Abstract
The ionizing irradiation mitigator MMS350 prolongs survival of mice treated with total-body irradiation and prevents radiation-induced pulmonary fibrosis when added to drinking water at day 100 after thoracic irradiation. The effects of MMS350 on hematopoiesis in long-term bone marrow culture and on the radiobiology of derived bone marrow stromal cell lines were tested. Long-term bone marrow cultures were established from C57BL/6NTac mice and maintained in a high-humidity incubator, with 7% C02 and the addition of 100 μM MMS350 at the weekly media change. Over 10 weeks in culture, MMS350 had no significant effect on maintenance of hematopoietic stem cell production, or on nonadherent cells or colony-forming units of hematopoietic progenitor cells. Stromal cell lines derived from non MMS350-treated long-term cultures or control stromal cells treated with MMS350 were radioresistant in the clonogenic survival curve assay. MMS350 is a non-toxic, highly water-soluble radiation mitigator that exhibits radioprotective effects on bone marrow stromal cells.
Keywords: Bifunctional sulfoxide, MMS350, hematopoiesis
Long-term marrow cultures (1–2) have been used in the development of cell lines for the study of new radiation protectors and radiation mitigators for use in both radiation counter-terrorism applications and in the protection of normal tissues during clinical radiotherapy (3–8). New techniques for in vitro screening of radiation dose modifiers have been applied (9–13). We recently reported a novel, highly watersoluble bifunctional sulfoxide, MMS350 (14), which, when administered in drinking water, reduces toxicity from total-body irradiation, improving survival (15). MMS350 treatment also reduces the onset and severity of radiation-induced pulmonary fibrosis in a mouse model (15). Furthermore, mice treated with MMS350 demonstrated lower migration to the lungs of bone marrow-origin mesenchymal stem cell progenitors of pulmonary radiation fibrosis (15).
In the present studies, we determined whether the addition of MMS350 to long-term bone marrow cultures resulted in a significant or detectable alteration in hematopoiesis. We also tested the capacity of MMS350 to modify the radiation survival curve in a clonogenic survival curve assay of bone marrow stromal cell lines derived from MMS350-treated long-term cultures.
Materials and Methods
Long-term bone marrow cultures.
Long-term bone marrow cultures were established according to previously published methods (1–2, 10, 15–18). The contents of a femur and tibia of C57BL/6NTac mice were flushed into a 40 cm. square plastic flask in Dulbecco’s modified Eagle’s medium containing 25% fetal calf serum and 10−5 M hydrocortisone sodium hemisuccinate and antibiotics. Cultures were medium-changed weekly with removal of nonadherent cells and replacement with equal volume of fresh medium. Cultures were maintained in a high humidity incubator with 7% C02 and medium-changed weekly.
MMS350.
The synthesis and physiochemical properties (14), as well as some aspects of the biological profile (15) of the water-soluble bi-functional sulfoxide, MMS350, have previously been described. MMS350 was added at concentrations designed to produce a 100 μM final concentration in long-term bone marrow cultures, weekly at the time of medium change.
Hematopoietic colony-forming cell assays.
Nonadherent cells removed from long-term bone marrow cultures were tested for production of day 7 and day 14 colonies in secondary semi-solid medium culture according to published methods (2, 15, 16). Cells were grown for seven and 14 days and scored at both time points. The same plates were returned to incubators for a second scoring at 14 days after the initial scoring at seven days. Colonies were assayed for those single cell-derived with greater than 50 cells at either day 7 or day 14.
Bone marrow stromal cell lines.
Adherent cell monolayers from MMS350 treated or control bone marrow cultures were removed at 20 weeks, after cessation of hematopoiesis, by trypsination and subcultured according to published methods (10, 15, 16). Cell lines were maintained in Dulbecco’s modified Eagle’s medium supplemented with 20% fetal bovine serum, and passaged weekly. Cell lines were maintained at 37°C and maintained in 100 μM MMS350-supplemented medium (15) or control marrow stromal cell lines in the absence of added drug.
Clonogenic radiation survival curves.
The methods for calculation of the slope in the linear region of the survival curve (D0) and shoulder of the curve as characterized by extrapolation of the terminal straight line portion of the curve back to the abscissa (n) as indicators of radiation sensitivity for the bone marrow stromal cell lines in clonogenic radiation survival curves were calculated according to previously published methods (10). All experiments were repeated in triplicate, and radiation survival curves and calculated parameters were based on triplicate experiments.
Measurement of gene transcripts of irradiation-inducible transcription factors, growth factors, inflammatory cytokines, adhesion molecules, and radiation-protective enzymes by real-time polymerase chain reaction (RT-PCR).
RNA was extracted from mouse lung using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions and quantified using a spectrophotometer, and stored at −80°C (15). Reverse transcription of 2 μg of total RNA to complementary DNA (cDNA) was accomplished using the High Capacity cDNA Reverse Transcription Kit (Applied Bio systems, Foster City, CA, USA) according to the manufacturer’s protocol.
