Abstract
Purpose: To report a rare case of spontaneous suprachoroidal hemorrhage (SSCH) in a high myopia patient with rhegmatogenous retinal detachment (RRD) and successful treatment.
Methods: We present a case of SSCH that occurred in a 73 woman with high myopia with RRD and discuss the results of a systemic review of the literature published from 1999 to 2017.
Results: Phacoemulsification without intraocular lens implantation and vitrectomy combined with silicone oil injection was performed and retinal detachment and choroidal detachment were reattached after oil removed. In the literature review, we found that among a total of 36 patients (37 eyes), acute secondary glaucoma was a complication in 70.3% (26 eyes) of the cases, and over half of the cases (24 eyes, 64.9%) were treated with surgery. Eighteen cases (50%) were characterized by systemic hypertension and 21 cases (58.3%) had abnormal hemostasis. Age-related macular degeneration (ARMD) was the most common (12 eyes, 32.4%) ocular disease and was followed by glaucoma (7 cases, 18.9%). Visual acuity was classified as hand motion (HM) or worse in 25 eyes (out of 34 eyes, 73.5%) at initial presentation and in 25 eyes (out of 36 eyes, 69.4%) upon final examination. Nine cases experienced significant visual improvement, including six that underwent vitrectomy.
Conclusion: Advanced age, systemic anticoagulation, and hypertension are strong risk factors. RRD associated with massive SSCH is an extremely rare event. Vitrectomy and choroidal blood drainage can effectively remove suprachoroidal hemorrhage (SCH) and promote retinal reattachment in these eyes. However, the final visual prognosis usually remains poor.
Keywords: high myopia, rhegmatogenous retinal detachment, Spontaneous suprachoroidal hemorrhage, vitrectomy
Introduction
Suprachoroidal hemorrhage (SCH) is usually associated with ocular surgery or ocular trauma. Spontaneous suprachoroidal hemorrhage (SSCH) is much more unusual. In most reported spontaneous cases, the patients were predisposed to bleeding because of inherited blood dyscrasia, age-related macular degeneration (ARMD), or the use of systemic antithrombotic therapy (e.g., antiplatelet, anticoagulation, or thrombolytic agents) [1]. Here, we report a case of SSCH in a high myopia patient with rhegmatogenous retinal detachment (RRD) and choroidal detachment. We also review the relevant literature regarding predisposing factors, the clinical course, and visual outcomes with the aim of preventing the occurrence of SSCH and providing appropriate management strategies.
Case report
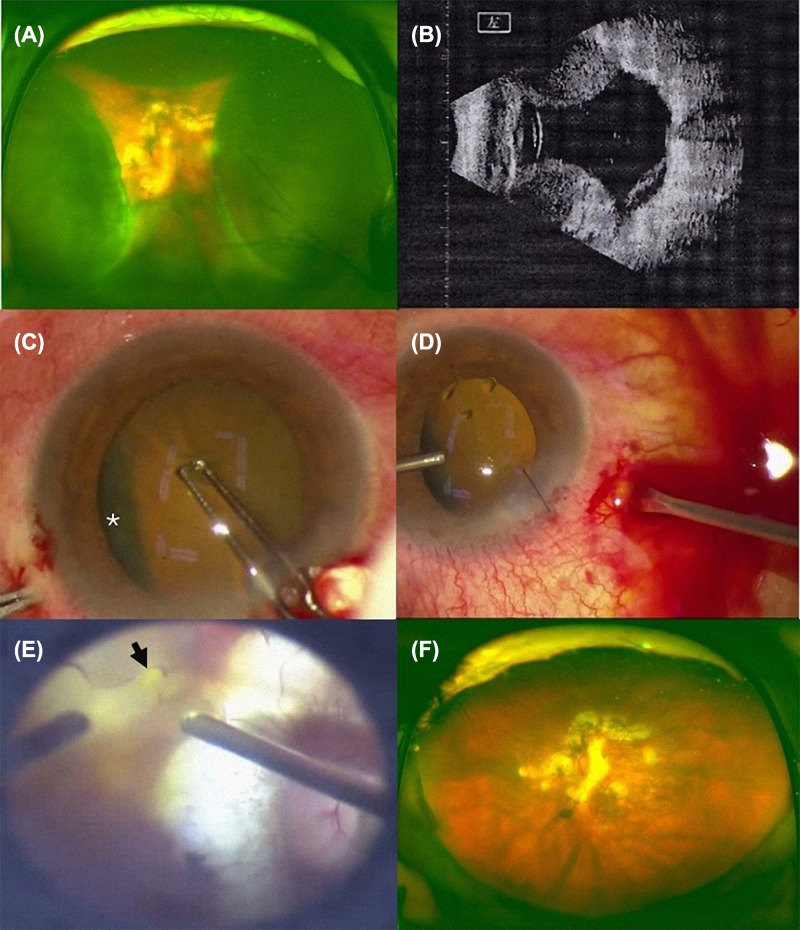
A 73-year-old Chinese female presented with blurred vision in the left eye for 50 days without any obvious inducement. Her medical history included high myopia for more than 50 years, but she had never had diabetes, hypertension, or other systemic illness. On admission, her blood pressure was 100/60 mmHg, her visual acuity was light perception, and her intraocular pressure (IOP) was 5 mmHg in the left eye. An ocular examination performed on the left eye showed a flare and was positive for cells in the anterior chamber. A fundus examination and B-ultrasound revealed retinal detachment with choroidal detachment (Figure 1A, B).
