Abstract
Aim:
We investigated whether homologous recombinant deletion of the endothelial cell-specific protein Von Willebrand factor (vWF) affected hematopoiesis in longterm bone marrow cultures, and irradiation induction of pulmonary fibrosis/organizing alveolitis.
Materials and Methods:
We established long-term bone marrow cultures from vWF−/− (C57BL/6 background) and vWF+/+ littermate mice. Non-adherent cells removed weekly were tested for formation of multi-lineage hematopoietic stem cells forming colonies at 7 and 14 days in secondary semi-solid medium cultures. Irradiation fibrosis in the lungs of 20-Gy thoracic irradiated mice was quantitated and scored.
Results:
Hematopoiesis was increased over 20 weeks in vWF−/− compared to vWF+/+ cultures in production of non-adherent cells, and cells forming colonies at 7 or 14 days in secondary semi-solid medium culture. The irradiated lungs showed no increased fibrosis.
Conclusion:
Absence of vWF increases hematopoiesis in long-term bone marrow cultures and has a protective effect in irradiated lungs.
Keywords: Von Willebrand factor, continuous marrow culture, longevity of hematopoiesis
The endothelial cell-specific protein Von Willebrand factor (vWF) is involved in the intrinsic pathway of blood clotting through its interaction with factor IX and blood platelets (1–23). Furthermore, vWF has been shown to be an adhesion molecule expressed on the surface of endothelial cells during their interaction with pathogenic microbes, as well as in migrating normal and metastasized malignant cells (1,4).
Recent studies have demonstrated up-regulation of expression of RNA for vWF in the pulmonary endothelial cells of the irradiated C57BL/6J mouse lung (5–7). Stabilization of vWF and other endothelial cell-specific molecules in C57BL/6J mice between the acute radiation pneumonitis phase and late fibrosis phase suggests that stable vWF expression might be a biomarker of continuous oxidative stress of the lung, leading to accumulation of biochemical alterations and the initiation of fibrosis. Migration into the irradiated lungs of bone marrow stromal cell progenitors of the cells forming lung fibrosis, and recent demonstration of their migration through the circulation (5), suggests that pulmonary endothelial cells might be the first target for entry into the lung microenvironment of circulating mesenchymal stem cells, potentially hematopoietic stem cells, and cancer-initiating cells.
A biological organ culture system by which to test the effects of endogenous or exogenous oxidative stress is the mouse long-term bone marrow culture (LTBMC) system (8–10). The absence of intrinsic mediators of oxidative stress such as manganese superoxide dismutase (11) or senescent prone mice with ROS handling defects (12) have been shown to limit hematopoiesis in long-term culture. In contrast, genetic modifications leading to decreased production of ROS-generating proteins, including nitroxide synthase-1 (13), and Mothers Against Decapentaplegic homolog 3 (SMAD3) (14), a mediator of transforming growth factor-β signaling, have been shown to increase hematopoiesis in LTBMC.
The oxidative stress of continuous bone marrow culture is associated with the use of a high oxygen incubator and the continuous production of byproducts of metabolism in the culture medium such that acidosis is detected prior to weekly medium changes. The adherent microenvironment of long-term bone marrow cultures is known to contain fibroblasts (mesenchymal stem cells), endothelial cells, macrophages, and the progenitors of osteoblasts. We sought to determine whether absence of vWF in the adherent layer of long-term cultures adversely affected hematopoiesis. A defective interaction between adherent layer stromal cells and adherent hematopoietic colony-forming cells (recognized as cobblestone islands) would suggest a deleterious role of deletion of vWF in long-term culture. In addition lungs of vWF−/− mice might not attract circulating bone marrow stromal cells if vWF was missing from their endothelial cells.
Materials and Methods
Mice.
C57BL/6J vWF−/− and control littermate vWF+/+ mice were obtained from Denisa D. Wagner, Ph.D., Professor of Pediatrics, Harvard Medical School, Boston, MA, USA. Animals were housed four per cage according to the University of Pittsburgh Institutional Animal Care and Use Committee regulations and fed standard laboratory chow with hyperchlorinated drinking water.
Continuous bone marrow cultures.
