published in the last decade, and 59 of the 68 studies contained several experimental groups of grafts. Nine different types of scaffolds were used (Supporting Information Fig. S6) and the ratios of acellular versus cellular TEVGs ranges from 0.28 to 1 between those scaffold types (data not shown). Six different animal species were used in the 68 studies, yet since animal model does not qualify as a parameter for clinical translation, we did not include it in the regression model, but maintained it as a parameter for study quality assessment (i.e., similarity to human physiology). Ninety percentage of TEVGs were tested in an artery circuit, whereas only 9% and 1% were tested in arterio‐venous and venous circuits, respectively. Thirty‐seven studies used antithrombotic treatment, but overall patency (100; 77.1; 33.7 [median; mean; SE]) remained nonsignificant (p = .3978) from studies not using this treatment (100; 74.5; 37.9 [median; mean; SE]). The median graft diameter of all TEVGs was 4 mm and ranged from 2 to 6 mm. When divided into three logical diameter bins to obtain sufficient number of studies and grafts in each bin, the median patency was 63.5%, 89%, and 100% for TEVGs with a median diameter of 3 mm, 4 mm, and 5 mm, respectively (Supporting Information Fig. S12). The median graft length was 5 cm and ranged from 1 to 25 cm, where the length of 510 of 873 analyzed TEVGs exceeds the diameter by a factor 10, as recommended by others to obtain a reliable patency measure 13. The median of follow‐up was 56 days, and ranged from many short‐term studies lasting 1 month or less (370 grafts) to longer, more clinically relevant 20 studies evaluated for >6 months (136 grafts). Most grafts, however, were evaluated in the time frame 1 day to 6 months (736 grafts). The median TEVG patency was 83% (n = 873) for a median follow‐up time of 56 days (95% confidence interval: [42, 60]).
Study Quality
To evaluate the presence of bias, we established our own risk of bias assessment tool (Supporting Information Fig. S9) according to what has been recommended by others 21. We scored studies according to three categories: high risk of bias (equal to 3), medium risk of bias (equal to 2), and low risk of bias (equal to 1). Accumulation of the total score for each study is not recommended since rating of individual categories is inappropriate 21. Yet, by summing scores across studies and determining the median score for each category (Fig. 2A), we found that only conflicting interests were prone to high bias (median: 3). In contrast, animal model (relevance to human), follow‐up (relevance to clinical scenario), patency assessment, cointerventions (e.g., antithrombotic treatment and treatment of unexpected complication during or after surgery), controls (proper controls), consistency, and graft length were mostly affected by medium bias across studies (median: 2), whereas drop‐out and secondary methods (use of supportive methods) were at a low risk of bias (median: 1; Fig. 2A).
Figure 2.
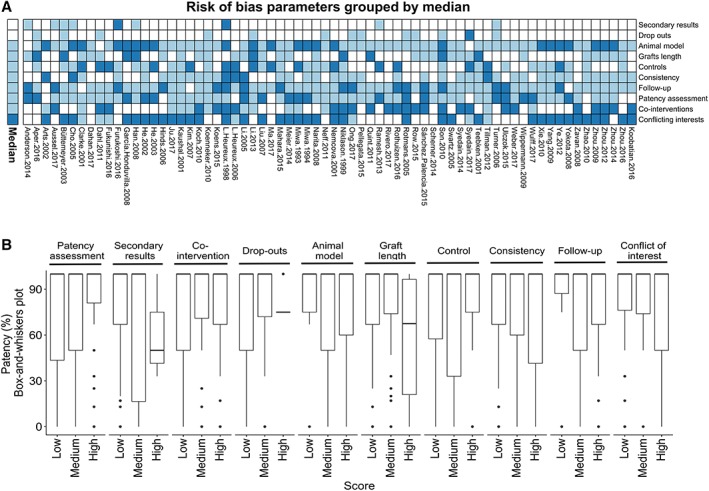
Risk of bias (RoB) analysis. (A): Heatmap of RoB for all included studies. White, light blue, and dark blue indicates low, medium, and high RoB, respectively. The annotation bar to the left indicates the median for each RoB category. The order of the rows (RoB categories) denotes the median for each category, low to high from the top. (B): Boxplots (box‐and‐whiskers, i.e., the median, hinges, whiskers, and outliers) of patency distribution among RoB categories for each score (low, medium, or high). Dunn's test of multiple comparison was used to test low and medium groups against high RoB, to identify RoB categories associated with high patency as indicated by p‐value.
We determined whether patency was different between low, medium and high risk of bias groups within each category using Dunn's test of multiple comparison following Kruskal–Wallis tests. These data revealed that a higher risk of bias results in an improved patency for only patency assessment and the use of appropriate control, whereas no evidence of association was found for other risk of bias categories (Fig. 2B).
Meta‐Analysis of Extracted Data
In order to assess the effects of explanatory variables on the patency of the graft, we fitted a binary logistic regression model using patency (patent and occluded coded numerically as 1 and 0, respectively) as the dependent variable (Fig. 3A, 3B). For the regression analysis, we initially used the candidate explanatory variables: length, diameter, recellularization, scaffold type, surface modification, and preconditioning. Yet, for none of the scaffold types (Supporting Information Fig. S6), the coefficients were statistically significantly different from the coefficient of the reference type (decellularized; .17 < p < .92). Moreover, a likelihood ratio test (data not shown) suggested that the variable scaffold type did not significantly improve the model fit. This suggests that the scaffold types (Supporting Information Fig. S6) investigated in the 68 studies are very similar in functionality and difficult to distinguish in terms of resulting patency. Consequently, scaffold type as a variable did not enter into the final model.
