The objective of this study was to elucidate the genetic and evolutionary relatedness of blaCMY-2- and blaSHV-12-carrying IncI1-Iγ plasmids. Phylogenomic analysis based on core genome alignments and gene presence/absence was performed for different IncI1-Iγ sequence types (STs).
KEYWORDS: ISEcp1, IS1294, IS26, Tn1721, sugE, blc, deoR, Salmonella Paratyphi B var. Java, S. Heidelberg, broiler, chicken
ABSTRACT
The objective of this study was to elucidate the genetic and evolutionary relatedness of blaCMY-2- and blaSHV-12-carrying IncI1-Iγ plasmids. Phylogenomic analysis based on core genome alignments and gene presence/absence was performed for different IncI1-Iγ sequence types (STs). Most IncI1-Iγ/ST12 and IncI1-Iγ/ST231 plasmids had near-identical core genomes. The data suggest that widely occurring blaCMY-2-carrying IncI1-Iγ/ST12 plasmids originate from a common ancestor. In contrast, blaSHV-12 was inserted independently into different IncI1-Iγ/ST231-related plasmids.
TEXT
Plasmid-encoded extended-spectrum and AmpC β-lactamases (ESBL/pAmpC) are the dominant causes of resistance to extended-spectrum cephalosporins in Enterobacteriaceae (1–3). Poultry and poultry products have been considered reservoirs of ESBL/pAmpC-producing Salmonella enterica and Escherichia coli (2–9). ESBL/pAmpC-carrying plasmids can be classified in different incompatibility groups, including IncI1-Iγ (10, 11). IncI1-Iγ plasmids harboring ESBL/pAmpC are dominant in S. enterica and E. coli originating from poultry in multiple countries (4, 12–17). Using plasmid multilocus sequence typing (pMLST) (18, 19), specific ESBL/pAmpC variants were found to be associated with particular IncI1-Iγ STs (12, 13, 16, 17). blaCMY-2 carriage has been associated with IncI1-Iγ/ST12 in isolates from poultry (12–14, 16, 17, 20). In contrast, blaSHV-12 has been described in multiple IncI1/STs in isolates originating from humans, animals (mainly poultry), and the environment (20–23). However, a resolution higher than the nucleotide sequences of the five housekeeping genes in the pMLST scheme is required to identify the evolutionary relatedness of plasmids belonging to the same ST (4, 17, 24). The objective of the present study was to elucidate the genetic and evolutionary relatedness of blaCMY-2- and blaSHV-12-carrying IncI1-Iγ plasmids within the same pMLSTs using whole-genome sequence (WGS)-based phylogenetic analysis.
Sequences of IncI1-Iγ plasmids originating from previous characterization of ESBL/pAmpC-carrying strains from Colombian baseline studies in poultry were selected. All sequences of blaCMY-2-carrying (n = 20) and blaSHV-12-carrying (n = 4) IncI1-Iγ plasmids from Salmonella (17) and all available blaCMY-2-carrying (n = 15) and blaSHV-12-carrying IncI1-Iγ plasmids (n = 4) from E. coli (16) were included. Plasmid sequences from Salmonella were characterized using Illumina WGS and electroporation of reference plasmids as previously described (17). For E. coli, previously transformed E. coli DH10B cells harboring blaCMY-2 and blaSHV-12 on IncI1-Iγ plasmids were subjected to Illumina WGS for the present study (16). Chromosomal contigs were detected and removed using BLAST as previously described for Salmonella (17). In addition, the allele sequences of IncI1-Iγ STs (https://pubmlst.org/plasmid/) encountered more than once in the selection of plasmids described above were concatenated as separate sequences for each allele in a single FASTA file and used as a query for the nucleotide database using BLAST (last accessed 29 May 2018). E. coli-derived plasmid sequences of two publications were used to include additional IncI1-Iγ/ST12 plasmids (n = 12) from Europe (see Table S1C in the supplemental material) (12, 13). Overall, ESBL/pAmpC gene variants and plasmids were characterized in silico with ResFinder 2.1 (25), PlasmidFinder 1.3, and pMLST 1.4 (19). A summary of all included plasmids is given in Table 1. The plasmid STs that were found repeatedly in Salmonella and E. coli from Colombian poultry were IncI1-Iγ/ST12 and ST231. From GenBank, 28 plasmids belonged to IncI1-Iγ/ST12 or ST12 single-locus variants (SLVs), and 5 plasmids belonged to IncI1-Iγ/ST231 or ST231 SLVs. Plasmids from GenBank originated from different S. enterica serovars and E. coli. Information regarding the source, isolation year, and in silico characterization of all plasmids (19, 25) and strains (26, 27) is shown in Table S1. The genome sequences of transformed E. coli DH10B strains harboring plasmids from Salmonella and E. coli from Colombia, which were used for reference, were submitted to the European Nucleotide Archive (ENA) under project numbers PRJEB23610 and PRJEB29690, respectively.