In subsequent steps, expression of specific RNA moieties (included: GPDH (Gen-Bank: NM_008084.2), GUSB (Gen-Bank: NM_010368.1), NFkβ (Gen-Bank: NM_199267.2), TNF-α (Gen-Bank: NM_013693.2), Nrf2 (Gen-Bank: NM_010902.3) (21), NFkβ (Gen-Bank: NM_008689.2), SP-1 (Gen-Bank: NM_013672.2), API (Gen-Bank: NM_001243043.1), Lysl Ox (Gen-Bank: NM_001178102.1), TGFβl (Gen-Bank: NM_011577.1), VEGFa (Gen-Bank: NM_001025250.3), IL-la (Gen-Bank: NM_010554.4), FGF1 (Gen-Bank: NM_010197.3), IFNγ (Gen-Bank: NM_008337.3), IL-6 (Gen-Bank: NM_031168.1), FAP (Gen-Bank: NM_007986.2), vWF (Gen-Bank: NM_011708.3), CTGF (Gen-Bank: NM_010217.2), MnSOD (Gen-Bank: NM_013671.3), p53 (Gen-Bank: NM_001168250), p21 (Gen-Bank: NM_001111099), Gadd45 (Gen-Bank: NM_007836.1), TLR4 (Gen-Bank: NM_021297.2), RAD51 (Gen-Bank: NM_011234.4), TLR1 (Gen-Bank: NM_030682.2), TLR2 (Gen-Bank: NM_011905.3), TLR5 (Gen-Bank: NM_016928.2), TLR6 (Gen-Bank: NM_011604.3), TLR7 (Gen-Bank: NM_133211.3), and epigenetic reader proteins BRD1 (Gen-Bank: AK149714.1), BRD2 (Gen-Bank: AB010246.1), BRD3 (Gen-Bank: AB206708.2), and BRD4 (Gen-Bank: AF273217.1). Collagen la (Gen-Bank: AK132180.1). Each was quantitated by RT-PCR. Ninety-six well plates were prepared with 10 μl of Taqman Gene Expression Master mix, 5 μl of RNase-free water, 1 μl of the corresponding Taqman Gene Expression probe, and 4 μl of cDNA (totaling 2 μg cDNA) using the Eppendorf epMotion 5070 automated pipetting system (Eppendorf, Westbury, NY, USA). The cDNA was amplified with 40 cycles of 95°C (dénaturation) for 15 s and 60°C (annealing and elongation) for 1 min using the Eppendorf Realplex2 Mastercycler (15, 18).
Data for each gene transcript were normalized by calculating the differences (ΔCt) from the Ct-Gusb and Ct-target genes. The relative increase or decrease in expression was calculated by comparing the reference gene with the target gene (ΔΔCt.) and using the formula for relative expression (=2ΔΔCt). Subsequently, (ΔΔCt) levels were compared and p-values were calculated using one-way ANOVA followed by Tukey’s multiple comparison tests. The results were presented as percent increase in RNA above baseline levels, which were adjusted to that of non-irradiated C57BL/6NTac stromal cells (15, 18). Baseline transcript levels were standardized to that of Gapdh.
Statistical analysis.
The single-hit multi-target model was used to analyze the in vitro radiation survival curves. Two parameters of the model, namely D0 (final slope representing multiple-event killing) and n (extrapolation number measuring width of the shoulder on the clonogenic radiation survival curve), were calculated (5). Results for D0 and n in each group were summarized as the mean±standard error (SEM) from multiple measurements and compared between groups with the two-sided two-sample t-test.
For data of long-term bone marrow cultures, weekly cobblestone island numbers; non-adherent cell numbers; percentage confluence of adherent cells; day-7 and day-14 colony-forming cell counts at each weekly harvest were counted, as described elsewhere (15, 16). At each week, the cobblestone numbers were summarized as mean±standard deviation in the MMS350-treated group and the control group, and p-values were calculated with the two-sided two-sample t-test to compare these two groups. Similar calculations and tests were performed for the non-adherent cell numbers and percentage confluence of adherent cells. The colony count data were also compared between MMS350-treated and control groups at each week with the two-sided two-sample t-test. As this was an exploratory study, p-values were not adjusted for multiple comparisons.
Results
Continuous bone marrow cultures treated with MMS350 exhibit unaltered longevity of hematopoiesis.