Figure 1. Clinical data of patient.
(A) Fundus examination revealed retinal detachment with choroidal detachment. (B) B-ultrasound revealed retinal detachment with choroidal detachment. (C) The choroidal detachment (white star) was clearly visible before phacoemulsification. (D) During the surgery, a copious, thick flux of blood flowed out of the 20G cannulas and was followed by a yellowish liquid. (E) A 1/5PD hole was found in the arch of the vascular arch below the macula (black arrow). (F) After surgical treatment, the retinal detachment and choroidal detachment were reduced.
All hematological and biochemical tests were within normal limits. After surgical contraindications were excluded, we performed a combined phacoemulsification without intraocular lens implantation and vitrectomy in combination with silicone oil injection. The choroidal detachment (Figure 1C, star) was clearly visible before phacoemulsification was performed. After phacoemulsification, 20G and 23G vitrectomy cannulas were placed 3.5 mm from the limbus. The 20G cannula was left open, and the infusion line was placed in the anterior chamber through a clear corneal paracentesis with a bottle height of 40 mmHg. As soon as the infusion line was opened, a copious, thick flux of blood flowed out of the 20G cannulas and was followed a yellowish liquid (Figure 1D). As the blood flow continued, the choroidal detachment visibly recessed. Though there was tight adhesion between the posterior vitreous and retina, the posterior vitreous cortex was completely separated from the inner surface of the retina. A 1/5PD hole was found in the arch of the vascular arch below the macula (Figure 1E, black arrow), and a full vitrectomy combined with intravitreal silicone oil tamponade was performed. After surgical treatment, the retinal detachment and choroidal detachment were reduced (Figure 1F). After treatment with a silicone oil tamponade, visual acuity was hand motion (HM) and counting fingers after 1 day and 1 month, respectively. After 3 months, the silicone oil was removed, and recurrent retinal detachment occurred because of proliferative vitreoretinopathy (PVR). A secondary surgery was performed to peel the preretinal membrane and apply silicon oil, and the silicone oil was removed 3 months later. Finally, the patient showed a good reattached choroidea and retina and achieved a visual acuity of 20/2000.
Materials and methods
A total of 29 articles (including 36 patients) published from December 1999 to March 2017 were reviewed and analyzed according to clinical course, risk factors, management, and final outcomes. Studies in which SSCH happened during or immediately after the surgery were excluded.
Including our case [1–28] (Table 1), 13 men and 23 women (37 eyes after 2 cases with missing data were excluded) were analyzed. One case had bilateral involvement for 1 year. The ages of the patients ranged from 27 to 90 years old [mean ± standard deviation (SD) 67.9 ± 13.6 years old]. The IOP on initial presentation ranged from 3 to 70 mmHg in 32 recorded eyes (mean ± SD 39.8 ± 14.7 mmHg), and four patients did not have a high IOP on the first visit prior to SSCH progression. Among five eyes without IOP data, high IOP was listed as >50 mmHg in one case, and two had corneal perforations that were assumed to have resulted from glaucoma. In all, acute glaucoma was noted in 28 eyes (75.7%). Two cases of low IOP (3–5 mmHg) were high myopia with RRD.
Table 1. Review of previous reports of sapantaneous choroidal hemorrhage.