Figure 3.
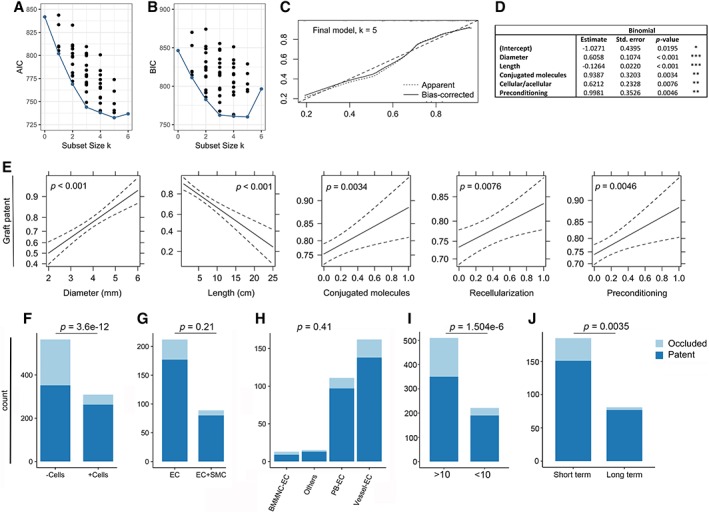
Binary logistic regression and exploratory data analysis. (A, B): The Akaike information criterion and the Bayesian information criterion for each of the 64 potential models are plotted according to number of explanatory variables (k). The “best” model for each subset size k is marked in red. (C): Bootstrap overfitting‐corrected lowest nonparametric calibration curve for the final model. (D): Final model. Signif. codes: “***” 0.001, “**” 0.01, “*” 0.05, “.” 0.1, “ ” 1. (E): Effects computed from the final model. In order to preserve the linear structure of the model while allowing for interpretation on a familiar scale, the effects are plotted on the scale of the linear predictor of explanatory variables, whereas the vertical axis is labeled on the scale of the dependent variable (i.e., the probability scale). Dotted lines indicate standard error. Association between patency at the time of follow‐up and (F) recellularization of the graft, (G) endothelial cells only, or endothelial cells and smooth muscle cells in combination seeded on the graft, (H) endothelial cell origin, (I) the length/diameter ratio, and (J) the duration of recellularization. The indicated p‐values computed by Monte Carlo simulation are from Pearson's chi‐squared test.
In contrast, the proposed final model (Fig. 3C) showed that graft length had a highly statistically significant (p < .001) negative effect on graft patency (Fig. 3D, 3E). Similarly, graft diameter had a highly statistically significant (p < .001) positive effect on graft patency (Fig. 3D, 3E), a result that was expected and in agreement with the general knowledge in the field that small diameter vessels are more prone to being occluded but here verified at a meta‐analysis level. Remarkably, according to our model, recellularization (p = .0076), surface modification with conjugated molecules (p = .0034), and preconditioning (p = .0046) also appear to have statistically significant positive effects on graft patency (Fig. 3D, 3E).
Exploratory Data Analysis of Meta‐Data
To analyze the variables with an impact on graft patency in detail, we performed exploratory analyses. Yet, since the results for graft surface modifications with conjugated molecules (Supporting Information Fig. S5) and graft preconditioning, derived from a relative low number of studies that each embraces various approaches for these two variables, respectively, we speculated that further exploratory analysis would be inadequate currently. Instead, we chose to analyze in detail the impact of graft length and recellularization as well as the cell type and origin of the recellularized cells used on graft patency.
Recellularized TEVGs accounted for 309 (73 TEVG groups/30 studies) of the 873 TEVGs analyzed. As compared with acellular TEVGs, recellularized grafts were more prone to being patent (χ 2 = 48.333, df = 1, p < .001; Fig. 3F). In the group of recellularized TEVGs, 220 TEVGs (20 studies) were seeded with endothelial cells in the luminal space, whereas 89 TEVGs (13 studies) were seeded with endothelial cells on the inside and smooth muscle cells on the outside. Interestingly, our data showed that TEVGs with endothelial cells alone exhibited a patency that was not statistically significantly different (χ 2 = 1.5747, df = 1, p = .2095) from the patency in the group with both endothelial and smooth muscle cells (Fig. 3G). Furthermore, extracting meta‐data on the origin of used endothelial cells, we found TEVGs to be seeded with endothelial cells derived from large blood vessel (162 TEVGs), peripheral blood (111 TEVGs), bone marrow (13 TEVGs) and other organs such as fat (14 TEVGs). Yet, no statistically significant difference (χ 2 = 3.0996, p = .4093) was revealed in patency between TEVGs with endothelial cells of different origins (Fig. 3H). Similar results were obtained for smooth muscle cells (data not shown). Also, we found no evidence of any association (χ 2 = 0, p = 1; without continuity correction, p = .9666) between patency and the stem cell/progenitor state of the endothelial origin (data not shown). Noteworthy, the median recellularization time was 2 hours for patent TEVGs (Supporting Information Fig. 8), but when recellularization time was divided into two natural bins: short‐term (0.16–6 hours) and long‐term (46–240 hours), we found that long‐term recellularization was better (χ 2 = 8.3105, p = .0040) in terms of a higher fraction of patent TEVGs (Fig. 3J).