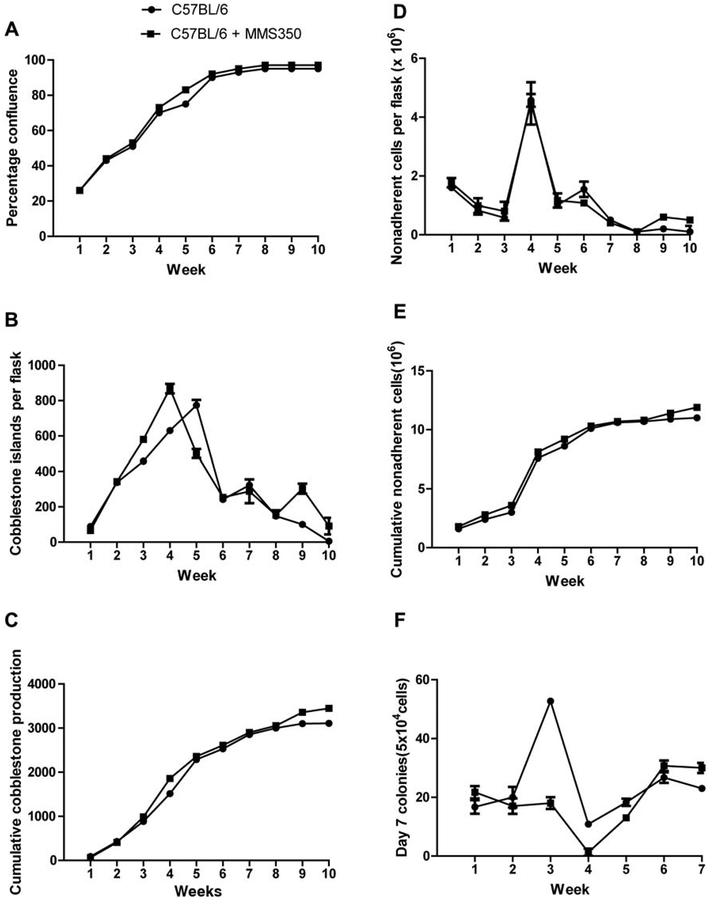
As shown in Figure 1, long-term bone marrow cultures maintained in MMS350 at 100 μM exhibited no significant increase or decrease in confluence of the adherent layer (Figure 1A), weekly (Figure 1B), and cumulative (Figure 1C) production of cobblestone islands (indicating adherent hematopoietic islands), and in weekly and cumulative production of nonadherent cells forming day 7 colonies in secondary semi-solid medium culture (Figure 1F and G), or weekly or cumulative production of day-14 colony forming hematopoietic progenitor cells (Figure 1H and I). Cells capable of forming greater than 50 cell colonies at day 7 or day 14 in secondary semi-solid medium culture are a measure of the robustness of hematopoiesis in long-term bone marrow cultures (10, 15, 16). Tables I–V represent statistical analyses of the graphical data in Figure 1.
Figure 1.
Hematopoiesis in long-term bone marrow cultures treated with MMS350. Long-term bone marrow cultures were established according to the materials and methods section. Non-adherent cells were harvested weekly. Prior to non-adherent cell harvest, the adherent layer was assayed for percent of the surface area of the flask that was confluent (A). The cobblestone islands were scored and indicate hematopoietic stem cells in the adherent layer (B), cumulative cobblestone islands (C), non-adherent cells removed weekly per flask (D), and cumulative non-adherent cells per flask, (E) were determined. Non-adherent cells were then assayed for hematopoietic colony forming cells. Non-adherent cells were plated in secondary semi-solid medium and colony-forming cells of greater than 50 cells per colony were scored weekly on day 7 (F), and cumulative (G). Plates were again scored for those forming colonies at day 14 weekly (H) and cumulative (I). Statistics are shown in Tables I–V.
Table I.
Hematopoiesis in MMS350-treated long-term bone marrow cultures: Confluence of adherent layer. Analysis of weekly percent confluence of adherent cells, where data are summarized as mean ± standard deviation, n is the number of flasks used, and the p-valuefor comparison between the two groups with the two-sided two sample t-test. Significant p-values are shown in bold. All data in Tables I–V represent statistical analysis of the graphical data in Figure 1.
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Control | 26.25±1.77 (n=2) | 42.50±0.00 (n=2) | 51.25±1.77 (n=2) | 70.00±0.00 (n=2) | 75.00±0.00 (n=2) | 90.00±0.00 (n=2) | 93.75±1.77 (n=2) | 95.00±0.00 (n=2) |
| MMS350 | 26.25±5.30 (n=2) | 43.75±1.77 (n=2) | 52.50±3.54 (n=2) | 72.50±0.00 (n=2) | 82.50±0.00 (n=2) | 90.00±0.00 (n=2) | 90.00±0.00 (n=2) | 95.00±0.00 (n=2) |
| p-Value | 1.0000 | 0.5000 | 0.6985 | <0.0001 | <0.0001 | 1.0000 | 0.2048 | 1.0000 |
Table V.
Hematopoiesis in MMS350-treated long-term bone marrow cultures: Day-14 CFU-GEMM.
| Group | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Control | 112.67±16.44 (n=3) | 31.67±3.79 (n=3) | 121.67±5.69 (n=3) | 28.67±4.16 (n=3) | 51.00±2.65 (n=3) |
| MMS350 | 105.33±16.62 (n=3) | 20.33±1.53 (n=3) | 59.67±4.51 (n=3) | 24.67±3.06 (n=3) | 36.33±2.08 (n=3) |
| p | 0.6158 | 0.0086 | 0.0001 | 0.2508 | 0.0017 |
| group | 6 | 7 | |||
| Control | 38.33±1.53 (n=3) | 57.33±6.81 (n=3) | |||
| MMS350 | 35.33±1.53 (n=3) | 46.67±5.51 (n=3) | |||
| p | 0.0739 | 0.1025 |
Analysis of day 14 colony counts at each week, where data are summarized as mean±standard deviation, n is the sample size, and p is the p-value for comparison between the two groups with the two-sided two sample ί-test. Significant p-values are shown in bold.