TABLE 1.
Inventory of sequenced IncI1-Iγ plasmids from Colombian poultry and previous reports used for detailed phylogenetic comparisons and analysis of the genetic environment
| ESBL/pAmpC |
n and pMLST of sequenced IncI1-Iγ plasmids from: |
|
|---|---|---|
| Colombian poultry | GenBank or previously publishedd | |
| blaCMY-2 | 32 ST12, 2 SLV ST12,c 1 ST231 | 29 ST12, 8 ST2,e 5 SLV ST12,c 3 ST23,e 1 ST20,e 1 ST265e |
| blaSHV-12 | 4 ST231, 1 ST12, 1 ST26, 1 SLV ST26,c 1 ST230f | 1 ST95,e 1 ST178,g 1 ST231 |
| Other bla genesa | 5 ST12, 1 ST107,f 1 ST131,f 1 ST270f | |
| No bla genesb | 1 ST12, 1 SLV ST12,c 1 ST230f | |
| Total | 43 | 61 |
bla genes other than blaCMY-2 and blaSHV-12.
No bla genes detected with ResFinder.
SLV due to incomplete match or missing 1 allele from the pMLST scheme.
Sequences of plasmids listed in this column were obtained based on the allele sequences of the highly prevalent IncI1-Iγ/ST12 and ST231 from GenBank and publications from Europe.
Selected for analysis of the genetic environment of blaCMY-2/blaSHV-12.
SLV of ST231.
SLV of ST12.
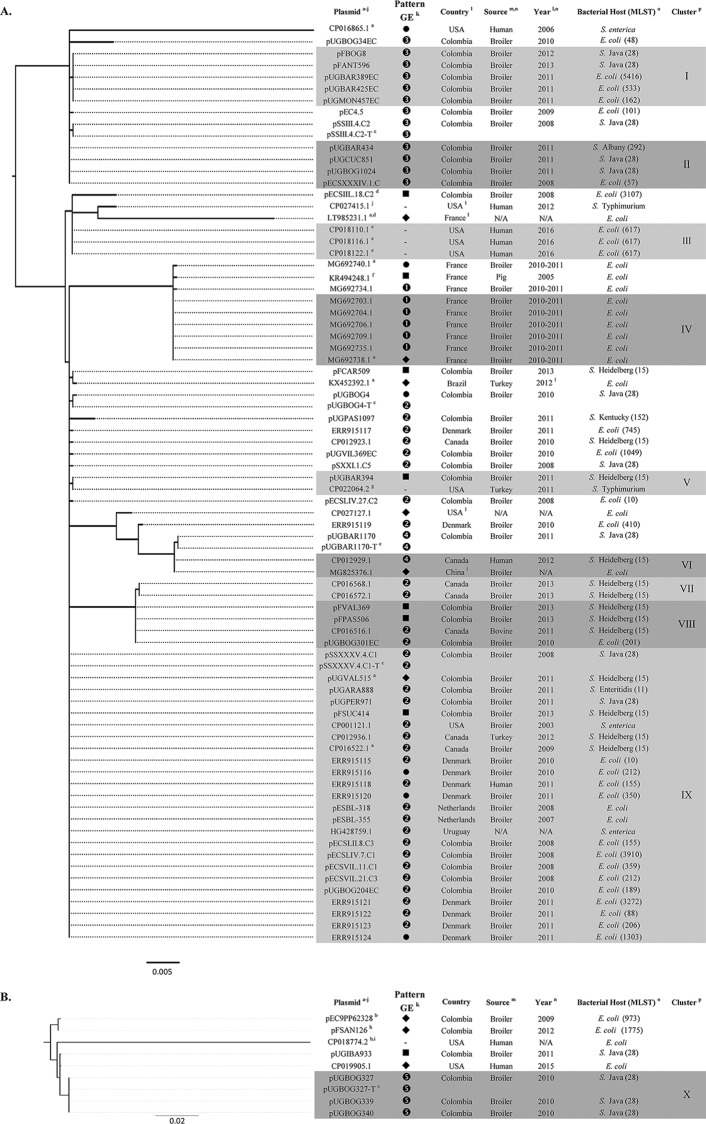
Phylogenomic reconstruction was based on core plasmid genome alignments using Parsnp v1.2 (28). Phylogenetic maximum-likelihood (midpoint-rooted) trees were constructed using FastTree2 v2.1.8 (29). Gene presence/absence maximum-likelihood trees were built by annotating the plasmid genomes using Prokka v.1.13 (30) followed by orthology predictions using Roary (31). The resulting gene presence/absence data were encoded as binary values, and trees were constructed using RAxML v.8.2.4 (32) with the BINCAT model. Genome annotations were used to describe the genetic environment of bla genes. Visualization of the trees was made with FigTree (http://tree.bio.ed.ac.uk/software/figtree/). The core genome of the resulting tree based on IncI1-Iγ/ST12-related plasmids was 40,056 bp (∼40% of the plasmid genome) (see Fig. S1 in the supplemental material). The sublineage of IncI1-Iγ/ST12 and ST12 SLVs is shown in Fig. 1A. Most IncI1-Iγ/ST12 plasmids carried blaCMY-2 and originated from samples from poultry (Fig. 1A). Although frequently reported (12–14, 24, 33, 34), detailed genomic relatedness of blaCMY-2-carrying IncI1-Iγ/ST12 plasmids originating from multiple countries and sources has not been assessed. In this study, several plasmids