The final model suggests that graft length has a negative effect on graft patency, whereas diameter has a positive effect on graft patency (Fig. 3D, 3E). This is important, since it has been suggested that the TEVG length should exceed the diameter by a factor of 10 to avoid false positive patency 13. However, we found that grafts not meeting this criterion were significantly (χ 2 = 29.275, df = 1, p < .001) more likely to be patent (Fig. 3I). In this regard, it is also worth noting that the length, the diameter, or both were not reported for 142 TEVGs. Finally, the 68 studies used nine different approaches to test mechanical properties of the TEVGs (Supporting Information Fig. S7), a key specification of the TEVG strength and another valuable read for the TEVG quality in addition to in vivo patency. Based on a correlation analysis, we found that burst pressure and suture retention tests, which were the two most commonly used methods, expectably correlated (r = .48, p < .001 and r = .31, p = .0015, respectively) with in vivo patency (Supporting Information Fig. S7).
Discussion
To the best of our knowledge, we here provide the first‐ever meta‐analysis showing that recellularization, surface modification, and preconditioning all improve TEVG patency in a clinically relevant setting. The observed median patency of 83% was relatively high, but we also identified that many of the preclinical studies, even though performed in large animals, uses TEVG lengths (median: 5 cm) and follow‐up (median: 56 days) that are inadequate for clinical use and thus cannot be directly compared with the 10‐year 60% patency known for autologous grafts.
As discussed in several reviews 1, 11, 12, 13 and as reflected systematically in our study, various approaches have been used to generate TEVGs. Still, the field is in its infancy with reference to the few clinical translations 8, 9, 10, which were identified by our search strategy. Therefore, we consider the heterogeneity of TEVG studies as an advantage, since tremendous progress emerges every day for scaffold preparation and bioreactor developments, which likely will benefit the field. Yet, the large heterogeneity of approaches used also complicates progress evaluation, where to continue, and what approaches to eliminate. Since our results are based on an unbiased systematical approach reflecting the field's status and not only a single study, they may be used to accelerate such decisions.
Specifically, we found that acellular TEVGs as compared with recellularized grafts were less prone to being patent (62.4% versus 85.1%, respectively), supporting for the first‐time previous studies 47, 62, 92, 93 on a meta‐analysis level. Moreover, our analysis pinpoints that the use of smooth muscle cells in addition to endothelial cells do not provide an immediate benefit to the patency. Thus, future studies may focus more specifically on generating TEVGs with endothelial cells alone, and here improve the specific condition for achieving a mature continuous sheet of fully functioning endothelial cells. Based on our data, preconditioning in emerging bioreactors will likely enhance this process, and it will be interesting in the future to do specific exploratory meta‐analysis of this parameter, when more studies exploiting this approach have been performed. Yet, our data do suggest that long‐term (several days) recellularization time prior to in vivo analysis is beneficial, but it is appropriate to speculate that recellularization duration may be reduced with the use of preconditioning bioreactors. In contrast, the physiological source of the endothelial cell does not seem to have a major impact on the patency according to our analysis. Thus, easy accessibility and clinically relevant cell numbers for generating TEVGs of 20–60 cm seem more important in relation to clinical translation of the approach than the origin for endothelial cell function itself. However, it is important to emphasize that we did not distinguish between the methods used for obtaining the cells. It is thus likely that some endothelial cell preparations are purer than others, and therefore result in a better fully functioning endothelium. We also did not distinguish between autologous, allogenic, and xenogenic cell preparations, which may elicit different host immune responses and hereby impact patency. Yet, we did speculate that an immature origin using circulating‐derived or bone marrow‐derived endothelial progenitors could be more beneficial for the patency than using mature blood vessel derived endothelial cells, but did not find a relationship. Systemic antithrombotic treatment was not included in the meta‐analysis as an explanatory variable but included as part of cointerventions in the risk of bias analysis, since it is a standard treatment for humans undergoing bypass surgery. Yet, based on the 36 animal studies using antithrombotic treatment herein, we indeed did not find any major impact on patency as otherwise could have been expected. Yet, antithrombotic modifications used for scaffold surface modifications did turn out to have an immediate positive effect on TEVG patency in our analysis. However, we consider that the number of studies using surface modification still is too low to make any robust conclusions and in addition embraces several types of modifications. Overall, we did not observe that any of the scaffold types were statistically significantly different from the reference type (decellularized), and thus the type of scaffold used may be less important. Yet, we can only speculate if another reference type would change the result, but we chose, prior to analysis, decellularized vessel as the reference type since this scaffold is closest to native vessels. Moreover, new scaffolds are developed continuously, and we may in the field not have identified the most optimal one yet.