Hematopoietic cell colonies from MMS350-treated cultures appeared identical morphologically to those of cultures that were not treated with MMS350. These results establish that MMS350 does not alter hematopoiesis in long-term culture.
Bone marrow stromal cell lines derived from MMS350-treated culture demonstrate radioresistance.
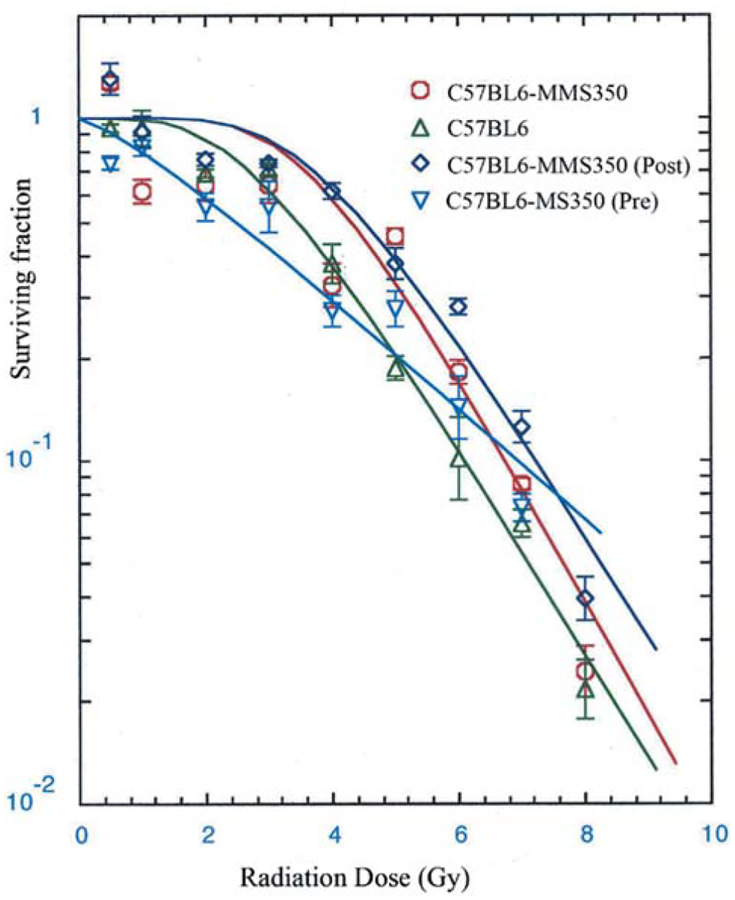
As shown in Figure 2, bone marrow stromal cell lines derived from MMS350-treated long-term cultures exhibited intrinsic radiation resistance as reflected in D0 and n. Furthermore, bone marrow stromal cell lines derived from untreated cultures, when treated with MMS350 for the clonogenic radiation survival curve assay also demonstrated increased radioresistance (Table VI).
Figure 2.
Clonogenic radiation survival curves of bone marrow stromal cell lines derived from MMS350-treated long-term marrow cultures. Adherent cells removed from week 10 long-term marrow cultures were cultured according to previously published methods and clonal cell lines established. Radiation survival curves of those cells removed from longterm marrow cultures derived from mice treated with MMS350 in vivo then treated with MMS350 in vitro, and maintained in MMS350 (100 μM) continually for 10 weeks, and in secondary stromal cell cultures derived at 10 weeks were irradiated in clonogenic survival curve assays according to published methods. Clonal cell lines from control cultures were also derived and radiation survival curves carried-out as described in the methods section for cells treated with MMS350 at the time of irradiation and for 7 days thereafter. The D0 and ñ evaluation of stromal cells treated freshly with MMS350 were compared to those continuously cultured with MMS350. D0 and n are shown in Table VI.
Table VI.
Radio sensitivity of stromal cell line derived from long term bone marrow cultures established from MMS350 treated mice.
| Cell Line | D0 (Gy) | n |
|---|---|---|
| C57BL/6NTac (B6) | 1.41±0.01 | 4.8±0.6 |
| C57BL/6NTac/MMS350 | 1.46±0.16 | 13.2±5.1 |
| (p=0.8058) | (p=0.0208) | |
| B6 + MMS350 | 1.38±0.07 | 22.9±8.0 |
| Post Irradiation | (p=0.6733) | (p=0.0038) |
| MMS350 + B6 | 2.69±0.04 | 2.1±0.7 |
| Pre Irradiation | (p=0.0011) | (p=0.0574) |
p-Value is comparison with C57BL/6NTac (B6). Single-hit, multi-target model of calculation used.
Effect of MMS350 on gene transcript levels in bone marrow stromal cell lines.