| Reference (year) | Age (year) | Sex | Eye | MH | OH | Anticoagulant agent | Initial VA | Initial IOP (mmHg) | Management | Final VA | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Wong [2], 1999 | 76 | M | R | HTN, IHD, DM | IOL | Heparin | CF | 60 | Medications | NLP |
| 2. | Ophir [3], 2001 | 90 | F | R | IHD, DM | Glaucoma | None | NLP | Perforation | Eviseration | – |
| 3. | Chen [1], 2001 | 57 | M | R | HTN, CBE, DM | AMD | None | HM | 50 | Medicaions | NLP |
| 78 | M | L | CAD | AMD | None | NLP | 50 | Sclerotomy | NLP | ||
| 55 | M | R | HTN | AMD | None | NLP | 42 | Sclerotomy | HM | ||
| 67 | F | L | HTN, DM, VHC | AMD | None | NA | 70 | Sclerotomy | N/A | ||
| 4. | Dandekar [4], 2001 | 62 | F | L | RHD | None | Warfarin | NA | NA | Medications | LP |
| 5. | Neudorfer [5], 2002 | 84 | F | L | IHD, | IOL | Enoxaparin | CF | 70 | Medications | 20/100 |
| 6. | Knox [6], 2002 | 81 | F | R | A-Fib | AMD | Warfarin | LP | 36 | Iridotomy | NLP |
| 7. | Goldsmith [7], 2003 | 74 | F | L | HTN, CRF, AL | Glaucoma | None | NLP | Perforation | Evisceration | – |
| 8. | Chak [8], 2003 | 78 | F | L | HTN | High myopia | Aspirin | 6/12 | 20 | Medications | 6/12 |
| 9. | Hammam [9], 2003 | 65 | M | R | None | None | None | 6/24 | 38 | Medications | 6/5 |
| 10. | Yang [10], 2003 | 84 | M | R | HTN | AMD | None | HM | 43 | Sclerotomy | NLP |
| 84 | F | R | HTN, A-Fib | AMD, IOL | Warfarin | HM | 26 | Sclerotomy | NLP | ||
| 66 | F | L | MVR | AMD | Warfarin | LP | 40 | Sclerotomy | NLP | ||
| 85 | M | L | HTN | AMD | None | HM | 18 | Sclerotomy | NLP | ||
| 79 | F | L | HTN, A-Fib | AMD, IOL | Warfarin | LP | 29 | Sclerotomy | HM | ||
| 11. | Maguluri [11], 2005 | 52 | M | R | Hemophilia | None | None | HM | 27 | PPV, PPL, enucleation | – |
| 12. | Barsam [12], 2005 | 86 | F | R | DM, IHD | Glaucoma, IOL | Aspirin | NLP | >50 | Medications | HM |
| 13. | Saeed [13], 2007 | 27 | F | L | DM, hemodialysis | DR, IOL | tPA | CF | 48 | PPV, SO | 6/12 |
| 14. | Lee [14], 2007 | 81 | F | L | HTN, | None | Aspirin, plavix | NLP | 58 | Enucleation | – |
| 15. | Tajika [15], 2008 | 32 | M | L | CRF | None | None | 0.01 | 34 | PPV | 0.8 |
| 16. | Chandra [16], 2009 | 84 | M | L | HTN, CBS, PE | AMD, Glaucoma | Warfarin | NLP | 44 | Medications | NLP |
| 17. | Fukuchi [17], 2009 | 46 | F | L | HTN | NA | NA | NA | 55 | Medications | NA |
| 18. | Chen [18], 2009 | 86 | M | R | IHD, DVT | Glaucoma, AMD | Aspirin | NLP | 45 | Sclerotomy, evisceration | – |
| 19. | Lim [19], 2011 | 75 | F | L | Aortic aneurysms | Hypermetropic | None | HM | 70 | Paracentesis, enucleation | – |
| 20. | Nadarajah [20], 2012 | 71 | F | NA | HLD, HTN | Glaucoma | NA | LP | NA | Sclerotomy | 6/48 |
| 21. | Nguyen [21], 2012 | 24 | F | L | Cystic fibrosis, DM | DR | Warfarin, heparin | HM | 34 | Medications | NLP |
| 22. | Kim [22], 2013 | 53 | F | R | PD, HTN, CRF, DM | Vitrectomized | None | CF | 19 | Sclerotomy | 20/40 |
| 23. | Srikanth [23], 2013 | 57 | F | R | DLD | NA | NA | NLP | 44 | Perforaion, evisceration | – |
| 24. | Zhang [24], 2014 | 59 | F | R | None | High myopia | None | LP | 3 | Phaco, PPV, SO | CF |
| 61 | F | R | None | High myopia | None | HM | 5 | PPV, SO | 20/400 | ||
| 25. | Andreatta [25], 2016 | 90 | F | L | A-Fib | AMD | Warfarin | HM | 55 | Medications | LP |
| 26. | Hsiao [26], 2016 | 64 | M | R | HTN, BS, | None | Clopidogrel bisulfate | NLP | 59 | Iridotomy, sclerotomy | NLP |
| 27. | Albert [27], 2017 | 62 | F | R, L | HTN, DM, HLD | Glaucoma | None | 20/25 (R), 20/200 (L) | 17 | Medications | 20/40(R), 20/20(L) |
| 28. | Atdignysx [28], 2017 | 68 | F | L | HTN, AL, HF | None | Warfarin | LP | 50 | Sclerotomy | NLP |
MH: medical history; OH: ocular history; VA: visual acuity; IOP: intraocular pressure; M: male; F: female; R: right; L; left; HTN, hypertension; IHD: ischemic heart disease; DM: diabetes mellitus; CAD: coronary arterial disease; CBE: cerebrovascular episode; VHC: viral hepatitis C; NA: not available; RHD: rheumatic heart disease; A-Fib: atrial fibrillation; MVR: mitral valve replacement; CRF: chronic renal failure; BS: brainstem stroke; AL: arteriosclerosis; AMD: age-related macular degeneration; tPA: tissue plasminogen activator; CBS: cardiac bypass surgery; PE: pulmonary embolus; IOL: intraocular lens; DR: diabetic retinopathy; HLD: hyperlipidemia; DVT: deep vein thrombosis; PD: peritoneal dialysis; DLD: decompensated liver disease; HF: heart failture; CF: count fingers; HM: hand motions; LP: light perception; NLP: no light perception; PPV: pars plana vitrectomy; PPL: pars plana lentectomy; SO: silicone oil.