Regarding follow‐up time, we showed that the included studies have a median of 56 days, which is clinically inadequate. Importantly, this relative short follow‐up does not generally allow for hyperplasia induced occlusions to occur 94. Thus, the relative high patency reported in large animal studies might be exaggerated, and whether this is comparable to the overall patency of 60% obtained after 10 years in humans when using autologous grafts remains questionable 14. Indeed, we did not observe an association between follow‐up time and patency in the timeframe used for animals (data not shown), yet again this could be due to the short evaluation time of most studies. Likewise, the median TEVG length in the animal studies was 5 cm, which seems irrelevant in relation to most clinical bypass procedures that often requires >20 cm. Most likely this reflects different anatomical targets in the animal settings for the TEVG tested (of which we had no restrictions for inclusion in our analysis). However, shorter grafts are suggested to be more prone to being patent 34 and our study for the first time adds meta‐analyses to support this statement. Thus, we can only speculate if the relative high patency obtained for the short TEVGs in animal trials can be recapitulated if the TEVG length is increased to clinical dimensions. Thus, future studies addressing longer TEVGs will be valuable. Moreover, careful reporting on the length and diameter is also desired to enable robust comparisons of TEVG patency. We encountered 12 studies with insufficient reporting on length. On a similar note, it is important to emphasize that the animals used are normal, healthy individuals, whereas patients most often suffer various vascular defects and diseases that may further lower patency of TEVGs. One advantage of the large animal studies is, however, the possibility for performing subsequent explant analysis of the in vivo tested TEVGs. We did not focus on those results but found that the majority of studies (66) did perform additional explant analysis, which clearly supports the robustness of the corresponding in vivo patency measurements. Another important analysis is in vitro mechanical testing that at least for burst pressure and suture retention herein correlates with in vivo patency. Thus, these mechanical testing methods that are less complex and more easily performed than large animal studies may further help researchers predict which TEVGs that indeed will perform in vivo and thereby prevent time consuming in vivo studies on low quality TEVGs. For obvious ethical restrictions the follow‐up in clinical studies are based on the physiology of the patient (i.e., body weight), physiological complications arising post implantation, and in vivo patency assessed by for example, Doppler ultrasound. Thus, clinical studies may be biased by secondary and undiagnosed effects, which may result in a lower patency. Both ultrasound and palpation are generally considered subjective measures for in vivo patency assessment, whereas angiography is more reliable 95, 96. In the included animal studies, 32 of the studies used ultrasound alone, whereas nine studies used angiography or both. Indeed, high risk of bias for patency assessment was encountered to be associated with a higher patency. During our systematic reviewing process, we identified that one major issue in the field might be that patency is reported as a binary measure. This eliminates the possibility to distinguish partly occluded scaffolds, which could be of benefit for the field, but at the moment is limited by the available methods. Indeed, the secondary analysis performed in large animal studies enables a more robust and detailed analysis of graded patencies, which could support reported patency.
Finally, we cannot emphasize enough that the present analysis is restricted to the data collected for the present study and to the statistical evaluation methods chosen herein. In particular, we cannot exclude that a few eligible studies have been unnoticed by our search strategy, and we apologize to authors of those studies. Also, it is likely that other factors than those analyzed and discussed here have an impact on the patency. Such factors include but are not limited to the addition of specific growth and differentiation factors to culture medium, as well as under what conditions cells are seeded and cultured, that is, single‐perfusion or bi‐perfusion and static or 3D rotation, oxygen tensions, and so on.
Conclusion
Thus, in conclusion our data underscore that recellularization of the TEVG with endothelial cells inside the lumen for several days is a prerequisite with the type of scaffolds considered hereto and preconditioning and scaffold surface modification may further benefit patency. Moreover, our data suggest that more standard guidelines for testing and reporting TEVGs in large animals should be considered at least with regards to TEVG length, follow‐up, patency assessment, and the use of appropriate controls.
Author Contributions
I.S.: collection of data, data analysis and interpretation, manuscript writing, final approval of manuscript; H.J.B.: collection of data, data analysis and interpretation, final approval of manuscript; E.B.H.: collection of data, data analysis including statistics, manuscript writing and editing, final approval of manuscript; C.D.J.: data analysis including statistics, manuscript editing, final approval of manuscript; J.S.L.: data interpretation, manuscript writing and editing, final approval of manuscript; D.C.A.: conception and design, collection of data, data analysis and interpretation, manuscript writing, final approval of manuscript, financial support.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Data Availability Statement
The data that support the findings of this study are available in the main article and in the supplemental material available online or may be obtained from the corresponding author upon reasonable request.
Supporting information
Appendix S1: Supplemenatry Information
Acknowledgments
We acknowledge all authors who provided further details on published studies, enabling our analysis to be as optimal as possible. This study was partly supported by a strategic grant from Odense University Hospital (OUH), research finance from Department of Clinical Biochemistry and Pharmacology (OUH), and a grant from the Danish Innovation Foundation (#7051‐00001A).