with an identical core genome were identified (clusters I to IX, Fig. 1A). Cluster IX included plasmids from European and American countries, which showed high similarity between Salmonella- and E. coli-derived plasmids. The gene presence/absence phylogeny grouped most of the plasmids from ST12 and SLVs in a sublineage within the tree (MG825376.1 to ERR915116) (see Fig. S2 in the supplemental material). blaCMY-2-carrying IncI1-Iγ/ST12 plasmids from nonpoultry sources, such as other livestock species and humans, were also found (Fig. 1A). These findings underscore the potential of IncI1-Iγ plasmids to be transferred in strains from Salmonella and E. coli outside the poultry environment (13, 35–38). The genetic environment of blaCMY-2 in most IncI1-Iγ/ST12 plasmids and ST12 SLVs was similar and characterized upstream by insertion sequence ISEcp1 and downstream by blc and sugE (Fig. 1A and Fig. S3A in the supplemental material). IS1294 (39) and IS26 were found upstream of blaCMY-2 in non-ST12 plasmids (see Fig. S4 in the supplemental material).
FIG 1.
Phylogenetic tree based on core genome of closely related blaCMY-2-carrying IncI1-Iγ/ST12 plasmids and its SLVs (A) and blaSHV-12-carrying IncI1-Iγ/ST231 and its SLVs (B). aSLVs of IncI1-Iγ/ST12. bSLVs of IncI1-Iγ/ST231. cTransformed plasmids from Colombian Salmonella included as reference. dCarrying blaSHV-12. eCarrying blaTEM1B-like. fCarrying blaCMY-2 together with blaCTX-M-1. gCarrying blaCMY-2-like. hCarrying blaCMY-2. iCarrying blaTEM1A. jCarrying no bla genes. kThe patterns of the genetic environment (GE) with their designated numbers can be found in Fig. S3 in the supplemental material. ⧫, unique pattern; ●, genetic environment of CMY-2 was characterized by blaCMY-2-blc-sugE and for SHV-12 by blaSHV-12-deoR (Fig. S3); -, carrying no blaCMY-2 or blaSHV-12 genes. lData from information accompanying the sequence submission in GenBank but not specifically found in the metadata fields. mDetails of the source listed in this column are available in Table S1 in the supplemental material. nN/A, data not available. oStrain MLST was added when information or complete sequence of strains was available. pClusters I to X of plasmids referred to in the manuscript are grouped in shaded boxes. Scale bars at the bottom of the phylogenetic trees represent nucleotide substitutions per site.