LTBMCs were established according to published methods (8–10). Briefly the bone marrow of a femur and tibia of an adult C57BL/6J mice were flushed into a T-25 Corning plastic tissue culuture flask, in 12 ml Fisher’s medium supplemented with 25% horse serum and 10−5 M hydrocortisone hemi-succinate. Cultures were maintained in a high humidity incubator at 37°C with weekly replacement of 50% of the volume with fresh medium. After week 5, the horse serum was replaced with 25% fetal bovine serum (FBS).
Measurements of hematopoiesis.
The adherent layer of LTBMCs was scored for the percentage of the surface area covered by adherent cells (percentage confluence), and the number of flattened adherent colonies of hematopoietic cells i.e. ‘cobblestone islands’ per flask. Half of the non-adherent cells were removed weekly, counted and transferred to semi-solid methylcellulose supplemented with granulocyte macrophage-colony stimulating factor (GM-CSF), granulocyte colony stimulating factor (G-CSF), interleukin 3 (IL3), and other growth factors (Stem Cell Technology, Vancouver, BC, Canada), as published elsewhere (10). Subcultures were maintained in a high-humidity incubator for seven days and scored for the number of colonies greater than or equal to 50 cells per colony, then returned to the incubator, and scored again on day 14 for the number of more primitive cells which from colonies at later timepoints according to published methods (15).
Hematopoietic cell colony-forming assays:
Non-adherent cells were removed from the LTBMC flasks and suspended at l×lO6 cells/ml with 5×l04 cells/dish were plated in triplicate in semi-solid medium consisting of methylcellulose in Iscove’s modified Dulbecco’s medium (IMDM) 10% FBS, 10% bovine serum albumin (BSA), L-glutamine, 3 U/ml erythropoietin, and 2-mercaptoethanol (Stem Cell Technology, Vancouver, BC, CA). WEHI-3 conditioned medium was added as a source of I1–3 (15). Colony-forming unit granulocyte-macrophage (CFU-GM) of 50 cells or greater were counted on days 7 and 14 after plating. Fresh marrow colonies were subdivided for CFU-GM, burst forming unit erythroid (BFUe) and colony-forming unit granulocyte-erythroid-megakaryocyte-macrophage (CFU-GEMM)
Quantitation of pulmonary fibrosis in lungs of irradiated mice.
vWF+/+ and vWF−/− mice were irradiated to 20 Gy to the pulmonary cavity with the remaining portion of the body shielded from the irradiation. The mice were sacrificed 200 days after irradiation at which time the lungs were expanded in Optimal Cutting Temperature compound (OCT), excised, frozen in OCT, sectioned and stained with hematoxylin and eosin (H&E) and Masson’s Trichrome Stain. The sections were examined microscopically and the percent of the lung exhibiting fibrosis calculated as published elsewhere (15). The percentage of the lung displaying fibrosis was scored in 100 high-powered fields in 10 slides per lobe (five lobes) for each of five vWF+/+ and five vWF−/− mice.
Statistical analysis.
For LTBMC data, weekly cobblestone island numbers, non-adherent cell numbers (×105), percentage confluence of adherent cells, number of day 7 colonies and day 14 colonies at each weekly harvest were counted as described elsewhere (15). Data are summarized as the mean±standard deviation, n is the number of mice used, and p-values were calculated with the two-sided two-sample Mest comparing vWF+/+ to vWF−/− mice. A p-value of less than 0.05 was considered as significant. As this was an exploratory study, p-values were not adjusted for multiple comparisons (13).
Results
Continuous hematopoiesis in vWF−/− LTBMCs is equivalent to that in cultures from wild-type mice.