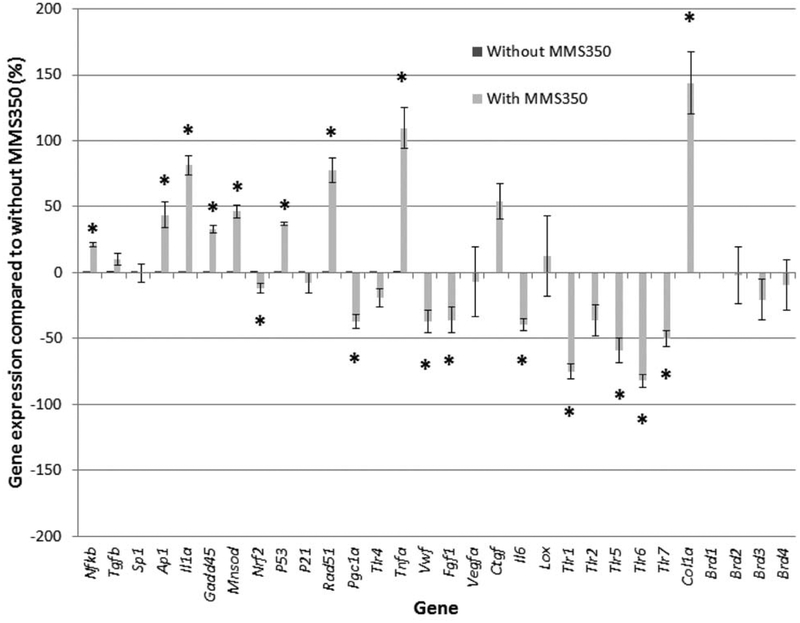
We tested the relative levels of expression of representative genes included in the oxidative stress responses of long-term culture, senescence, and inflammation on the MMS350-treated stromal cell line derived from continuously MMS350-treated cells compared to the control bone marrow stromal cell line (Figure 3, Table VII). MMS350-treated cells exhibited significant increase in RNA levels for Nfkb, Apl, Ilia, Gadd45, Sod2, P53, Rad51, Infa, and Colla. There were significant decreases in Nrf2, Rad51, Tnfa, Pgcla, Vwf, Fgf1, 116, Tlr1, Tlr5, Tlr6, and Tlr7. These gene transcript patterns were not correlated with any specific drug-induced toxicity to marrow stromal cells, as they have been detected in other mouse genotypes as baseline levels (10).
Figure 3.
Effect of continuous MMS350 treatment on gene transcript expression in bone marrow stromal cell lines. Bone marrow stromal cell lines were tested by RT-PCR for gene transcript levels as described in the Materials and Methods. Transcripts are expressed as percent change in expression, compared to untreated bone marrow stromal cells. Statistics are shown in Table VII. *indicates a significant difference (p<0.05) comparing without MMS350 to the one with MMS350.
Table VII.
Relative gene expression in bone marrow stromal cell line maintained in MMS350. Data summary and group comparisons for the expression of the 30 genes in B6 stromal+/+ cells with or without MMS350, where n is the number of repetitions, p-Value for the comparison between the group with permanent MMS350 and the group without. Data are from Figure 3.
| Continuous MMS350 | ||||
|---|---|---|---|---|
| Gene name | Gene symbol | With | Without | p-Value |
| NFKb | Nfkb | 21.29±2.53 (n=3) | 1.25±1.09 (n=3) | 0.0002 |
| TGFb | Tgfb | 10.15±9.28 (n=4) | 0.25±0.017 (n=3) | 0.1224 |
| SP1 | Sp1 | −0.48±14.39 (n=4) | 0.13±0.13 (n=3) | 0.9374 |
| API | Ap1 | 43.86±20.20 (n=4) | 0.93±1.10 (n=3) | 0.0236 |
| IL-1a | Il1a | 81.48±14.52 (n=4) | 0.81±1.13 (n=3) | 0.0015 |
| Gadd45 | Gadd45 | 32.72±5.45 (n=4) | 0.15±0.20 (n=5) | 0.0013 |
| MnSOD | Sod2 | 46.50±9.57 (n=4) | 0.35±0.27 (n=5) | 0.0024 |
| NRF2 | Nrf2 | −12.17±7.35 (n=4) | 0.26±0.26 (n=3) | 0.0429 |
| P53 | P53 | 36.95±1.59 (n=3) | 0.87±1.22 (n=3) | <0.0001 |
| P21 | P21 | −7.84±16.04 (n=4) | 0.66±0.84 (n=5) | 0.3671 |
| Rad51 | Rad51 | 77.56±18.73 (n=4) | 0.12±0.11 (n=3) | 0.0037 |
| PGC-1A | Pvc1a | −37.03±10.69 (n=4) | 0.19±0.08 (n=3) | 0.0061 |
| TLR-4 | Tlr4 | −19.03±14.07 (n=4) | 0.05±0.03 (n=3) | 0.0730 |
| TNF-A | Tnfa | 109.69±31.44 (n=4) | 1.70±1.35 (n=3) | 0.0062 |
| vWF | Vwf | −37.38±14.72 (n=3) | 0.49±0.28 (n=3) | 0.0468 |
| fgf1 | Fgf1 | −35.92±20.04 (n=4) | 0.39±0.50 (n=3) | 0.0361 |
| vegfa | Vegfa | −6.72±53.19 (n=4) | 0.16±0.16 (n=3) | 0.8127 |
| ctgf | Ctgf | 53.95±23.04 (n=3) | 0.10±0.10 (n=3) | 0.0560 |
| il6 | Il6 | −39.43±8368 (n=4) | 0.04±0.02 (n=3) | 0.0028 |
| lox | Lox | 12.85±60.88 (n=4) | 0.30±0.33 (n=3) | 0.7078 |
| tlr1 | Tlr1 | −75.01±10.16(n=3) | 2.36±1.66 (n=3) | 0.0002 |
| tlr2 | Tlr2 | −36.33±20.60 (n=3) | 0.40±0.34 (n=3) | 0.0908 |
| tlr5 | Tlr5 | −59.00±18.84 (n=4) | 0.47±0.36 (n=3) | 0.0080 |
| tlr6 | Tlr6 | −82.12±9.83 (n=4) | 0.57±0.40 (n=3) | 0.0004 |
| tlr7 | Tlr7 | −50.12±12.08 (n=4) | 1.48±0.43 | 0.0033 |
Discussion
The development of small-molecule radiation protectors and radiation mitigators is a high priority for both the radiation counter-measures program and in clinical radiotherapy (8). Research in this area has uncovered multiple classes of agents with a capacity for either a direct or indirect action on tissues, organs, and organ systems. For example, GS-nitroxides, including JP4–039, have been shown to have a direct effect on radiation protection and radiation mitigation by localization of the drug to the mitochondria followed by stabilization of the mitochondrial membrane (8). Furthermore, the direct action of GS-nitroxides has been localized to cells that exhibited resistance to radiation, including hematopoietic progenitor cells, epithelial cells of the oral cavity, and supportive bone marrow stromal cells of the hematopoietic microenvironment. In contrast, radiation-protective agents, glyburide and carbamazepine have been shown to have systemic radioprotective effects when administered to mice prior to or following total-body irradiation, but do not show cell-specific radioprotective capacity with respect to radiation survival curves for freshly-harvested human umbilical cord blood hematopoietic progenitor cells (13). Thus, the mechanism of action of this latter category of radioprotectors appears to be indirect and may reflect secondary responses of the experimental animal to stimulation of other pathways by drug administration.