The core genome of the tree based on IncI1-Iγ/ST231-related plasmids was 32,789 bp (∼32% of the plasmid genome) (see Fig. S5 in the supplemental material). The sublineage of IncI1-Iγ/ST231 and related ST231 SLVs is shown in Fig. 1B. The phylogeny based on gene presence/absence of ST231-related plasmids confirmed phylogenetic distance between the plasmids from Colombian Salmonella and E. coli. Thus, no evidence of the exchange of blaSHV-12-carrying plasmids between these bacterial species was observed (see Fig. S6 in the supplemental material). In contrast, the plasmids from Colombian Salmonella and one from E. coli from a human in the United States were found to be closely related, at both the core genome and gene content levels. In this case, these plasmids may be derived from a common ancestor. Despite differences in core genome and gene presence/absence, the genetic environment of blaSHV-12 in all IncI1-Iγ/ST231 and SLVs was characterized upstream by IS26 and downstream by deoR (see Fig. S7 in the supplemental material). This pattern of genetic environment was found repeatedly (Fig. 1B and Fig. S3B). However, the results of ST231-related plasmids have to be interpreted with care, given the limited number of plasmids available for phylogenetic analysis.
In conclusion, WGS-based analysis supports the hypothesis that blaCMY-2-carrying IncI1-Iγ/ST12 plasmids in Salmonella and E. coli likely originated from a common ancestor. As previously suggested, the source of the contamination with these plasmids may be related to similar practices in poultry trade and farming (40, 41). blaSHV-12 in association with IS26 was likely introduced independently in different lineages within IncI1-Iγ/ST231. More observations are needed to better understand the transmission of blaSHV-12 in ST231 plasmids.
Supplementary Material
ACKNOWLEDGMENTS
We thank Birgitta Duim, Arjen Timmerman, Mirlin Spaninks, and Alice Wegener from Utrecht University for assistance in obtaining the WGS of strains.
WHO-AGISAR is acknowledged for facilitating the exchange of researchers and knowledge between research groups.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02546-18.
REFERENCES
- 1.Jacoby GA. 2009. AmpC β-lactamases. Clin Microbiol Rev 22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ewers C, Bethe A, Semmler T, Guenther S, Wieler LH. 2012. Extended-spectrum β-lactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: a global perspective. Clin Microbiol Infect 18:646–655. doi: 10.1111/j.1469-0691.2012.03850.x. [DOI] [PubMed] [Google Scholar]
- 3.Dorado-García A, Smid JH, van Pelt W, Bonten MJM, Fluit AC, van den Bunt G, Wagenaar JA, Hordijk J, Dierikx CM, Veldman KT, de Koeijer A, Dohmen W, Schmitt H, Liakopoulos A, Pacholewicz E, Lam T, Velthuis AG, Heuvelink A, Gonggrijp MA, van Duijkeren E, van Hoek A, de Roda Husman AM, Blaak H, Havelaar AH, Mevius DJ, Heederik D. 2018. Molecular relatedness of ESBL/AmpC-producing Escherichia coli from humans, animals, food and the environment: a pooled analysis. J Antimicrob Chemother 73:339–347. doi: 10.1093/jac/dkx397. [DOI] [PubMed] [Google Scholar]