References
- 1. Seifu DG, Purnama A, Mequanint K et al. Small‐diameter vascular tissue engineering. Nat Rev Cardiol 2013;10:410–421. [DOI] [PubMed] [Google Scholar]
- 2. Kannan RY, Salacinski HJ, Butler PE et al. Current status of prosthetic bypass grafts: A review. J Biomed Mater Res B Appl Biomater 2005;74:570–581. [DOI] [PubMed] [Google Scholar]
- 3. McBane JE, Sharifpoor S, Labow RS et al. Tissue engineering a small diameter vessel substitute: Engineering constructs with select biomaterials and cells. Curr Vasc Pharmacol 2012;10:347–360. [DOI] [PubMed] [Google Scholar]
- 4. Hoenig MR, Campbell GR, Rolfe BE et al. Tissue‐engineered blood vessels: Alternative to autologous grafts? Arterioscler Thromb Vasc Biol 2005;25:1128–1134. [DOI] [PubMed] [Google Scholar]
- 5. Baguneid M, de Mel A, Yildirimer L et al. In vivo study of a model tissue‐engineered small‐diameter vascular bypass graft. Biotechnol Appl Biochem 2011;58:14–24. [DOI] [PubMed] [Google Scholar]
- 6. Desai M, Seifalian AM, Hamilton G. Role of prosthetic conduits in coronary artery bypass grafting. Eur J Cardiothorac Surg 2011;40:394–398. [DOI] [PubMed] [Google Scholar]
- 7. Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science 1986;231:397–400. [DOI] [PubMed] [Google Scholar]
- 8. Lawson JH, Glickman MH, Ilzecki M et al. Bioengineered human acellular vessels for dialysis access in patients with end‐stage renal disease: Two phase 2 single‐arm trials. Lancet 2016;387:2026–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olausson M, Kuna VK, Travnikova G et al. In vivo application of tissue‐engineered veins using autologous peripheral whole blood: A proof of concept study. EBioMedicine 2014;1:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wystrychowski W, Cierpka L, Zagalski K et al. Case study: First implantation of a frozen, devitalized tissue‐engineered vascular graft for urgent hemodialysis access. J Vasc Access 2011;12:67–70. [DOI] [PubMed] [Google Scholar]
- 11. Cleary MA, Geiger E, Grady C et al. Vascular tissue engineering: The next generation. Trends Mol Med 2012;18:394–404. [DOI] [PubMed] [Google Scholar]
- 12. Song H‐HG, Rumma RT, Ozaki CK et al. Vascular tissue engineering: Progress, challenges, and clinical promise. Cell Stem Cell 2018;22:340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomas LV, Lekshmi V, Nair PD. Tissue engineered vascular grafts—Preclinical aspects. Int J Cardiol 2013;167:1091–1100. [DOI] [PubMed] [Google Scholar]
- 14. de Vries MR, Simons KH, Jukema JW et al. Vein graft failure: From pathophysiology to clinical outcomes. Nat Rev Cardiol 2016;13:451–470. [DOI] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol 2009;62:e1–e34. [DOI] [PubMed] [Google Scholar]
- 16. Software Csr. Veritas Health Innovation.
- 17. Isenberg BC, Williams C, Tranquillo RT. Small‐diameter artificial arteries engineered in vitro. Circ Res 2006;98:25–35. [DOI] [PubMed] [Google Scholar]
- 18. L'Heureux N, Dusserre N, Marini A et al. Technology insight: The evolution of tissue‐engineered vascular grafts—From research to clinical practice. Nat Clin Pract Cardiovasc Med 2007;4:389–395. [DOI] [PubMed] [Google Scholar]
- 19. Koobatian MT, Row S, Smith RJ Jr et al. Successful endothelialization and remodeling of a cell‐free small‐diameter arterial graft in a large animal model. Biomaterials 2016;76:344–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Conte MS, Mann MJ, Simosa HF et al. Genetic interventions for vein bypass graft disease: A review. J Vasc Surg 2002;36:1040–1052. [DOI] [PubMed] [Google Scholar]
- 21. Viswanathan M, Ansari MT, Berkman ND et al. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. Methods guide for comparative effectiveness reviews: Publication no. 12‐EHC047‐EF; 2012.
- 22. Benjamini Y, Hochberg Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J R Stat Soc B Met 1995;57:289–300. [Google Scholar]
- 23. Allison PD. Missing Data. SAGE Publications, University of Pennsylvania, USA, 2001. [Google Scholar]
- 24. R Core Team . R: A Language and Environment for Statistical Computing; 2018.
- 25. Anderson DEJ, Glynn JJ, Song HK et al. Engineering an endothelialized vascular graft: A rational approach to study design in a non‐human primate model. PLoS One 2014;9:e115163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aper T, Wilhelmi M, Gebhardt C et al. Novel method for the generation of tissue‐engineered vascular grafts based on a highly compacted fibrin matrix. Acta Biomater 2016;29:21–32. [DOI] [PubMed] [Google Scholar]
- 27. Arts CHP, Hedeman Joosten PPA, Blankensteijn JD et al. Contaminants from the transplant contribute to intimal hyperplasia associated with microvascular endothelial cell seeding. Eur J Vasc Endovasc Surg 2002;23:29–38. [DOI] [PubMed] [Google Scholar]
- 28. Aussel A, Thébaud NB, Bérard X et al. Chitosan‐based hydrogels for developing a small‐diameter vascular graft: in vitro and in vivo evaluation. Biomed Mater 2017;12:065003. [DOI] [PubMed] [Google Scholar]