In the case of GS-nitroxides, principally JP4–039, administration of the drug to long-term bone marrow cultures has been shown to increase the duration of hematopoiesis (18), as well as having direct effects on both bone marrow stromal cells and hematopoietic progenitor cells, inducing radioresistance and having radiation-mitigation properties. The mechanism of action of JP4–039 appears to be consistent across a broad range of biological assays and suggests direct effects on multiple cell types. One problem with JP4–039 and other GS-nitroxides has been their relative insolubility, making intra-oral administration a challenge (7, 12).
In the present studies, we tested the biological properties of MMS350, a highly water-soluble bi-functional sulfoxide recently shown to be a potent radiation protector and mitigator for cells in culture, and which is also quite effective as a radiation mitigator in vivo (14–15). MMS350 is particularly attractive for use as a radiation mitigator because it also reduces irradiation-induced late effects, principally fibrosis, in a model for radiation pulmonary fibrosis (15). In the present studies, the addition of MMS350 weekly to long-term bone marrow cultures (16–17) resulted in no significant increase in production of hematopoietic cells, or longevity of the bone marrow cultures. This result may be attributable to the rapid uptake or metabolism of MMS350 in culture compared to a slower metabolism of JP4–039 in long-term culture. Further studies will be required to confirm this possibility.
Similar to data with JP4–039, MMS350-treated bone marrow stromal cells exhibited radiation resistance and radiation mitigation in vitro in clonogenic survival curve assay (18). These results were consistent with other experiments showing an increase in antioxidant stores within MMS350-treated cells, decrease in apoptosis of irradiated cells, and rapid time of repair of DNA doublestrand breaks by the comet assay (10). These findings were made in both bone marrow stromal cell lines derived from MMS350-treated long-term bone marrow cultures and in freshly, acutely-treated bone marrow stromal cell lines from control long-term bone marrow cultures first exposed to MMS350 in the immediate radiation survival curve assay. The results establish that MMS350 is a chronic and acute small-molecule radiation protector with respect to bone marrow stromal cells derived from long-term bone marrow cultures.
These results provide strong evidence that MMS350 treatment conferred radioresistance to bone marrow stromal cells. The absence of a detectable increase in hematopoietic stem cell numbers in long-term bone marrow cultures hematopoiesis and the induction of radioresistance of marrow stromal cells suggest a protective effect on components of the hematopoietic microenvironment, rather than on hematopoietic stem cells.
The mechanism of radiation protection of MMS350 in stromal cells in the clonogenic survival curve assay is not known. The lack of detectable improvement in hematopoiesis in long-term bone marrow cultures suggests that the effect of MMS350 on hematopoiesis was indirect through the bone marrow stromal cells. Radioprotection of bone marrow stromal cells in this experiment was separated from enhanced support of hematopoietic cells in long-term bone marrow culture. This result might be explained by a smaller enhancement of expression of adhesion molecules in MMS350-treated bone marrow stromal cells in long-term culture, compared to that observed with JP4–039 stimulation of hematopoiesis. Unlike results of cultures treated with JP4–039 (18), the stromal cell lines derived from cultures treated with MMS350 were not different from those of cultures of standard media. Further studies will be required to elucidate the differences in the response of bone marrow stromal cells from long-term cultures to JP4–039 compared to MMS350, and these studies may elucidate a different pattern of hematopoiesis enhancement. The results establish that these two different categories of small-molecule radiation protectors/mitigator agents, GS-nitroxide and bi-functional sulfoxide, have different effects on hematopoiesis in long-term bone marrow cultures. Further studies will be required to determine the molecular mechanisms of action of these two radiation protector and mitigator drugs.