- 4.de Been M, Lanza VF, de Toro M, Scharringa J, Dohmen W, Du Y, Hu J, Lei Y, Li N, Tooming-Klunderud A, Heederik DJJ, Fluit AC, Bonten MJM, Willems RJL, de la Cruz F, van Schaik W. 2014. Dissemination of cephalosporin resistance genes between Escherichia coli strains from farm animals and humans by specific plasmid lineages. PLoS Genet 10:e1004776. doi: 10.1371/journal.pgen.1004776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antunes P, Mourão J, Campos J, Peixe L. 2016. Salmonellosis: the role of poultry meat. Clin Microbiol Infect 22:110–121. doi: 10.1016/j.cmi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Vincent C, Boerlin P, Daignault D, Dozois CM, Dutil L, Galanakis C, Reid-Smith RJ, Tellier PP, Tellis PA, Ziebell K, Manges AR. 2010. Food reservoir for Escherichia coli causing urinary tract infections. Emerg Infect Dis 16:88–95. doi: 10.3201/eid1601.091118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasman H, Mevius D, Veldman K, Olesen I, Aarestrup FM. 2005. β-Lactamases among extended-spectrum β-lactamase (ESBL)-resistant Salmonella from poultry, poultry products and human patients in The Netherlands. J Antimicrob Chemother 56:115–121. doi: 10.1093/jac/dki190. [DOI] [PubMed] [Google Scholar]
- 8.Dierikx C, van der Goot J, Fabri T, van Essen-Zandbergen A, Smith H, Mevius D. 2013. Extended-spectrum-β-lactamase- and AmpC-β-lactamase-producing Escherichia coli in Dutch broilers and broiler farmers. J Antimicrob Chemother 68:60–67. doi: 10.1093/jac/dks349. [DOI] [PubMed] [Google Scholar]
- 9.EFSA Panel on Biological Hazards (BIOHAZ). 2011. Scientific opinion on the public health risks of bacterial strains producing extended-spectrum β-lactamases and/or AmpC β-lactamases in food and food-producing animals. Efsa J 9:2322. doi: 10.2903/j.efsa.2011.2322. [DOI] [Google Scholar]
- 10.Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother 53:2227–2238. doi: 10.1128/AAC.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozwandowicz M, Brouwer MSM, Fischer J, Wagenaar JA, Gonzalez-Zorn B, Guerra B, Mevius DJ, Hordijk J. 2018. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J Antimicrob Chemother 73:1121–1137. doi: 10.1093/jac/dkx488. [DOI] [PubMed] [Google Scholar]
- 12.Smith H, Bossers A, Harders F, Wu G, Woodford N, Schwarz S, Guerra B, Rodríguez I, Van Essen-Zandbergen A, Brouwer M, Mevius D. 2015. Characterization of epidemic IncI1 plasmids harboring ambler class A and C genes in Escherichia coli and Salmonella enterica from animals and humans. Antimicrob Agents Chemother 59:5357–5365. doi: 10.1128/AAC.05006-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen KH, Bortolaia V, Nielsen CA, Nielsen JB, Schonning K, Agerso Y, Guardabassi L. 2016. Host-specific patterns of genetic diversity among IncI1-Iγ and IncK plasmids encoding CMY-2 β-lactamase in Escherichia coli isolates from humans, poultry meat, poultry, and dogs in Denmark. Appl Environ Microbiol 82:4705–4714. doi: 10.1128/AEM.00495-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folster JPP, Pecic G, Singh A, Duval B, Rickert R, Ayers S, Abbott J, McGlinchey B, Bauer-Turpin J, Haro J, Hise K, Zhao S, Fedorka-Cray PJJ, Whichard J, McDermott P. 2012. Characterization of extended-spectrum cephalosporin–resistant Salmonella enterica serovar Heidelberg isolated from food animals, retail meat, and humans in the United States 2009. Foodborne Pathog Dis 9:638–645. doi: 10.1089/fpd.2012.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leverstein-van Hall MA, Dierikx CM, Cohen Stuart J, Voets GM, van den Munckhof MP, van Essen-Zandbergen A, Platteel T, Fluit AC, van de Sande-Bruinsma N, Scharinga J, Bonten MJM, Mevius DJ. 2011. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin Microbiol Infect 17:873–880. doi: 10.1111/j.1469-0691.2011.03497.x. [DOI] [PubMed] [Google Scholar]