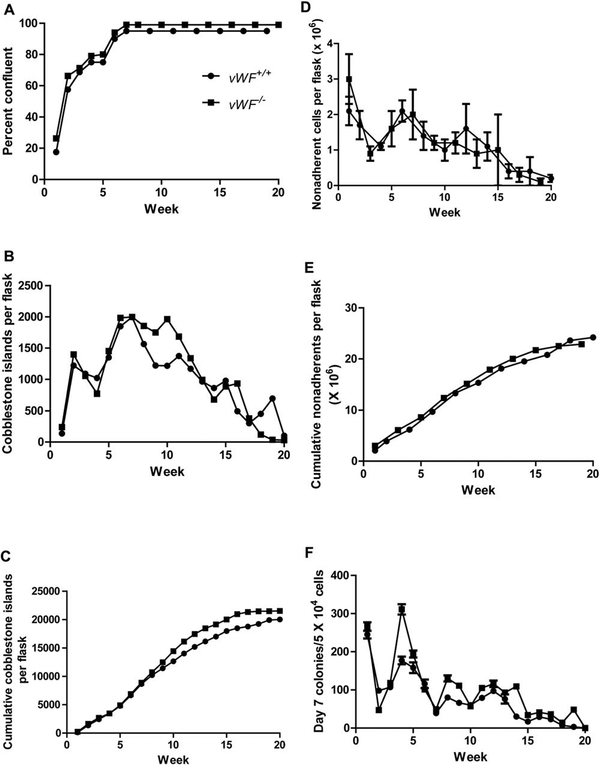
As shown in Figure 1A, the adherent layer of LTBMCs from vWF−/− mice reached confluence and maintained steady confluence for 20 weeks, indistinguishable from that of wild-type culture. These results established that the absence of vWF did not adversely affect the attachment and adherence of bone marrow stromal cells, nor did it result in detectable toxicity such as sloughing of the adherent layer in marrow cultures. Such sloughing has been detected in the presence of toxins in some serum lots used for LTBMCs. As shown in Figure IB, cobblestone islands were scored weekly, and the numbers were detected at a frequency comparable to that in LTBMCs from wild-type vWF+/+ mice. Cumulative confluence and production of cobblestone islands was equivalent between genotypes (Figure 1A–1C).
Figure 1.
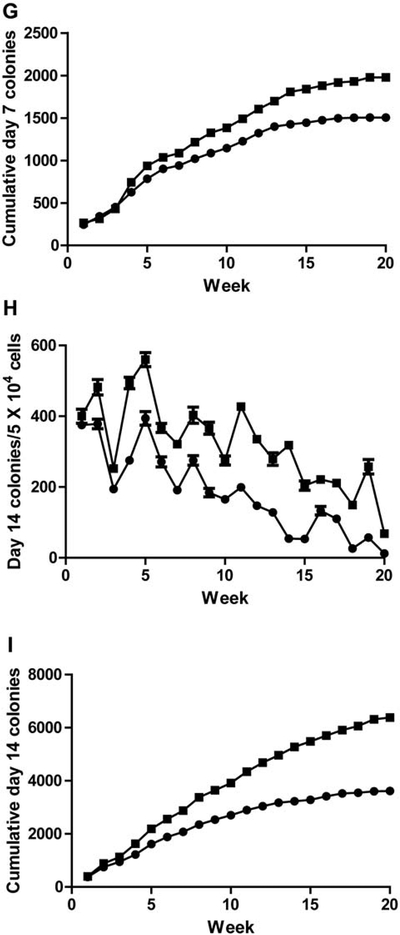
Long-term marrow cultures from Von Willebrand Factor (vWF−/− compared to vWF+/+ mice: A: percentage confluence; B: cobblestone islands produced; C: cumulative cobblestone islands; D: weekly non-adherent cells produced; E: cumulative nonadherent cells produced; F : weekly day-7 colony-forming unit granulocyte-erythroid-megakaryocyte-macrophage (CFU-GEMM); G: cumulative day-7 CFU-GEMM; H: weekly day-14 CFU-GEMM; I: cumulative day-14 CFU-GEMM.
Non-adherent cell production by LTBMCs of vWF−/− mice was measured weekly. As shown in Figure ID, the release of non-adherent cells into the non-adherent layer of marrow cultures was comparable in vWF−/− LTBMCs on a weekly basis compared to that detected in wild-type marrow cultures. Fluctuation in cell numbers has been reported with LTBMCs, and the results were consistent with that previously published for C57BL/6J mouse marrow cultures compared to other mouse strains and stocks (10). Cumulative production of non-adherent cells was equivalent between mouse genotypes (Figure IE).