- 29. Büttemeyer R, Mall JW, Paulitschke M et al. In a pig model ePTFE grafts will sustain for 6 weeks a confluent endothelial cell layer formed in vitro under shear stress conditions. Eur J Vasc Endovasc Surg 2003;26:156–160. [DOI] [PubMed] [Google Scholar]
- 30. Cho S‐W, Lim SH, Kim I‐K et al. Small‐diameter blood vessels engineered with bone marrow‐derived cells. Ann Surg 2005;241:506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clarke DR, Lust RM, Sun YS et al. Transformation of nonvascular acellular tissue matrices into durable vascular conduits. Ann Thorac Surg 2001;71:S433–S436. [DOI] [PubMed] [Google Scholar]
- 32. Dahan N, Sarig U, Bronshtein T et al. Dynamic autologous reendothelialization of small‐caliber arterial extracellular matrix: A preclinical large animal study. Tissue Eng Part A 2017;23:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dahl SLM, Kypson AP, Lawson JH et al. Readily available tissue‐engineered vascular grafts. Sci Transl Med 2011;3:68ra69. [DOI] [PubMed] [Google Scholar]
- 34. Fukunishi T, Best CA, Sugiura T et al. Tissue‐engineered small diameter arterial vascular grafts from cell‐free nanofiber PCL/chitosan scaffolds in a sheep model. PLoS One 2016;11:e0158555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. García‐Honduvilla N, Domínguez B, Pascual G et al. Viability of engineered vessels as arterial substitutes. Ann Vasc Surg 2008;22:255–265. [DOI] [PubMed] [Google Scholar]
- 36. Han B‐S, Fan C‐Y, Liu S‐H et al. Surface heparinization and blood compatibility modification of small intestinal submucosa for small‐caliber vascular regeneration with hypothermic plasma technique. J Clin Rehab Tissue Eng Res 2008;12:2753–2756. [Google Scholar]
- 37. He H, Matsuda T. Arterial replacement with compliant hierarchic hybrid vascular graft: Biomechanical adaptation and failure. Tissue Eng 2002;8:213–224. [DOI] [PubMed] [Google Scholar]
- 38. He H, Shirota T, Yasui H et al. Canine endothelial progenitor cell‐lined hybrid vascular graft with nonthrombogenic potential. J Thorac Cardiovasc Surg 2003;126:455–464. [DOI] [PubMed] [Google Scholar]
- 39. Hinds MT, Rowe RC, Ren Z et al. Development of a reinforced porcine elastin composite vascular scaffold. J Biomed Mater Res A 2006;77:458–469. [DOI] [PubMed] [Google Scholar]
- 40. Ju YM, Ahn H, Arenas‐Herrera J et al. Electrospun vascular scaffold for cellularized small diameter blood vessels: A preclinical large animal study. Acta Biomater 2017;59:58–67. [DOI] [PubMed] [Google Scholar]
- 41. Kaushal S, Amiel GE, Guleserian KJ et al. Functional small‐diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med 2001;7:1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim WS, Seo JW, Rho JR et al. Histopathologic changes of acellularized xenogenic carotid vascular grafts implanted in a pig‐to‐goat model. Int J Artif Organs 2007;30:44–52. [DOI] [PubMed] [Google Scholar]
- 43. Koch S, Flanagan TC, Sachweh JS et al. Fibrin‐polylactide‐based tissue‐engineered vascular graft in the arterial circulation. Biomaterials 2010;31:4731–4739. [DOI] [PubMed] [Google Scholar]
- 44. Koenneker S, Teebken OE, Bonehie M et al. A biological alternative to alloplastic grafts in dialysis therapy: Evaluation of an autologised bioartificial haemodialysis shunt vessel in a sheep model. Eur J Vasc Endovasc Surg 2010;40:810–816. [DOI] [PubMed] [Google Scholar]
- 45. Koens MJW, Krasznai AG, Hanssen AEJ et al. Vascular replacement using a layered elastin‐collagen vascular graft in a porcine model: One week patency versus one month occlusion. Organogenesis 2015;11:105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. L'Heureux N, Dusserre N, Konig G et al. Human tissue‐engineered blood vessels for adult arterial revascularization. Nat Med 2006;12:361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. L'Heureux N, Pâquet S, Labbé R et al. A completely biological tissue‐engineered human blood vessel. FASEB J 1998;12:47–56. [DOI] [PubMed] [Google Scholar]
- 48. Li C, Hill A, Imran M. In vitro and in vivo studies of ePTFE vascular grafts treated with P15 peptide. J Biomater Sci Polym Ed 2005;16:875–891. [DOI] [PubMed] [Google Scholar]
- 49. Li Q, Huang C, Xu Z et al. The fetal porcine aorta and mesenteric acellular matrix as small‐caliber tissue engineering vessels and microvasculature scaffold. Aesthetic Plast Surg 2013;37:822–832. [DOI] [PubMed] [Google Scholar]
- 50. Liu JY, Swartz DD, Peng HF et al. Functional tissue‐engineered blood vessels from bone marrow progenitor cells. Cardiovasc Res 2007;75:618–628. [DOI] [PubMed] [Google Scholar]
- 51. Ma X, He Z, Li L et al. Development and in vivo validation of tissue‐engineered, small‐diameter vascular grafts from decellularized aortae of fetal pigs and canine vascular endothelial cells. J Cardiothorac Surg 2017;12:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mahara A, Somekawa S, Kobayashi N et al. Tissue‐engineered acellular small diameter long‐bypass grafts with neointima‐inducing activity. Biomaterials 2015;58:54–62. [DOI] [PubMed] [Google Scholar]