- 16.Castellanos LR, Donado-Godoy P, León M, Clavijo V, Arevalo A, Bernal JF, Timmerman AJ, Mevius DJ, Wagenaar JA, Hordijk J. 2017. High heterogeneity of Escherichia coli sequence types harbouring ESBL/AmpC genes on IncI1 plasmids in the Colombian poultry chain. PLoS One 12:e0170777. doi: 10.1371/journal.pone.0170777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castellanos LR, van der Graaf-van Bloois L, Donado-Godoy P, León M, Clavijo V, Arévalo A, Bernal JF, Mevius DJ, Wagenaar JA, Zomer A, Hordijk J. 2018. Genomic characterization of extended-spectrum cephalosporin-resistant Salmonella enterica in the Colombian poultry chain. Front Microbiol 9:2431. doi: 10.3389/fmicb.2018.02431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Fernández A, Chiaretto G, Bertini A, Villa L, Fortini D, Ricci A, Carattoli A. 2008. Multilocus sequence typing of IncI1 plasmids carrying extended-spectrum β-lactamases in Escherichia coli and Salmonella of human and animal origin. J Antimicrob Chemother 61:1229–1233. doi: 10.1093/jac/dkn131. [DOI] [PubMed] [Google Scholar]
- 19.Carattoli A, Zankari E, García-Fernández A, Larsen MV, Lund O, Villa L, Aarestrup FM, Hasman H. 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Accogli M, Fortini D, Giufrè M, Graziani C, Dolejska M, Carattoli A, Cerquetti M, Giufre M, Graziani C, Dolejska M, Carattoli A, Cerquetti M. 2013. IncI1 plasmids associated with the spread of CMY-2, CTX-M-1 and SHV-12 in Escherichia coli of animal and human origin. Clin Microbiol Infect 19:E238–E240. doi: 10.1111/1469-0691.12128. [DOI] [PubMed] [Google Scholar]
- 21.Liakopoulos A, Mevius D, Ceccarelli D. 2016. A review of SHV extended-spectrum β-lactamases: neglected yet ubiquitous. Front Microbiol 7:1374. doi: 10.3389/fmicb.2016.01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones-Dias D, Manageiro V, Martins AP, Ferreira E, Caniça M. 2016. New class 2 integron In2-4 among IncI1-positive Escherichia coli isolates carrying ESBL and PMAβ genes from food animals in Portugal. Foodborne Pathog Dis 13:36–39. doi: 10.1089/fpd.2015.1972. [DOI] [PubMed] [Google Scholar]
- 23.Jones-Dias D, Manageiro V, Caniça M. 2016. Influence of agricultural practice on mobile bla genes: IncI1-bearing CTX-M, SHV, CMY and TEM in Escherichia coli from intensive farming soils. Environ Microbiol 18:260–272. doi: 10.1111/1462-2920.13021. [DOI] [PubMed] [Google Scholar]
- 24.Carattoli A, Villa L, Fortini D, García-Fernández A. 2018. Contemporary IncI1 plasmids involved in the transmission and spread of antimicrobial resistance in Enterobacteriaceae. Plasmid 10.1016/j.plasmid.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida CE, Kruczkiewicz P, Laing CR, Lingohr EJ, Gannon VPJ, Nash JHE, Taboada EN. 2016. The Salmonella in silico typing resource (SISTR): an open web-accessible tool for rapidly typing and subtyping draft Salmonella genome assemblies. PLoS One 11:e0147101. doi: 10.1371/journal.pone.0147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Achtman M, Wain J, Weill FX, Nair S, Zhou Z, Sangal V, Krauland MG, Hale JL, Harbottle H, Uesbeck A, Dougan G, Harrison LH, Brisse S. 2012. Multilocus sequence typing as a replacement for serotyping in Salmonella enterica. PLoS Pathog 8:e1002776. doi: 10.1371/journal.ppat.1002776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treangen TJ, Ondov BD, Koren S, Phillippy AM. 2014. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol 15:524. doi: 10.1186/s13059-014-0524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price MN, Dehal PS, Arkin AP. 2010. FastTree 2 – approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 31.