Colony-forming progenitor cells detected as those forming colonies of 50 or more cells at day 7, and at day 14 (Table II) were compared between LTBMCs of each genotype. As shown in Figure IF and Table I, weekly and cumulative production (Figure 1G) of non-adherent cells forming colonies at day 7 was increased in vWF−/− LTBMCs. The results for cells forming more primitive colonies at day 14 was also similar, showing increases in vWF−/− cultures, when scoring for weekly (Figure 1H and Table II) and cumulative production (Figure II). These results established that multiple parameters of hematopoiesis were not adversely affected with respect to longevity of in vitro LBTMCs by absence of the vWF protein.
Table II.
Analysis of hematopoiesis in long-term bone marrow cultures (LTBMCs) from Von Willebrand factor (vWF−/−) mice: Day-14 colony-forming unit granulocyte-erythroid-megakaryocyte-macrophage (CFU-GEMM). The day-14 colony counts at each week are shown, where data are summarized as mean±standard deviation, n is the sample size, and p-value is for comparison to C57BL/6 group using the two-sided two sample t-test. Significant p-values less than 0.05 are shown in bold.
| Group | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 |
|---|---|---|---|---|---|
| vWF−/− | 399.3±14.0 (n=3) | 482.7±15.6 (n=3) | 245.0±12.1 (n=3) | 483.3±22.6 (n=3) | 547.7±25.5 (n=3) |
| C57BL/6 | 374.7±10.1 (n=3) | 377.7±13.5 (n=3) | 194.3±8.0 (n=3) | 274.7±9.7 (n=3) | 394.0±19.1 (n=3) |
| p-Value | 0.0688 | 0.0009 | 0.0038 | 0.0001 | 0.0011 |
| Group | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 |
| vWF−/− | 367.7±9.1 (n=3) | 314.7±11.4 (n=3) | 402.7±16.0 (n=3) | 364.7±12.2 (n=3) | 275.0±9.0 (n=3) |
| C57BL/6 | 271.0±14.1 (n=3) | 190.7±7.5 (n=3) | 274.7±13.5 (n=3) | 184.0±12.0 (n=3) | 164.7±6.0 (n=3) |
| p-Value | 0.0006 | 0.0001 | 0.0005 | 0.0001 | 0.0001 |
| Group | Week 11 | Week 12 | Week 13 | Week 14 | Week 15 |
| vWF−/− | 427.0±9.0 (n=3) | 335.3±7.0 (n=3) | 278.3±12.5 (n=3) | 321.7±6.7 (n=3) | 203.3±9.5 (n=3) |
| C57BL/6 | 198.7±9.5 (n=3) | 146.7±8.0 (n=3) | 128.3±8.1 (n=3) | 54.0±5.6 (n=3) | 52.7±6.0 (n=3) |
| p-Value | <0.0001 | <0.0001 | 0.0001 | <0.0001 | <0.0001 |
| Group | Week 16 | Week 17 | Week 18 | Week 19 | Week 20 |
| vWF−/− | 220.3±6.5 (n=3) | 211.7±9.1 (n=3) | 149.7±7.1 (n=3) | 255.7±14.6 (n=3) | 68.0±6.6 (n=3) |
| C57BL/6 | 133.3±12.0 (n=3) | 109.7±4.0 (n=3) | 25.7±5.5 (n=3) | 57.0±7.5 (n=3) | 11.7±2.1 (n=3) |
| p-Value | 0.0004 | 0.0001 | <0.0001 | <0.0001 | 0.0001 |
Table I.
Analysis of hematopoiesis in long-term bone marrow cultures (LTBMCs) for Von Willebrand factor (vWF−/−) mice: Day-7 colony-forming unit granulocyte-erythroid-megakaryocyte-macrophage (CFU-GEMM). The day-7 colony counts at each week are shown, where data are summarized as mean±standard deviation, n is the sample size, and the p-value is for comparison to C57BL/6 group using the two-sided two sample t-test. Significant p-values of less than 0.05 are shown in bold.