Table II.
Hematopoiesis in MMS350-treated long-term bone marrow cultures: Cobblestone islands. Data are summarized as mean±standard deviation, n is the number of flasks used, and the p-value for comparison between the two groups with the two-sided two sample t-test. Significant p-values are shown in bold.
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Control | 89.00±24.04 (n=2) | 338.00±24.04 (n=2) | 458.00±24.04 (n=2) | 630.00±2.83 (n=2) | 774.00±29.70 (n=2) | 242.00±14.14 (n=2) | 322.00±5.66 (n=2) | 143.00±24.04 (n=2) |
| MMS350 | 66.00±8.49 (n=2) | 343.00±7.07 (n=2) | 580.00±35.36 (n=2) | 868.00±18.38 (n=2) | 501.50±6.36 (n=2) | 254.00±5.66 (n=2) | 255.00±141.42 (n=2) | 153.00±4.24 (n=2) |
| p-Value | 0.3302 | 0.8043 | 0.0563 | 0.0030 | 0.0062 | 0.3811 | 0.5721 | 0.6210 |
Table III.
Hematopoiesis in MMS350-treated long-term bone marrow cultures: Non-adherent cells produced. Analysis of weekly non-adherent cell number data, where data are summarized as mean±standard deviation, n is the number of flasks used, and p-value for comparison between the two groups with the two-sided two sample t-test. Significant p-values are shown in bold.
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Control | 2009.25±18.38 (n=2) | 1049.38±38.36 (n=2) | 727.63±5.83 (n=2) | 6415.38±1231.96 (n=2) | 1137.63±178.01 (n=2) | 1934.00±590.43 (n=2) | 1207.13±276.66 (n=2) | 287.00±84.85 (n=2) |
| MMS350 | 2210.75±93.34 (n=2) | 1238.38±316.96 (n=2) | 995.63±468.64 (n=2) | 5592.00±919.95 (n=2) | 1463.88±38.36 (n=2) | 1644.75±184.55 (n=2) | 1017.50±7.07 (n=2) | 422.00±91.22 (n=2) |
| p-Value | 0.0957 | 0.4906 | 0.5671 | 0.5279 | 0.1268 | 0.5764 | 0.5098 | 0.2651 |
Table IV.
Hematopoiesis in MMS350-treated long-term bone marrow cultures: Day-7 Colony Forming Unit-Granulocyte, Erythrocyte, Monocyte, Megakaryocyte. Analysis of day-7 colony counts at each week, where data are summarized as mean±standard deviation, n is the sample size, and p-value for comparison between the two groups with the two-sided two sample t-test. Significant p-Values are shown in bold.
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Control | 16.67±2.31 (n=3) | 20.00±3.46 (n=3) | 52.67±1.15 (n=3) | 10.67±1.15 (n=3) | 18.33±2.08 (n=3) | 26.67±3.06 (n=3) | 23.00±1.00 (n=3) |
| MMS350 | 21.67±2.08 (n=3) | 17.00±2.65 (n=3) | 18.00±2.00 (n=3) | 11.33±2.08 (n=3) | 13.00±1.00 (n=3) | 30.67±3.06 (n=3) | 30.00±3.00 (n=3) |
| p- Value | 0.0495 | 0.2991 | <0.0001 | 0.6530 | 0.0161 | 0.1841 | 0.0186 |
Acknowledgements
Supported by research grant U19A168021 from the National Institute of Allergy and Infectious Diseases/National Institutes of Health NIAID/NIH.