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MTG, Fookes M, Falush D, Keane JA, Parkhill J. 2015. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edirmanasinghe R, Finley R, Parmley EJ, Avery BP, Carson C, Bekal S, Golding G, Mulvey MR. 2017. A whole-genome sequencing approach to study cefoxitin-resistant Salmonella enterica serovar Heidelberg isolates from various sources. Antimicrob Agents Chemother 61:e01919-16. doi: 10.1128/AAC.01919-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baron S, Le Devendec L, Touzain F, Jouy E, Lucas P, de Boisséson C, Larvor E, Kempf I. 2018. Longitudinal study of Escherichia coli plasmid resistance to extended-spectrum cephalosporins in free-range broilers. Vet Microbiol 216:20–24. doi: 10.1016/j.vetmic.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Roer L, Overballe-Petersen S, Hansen F, Johannesen TB, Stegger M, Bortolaia V, Leekitcharoenphon P, Korsgaard HB, Seyfarth AM, Mossong J, Wattiau P, Boland C, Hansen DS, Hasman H, Hammerum AM, Hendriksen RS. 2019. ST131 fimH22 Escherichia coli isolate with a blaCMY-2/IncI1/ST12 plasmid obtained from a patient with bloodstream infection: highly similar to E. coli isolates of broiler origin. J Antimicrob Chemother 74:557–560. doi: 10.1093/jac/dky484. [DOI] [PubMed] [Google Scholar]
- 36.Sidjabat HE, Seah KY, Coleman L, Sartor A, Derrington P, Heney C, Faoagali J, Nimmo GR, Paterson DL. 2014. Expansive spread of IncI1 plasmids carrying blaCMY-2 amongst Escherichia coli. Int J Antimicrob Agents 44:203–208. doi: 10.1016/j.ijantimicag.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 37.Tagg KA, Ginn AN, Jiang X, Ellem J, Partridge SR, Iredell JR. 2015. Distribution of acquired AmpC β-lactamase genes in Sydney, Australia. Diagn Microbiol Infect Dis 83:56–58. doi: 10.1016/j.diagmicrobio.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Harris PNA, Ben Zakour NL, Roberts LW, Wailan AM, Zowawi HM, Tambyah PA, Lye DC, Jureen R, Lee TH, Yin M, Izharuddin E, Looke D, Runnegar N, Rogers B, Bhally H, Crowe A, Schembri MA, Beatson SA, Paterson DL, Harris BT, Lorenc P, McNamara J, Underwood N, Eisenmann J, Stewart J, Henderson A, Ali J, Chiang D, Hwa SS, Kang Y, Pei OS, Ying D, Holland U, Korman T. 2018. Whole genome analysis of cephalosporin-resistant Escherichia coli from bloodstream infections in Australia, New Zealand and Singapore: high prevalence of CMY-2 producers and ST131 carrying blaCTX-M-15 and blaCTX-M-27. J Antimicrob Chemother 73:634–642. doi: 10.1093/jac/dkx466. [DOI] [PubMed] [Google Scholar]
- 39.Tagg KA, Iredell JR, Partridge SR. 2014. Complete sequencing of IncI1 sequence type 2 plasmid pJIE512b indicates mobilization of blaCMY-2 from an IncA/C plasmid. Antimicrob Agents Chemother 58:4949–4952. doi: 10.1128/AAC.02773-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dierikx CM, Van Der Goot JA, Smith HE, Kant A, Mevius DJ. 2013. Presence of ESBL/AmpC -producing Escherichia coli in the broiler production pyramid: a descriptive study. PLoS One 8:e79005. doi: 10.1371/journal.pone.0079005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nilsson O, Börjesson S, Landén A, Bengtsson B. 2014. Vertical transmission of Escherichia coli carrying plasmid-mediated AmpC (pAmpC) through the broiler production pyramid. J Antimicrob Chemother 69:1497–1500. doi: 10.1093/jac/dku030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.