| Group | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 |
|---|---|---|---|---|---|
| vWF−/− | 266.0±11.1 (n=3) | 47.3±5.0 (n=3) | 118.0±3.0 (n=3) | 311.0±13.5 (n=3) | 194.0±9.2 (n=3) |
| C57BL/6 | 245.3±10.1 (n=3) | 98.0±6.0 (n=3) | 107.3±5.5 (n=3) | 176.7±10.1 (n=3) | 157.7±14.0 (n=3) |
| p-Value | 0.0756 | 0.0004 | 0.0421 | 0.0002 | 0.0199 |
| Group | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 |
| vWF−/− | 100.7±6.5 (n=3) | 48.7±7.0 (n=3) | 130.0±8.0 (n=3) | 111.3±7.0 (n=3) | 57.3±5.5 (n=3) |
| C57BL/6 | 116.0±11.1 (n=3) | 39.0±6.0 (n=3) | 79.7±7.1 (n=3) | 66.0±6.0 (n=3) | 59.7±7.5 (n=3) |
| p-Value | 0.1085 | 0.1441 | 0.0012 | 0.0011 | 0.6866 |
| Group | Week 11 | Week 12 | Week 13 | Week 14 | Week 15 |
| vWF−/− | 105.3±4.5 (n=3) | 115.7±8.5 (n=3) | 92.7±6.5 (n=3) | 109.0±5.0 (n=3) | 33.7±3.5 (n=3) |
| C57BL/6 | 79.3±4.5 (n=3) | 96.7±5.0 (n=3) | 76.3±12.0 (n=3) | 29.7±4.0 (n=3) | 17.0±3.0 (n=3) |
| p-Value | 0.0021 | 0.0291 | 0.1072 | <0.0001 | 0.0033 |
| Group | Week 16 | Week 17 | Week 18 | Week 19 | Week 20 |
| vWF−/− | 40.7±4.5 (n=3) | 36.0±4.0 (n=3) | 14.0±2.0 (n=3) | 47.7±6.0 (n=3) | 0.0±0.0 (n=3) |
| C57BL/6 | 29.3±4.2 (n=3) | 23.0±5.0 (n=3) | 6.7±3.1 (n=3) | 2.7±2.5 (n=3) | 0.0±0.0 (n=3) |
| p-Value | 0.0329 | 0.0245 | 0.0254 | 0.0003 | 1.0000 |
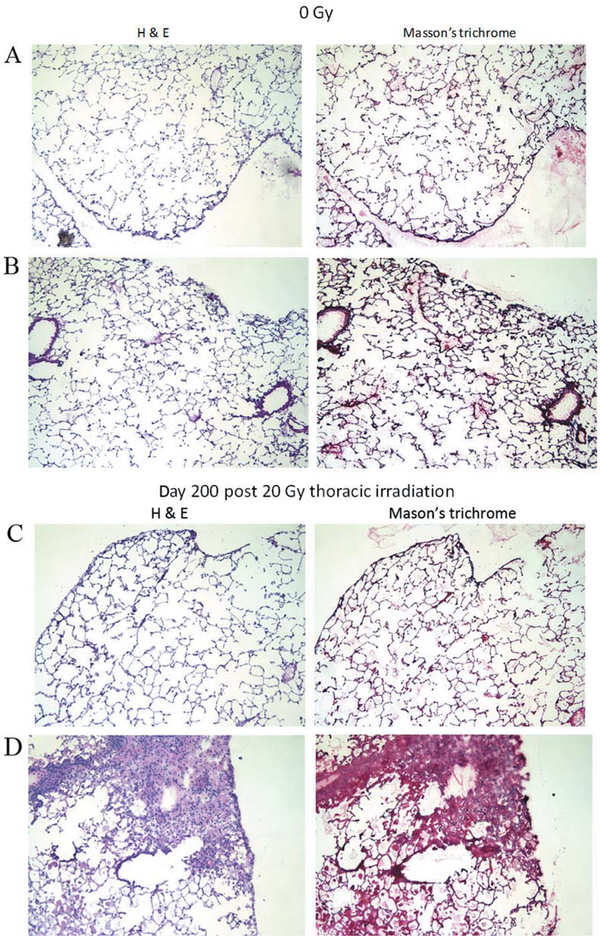
Figure 2.