References
- 1.Greenberger JS: Sensitivity of corticosteroid-dependent, insulin-resistant lipogenesis in marrow preadipocytes of mutation diabetic-obese mice. Nature 275: 752–754, 1978. [DOI] [PubMed] [Google Scholar]
- 2.Sakakeeny MA and Greenberger JS: Granulopoiesis longevity in continuous bone marrow cultures and factor dependent cell line generation: Significant variation among 28 inbred mouse strains and outbred stocks. J Natl Cancer Inst 68: 305–317, 1982. [PubMed] [Google Scholar]
- 3.Lechpammer S, Epperly MW, Zhou S, Nie S, Glowacki J and Greenberger JS: Antioxidant pool regulated adipocyte differentiation Sod2−/−bone marrow stromal cells. Exp Hematol 33: 1201–1208,2005. [DOI] [PubMed] [Google Scholar]
- 4.Epperly MW, Cao S, Zhang X, Franicola D, Kanai AJ, Greenberger EE and Greenberger JS: Increased longevity of hematopoiesis in continuous bone marrow cultures derived from mtNOS−/− homozygous recombinant negative mice correlates with increased radioresistance of hematopoietic and bone marrow stromal cells. Exp Hematol 35: 137–145, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Rajagopalan MS, Gupta K, Epperly MW, Franicola D, Zhang X, Wang H, Zhao H, Tyurin VA, Kagan VE, Wipf P, Kanai A and Greenberger JS: The mitochondria-targeted nitroxide JP4–039 augments potentially lethal irradiation damage repair. In Vivo 23: 717–726, 2009. [PMC free article] [PubMed] [Google Scholar]
- 6.Rajagopalan MS, Stone B, Rwigema J-C, Salimi U, Epperly MW, Goff J, Franicola D, Dixon T, Cao S, Zhang X, Buchholz BM, Bauer AJ, Choi S, Bakkenist C, Wang H and Greenberger JS: Intraesophageal manganese superoxide dismutase-plasmid liposomes ameliorates novel total body and thoracic irradiation sensitivity of homologous deletion recombinant negative nitric oxide synthase-1 (NOS1−/−) mice. Radiat Res 174: 297–312, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goff JP, Epperly MW, Shields D, Wipf P, Dixon T, and Greenberger JS: Radiobiologie effects of GS-nitroxide (JP4–039) in the hematopoietic syndrome. In Vivo 25: 315–324, 2011. [PMC free article] [PubMed] [Google Scholar]
- 8.Rwigema J-CM, Beck B, Wang W, Doemling A, Epperly MW, Shields D, Franicola D, Dixon T, Frantz M-C, Wipf P, Tyurina Y, Kagan VE, Wang H and Greenberger JS: Two strategies for the development of mitochondrial-targeted small molecule radiation damage mitigators. Int J Radiat Oncol Biol Phys 80(3): 860–868, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Sullivan R, Goff J, Shields D, Epperly M, Greenberger JS and Glowacki J: Cell biologic parameters of accelerated osteoporosis in SAMP6 mice are demonstrated in long-term bone marrow culture senescence and in the biology of bone marrow stromal cell lines. Exp Hematol 40: 499–509, 2012.22326715 [Google Scholar]
- 10.Berhane H, Epperly MW, Goff J, Kalash R, Cao S, Franicola D, Zhang X, Shields D, Houghton F, Wang H, Sprachman M, Wipf P, Li S, Gao X, Parmar K and Greenberger JS: Radiobiologie differences between bone marrow stromal and hematopoietic progenitor cell lines from Fanconi Anemia (Fancd2−/−) mice. Radiat Res 181: 76–89, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernard ME, Kim H, Berhane H, Epperly MW, Franicola D, Zhang X, Houghton F, Shields D, Wang H, Bakkenist CJ, Frantz M-C, Wipf P and Greenberger JS: GS-nitroxide (JP4–039) mediated radioprotection of human Fanconi anemia cell lines. Radiat Res 176: 603–612, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H, Bernard ME, Epperly MW, Shen H, Amoscato A, Dixon TM, Doemling AS, Li S, Gao X, Wipf P, Wang H, Zhang X, Kagan VE, and Greenberger JS: Amelioration of radiation esophagitis by orally administered p53/mdm2/mdm4 inhibitor (BEB55) or GS-nitroxide. In Vivo 25(6): 841–849, 2011. [PMC free article] [PubMed] [Google Scholar]
- 13.Goff J, Shields D, Wang H, Skoda E, Sprachman M, Wipf P, Lazo J, Atkinson J, Kagan V, Epperly M and Greenberger JS: Evaluation of ionizing irradiation protectors and mitigators using clonogenic survival of human umbilical cord blood hematopoietic progenitor cells. Exp Hematol 41(11): 957–966, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprachman MM and Wipf P: A bifunctional dimethylsulfoxide substitute enhances the aqueous solubility of small organic molecules. Assay Drug Dev Technol 10(3): 269–277, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalash R, Epperly MW, Goff J, Dixon T, Sprachman MM, Zhang X, Shields D, Cao S, Wipf P, Franicola D, Berhane H and Greenberger JS: Amelioration of irradiation pulmonary fibrosis by a water-soluble bifunctional sulfoxide radiation mitigator (MMS350). Radiat Res 180: 474–490, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epperly M, Cao S, Shields D, Franicola D, Zhang X, Wang H, Friedlander R and Greenberger JS: Increased hematopoiesis in continuous marrow cultures and radiation resistance of marrow stromal cells from aspase-1 knockout mice. In Vivo 27: 419–430, 2013. [PMC free article] [PubMed] [Google Scholar]
- 17.Epperly M, Cao S, Wang H and Greenberger JS: Unaltered hematopoietic longevity in continuous bone marrow cultures from Nrf2 homozygous deletion recombinant (knockout) mice. In Vivo 27: 571–582,2013. [PMC free article] [PubMed] [Google Scholar]
- 18.Epperly MW, Franicola D, Cao S, Holt D, Shinde A, Goff J, Shields D, Wipf P, Wang H and Greenberger JS: Increased longevity of GS-nitroxide (JP4–039) treated mouse long-term bone marrow cultures and radioresistance of derived bone marrow stromal cell lines. In Vivo (submitted manuscript). [PMC free article] [PubMed] [Google Scholar]