Table 2 summarizes the systemic factors associated with the development of SSCH. Hypertension was the most frequent systemic disease (18 cases, 50%) and was followed by cardiovascular or cerebrovascular disease (17 cases, 47.2%) and diabetes mellitus (9 cases, 25%). The Valsalva maneuver was noted in four cases (10.8%) prior to the episode. Chronic renal failure (CRF, 3 cases), decompensated liver disease (1 case), hemodialysis (1 case), peritoneal dialysis (1 case) and hemophilia (1 case) might have contributed to abnormal hemostasis. Anticoagulant, antiplatelet, and thrombolytic agents were used in 17 cases (47.2%) and included warfarin (9 cases), aspirin (4 cases), heparin (3 cases), clopidogrel (1 case), plavix (1 case) and a tissue plasminogen activator (tPA, 1 case). A combination of two anticoagulants was noted in two cases (Table 1). Table 2 also shows the associated ocular diseases. The most common factor was ARMD (12 eyes, 32.4%), which was followed by glaucoma (7 eyes, 18.9%) and pseudophakia (6 eyes, 16.2%). High myopia (3 eyes, 8.1%) and diabetic retinopathy (1 eye, 2.7%) were seldom associated.
Table 2. Summary of previous reports of sapantaneous choroidal hemorrhage.
| Characteristics | Values |
|---|---|
| Demographic data | n |
| Sex (M/F) | 13/23 |
| Eye (R/L) | 17/20 |
| Average age (year ± SD) | 67.9 ± 16.7 |
| Age range (year) | 27-90 |
| Medical history | n (%) |
| Hypertension | 18 (50) |
| Diabetes mellitus | 9 (25) |
| Ischemic heart disease | 5 (13.9) |
| Atrial fibrillation | 4 (11.1) |
| Chronic renal failure | 3 (8.3) |
| Arteriosclerosis | 2 (5.6) |
| Hyperlipidemia | 2 (5.6) |
| Hemophilia | 1 (2.8) |
| Hemodialysis | 1 (2.8) |
| Aortic aneurysms | 1 (2.8) |
| Cystic fibrosis | 1 (2.8) |
| Cerebrovascular episode | 1 (2.8) |
| Coronary arterial disease | 1 (2.8) |
| Viral hepatitis C | 1 (2.8) |
| Rheumatic heart disease | 1 (2.8) |
| Mitral valve replacement | 1 (2.8) |
| Deep vein thrombosis | 1 (2.8) |
| Peritoneal dialysis | 1 (2.8) |
| Decompensated liver disease | 1 (2.8) |
| Brainstem stroke | 1 (2.8) |
| None | 3 (8.3) |
| Anticoagulant agent | n (%) |
| Warfarin | 9 (25) |
| Aspirin | 4 (11.1) |
| Heparin | 3 (8.3) |
| Tissue plasminogen activator | 1 (2.8) |
| Clopidogrel bisulfate | 1 (2.8) |
| Plavix | 1 (2.8) |
| None | 16 (44.4) |
| Not available | 3 (8.3) |
| Ocular history | n (%) |
| Age-related macular degeneration | 12 (32.4) |
| Glaucoma | 7 (18.9) |
| Pseudophakia | 6 (16.2) |
| High myopia | 3 (8.1) |
| Hypermetropic | 1 (2.7) |
| Vitrectomized | 1 (2.7) |
| Diabetic retinopathy | 1 (2.7) |
| None | 9 (24.3) |
| Not available | 2 (5.4) |
| Treatment | n (%) |
| Conservative treatment | 13 (35.1) |
| Sclerotomy | 11 (29.7) |
| Eviseration/enucleation | 7 (18.9) |
| Vitrectomy | 4 (10.8) |
| Laser iridotomy | 2 (5.4) |
| Paracentesis | 1 (2.7) |
M: male;F: female; R: right; L: left; SD: standard deviation.
In addition to m edication, 24 eyes (64.9%) received surgical intervention (Table 2). Blood drainage with sclerotomy or vitrectomy was performed in 15 eyes (40.5%) from the 2nd day to over 30 days after the episode. Laser iridotomy performed in 2 cases (5.4%), while paracentesis was performed in one case (2.7%) to reduce IOP. Two cases (5.4%) had subsequent global perforations, and one of these was treated with evisceration. The other five eyes underwent evisceration or enucleation due to corneal perforation or pain or recurrent SSCH following drainage.