- 53. Meier LA, Syedain ZH, Lahti MT et al. Blood outgrowth endothelial cells alter remodeling of completely biological engineered grafts implanted into the sheep femoral artery. J Cardiovasc Transl Res 2014;7:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miwa H, Matsuda T. An integrated approach to the design and engineering of hybrid arterial prostheses. J Vasc Surg 1994;19:658–667. [DOI] [PubMed] [Google Scholar]
- 55. Miwa H, Matsuda T, Tani N et al. An in vitro endothelialized compliant vascular graft minimizes anastomotic hyperplasia. ASAIO J 1993;39:M501–M505. [PubMed] [Google Scholar]
- 56. Narita Y, Kagami H, Matsunuma H et al. Decellularized ureter for tissue‐engineered small‐caliber vascular graft. J Artif Organs 2008;11:91–99. [DOI] [PubMed] [Google Scholar]
- 57. Neff LP, Tillman BW, Yazdani SK et al. Vascular smooth muscle enhances functionality of tissue‐engineered blood vessels in vivo. J Vasc Surg 2011;53:426–434. [DOI] [PubMed] [Google Scholar]
- 58. Nemcova S, Noel AA, Jost CJ et al. Evaluation of a xenogeneic acellular collagen matrix as a small‐diameter vascular graft in dogs—Preliminary observations. J Invest Surg 2001;14:321–330. [DOI] [PubMed] [Google Scholar]
- 59. Niklason LE, Gao J, Abbott WM et al. Functional arteries grown in vitro. Science 1999;284:489–493. [DOI] [PubMed] [Google Scholar]
- 60. Ong CS, Fukunishi T, Liu RH et al. Bilateral arteriovenous shunts as a method for evaluating tissue‐engineered vascular grafts in large animal models. Tissue Eng Part C Methods 2017;23:728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pellegata AF, Dominioni T, Ballo F et al. Arterial decellularized scaffolds produced using an innovative automatic system. Cells Tissues Organs 2015;200:363–373. [DOI] [PubMed] [Google Scholar]
- 62. Quint C, Kondo Y, Manson RJ et al. Decellularized tissue‐engineered blood vessel as an arterial conduit. Proc Natl Acad Sci USA 2011;108:9214–9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ramesh B, Mathapati S, Galla S et al. Crosslinked acellular saphenous vein for small‐diameter vascular graft. Asian Cardiovasc Thorac Ann 2013;21:293–302. [DOI] [PubMed] [Google Scholar]
- 64. Rivero KTV, Escobar JJ, Forero SDG et al. New regenerative vascular grafts for hemodialysis access: Evaluation of a preclinical animal model. J Invest Surg 2018;31:192–200. [DOI] [PubMed] [Google Scholar]
- 65. Rothuizen TC, Damanik FFR, Lavrijsen T et al. Development and evaluation of in vivo tissue engineered blood vessels in a porcine model. Biomaterials 2016;75:82–90. [DOI] [PubMed] [Google Scholar]
- 66. Rotmans JI, Heyligers JMM, Verhagen HJM et al. In vivo cell seeding with anti‐CD34 antibodies successfully accelerates endothelialization but stimulates intimal hyperplasia in porcine arteriovenous expanded polytetrafluoroethylene grafts. Circulation 2005;112:12–18. [DOI] [PubMed] [Google Scholar]
- 67. Row S, Peng H, Schlaich EM et al. Arterial grafts exhibiting unprecedented cellular infiltration and remodeling in vivo: The role of cells in the vascular wall. Biomaterials 2015;50:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sánchez‐Palencia DM, Navarro J, Araque JC et al. Effects of fabrication on early patency and regeneration of small intestinal submucosa vascular grafts. ASAIO J 2015;61:596–604. [DOI] [PubMed] [Google Scholar]
- 69. Scherner M, Reutter S, Klemm D et al. In vivo application of tissue‐engineered blood vessels of bacterial cellulose as small arterial substitutes: Proof of concept? J Surg Res 2014;189:340–347. [DOI] [PubMed] [Google Scholar]
- 70. Son KH, Fang YH, Choi YJ et al. Evaluation of the hemodynamics of a tissue‐engineered hybrid graft. Artif Organs 2010;34:E17–E21. [DOI] [PubMed] [Google Scholar]
- 71. Swartz DD, Russell JA, Andreadis ST. Engineering of fibrin‐based functional and implantable small‐diameter blood vessels. Am J Physiol Heart Circ Physiol 2005;288:H1451–H1460. [DOI] [PubMed] [Google Scholar]
- 72. Syedain ZH, Graham ML, Dunn TB et al. A completely biological “off‐the‐shelf” arteriovenous graft that recellularizes in baboons. Sci Transl Med 2017;9:eaan4209. [DOI] [PubMed] [Google Scholar]
- 73. Syedain ZH, Meier LA, Lahti MT et al. Implantation of completely biological engineered grafts following decellularization into the sheep femoral artery. Tissue Eng Part A 2014;20:1726–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Teebken OE, Pichlmaier AM, Haverich A. Cell seeded decellularised allogeneic matrix grafts and biodegradable polydioxanone‐prostheses compared with arterial autografts in a porcine model. Eur J Vasc Endovasc Surg 2001;22:139–145. [DOI] [PubMed] [Google Scholar]
- 75. Tillman BW, Yazdani SK, Neff LP et al. Bioengineered vascular access maintains structural integrity in response to arteriovenous flow and repeated needle puncture. J Vasc Surg 2012;56:783–793. [DOI] [PubMed] [Google Scholar]
- 76. Turner NJ, Murphy MO, Kielty CM et al. α2(VIII) collagen substrata enhance endothelial cell retention under acute shear stress flow via an α2β1 integrin‐dependent mechanism: An in vitro and in vivo study. Circulation 2006;114:820–829. [DOI] [PubMed] [Google Scholar]