Histopathological analysis of lungs from Von Willebrand Factor (vWF−/−) mice irradiated with 20 Gy to the thorax. Histopathology of lungs removed at 200 days after 20 Gy thoracic irradiation ofvWF−/− mice (xlOO). A-D: Hematoxylin and eosin (H&E) staining on the left, and Masson’s trichrome stains for collagen on the right for each of four mice. Sections from two nonirradiated vWF−/− mice are shown in A and B, while sections from two 20 Gy irradiated vWF^-mice are shown in C and D. Inflammatory cell infiltrates can be seen on the right in D. No mouse lung exhibited collagen deposition.
The present results establish that homologous recombinant deletion of the gene for vWF, which would result in lack of production of vWF, does not adversely affect continuous hematopoiesis in LBTMCs.
Radiation fibrosis is reduced in lungs of vWF−/− mice irradiated with 20 Gy to the thorax.
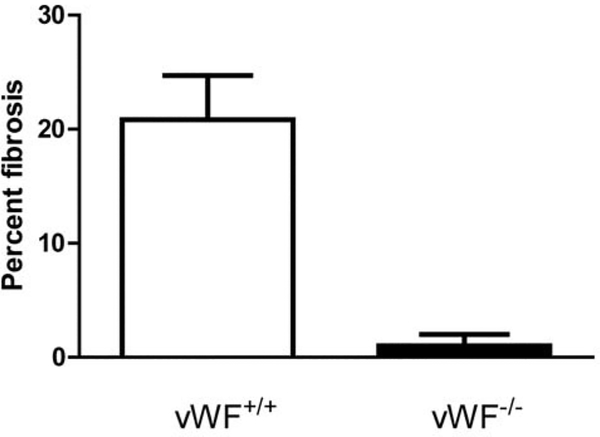
Mice were irradiated to the thoracic cavity as published elsewhere (6) and subgroups sacrificed at day 200 following irradiation. The percentage lung replaced by fibrosis in vWF−/− was compared to that in vWF+/+ mice. The results showed no evidence of fibrosis in vWF−/− mouse lungs. Representative photographs of fibrotic areas in the lungs of two unirradiated and two irradiated vWF−/− mice at day 200 are shown in Figure 2. These data are presented in quantitative form in Figure 3.
Figure 3.
Quantitation of pulmonary fibrosis in the lungs of Von Willebrand Factor (vWF−/−) and wild-type mice irradiated with 20 Gy to the thorax. vWF+/+ and vWF−/− mice were irradiated to 20 Gy to the pulmonary cavity. The mice were sacrificed 200 days after irradiation. The lungs were removed, frozen in Optimal Cutting Temperature compound, sectioned, stained with hematoxylin and eosin, and the percentage fibrosis determined in 100 high-powered fields examined in each of 10 slides per lung lobe (five lobes per mouse), five mice per group.
Discussion
The present results establish that homologous recombinant deletion of vWF does not adversely affect hematopoiesis in long-term culture and increases several parameters of robustness of hematopoiesis. Several parameters reflecting the stability of the hematopoietic microenvironment were shown to be indistinguishable in cultures from vWF−/− compared to wild-type mice. These included confluence of the adherent layer and maintenance of cobblestone islands; however, longevity of the production of non-adherent cells was not increased in LTBMCs from vWF−/− mouse marrow. These data establish that absence of vWF, a major adhesion molecule of the hematopoietic microenvironment, specifically in endothelial cells, does not adversely affect the interaction between hematopoietic stem cells and the bone marrow microenvironment in vitro.
Thoracic irradiation of C57BL/6J mice produces radiation-induced lung fibrosis after a radiation pneumonitis phase and latent period during which vWF and other endothelial-specific gene transcripts have been shown to be elevated (6). In contrast, C3H/HeNHsd mice, which do not develop radiation-induced fibrosis showed continuous maintenance of transcripts for acute radiation pneumonitis and no specific down-regulation or latent period (7). Endothelial cells have been shown to respond in vitro and in vivo to irradiation by up-regulation of vWF (1–3). The endothelial cell is known to be critically-involved in the pathophysiology of radiation damage to multiple organs, including the intestine (16, 17), lung (4–6), and central nervous system. A common component of endothelial responses to irradiation has been shown to be up-regulation of gene transcripts associated with the stress response, as well as those associated with adhesion of circulating inflammatory cells (4, 18–21).