At the initial examination, visual acuity was HM or worse in 25 of the 34 recorded eyes (73.5%), including 10 eyes (28.6%) that presented with no light perception. Finally, the final visual acuity was HM or worse in 25 eyes (of 36 eyes, 69.4%), and no light perception was found in 20 eyes (55.6%), including six phthitic eyes and seven anophthalmic cases. Nine cases experienced significant visual improvement, and six of these improved after vitrectomy. Fair visual acuity on presentation was noted in only two cases (20/25, 6/12 and 20/40, 6/12 following medical therapy).
Discussion
SCH is a vision-threatening complication associated with ocular trauma or certain surgical procedures, such as cataract extraction, glaucoma filtering surgery, penetrating keratoplasty, and vitreoretinal surgery [29–33]. However, SSCH is uncommon and has previously been described in only isolated case reports. The recognized risk factors for SSCH include systemic diseases treated with anticoagulants [6,7] and ARMD treated with anticoagulants [1,2,10]. RRD associated with SSCH is rare.
The exact cause for the development of SSCH in RRD patients is unclear. Speaker et al. [34] were the first to publish a report (in 1991) showing that increased axial length is a significant independent risk factor for SCH in intraocular surgery because a longer axial length causes choroidal vascular fragility to increase. Hypertension, arteriosclerosis, and advanced age have been widely reported to be systemic risk factors for SCH in a surgical setting, but the association between these risk factors and spontaneous hemorrhage is unknown [35,36]. In 2003, Chak and Williamson [8] reported a case of SSCH with high myopia and aspirin and suggested that high myopia and using anticoagulants may be risk factors for SSCH. Zhang et al. [24] reported six cases of RRD and massive SSCH in eyes that were all associated with high myopia and increased ocular length. In our study, this patient had no history of systemic disease but was associated with an increased axial length of more than 30 mm. Therefore, the risk factor that is suspected to have predisposed this patient to massive SSCH in RRD may be a long axial length. An increased axial length may also be related to increased choroidal vascular fragility. Severe myopia was one of the main risk factors for RRD associated with choroidal detachment. Moreover, a dramatic drop in IOP increase the risk of developing SSCH in patients with RRD associated with choroidal detachment myopia. Usually, massive SCH leads to a sharp increase in IOP due to the increase in ocular contents [1,3,5,12,18]. In the present study, choroidal detachment was caused by retinal detachment in patients with high myopia, and SCH was caused by low IOP and fragile vascular elasticity, so that there was no increase in IOP in this patient.
Surgical drainage of SCH without RRD has been reported in a few isolated papers, but the clinical effects and optimal timing of this surgical intervention remains uncertain [10,20,37,38]. Yang proposed that surgical intervention may have value for relieving pain and elevated IOP but has not been shown to be beneficial for visual outcomes. In our review, at the initial examination, the patients’ visual acuity was HM or worse in 25 of the 34 recorded eyes (73.5%), including 10 eyes (28.6%) that presenting with no light perception. In the end, the final visual acuity was HM or worse in 25 eyes (of 36 eyes, 69.4%), and no light perception was found in 20 eyes (55.6%), including 6 phthitic eyes and seven anophthalmic cases. Nine cases experienced significant visual improvement, and six of these improved after vitrectomy. Fair visual acuity on presentation was noted in only two cases (20/25, 6/12 and 20/40, 6/12 following medical therapy). Our patient was associated with RRD and high myopia. These basic lesions can themselves also cause vision loss. We took advantage of 20G and 23G vitrectomy cannulas to ensure sclerotomies of known and reliable diameter and consistent patency throughout all surgical maneuvers. During infusion and vitrectomy, the sclerotomies remained functional and permitted continuous blood flow out of the suprachoroidal space. This method has also been described in other studies that used 23G or 25G cannulas [39]. The use of 20G and 23G cannulas also allows the very quick, safe, and easy closure of the sclerotomy when needed. After vitrectomy, suprachoroidal blood drainage, photocoagulation, phacoemulsification and silicon oil tamponade, and retina reattachment are performed. PVR causes retinal detachment after oil extraction. In a secondary surgery preretinal membrane peeling and silicon oil retention were performed. Finally, this patient showed a good reattached choroidea and retina and achieved a visual acuity of 20/2000 after the silicone oil was removed. We hypothesize that the PVR was caused by two factors. First, tight adhesion between the posterior vitreous membrane and retina makes the posterior vitreous cortex difficult to clear. Second, massive SCH can also stimulate PVR development.
There is some disagreement regarding the timing of surgery. Some authors suggest waiting 10–14 days for the clot to liquefy, whereas others advocate early surgical intervention to achieve better anatomical and visual outcomes [40]. Waiting for spontaneous resolution can result in retinal detachment when there is vitreous incarceration, which can in these cases lead to a poor visual prognosis. In cases of extensive hemorrhage, patients have sustained vision loss from chronic atrophy or phthisis bulbi in the absence of prompt surgical intervention. Prompt drainage may provide the best chance for maintaining useful vision. In some studies, the mean time interval was 11 days (range, 6–20 days) [40,41]. Generally, a longer duration of appositional SCH has been shown to result in poorer visual outcomes [42]. In accordance with previous studies, we also suggest that the time interval should not exceed 14 days.