- 77. Ulczok R, Milewski K, Bis J et al. A novel peritoneum derived vascular prosthesis formed on a latex catheter in an SDF‐1 chemokine enriched environment: A pilot study. Int J Artif Organs 2015;38:89–95. [DOI] [PubMed] [Google Scholar]
- 78. Weber C, Reinhardt S, Eghbalzadeh K et al. Patency and in vivo compatibility of bacterial nanocellulose grafts as small‐diameter vascular substitute. J Vasc Surg 2018;68:177S–187S.e1. [DOI] [PubMed] [Google Scholar]
- 79. Wippermann J, Schumann D, Klemm D et al. Preliminary results of small arterial substitute performed with a new cylindrical biomaterial composed of bacterial cellulose. Eur J Vasc Endovas Surg 2009;37:592–596. [DOI] [PubMed] [Google Scholar]
- 80. Wulff B, Stahlhoff S, Vonthein R et al. Biomimetic heparan sulfate‐like coated ePTFE grafts reduce in‐graft neointimal hyperplasia in ovine carotids. Ann Vasc Surg 2017;40:274–284. [DOI] [PubMed] [Google Scholar]
- 81. Xie X, Eberhart A, Guidoin R et al. Five types of polyurethane vascular grafts in dogs: The importance of structural design and material selection. J Biomater Sci Polym Ed 2010;21:1239–1264. [DOI] [PubMed] [Google Scholar]
- 82. Yang D, Guo T, Nie C et al. Tissue‐engineered blood vessel graft produced by self‐derived cells and allogenic acellular matrix: A functional performance and histologic study. Ann Plast Surg 2009;62:297–303. [DOI] [PubMed] [Google Scholar]
- 83. Ye L, Wu X, Duan H‐Y et al. The in vitro and in vivo biocompatibility evaluation of heparin‐poly(ε‐caprolactone) conjugate for vascular tissue engineering scaffolds. J Biomed Mater Res A 2012;100:3251–3258. [DOI] [PubMed] [Google Scholar]
- 84. Yokota T, Ichikawa H, Matsumiya G et al. In situ tissue regeneration using a novel tissue‐engineered, small‐caliber vascular graft without cell seeding. J Thorac Cardiovasc Surg 2008;136:900–907. [DOI] [PubMed] [Google Scholar]
- 85. Zavan B, Vindigni V, Lepidi S et al. Neoarteries grown in vivo using a tissue‐engineered hyaluronan‐based scaffold. FASEB J 2008;22:2853–2861. [DOI] [PubMed] [Google Scholar]
- 86. Zhao Y, Zhang S, Zhou J et al. The development of a tissue‐engineered artery using decellularizedscaffold and autologous ovine mesenchymal stem cells. Biomaterials 2010;31:296–307. [DOI] [PubMed] [Google Scholar]
- 87. Zhou M, Liu Z, Liu C et al. Tissue engineering of small‐diameter vascular grafts by endothelial progenitor cells seeding heparin‐coated decellularized scaffolds. J Biomed Mater Res B Appl Biomater 2012;100:111–120. [DOI] [PubMed] [Google Scholar]
- 88. Zhou M, Liu Z, Wei Z et al. Development and validation of small‐diameter vascular tissue from a decellularized scaffold coated with heparin and vascular endothelial growth factor. Artif Organs 2009;33:230–239. [DOI] [PubMed] [Google Scholar]
- 89. Zhou M, Qiao W, Liu Z et al. Development and in vivo evaluation of small‐diameter vascular grafts engineered by outgrowth endothelial cells and electrospun chitosan/poly(ε‐caprolactone) nanofibrous scaffolds. Tissue Eng Part A 2014;20:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhou X, Zhao B, Wang J et al. Engineered vascular graft using nanoscale decellularized arteries modified with controlled‐release heparin and vascular endothelial growth factor. J Biomater Tissue Eng 2016;6:870–882. [Google Scholar]
- 91. Furukoshi M, Moriwaki T, Nakayama Y. Development of an in vivo tissue‐engineered vascular graft with designed wall thickness (biotube type C) based on a novel caged mold. J Artif Organs 2016;19:54–61. [DOI] [PubMed] [Google Scholar]
- 92. Peng H, Schlaich EM, Row S et al. A novel ovine ex vivo arteriovenous shunt model to test vascular implantability. Cells Tissues Organs 2012;195:108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Schneider PA, Hanson SR, Price TM et al. Preformed confluent endothelial cell monolayers prevent early platelet deposition on vascular prostheses in baboons. J Vasc Surg 1988;8:229–235. [PubMed] [Google Scholar]
- 94. Van Tricht I, De Wachter D, Tordoir J et al. Hemodynamics and complications encountered with arteriovenous fistulas and grafts as vascular access for hemodialysis: A review. Ann Biomed Eng 2005;33:1142–1157. [DOI] [PubMed] [Google Scholar]
- 95. Meyer M, Geiger N, Benck U et al. Imaging of patients with complex hemodialysis arterio‐venous fistulas using time‐resolved dynamic CT angiography: Comparison with duplex ultrasound. Sci Rep 2017;7:12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Madycki G, Gabriel M, Hawro P et al. Duplex Doppler ultrasound examination of carotid and vertebral arteries: Guidelines of the Polish Society for Vascular Surgery. Kardiol Pol 2014;72:288–309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplemenatry Information
Data Availability Statement
The data that support the findings of this study are available in the main article and in the supplemental material available online or may be obtained from the corresponding author upon reasonable request.