In the present studies, we utilized a culture system in which interaction and attachment of hematopoietic cells with stromal cells can be studied for months in vitro. Interaction of hematopoietic and stromal cells is known to be critical for hematopoiesis. Long-term marrow culture from mice with defects in management of oxidative stress found in a high oxygen incubator has been shown to reduce the longevity of hematopoiesis (11). Similarly, genetic defects associated with senescence (12), and cultures from other mice with deletion of negative regulatory critical components 14) have been shown to modulate the longevity of hematopoiesis. Finally, intrinsic genetic variation between mouse strains has been shown to increase longevity of hematopoiesis (10).
The absence of a deleterious effect of homologous deletion of vWF with respect to hematopoiesis in LBTMCs was compared to the effect of this vW7−/− genotype on induction of radiation fibrosis in C57BL/6J mice. The results demonstrated no evidence of fibrosis or collagen deposition in lungs after irradiation of vWF−/− C57BL/6J with 20 Gy to the thorax. Distinct foci of increased number of macrophages were found throughout the lung tissues of both C57BL/6J controls and vWF−/− mice. Increased numbers of heterogenous populations of other inflammatory cells, including granulocytes and lymphocytes, were present throughout the lungs of both strains. Further studies are required to determine the effect of the lack of the vWF gene product on the function of other organ systems in vivo and in vitro.
Acknowledgements
This article was supported by the following grants: NIH R01-CA119927–11, R01-HL-094488–02, and NIH/NIAID 1U19A168021–06. This project used the UPCI Animal Facility that is supported in part by award P30CA047904
References
- 1.Jahroudi N, Ardekani AM and Greenberger JS: Ionizing irradiation increases transcription of the vWF gene in endothelial cells. Blood 88(10): 3801–3814, 1996. [PubMed] [Google Scholar]
- 2.Jahroudi N, Ardekani AM and Greenberger JS: An NFl-like protein functions as repressor of vWF promoter. J Biol Chem 271(35): 21413–21421, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Ardekani AM, Greenberger JS and Jahroudi N: Endothelial specific activity of the vWF promoter is regulated by two repressor elements. J Thromb Hemost 80: 488–494, 1998. [PubMed] [Google Scholar]
- 4.Epperly MW, Sikora CA, Defilippi SJ, Gretton JE, Bar-Sagi D, Carlos T, Guo HL and Greenberger JS: Pulmonary irradiation-induced expression of VCAM-1 and ICAM-1 is decreased by MnSOD-PL gene therapy. Biol Blood Bone Marrow Transplant 8(4): 175–187,2002. [DOI] [PubMed] [Google Scholar]
- 5.Kalash R, Berhane H, Goff J, Houghton F, Epperly MW, Dixon T, Zhang X, Sprachman MM, Wipf P, Franicola D, Wang H and Greenberger JS: Thoracic irradiation effects on pulmonary endothelial compared to alveolar type II cells in fibrosis prone C57BL/6NTac mice. In Vivo 27: 291–298, 2013. [PMC free article] [PubMed] [Google Scholar]
- 6.Kalash R, Epperly MW, Goff J, Dixon T, Sprachman MM, Zhang X, Shields D, Cao S, Wipf P, Franicola D, Berhane H and Greenberger JS: Amelioration of irradiation pulmonary fibrosis by a water-soluble bi-functional sulfoxide radiation mitigator (MMS350). Radiat Res 180: 474–490, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalash R, Berhane H, Epperly MW, Goff J, Dixon T, Zhang X, Franicola D and Greenberger JS: Differences in irradiated lung gene transcription between fibrosis-prone C57BL/6NHsd and fibrosis-resistant C3H/HeNHsd mice. In Vivo 28(2): 147–171, 2014 [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberger JS: Sensitivity of corticosteroid-dependent, insulin-resistant lipogenesis in marrow preadipocytes of mutation diabetic-obese mice. Nature 275: 752–754, 1978. [DOI] [PubMed] [Google Scholar]