The literature review presented in this work indicates that SSCH is highly associated with hypertension, systemic anticoagulation, and ARMD. If medication cannot be withheld, general practitioners or cardiologists should consult an ophthalmologist for a complete ophthalmic examination prior to treatment, and they should also inform their patients of the possible risk factors associated with SSCH. In the case of patients with intractable pain, surgery should be performed. The limitations of our review include its retrospective nature, the lack of a control group, and the limited number of cases. The definite relative risk of each factor therefore could not be clearly determined.
Conclusions
Overall, RRD associated with massive SSCH is an extremely rare event. The most common risk factor is long axial length. Vitrectomy and choroidal blood drainage can effectively remove SCH and promote retinal reattachment in these eyes. However, the final visual acuities are generally poor.
Availability of data and material
All data supporting our findings will be shared upon request, although the majority is contained within the manuscript.
Supporting information
Supplementary Video.
Acknowledgments
The authors would like to thank the ophthalmic staff at Xi’an No. 4 Hospital with their assistance in processing the article.
Abbreviations
- ARMD
age-related macular degeneration
- HM
hand motion
- IOP
intraocular pressure
- RRD
rhegmatogenous retinal detachment
- SCH
suprachoroidal hemorrhage
- SSCH
spontaneous suprachoroidal hemorrhage
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images, providing no identifying features are released.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Project of Science and Technology of Social Development Funding [grant number 2016SF-100]; Bureau-level scientific reasearch projects [grant number J201901013];and the Xi’an No. 4 Hospital Research Incubation Fund [grant number 2018LH-2].
Author Contribution
Xiquan Zhao designed the observation and performed the treatment of this patient. Fang Chai and Lu Zeng collected and analyzed the data. Fang Chai and Chunhua Li drafted the manuscript. All authors have read and approved the final version of the manuscript.
References
- 1.Chen S.N., Ho C.L., Ho J.D., Guo Y.H., Chen T.L. and Chen P.F. (2001) Acute angle-closure glaucoma resulting from spontaneous hemorrhagic retinal detachment in age-related macular degeneration: case reports and literature review. Jpn. J. Ophthalmol. 45, 270–275 10.1016/S0021-5155(00)00382-8 [DOI] [PubMed] [Google Scholar]
- 2.Wong J.S. (1999) Spontaneous suprachoroidal haemorrhage in a patient receiving low-molecular-weight heparin (fraxiparine) therapy. Aust. N. Z. J. Ophthalmol. 27, 433–434 10.1046/j.1440-1606.1999.00260.x [DOI] [PubMed] [Google Scholar]
- 3.Ophir A., Pikkel J. and Groisman G. (2001) Spontaneous expulsive suprachoroidal hemorrhage. Cornea 20, 893–896 10.1097/00003226-200111000-00025 [DOI] [PubMed] [Google Scholar]
- 4.Dandekar S.S. and Laidlaw D.A. (2001) Suprachoroidal haemorrhage after addition of clarithromycin to warfarin. J. R. Soc. Med. 94, 583–584 10.1177/014107680109401109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neudorfer M., Leibovitch I., Goldstein M. and Loewenstein A. (2002) Massive choroidal hemorrhage associated with low molecular weight heparin therapy. Blood Coagul. Fibrinolysis 13, 257–259 10.1097/00001721-200204000-00012 [DOI] [PubMed] [Google Scholar]
- 6.Knox F.A. and Johnston P.B. (2002) Spontaneous suprachoroidal haemorrhage in a patient with age-related macular degeneration on excessive anticoagulation therapy. Eye (Lond) 16, 669–670 10.1038/sj.eye.6700109 [DOI] [PubMed] [Google Scholar]
- 7.Goldsmith C. and Rene C. (2003) Massive spontaneous expulsive suprachoroidal haemorrhage in a blind glaucomatous eye treated with chronic topical steroid. Eye (Lond) 17, 439–440 10.1038/sj.eye.6700372 [DOI] [PubMed] [Google Scholar]
- 8.Chak M. and Williamson T.H. (2003) Spontaneous suprachoroidal haemorrhage associated with high myopia and aspirin. Eye (Lond) 17, 525–527 10.1038/sj.eye.6700388 [DOI] [PubMed] [Google Scholar]
- 9.Hammam T. and Madhavan C. (2003) Spontaneous suprachoroidal haemorrhage following a valsalva manoeuvre. Eye (Lond) 17, 261–262 10.1038/sj.eye.6700298 [DOI] [PubMed] [Google Scholar]
- 10.Yang S.S., Fu A.D., McDonald H.R., Johnson R.N., Ai E. and Jumper J.M. (2003) Massive spontaneous choroidal hemorrhage. Retina 23, 139–144 10.1097/00006982-200304000-00001 [DOI] [PubMed] [Google Scholar]
- 11.Maguluri S., Bueno C.L., Fuller I.B., Eagle R.C. Jr and Spell D.W. (2005) Delayed suprachoroidal hemorrhage and factor VIII deficiency. Am. J. Ophthalmol. 139, 195–197 10.1016/j.ajo.2004.07.005 [DOI] [PubMed] [Google Scholar]
- 12.Barsam A., Heatley C.J. and Herbert L. (2006) Spontaneous suprachoroidal hemorrhage secondary to thrombolysis for the treatment of myocardial infarction. Clin. Exp. Ophthalmol. 34, 177–179 10.1111/j.1442-9071.2006.01149.x [DOI] [PubMed] [Google Scholar]
- 13.Saeed M.U., Wong D., Heimann H. and Gibran S.K. (2007) Spontaneous progressive supra-choroidal haemorrhage in a patient undergoing haemodialysis. Graefes Arch. Clin. Exp. Ophthalmol. 245, 1741–1742 10.1007/s00417-007-0653-y [DOI] [PubMed] [Google Scholar]
- 14.Lee Y.J., Kang S.M. and Kang I.B. (2007) Acute angle-closure glaucoma from spontaneous massive hemorrhagic retinal detachment. Korean J. Ophthalmol. 21, 61–64 10.3341/kjo.2007.21.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tajika T., Yokozeki H., Ishimaru K., Naito T. and Shiota H. (2008) Rare case of choroidal hemorrhage complicated with hypertension due to chronic renal failure. J. Med. Invest. 55, 151–155 10.2152/jmi.55.151 [DOI] [PubMed] [Google Scholar]
- 16.Chandra A., Barsam A. and Hugkulstone C. (2009) spontaneous suprachoroidal haemorrhage: a case report. Cases J. 2, 185. 10.1186/1757-1626-2-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuchi T., Suda K., Matsuda H., Ueda J. and Abe H. (2009) Secondary acute angle closure with spontaneous suprachoroidal hemorrhage suspected by ultrasound biomicroscopic examination. Jpn. J. Ophthalmol. 53, 661–663 10.1007/s10384-009-0742-z [DOI] [PubMed] [Google Scholar]
- 18.Chen Y.Y., Chen Y.Y. and Sheu S.J. (2009) Spontaneous suprachoroidal hemorrhage associated with age-related macular degeneration and anticoagulation therapy. J. Chin. Med. Assoc. 72, 385–387 10.1016/S1726-4901(09)70393-4 [DOI] [PubMed] [Google Scholar]
- 19.Lim L.T., Agarwal P.K. and Rotchford A. (2011) Angle-closure glaucoma due to suprachoroidal hemorrhage secondary to disseminated intravascular coagulation. Semin. Ophthalmol. 26, 59–60 10.3109/08820538.2011.559517 [DOI] [PubMed] [Google Scholar]
- 20.Nadarajah S., Kon C. and Rassam S. (2012) Early controlled drainage of massive suprachoroidal hemorrhage with the aid of an expanding gas bubble and risk factors. Retina 32, 543–548 10.1097/IAE.0b013e31822058e9 [DOI] [PubMed] [Google Scholar]
- 21.Nguyen H.N. and Nork T.M. (2012) Massive spontaneous suprachoroidal hemorrhage in a young woman with cystic fibrosis and diabetes mellitus on anticoagulants. Retin. Cases Brief Rep. 6, 216–218 10.1097/ICB.0b013e3182378c1a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim M., Lee S.C. and Lee S.J. (2013) Abrupt spontaneous suprachoroidal hemorrhage post-23-gauge vitrectomy during peritoneal dialysis. Clin. Ophthalmol. 7, 1175–1179 10.2147/OPTH.S46787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srikanth K. and Kumar M.A. (2013) Spontaneous expulsive suprachoroidal hemorrhage caused by decompensated liver disease. Indian J. Ophthalmol. 61, 78–79 10.4103/0301-4738.107201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J., Zhu X.H. and Tang L.S. (2014) Rhegmatogenous retinal detachment associated with massive spontaneous suprachoroidal hemorrhage and prognosis of pars plana vitrectomy, Int. J. Ophthalmol. 7, 850–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andreatta W., Boukouvala S. and Bansal A. (2016) Combined Acute Haemolytic and Secondary Angle Closure Glaucoma following Spontaneous Intraocular Haemorrhages in a Patient on Warfarin. Case Rep. Ophthalmol. 7, 233–238 10.1159/000452440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsiao S.F., Shih M.H. and Huang F.C. (2016) Spontaneous suprachoroidal hemorrhage: case report and review of the literature. Taiwan J. Ophthalmol. 6, 36–41 10.1016/j.tjo.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung A.Y., David J.A. and Ober M.D. (2017) Spontaneous bilateral hemorrhagic choroidal detachments associated with malignant hypertension. Retin. Cases Brief Rep. 11, 175–179 10.1097/ICB.0000000000000322 [DOI] [PubMed] [Google Scholar]