- 9.Mauch P, Greenberger JS, Botnick LE, Hannon EC and Heilman S: Evidence for structured variation in self-renewal capacity within long-term bone marrow cultures. Proc Natl Acad Sei USA 77: 2927–2930, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakakeeny MA and Greenberger JS: Granulopoiesis longevity in continuous bone marrow cultures and factor-dependent cell line generation: Significant variation among 28 inbred mouse strains and outbred stocks. J Natl Cane Inst 68: 305–317, 1982. [PubMed] [Google Scholar]
- 11.Epperly MW, Epstein CJ, Travis EL and Greenberger JS: Decreased pulmonary radiation resistance of manganese superoxide dismutase (MnSOD)-deficient mice is corrected by human manganese superoxide dismutase-plasmid/liposome (SOD2-PL) intratracheal gene therapy. Radiat Res 154(4): 365–374,2000. [DOI] [PubMed] [Google Scholar]
- 12.O’Sullivan R, Goff J, Shields D, Epperly M, Greenberger JS and Glowacki J: Cell biologic parameters of accelerated osteoporosis in SAMP6 mice are demonstrated in long-term bone marrow culture senescence and in the biology of bone marrow stromal cell lines. Exp Hematol 40: 499–509, 2012.22326715 [Google Scholar]
- 13.Epperly MW, Cao S, Zhang X, Franicola D, Kanai AJ, Greenberger EE and Greenberger JS: Increased longevity of hematopoiesis in continuous bone marrow cultures derived from mtNos−/− homozygous recombinant negative mice correlates with increased radioresistance of hematopoietic and bone marrow stromal cells. Exp Hematol 35: 137–145, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Epperly MW, Goff J, Zhang X, Shields D, Wang H, Shen H, Franicola D, Bahnson A, Greenberger EE and Greenberger JS: Increased radioresistance, G2M checkpoint inhibition and impaired migratory capacity of bone marrow stromal cell lines derived from Smad3−/− mice. Radiat Res 165: 671–677, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Berhane H, Epperly MW, Goff J, Kalash R, Cao S, Franicola D, Zhang X, Shields D, Houghton F, Wang H, Wipf P, Parmar K and Greenberger JS: Radiobiologie differences between bone marrow stromal and hematopoietic progenitor cell lines from Fanconi anemia (Fancd2−/− mice. Radiat Res 181:76–89, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paris F, Fuks Z, Kang Z, Capodieci P, Juan G, Ehleiter D, Halmovitz-Friedman A, Cordon-Cardo C and Kolesnick R: Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293(5528): 293–297, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Fuks Z, Persaud R, Alfieri A, McLoughlin M, Ehleiter D, Schwartz JL, Seddon AP, Cordon-Cardo C and Haimovitz-Friedman A: Basic fibroblast growth factor protects endothelial cells against radiation-induced programmed cell death in vitro and in vivo. Cancer Res 54: 2582–2590, 1994. [PubMed] [Google Scholar]
- 18.Epperly MW, Travis EL, Sikora C and Greenberger JS: Magnesium superoxide dismutase (MnSOD) plasmid/liposome pulmonary radioprotective gene therapy: Modulation of irradiation-induced mRNA for IL-1, TNF-α, and TGF-β correlates with delay of organizing alveolitis/fibrosis. Biol Blood Marrow Transplant 5: 204–214, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Philips T: An ultrastructural study of the development of radiation injury in the lung. Radiology 87(1): 49–54, 1966. [DOI] [PubMed] [Google Scholar]
- 20.Witte L, Fuks Z, Haimovitz-Friedman A, Vlodavsky I, Goodman DS and Eldor A: Effects of irradiation on the release of growth factors from cultured bovine, porcine, and human endothelial cells. Cancer Res 49: 5066–5072, 1989. [PubMed] [Google Scholar]
- 21.Gaugier M, Squiban C, Van Der Meeren A, Bertho JM, Vandamme M and Mouthon MA: Late and persistent upregulation of intercellular adhesion molecule-1 (ICAM-1) expression by ionizing radiation in human endothelial cells in vitro. Int J Radiat Biol 72(2): 201–209, 1997. [DOI] [PubMed] [Google Scholar]