- 28.Akkan Aydoğmuş F.S., Serdar K., Kalayci D. and Çelik A. (2019) Spontaneous suprachoroidal hemorrhage associated with iatrogenic coagulopathy. Retin. Cases Brief Rep. 13, 174–175 [DOI] [PubMed] [Google Scholar]
- 29.Pollack A.L., McDonald H.R., Ai E., Johnson R.N., Dugel P.U., Folk J.. et al. (2001) Massive suprachoroidal hemorrhage during pars plana vitrectomy associated with Valsalva maneuver. Am. J. Ophthalmol. 132, 383–387 10.1016/S0002-9394(01)01049-2 [DOI] [PubMed] [Google Scholar]
- 30.Tabandeh H., Sullivan P.M., Smahliuk P. and Flynn H.W. Jr (1999) Suprachoroidal hemorrhage during pars plana vitrect outcomes. Ophthalmology 106, 236–242 10.1016/S0161-6420(99)90062-3 [DOI] [PubMed] [Google Scholar]
- 31.Wang L.C., Yang C.M., Yang C.H., Huang J.S., Ho T.C., Lin C.P.. et al. (2008) Clinical characteristics and visual outcome of non-traumatic suprachoroidal haemorrhage in Taiwan. Acta Ophthalmol. 86, 908–912 10.1111/j.1755-3768.2008.01266.x [DOI] [PubMed] [Google Scholar]
- 32.Chandra A., Xing W., Kadhim M.R. and Williamson T.H. (2014) Suprachoroidal hemorrhage in pars plana vitrectomy: risk factors and outcomes over 10 years. Ophthalmology 121, 311–317 10.1016/j.ophtha.2013.06.021 [DOI] [PubMed] [Google Scholar]
- 33.Mei H., Xing Y., Yang A., Wang J., Xu Y. and Heiligenhaus A. (2009) Suprachoroidal hemorrhage during pars plana vitrectomy in traumatized eyes. Retina 29, 473–476 10.1097/IAE.0b013e318196b189 [DOI] [PubMed] [Google Scholar]
- 34.Speaker M.G., Guerriero P.N., Met J.A., Coad C.T., Berger A. and Marmor M. (1991) A case-control study of risk factors for intraoperative suprachoroidal expulsive hemorrhage. Ophthalmology 98, 202–209 10.1016/S0161-6420(91)32316-9 [DOI] [PubMed] [Google Scholar]
- 35.Chu T.G. and Green R.L. (1999) Suprachoroidal hemorrhage. Surv. Ophthalmol. 43, 471–486 10.1016/S0039-6257(99)00037-5 [DOI] [PubMed] [Google Scholar]
- 36.Jin W., Xing Y., Xu Y., Wang W. and Yang A. (2014) Management of delayed suprachoriodal haemorrhage after intraocular surgery and trauma. Graefes Arch. Clin. Exp. Ophthalmol. 252, 1189–1193 10.1007/s00417-013-2550-x [DOI] [PubMed] [Google Scholar]
- 37.Mandelcorn E.D., Kitchens J.W., Fijalkowski N. and Moshfeghi D.M. (2014) Active aspiration of suprachoroidal hemorrhage using a guarded needle. Ophthalmic Surg. Lasers Imaging Retina 45, 150–152 10.3928/23258160-20140306-09 [DOI] [PubMed] [Google Scholar]
- 38.Kunjukunju N., Gonzales C.R. and Rodden W.S. (2011) Recombinant tissue plasminogen activator in the treatment of suprachoroidal hemorrhage. Clin. Ophthalmol. 5, 155–157 10.2147/OPTH.S16134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizzo S., Tartaro R., Faraldi F., Franco F., Finocchio L., Barca F.. et al. (2017) Two-stage surgery to manage massive suprachoroidal hemorrhage. Retina, 10.1097/IAE.0000000000001769[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Lavinsky F., Moisseiev J. and Levkovitch-Verbin H. (2013) The surgical management of massive intraoperative and postoperative suprachoroidal hemorrhage: anatomic and functional outcomes. Arq. Bras. Oftalmol. 76, 212–214 10.1590/S0004-27492013000400003 [DOI] [PubMed] [Google Scholar]
- 41.Meier P. and Wiedemann P. (2000) Massive suprachoroidal hemorrhage: secondary treatment and outcome. Graefes Arch. Clin. Exp. Ophthalmol. 238, 28–32 10.1007/s004170050005 [DOI] [PubMed] [Google Scholar]
- 42.Scott I.U., Flynn H.W. Jr, Schiffman J., Smiddy W.E., Murray T.G. and Ehlies F. (1997) Visual acuity outcomes among patients with appositional suprachoroidal hemorrhage. Ophthalmology 104, 2039–2046 10.1016/S0161-6420(97)30042-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting our findings will be shared upon request, although the majority is contained within the manuscript.